Abstract
The European seabass is one of the most important species of the Mediterranean, specifically Greece. Individuals with different numbers of vertebrae have been reported. This number ranges from 24 to 26 vertebrae. In this study a sample of 73 individual seabass were collected from fish farms and divided into three age groups. The first group included fingerling individuals, the second group, juvenile individuals and the third group, adult individuals. The number and the length of their vertebrae were measured by radiographs. The individuals were divided into subgroups according to their vertebrae number, and from each one the tenth vertebra was taken. Ca and P levels (%) of each tenth vertebra were measured by X-ray spectroscopy (EDS), and the Ca/P ratio was determined. Vertebrae length, Ca and P levels and Ca/P ratio were compared among age groups and among individuals with different numbers of vertebrae. It was shown that the European seabass’s vertebral column can be divided to three sections—cervical, abdominal and caudal—following the striped bass (Morone saxatilis) model.
1. Introduction
Since the late 1980s the European seabass has become increasingly important for Europe, and specifically for the Mediterranean region, with a steady increase in demand. In 2017, Europe produced 84,319 tons (t) of European seabass. Greece produced 48,000 t, which constituted more than half of the total European production and had a value of €251.5 million, with an average price of € 5.24 per kg [1]. Of the 48,000 t, 39,000 t were exported. In 2018 the production of European seabass in Greece was 41,500 t [1]. The European seabass is an actinopterygian, and more specifically, a neoteleost fish. Its skeletal system is divided into three parts: the axial skeleton or vertebral column, the cranial skeleton and the zonal skeleton [2,3]. It includes bones, cartilage, scales, teeth, connective tissue, fin rays, tendons and all the associated stem cells [2]. The axial skeleton is made up of bones and cartilage, acts as a support for body muscles, protects organs and plays an important role in the metabolism of calcium as a source of stored ions [4,5,6]. The axial skeleton is structurally varied from a cartilage that encloses the notochord to a fully ossified vertebral column in the teleosts. The vertebral column is formed by vertebrae, with each one consisting of the vertebral body, the neural arch and the hemal arch. The front two vertebrae, which are the atlas and the axis, are differentiated for head adhesion. At the other end of the vertebral column, the last vertebra, the undertail, has specially flattened bows for symmetrical adhesion to the tail fin rays [3,7]. The vertebral column of fish has traditionally been divided into just two distinct regions, the abdominal and caudal [8,9]. The abdominal vertebrae are located anterior to the anus and they generally bear ribs, while the caudal vertebrae are defined as the hemal spine-bearing vertebrae located posterior to the anus. According to Nowroozi et al. [7], in striped bass (Morone saxatilis) the vertebral column can be divided into three regions: the cervical (vertebrae 1–4), the abdominal (vertebrae 5–12) and the caudal (vertebrae 13–25). The vertebrae number in the European seabass ranges from 24 to 26, and the medial vertebral spaces range from 23 to 25 [10]. These meristic characteristics, like skeletal deformities, are known to be affected by genetic, environmental and nutritional factors [11]. European seabass larvae tend to develop 24 vertebrae when they are intensively reared; when they are reared in mesocosm systems they tend to develop 26 vertebrae [11].
Fish bones are cellular or acellular. They consist of cells (osteoblasts, osteocytes and bone lining cells), hydroxyapatite salts (almost 65% of the dry mass of bone) and a collagen fiber matrix [12,13]. Although the inorganic material of fish bone (calcium phosphate hydroxyapatite) is the same as a mammal’s bone, the organization is quite different in that it is often acellular [14]. It is generally accepted that bone strength can be estimated by the measurement of bone material content. From the existing literature, there is no evidence of differences in Ca and P levels relevant to differences in the number or size of bones. The determination of the vertebral Ca and P levels may provide a sensitive measure of bone mineral changes that complements existing studies [15,16] and may help to understand the changes occurring as a result of bone malformation or disease.
