Isolation, Culture, and Differentiation of Blastema Cells from the Regenerating Caudal Fin of Zebrafish
Abstract
1. Introduction
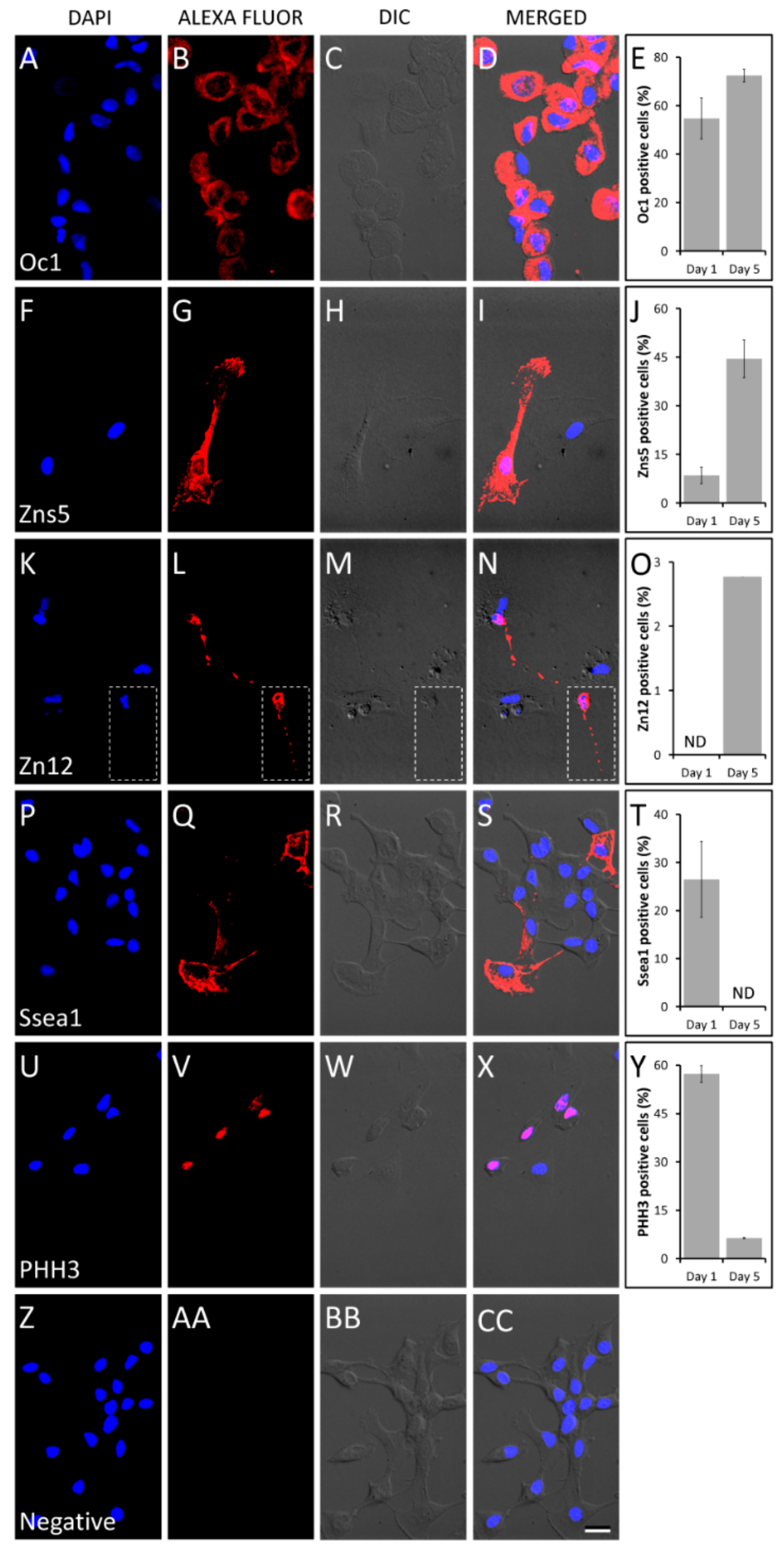
2. Results and Discussion