The purpose of the present study was to measure and compare vertebral morphological characteristics, the levels of Ca and P, and the Ca/P ratio in European seabass vertebrae between individuals with different age classes and different vertebrae numbers.
2. Results
Using X-ray images (Figure 1) the number of vertebrae from each fish was counted (Table 1). All three age groups (fingerlings, juveniles and adults) had individuals with different numbers of vertebrae. Fingerlings (45 individuals) had 17 individuals with 24 vertebrae (37.8%), 22 with 25 vertebrae (48.9%) and 6 with 26 vertebrae (13.3%). Juveniles (18 individuals) had 4 individuals with 24 vertebrae (22.2%), 8 with 25 vertebrae (44.5%) and 6 with 26 vertebrae (33.3%). Adults (10 individuals) had 3 individuals with 24 vertebrae (30%), 5 with 25 vertebrae (50%) and 2 with 26 vertebrae (20%).
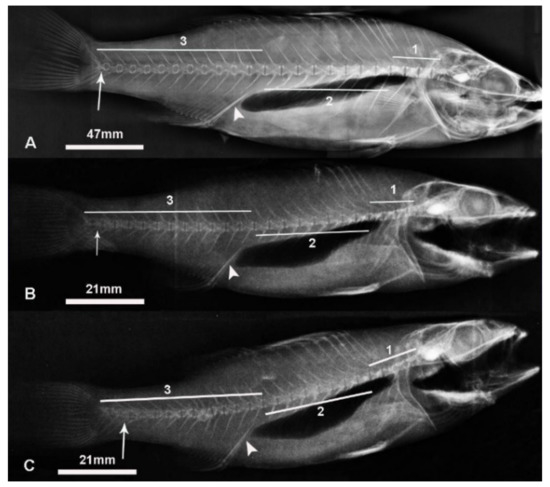
Figure 1.
X-ray of (A) adult European seabass with 24 vertebrae, (B) juvenile European seabass with 25 vertebrae and (C) juvenile European seabass with 26 vertebrae. Note: 1 is cervical region, 2 is abdominal region and 3 is caudal region. The arrowhead shows the interhaemal bone and the arrow shows the 24th vertebra. Individuals with different vertebrae numbers (24, 25 and 26 vertebrae) but within the same age group had similar mean total lengths (p > 0.05).

Table 1.
Mean total length (mm) of European seabass individuals grouped by age and total number of vertebrae.
We assumed that the vertebral column of the European seabass could be divided into three sections in accordance with the vertebral column of the linear European seabass [7]. The vertebral column of each fish was divided in three sections: cervical (vertebrae 1–4), abdominal (vertebrae 5–12) and caudal (vertebrae 13–last one) (Figure 1). In order to confirm this assumption, the mean length of each vertebral column section was measured using X-ray images (Table 2, Figure 2). A comparison of the mean vertebrae lengths from cervical to abdominal and caudal to abdominal from individuals within the same age group but within different vertebrae number groups revealed significant differences (p < 0.05) (Table 2). The mean vertebrae length of the vertebral column regions within the same age group but within different vertebrae number groups were similar (p > 0.05), with the only exception being the cervical region of the 24 vertebrae group of the fingerlings (Table 2). Finally, the length of the 24th vertebra, the last common one of the individuals with 24, 25 and 26 vertebrae, was measured (Table 3). No statistical differences were found among individuals of the same age (Table 3).

Table 2.
Mean length of vertebrae (mm) of European seabass individuals for each vertebral column region, grouped by age and total number of vertebrae.
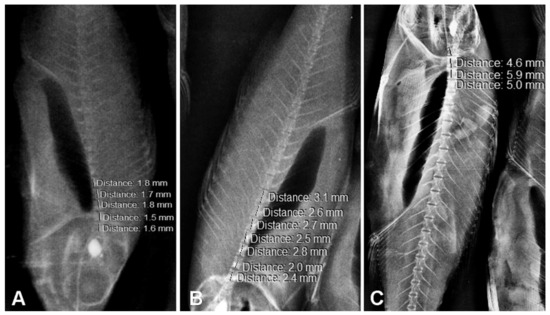
Figure 2.
X-rays and vertebrae length measurements of (A) fingerling European seabass, (B) juvenile European seabass and (C) adult European seabass. Note: The mean lengths of the 24th vertebra (for individuals with 24, 25 and 26 vertebrae) for individuals within the same age group were similar (p > 0.05).

Table 3.
Mean length of the 24th vertebra (mm) of European seabass individuals grouped by age and total number of vertebrae.
The EDS atomic percentage (At %) Ca and P levels and Ca/P ratios according to different age group and different total vertebrae number are shown in Table 4. Adults showed higher levels of intervertebral Ca and P than fingerlings and juveniles (p < 0.05). Fingerling and juvenile individuals with different total numbers of vertebrae (24, 25 or 26) had similar intervertebral Ca levels (p > 0.05). Juvenile individuals with different total numbers of vertebrae (24, 25 or 26) had similar intervertebral P levels (p > 0.05). Fingerlings with 26 vertebrae had almost twice the intervertebral P than fingerlings with 24 or 25 vertebrae. Adults with 25 vertebrae had significantly lower (p < 0.05) intervertebral P than adults with 24 or 26 vertebrae. Fingerling, juvenile and adult individuals with 24 vertebrae had similar Ca/P ratios (p > 0.05). Adults with 25 vertebrae had significantly higher Ca/P ratios (p < 0.05) than fingerlings and juveniles with 25 vertebrae. Fingerlings with 26 vertebrae had the lowest Ca/P ratio (0.73).

Table 4.
The Ca (% At) and P (% At) levels and Ca/P ratio of the 10th vertebra of the European seabass grouped by age and number of vertebrae.
3. Discussion
The vertebrae number in the European seabass ranges from 24 to 26, with most having 25 vertebrae [11,17,18]. According to Zuiten et al. [11], in intensively reared European seabass, 24, 25 and 26 vertebrae develop in 26.4%, 69.8% and 3.8% of the total reared population, respectively. Striped bass (Morone saxatilis) was found to have 24 vertebrae in most cases, but some individuals developed 25 vertebrae, with the extra vertebra added to the caudal area [7,19]. Our results were similar, as we identified 24 to 26 vertebrae in all three European seabass age groups (fingerlings, juveniles and adults), with most having 25 vertebrae.
The number of vertebrae in European seabass is not related to the presence of skeletal malformations; however, nutrition factors contribute to both vertebrae number and skeletal malformation [10,20]. In Dicentrarchus labrax and Sparus aurata, vitamin C deficiency is associated with skeletal anomalies [21]. In particular, vitamin C deficiency can lead to a decrease in the bone collagen content and therefore to the formation of spinal kyphosis, scoliosis and lordosis [22]. According to Darias et al. [10] European seabass fed with low vitamin C levels (<15 mg per kg of diet) may develop 24 or 25 vertebrae, whereas European seabass fed the highest vitamin C levels (<400 mg per kg of diet) may develop 24, 25 or 26 vertebrae. Our results confirm those of Darias et al. [10], as we detected 24 to 26 vertebrae in fingerlings, juveniles and adult European seabass fed 400–500 mg of vitamin C per kg of diet. The number of vertebrae seems also to be unrelated to total body length. Our results showed that individuals with different vertebrae numbers (24, 25 and 26 vertebrae) but of the same age group had similar body length. Darias et al. [10] also reported that the changes observed in European seabass body shape were not correlated with the variation of the number of vertebrae.