3. Materials and Methods
3.1. Fish Maintenance
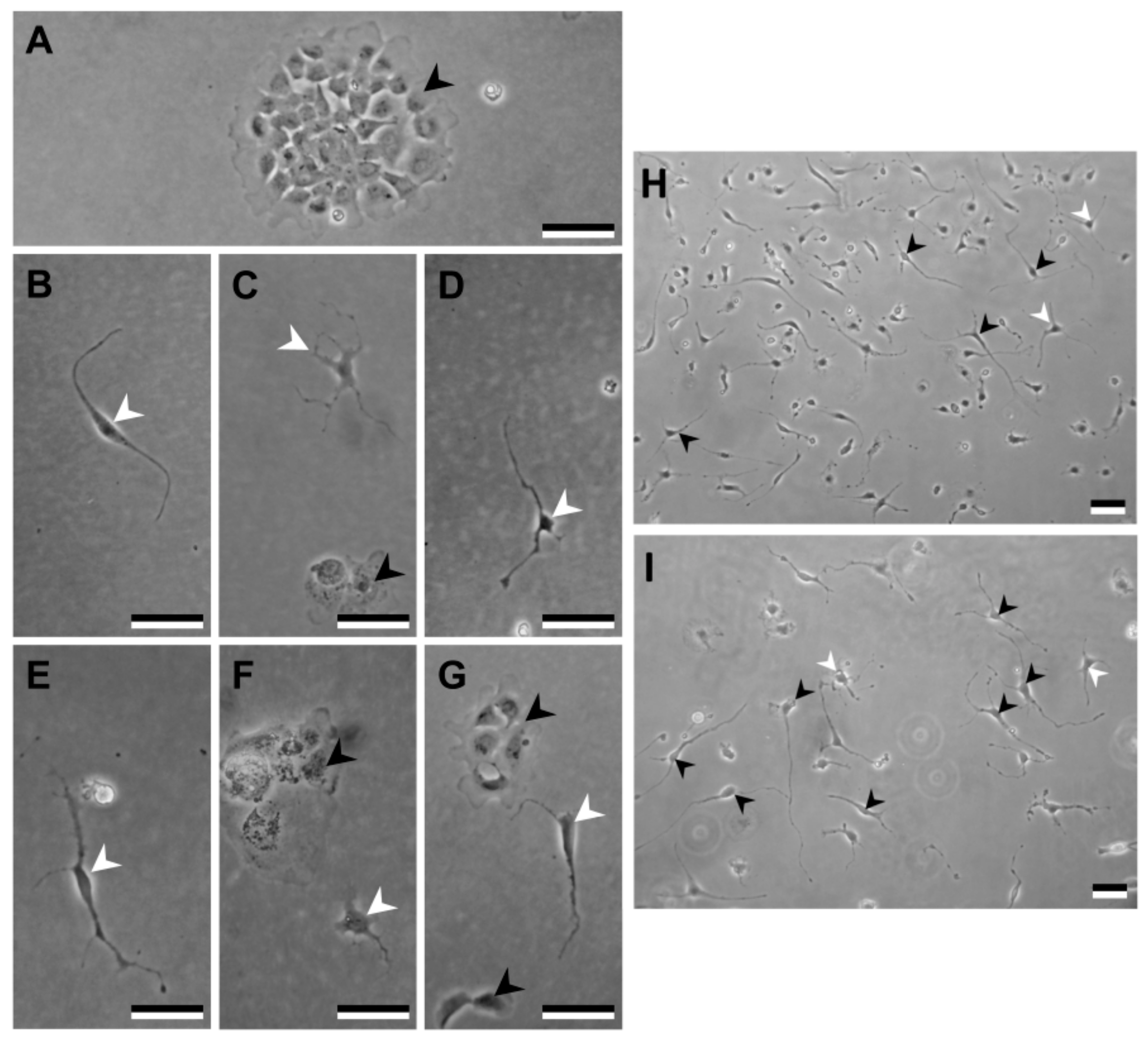
3.2. Primary Blastema Cells Isolation and Culture
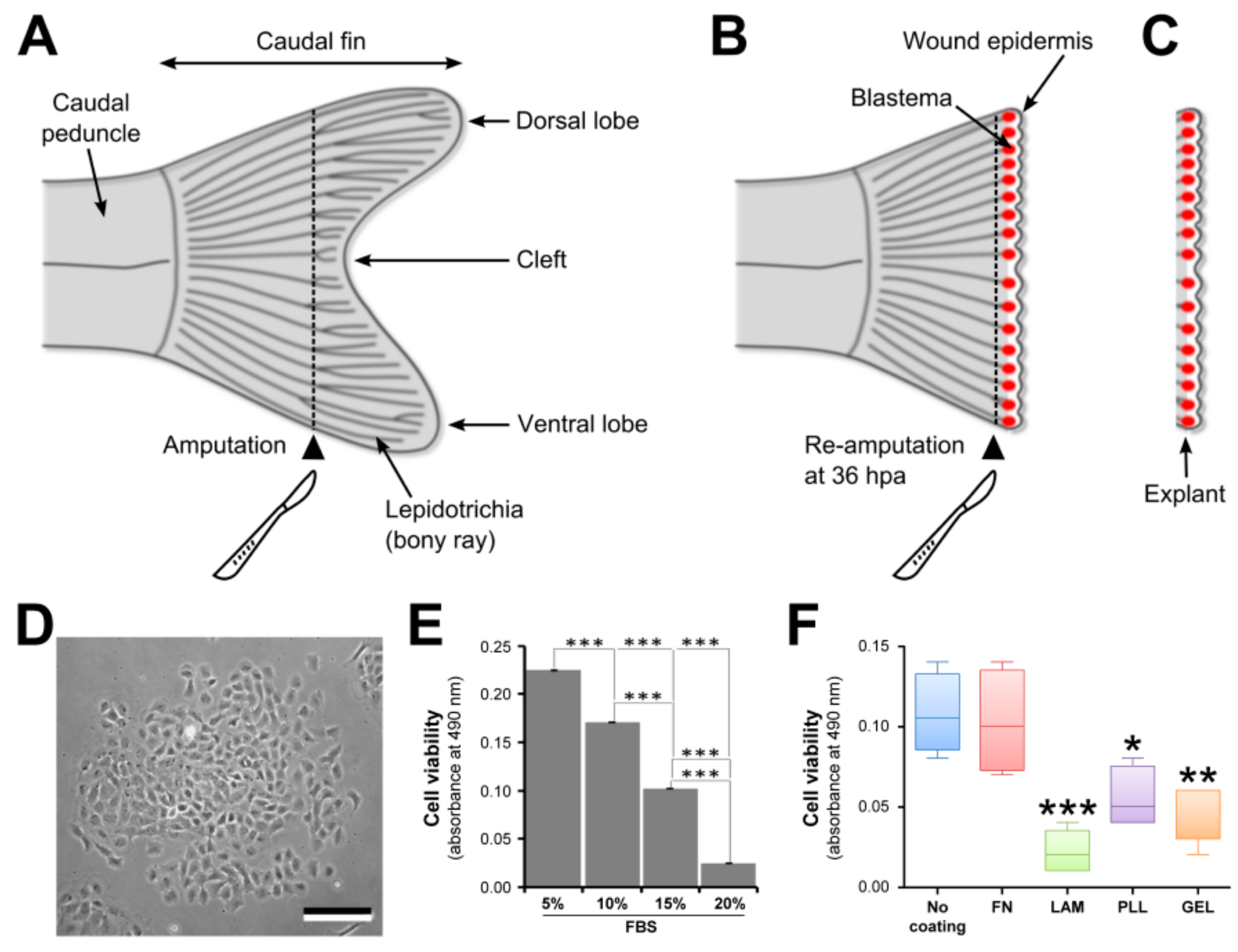
3.2.1. Amputation of the Caudal Fin
- Anesthetize the animals by exposing them to tricaine methanesulfonate (MS-222; 0.01% (w/v) in fish water; Sigma-Aldrich, St. Louis, MO, USA) for 4–6 min. Animals are anesthetized in batches of five fishes.