Our vertebral column morphology measurements detected three distinct vertebral column sections (cervical, abdominal and caudal), the same ones that Nowroozi et al. [7] detected for the striped bass (Morone saxatilis). Seabass and striped bass belong to the same family (Moronidae) and both species show a variability in their vertebrae number [7,11,17,18]. According to our results, cervical to abdominal and caudal to abdominal mean vertebrae length comparison from individual European seabass of the same age group (fingerlings, juveniles or adults) but of different vertebrae number groups (24, 25 or 26 vertebrae) revealed significant differences, suggesting that these three vertebral sections can be clearly distinguished. Same-age individuals with different vertebrae numbers had similar mean vertebrae lengths of the vertebral column regions (cervical, abdominal and caudal). This suggests that the division of the vertebral column into these three sections was not affected by the development of one more or one less vertebra. Darias et al. [10] concluded that European seabass larvae presenting one less vertebra generally lacked that vertebra from the cephalic region, whereas European seabass larvae with one extra vertebra added that vertebra to the caudal region. Our results support that both the addition and absence of a vertebra takes place in the caudal region. Between the head and the interhaemal bone there are 12 vertebrae. This number does not seem to be affected by the total vertebrae number of the vertebral column (24, 25 or 26 vertebrae). As the end of the abdominal section is the twelfth vertebrae, the one just before the interhaemal bone, we assume that the loss of one vertebra or the development of an extra one takes place in the caudal region. This conclusion can also be supported by the fact that in same age group European seabass with 24, 25 or 26 total vertebrae had similar lengths of the 24th vertebra. The 24th vertebra is the last common vertebra among individuals with different total vertebrae numbers.
In most vertebrates the skeleton represents a reservoir of Ca and P. Calcium and phosphorus are related to the development of the skeletal system, and the stability of the vertebrae is maintained by a solid phase of calcium phosphate [23]. A fish skeleton is made up of complex, metabolically active tissue (composed of bones and cartilage) that undergoes continuous remodeling throughout a fish’s life [24]. Fish can absorb calcium directly from water, so calcium deficiency is quite rare in fish. Phosphorus concentration in seawater is low, and thus diet is the main source of phosphorus [15,24]. Bone development and growth are highly dependent on phosphorus concentration in water as well as the availability of dietary phosphorus [24]. Phosphorus deficiency signs include the slow growth of the fish, slow bone calcification and the appearance of skeletal malformations [15]. According to Food and Agriculture Organization (FAO) [25] there is a definite lack of data on the requirements for minerals and trace elements for seabass. It has been estimated that 0.65% of the dry diet [26] is required to be phosphorus for the normal development of European seabass in all life stages and size classes. In our study the phosphorus level in the fed diet was twice of that required according to Oliva-Teles and Pimentel-Rodrigues [26] for the fingerlings and juveniles and three times for the adults, suggesting no phosphorus deficiency.
Many techniques can be used to measure the inorganic components of the bones and the Ca/P ratio (EDS, Auger electron spectroscopy, high photon flux X-ray beam) [5,25]. It has been reported [27,28] that the Ca/P ratio may provide greater reliability for the diagnosis of bone disorders than detecting the amounts of Ca and P. Different fish species may have different Ca/P ratios. Lall [15] reported that the calcium-to-phosphorus ratio in fish bones ranges from 0.7 to 1.6. The Ca/P ratio for sea bream individuals ranges from 1.79 to 2.36 [5,6,16]. According to Song et al. [29] (2017) the Ca/P ratio for Japanese seabass with a mean weight of 12.5 g ranged from 1.71 to 1.79. Fortes-Silva et al. [30] calculated a Ca/P ratio of approximately 1.16–1.33 in European seabass operculum. In Japanese seabass (Lateolabrax japonicus), a 11.88–16.50% vertebral Ca and a 6.00–7.90% vertebral P were detected. These amounts reflect a 1.98–2.09 Ca/P ratio [29]. Elsadin et al. [31], working with white grouper (Epinephelus aeneus), referred to an even higher Ca/P ratio of 2.6 in vertebrae. Our results for Ca/P ratios vary among different age groups and different vertebrae number groups. The Ca/P level was similar for fingerlings, juveniles and adult European seabass with 25 vertebrae (2.08, 2.78 and 1.95, respectively). The Ca/P levels of fingerlings, juveniles and adults with 24 vertebrae were also similar (2.26, 1.82 and 2.28, respectively). Fingerings with 26 vertebrae had the lowest Ca/P ratio, 0.73, which was in the range of Ca/P ratios that Lall [15] reported. Juveniles and adults with 26 vertebrae had similar Ca/P levels (1.90 and 1.96, respectively). Our results suggested that age did not affect the Ca/P ratio of European seabass with a normal number of vertebrae (25) or one less (24). The absorption of Ca and P through the water and food seemed also to be sufficient. In European seabass with 26 vertebrae, the Ca/P ratio seemed to increase with age, while the P accumulation in vertebrae seemed to be greater than the Ca level at the fingerling age. Bone mineralization is associated with hardness of the bones. The higher the Ca and P levels, the harder the bone. According to Driessens and Verbeeck [32], an increase in the Ca/P ratio in bones can be observed during the first stages of life in mammals. Bone Ca accretion or accumulation is also positive during the first years of life of mammals [32]. An almost fully mineralized skeleton is evident in sea bream larvae of 16 mm body length or at about 30 days post-hatching [33]. Fish vertebrae differ from mammal vertebrae and may contain appreciably less mineral [34], reflected by lower bone densities. A fish skeleton is made up of complex, metabolically active tissue that undergoes continuous remodeling throughout a fish’s life [24]. Fish bones may be cellular or acellular. Although the material of fish bone (calcium phosphate hydroxyapatite) is the same as mammal bone, the organization is quite different in that it is often acellular. The lack of Haversian systems, trabeculae, and the remodeling that accompanies cellularity imply significant differences in the ultrastructural organization of fish bone [6,14]. Bones, as biomaterials, are adapted to different loading situations and functions. Consequently, their composition may vary. The metabolism of acellular bone is poorly defined and remains an area that requires systemic investigation to better understand the skeletal development and growth of fish [24].
4. Materials and Methods
4.1. Fish Sampling
From two local fish farms, 73 individuals of Dincentrarchus labrax were obtained. Fish at the age of fingerlings and juveniles were obtained from the first one, while from the second one adults were obtained. For the study, ethical approval was not required as no live fish were used or cultured. The fish obtained from the fish farms were dead (the personnel of the fish farms killed the fish). The fish were transported in ice to the lab and divided into three age groups. Group 1 included fingerlings (45 fish, mean weight 5.27 ± 0.06 g, mean length 94.96 ± 0.9 mm, 5 months old), group 2 included juveniles (18 fish, mean weight 25.25 ± 1.05 g, mean length 143.79 ± 1.98 mm, 10 months old) and group 3 included adults (10 fish, mean weight 291.9 ± 29.68 g, mean length 319.43 ± 5 mm, 15 months old). The number of individuals in each group was sufficient for statistical analysis as it was similar or higher than previously reported research [5,6,7,19,35]. In the fish farms, fish were fed and kept as follows. Fingerlings and juveniles were fed to satiation with a commercial diet (55% crude protein, 15% fat, 1.6% P, 500 mg/kg of vitamin C and 350 mg/kg of vitamin E). Average water temperature during the sampling period was 20.6 °C, average pH was 7.5 and the average water dissolved oxygen was 8.6 mg/L. Adults were fed to satiation with a commercial diet (44% crude protein, 16% fat, 1.1% P, 750 mg/kg of vitamin C and 400 mg/kg of vitamin E). Average water temperature during the sampling period was 22.6 °C, average pH was 7.5 and the average water dissolved oxygen was 8.6 mg/L.
4.2. X-ray
Each individual fish was examined by X-ray (50 kV). Their total length, the number of vertebrae and vertebrae length were measured through Siemens syngo fastView program. Nowroozi et al. [7] suggested that the vertebral column of the striped bass (Morone saxatilis) can be divided in three sections: the cervical (vertebrae 1–4), the abdominal (vertebrae 5–12) and the caudal (vertebrae 13–last one). Using vertebrae length measurements we checked if this pattern could be applied to European seabass.
4.3. Ca and P Level Measurement
The tenth vertebra, which was considered as a representative sample, was taken from each individual fish and incinerated in order to measure calcium and phosphorus levels (%). For each vertebra three measurements were taken. Stoichiometric analysis of the vertebrae was done by energy dispersive spectroscopy (EDS). For this purpose, a scanning electron microscope (Jeol JSM-6510 LV, Ltd., Tokyo, Japan) equipped with an X-ray analyzer (x-act Oxford, Abingdon, United Kingdom) was used.
4.4. Statistical Analysis
For the statistical analysis the OriginPro8 program was used. All values are given as the means ± standard errors. The Shapiro–Wilk test or the Kolmogorov–Smirnov test, depending on the data number, was used for normality checking. One-way ANOVA was used for statistical comparison of total fish length, vertebrae length, Ca level, P level and Ca/P ratio. All the differences presented at the 0.05 level were considered significant [36].
5. Conclusions
Our results confirmed that the vertebrae number in the European seabass (Dincentrarchus labrax) ranges from 24 to 26, with most having 25 vertebrae. Direct measurements of Ca, P and Ca/P levels in vertebrae of European seabass showed that age seems not to affect the Ca/P ratio of European seabass with a normal number of vertebrae (25) or with one less (24). In European seabass with 26 vertebrae, the Ca/P ratio seems to increase with age. It was shown for the first time in this study that the seabass vertebral column can follow the linear seabass (Morone saxatilis) model and can be divided into three sections. Three distinct parts of the European seabass vertebral column were detected: the cervical (vertebrae 1–4), the abdominal (vertebrae 5–12) and the caudal (vertebrae 13–last one). The total body length of same-age seabass seems to be independent of the vertebrae number (24, 25 or 26).
Author Contributions
P.B. and S.Z. were involved in the conception of the idea, methodology design, and data analysis and interpretation. P.B., S.Z., N.V. and V.N. participated in the design of the methodology, sampling, and the laboratory work and data analysis. P.B. prepared the manuscript for publication and revision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Federation of Greek Maricultures. Annual Report 2019. Available online: https://www.fgm.com.gr (accessed on 20 March 2020).
- Genten, F.; Terwinghe, E.; Danguy, A. Atlas of Fish Histology; Science Publishers: Enfield, NH, USA, 2009; p. 219. [Google Scholar]
- Moutou, A. Fish Physiology; University of Thessaly: Larissa, Greece, 2015; p. 26. [Google Scholar]
- Groman, D.B. Histology of the Striped Bass; American Fisheries Society: Bethesda, MD, USA, 1982; p. 116. [Google Scholar]
- Berillis, P.; Panagiotopoulos, N.; Boursiaki, V.; Karapanagiotidis, I.T.; Mente, E. Vertebrae length and ultra-structure measurements of collagen fibrils and mineral content in the vertebrae of lordotic gilthead seabreams (Sparus aurata). Micron 2015, 75, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Boursiaki, V.; Theochari, C.; Zaoutsos, S.P.; Mente, E.; Vafidis, D.; Apostologamvrou, C.; Berillis, P. Skeletal Deformity of Scoliosis in Gilthead Seabreams (Sparus aurata): Association with Changes to Calcium-Phosphor Hydroxyapatite Salts and Collagen Fibers. Water 2019, 11, 257. [Google Scholar] [CrossRef]
- Nowroozi, B.N.; Harper, C.J.; De Kegel, B.; Adriaens, D.; Brainerd, E.L. Regional variation in morphology of vertebral centra and intervertebral joints in striped bass, Morone saxatilis. J. Morphol. 2012, 273, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Bond, C.E. Biology of Fishes, 2nd ed.; Saunders College Publishing: Philadelphia, PA, USA, 1996; p. 514. [Google Scholar]
- Grande, L.; Bemis, W.E. A comprehensive phylogenetic study of amiid fishes (Amiidae) based on comparative skeletal anatomy. An empirical search for interconnected patterns of natural history. J. Vertebr. Paleontol. 1998, 18 (Suppl. S1), 1–696. [Google Scholar] [CrossRef]
- Darias, M.J.; Mazurais, D.; Koumoundouros, G.; Le Gall, M.M.; Huelvan, C.; Desbruyeres, E.; Quazuguel, P.; Cahu, C.L.; Zambonino-Infante, J.L. Imbalanced dietary ascorbic acid alters molecular pathways involved in skeletogenesis of developing European sea bass (Dicentrarchus labrax). Comp. Biochem. Phys. A 2011, 159, 46–55. [Google Scholar] [CrossRef]
- Zouiten, D.; Ben Khemis, I.; Slaheddin Masmoudi, A.; Huelvan, C.; Cahu, C. Comparison of growth, digestive system maturation and skeletal development in sea bass larvae reared in an intensive or a mesocosm system. Aquac. Res. 2011, 42, 1723–1736. [Google Scholar] [CrossRef]
- Weiss, R.E.; Watabe, N. Studies on the biology of fish bone. III. Ultrastructure of osteogenesis and resorption in osteocytic (cellular) and anosteocytic (acellular) bones. Calcif. Tissue Int. 1979, 28, 43–56. [Google Scholar] [CrossRef]
- Mahamid, J.; Sharir, A.; Addadi, L.; Weiner, S. Amorphous calcium phosphate is a major component of the forming fin bones of zebrafish: Indications for an amorphous precursor phase. Proc. Natl. Acad. Sci. USA 2008, 105, 12748–12753. [Google Scholar] [CrossRef]
- Moss, M.L. Studies of the acellular bone of teleost fish. Cells Tissues Organs 1961, 46, 343–362. [Google Scholar] [CrossRef]
- Lall, S.P. The minerals. In Fish Nutrition, 3rd ed.; Halver, J.E., Hardy, R.W., Eds.; Academic Press Inc.: San Diego, CA, USA, 2002; pp. 259–308. [Google Scholar]
- Ozawa, M.; Suzuki, S. Microstructural development of natural hydroxyapatite originated from fish-bone waste through heat treatment. J. Am. Ceram. Soc. 2002, 85, 1315–1317. [Google Scholar] [CrossRef]
- Gravier, R. Les bars (loups) du Maroc atlantique Morone labrax (Linné) et Morone punctata (Bloch). Rev. Trav. l’Inst. Pêches Marit. 1961, 25, 281–292. [Google Scholar]
- Bouain, A. Étude des caracters morphologiques et anatomiques de Dicentrarchus labrax (Linné 1758) et Dicentrarchus punctatus (Bloch 1792) des côtes tunisiennes. Bull. Soc. Sci. Nat. Tunisie Nouv. Ser. 1977, 12, 57–68. [Google Scholar]
- Nowroozi, B.N.; Brainerd, E.L. X-ray motion analysis of the vertebral column during the startle response in striped bass, Morone saxatilis. J. Exp. Biol. 2013, 216, 2833–2842. [Google Scholar] [CrossRef]
- Berillis, P. Factors that can lead to the development of skeletal deformities in fishes: A review. J. Fish. Sciences.com 2015, 9, 17–23. [Google Scholar]
- Divanach, P.; Boglione, C.; Menu, B.; Koumoundouros, G.; Kentouri, M.; Cataudella, S. Abnormalities in finfish mariculture: An overview of the problem, causes and solutions. In Handbook of Contributions and Short Communications, Presented at the International Workshop on Seabass and Seabream Culture: Problems and Prospects, Verona, Italy, 16–18 October 1996; European Aquaculture Society (EAS): Oostende, Belgium; pp. 45–66.
- Lim, C.; Lowell, R.T. Pathology of the vitamin C syndrome in channel catfish (Ictalurus punctatus). J. Nutr. 1978, 108, 1137–1146. [Google Scholar] [CrossRef]
- Tzaphlidou, M. The role of collagen in bone structure: An image processing approach. Micron 2005, 36, 593–601. [Google Scholar] [CrossRef]
- Lall, S.P.; Lewis-McCrea, L.M. Role of nutrients in skeletal metabolism and pathology in fish—An overview. Aquaculture 2007, 267, 3–19. [Google Scholar] [CrossRef]
- FAO. Aquaculture Feed and Fertilizer Resources Information System. Available online: http://www.fao.org/fishery/affris/species-profiles/european-seabass/nutritional-requirements/en/ (accessed on 21 September 2020).
- Oliva-Teles, A.; Pereira, J.P.; Gouveia, A.; Gomes, E. Utilisation of diets supplemented with microbial phytase by seabass (Dicentrarchus labrax) juveniles. Aquat. Living Resour. 1998, 11, 255–259. [Google Scholar] [CrossRef]
- Bass, J.K.; Chan, G.M. Calcium nutrition and metabolism during infancy. Nutr. J. 2006, 22, 1057–1066. [Google Scholar] [CrossRef]
- Zaichick, V.; Tzaphlidou, M. Determination of calcium, phosphorus, and the calcium/phosphorus ratio in cortical bone from the human femoral neck by neutron activation analysis. Appl. Radiat. Isotopes. 2002, 56, 781–786. [Google Scholar] [CrossRef]
- Song, J.Y.; Zhang, C.X.; Wang, L.; Song, K.; Hu, S.C.; Zhang, L. Effects of dietary calcium levels on growth and tissue mineralization in Japanese seabass, Lateolabrax japonicus. Aquac. Nutr. 2017, 23, 637–648. [Google Scholar] [CrossRef]
- Fortes-Silva, R.; Sánchez-Vázquez, F.J.; Martínez, F.J. Effects of pretreating a plant-based diet with phytase on diet selection and nutrient utilization in European sea bass. Aquaculture 2011, 319, 417–422. [Google Scholar] [CrossRef]
- Elsadin, S.; Nixon, O.; Mozes, N.; Allon, G.; Gaon, A.; Tandler, A.; Koven, W. The effect of dissolved carbon dioxide (CO2) on the bone mineral content and on the expression of bone-Gla protein (BGP, Osteocalcin) in the vertebral column of white grouper (Epinephelus aeneus). Aquaculture 2019, 511, 634196. [Google Scholar] [CrossRef]
- Driessens, F.C.; Verbeeck, R.K. Biominerals; CRC Press: Boston, MA, USA, 1990; p. 440. [Google Scholar]
- Cruz, J.S. Estudio comparado del desarrollo embrionario y larvario del bocinegro (Pagrus pagrus) y de la sama de pluma (Dentex gibbosus). Ph.D. Thesis, University of Las Palmas de Gran Canaria, Las Palmas, Gran Canaria, Spain, 2006. [Google Scholar]
- Biltz, R.M.; Pellegrino, E.D. The chemical anatomy of bone: I. A comparativestudy of bone composition in sixteen vertebrates. J. Bone Jt. Surg. 1969, 51, 456–466. [Google Scholar] [CrossRef]
- Lu, K.L.; Rahimnejad, S.; Ji, Z.L.; Zhang, C.X.; Wang, L.; Song, K. Comparative analysis of vertebral transcriptome in Japanese seabass (Lateolabrax japonicus) fed diets with varying phosphorus/calcium levels. Comp. Biochem. Phys. A 2019, 230, 49–55. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis; Prentice-Hall: Bergen, NJ, USA, 1996; p. 663. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).