- Using blunt-end forceps, transfer anesthetized fish onto an inverted Petri dish (sterile, bacterial) placed on the stage of a stereomicroscope (e.g., MZ6 from Leica Microsystems, Wetzlar, Germany) and carefully deploy and flatten the caudal fin.
- Using a sterile scalpel (scalpel No. 4 and blade No. 24), amputate the caudal fin in two segments ahead of the cleft (see Figure 1A). Clean cut is achieved by positioning the blade tip on the Petri dish and by sectioning the fin in a single downward movement.
- Transfer fish with amputated fins back to their aquarium or tank.
- Repeat Steps 2–4 for the remaining animals.
- Maintain the animals in normal culture conditions until re-amputation.
3.2.2. Blastema Collection
- 7.
- At 36 h post-amputation (hpa), repeat Steps 1 and 2 for all animals.
- 8.
- Using a sterile scalpel, re-amputate the caudal fin one segment ahead of the first amputation plane (see Figure 1B). See Step 3 for making clean cuts.
- 9.
- Using sharpened forceps, collect the fin fragment containing the whole blastema (see Figure 1C) and transfer it into a Petri dish containing 3 mL of Leibovitz’s L-15 culture medium (Invitrogen, Carlsbad, CA, USA) supplemented with 5× antibiotics (100× solution containing 10,000 units of penicillin, and 10,000 µg of streptomycin per mL; Invitrogen) and 1× fungizone (100× solution containing 250 µg of amphotericin B per mL; Invitrogen) and allow it to bath for 15 min to avoid bacterial contamination.
- 10.
- Repeat Steps 8 and 9 for the remaining animals.
- 11.
- Transfer the blastemas into a sterile 1.5-mL microcentrifuge tube containing 1 mL of collagenase solution (0.125% (w/v) in PBS; Invitrogen).
- 12.
- Gently dissociate the blastemas by pipetting up and down (approximately 15 times using a 1000 µL micropipette) and place the tube in a rotary shaker for 10 min at room temperature (approximately 24–25 °C).
- 13.
- Centrifuge the tube for 3 min at 972 × g at 25 °C and gently discard the supernatant.
- 14.
- Add 0.5 mL of trypsin-EDTA solution (0.25% trypsin (Invitrogen) and 1.1 mM EDTA in phosphate-buffered saline (PBS, 1×, sterile); pH 7.4) and gently pipette up and down (using a 1000 µL micropipette) to resuspend the cells and then place the tube in a rotary shaker for 3–5 min at 25 °C.Almost all the blastemas are dissociated during collagenase and trypsin treatment.
- 15.
- Repeat Step 13 and gently resuspend the cells in 1 mL of L-15 medium.
- 16.
- Repeat Step 13 and gently resuspend the cells in 1 mL of L-15 medium supplemented with 5% fetal bovine serum (FBS; Sigma-Aldrich), 1× antibiotics, and 0.2× fungizone.
- 17.
- Count cells using a Neubauer counting chamber (BLAUBRAND) and seed 1.5 × 103 cells per well in a 96-well dish (Nunc, Roskilde, Denmark) coated with laminin, gelatin, fibronectin, or poly-L-lysine and 1 × 104 cells per well in a 24-well dish (Nunc) for cell culture.
- 18.
- Incubate enzymatically dissociated cell cultures in a 28 °C cell incubator (e.g., Galaxy 170 S from New Brunswick Scientific, Enfield, CT, USA).
3.2.3. Maintenance of Primary Cell Cultures
- 19.
- Observe daily the growth of primary cell cultures under an inverted microscope (e.g., Axiovert 25 from Zeiss, Oberkochen, Germany).
- 20.
- Renew culture medium every 3 days.
- 21.
- At confluence, cells are detached/dissociated with trypsin-EDTA and then split 1:2.
3.3. ECM Coating of Tissue Culture Dishes
3.4. Cell Viability
3.5. Extracellular Matrix Mineralization and Acridine Orange Staining
3.6. Cell Differentiation
3.7. Immunofluorescence Staining
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Compliance with Ethical Standards
References
- Akimenko, M.-A.; Mari-Beffa, M.; Becerra, J.; Géraudie, J. Old questions, new tools, and some answers to the mystery of fin regeneration. Dev. Dyn. 2003, 226, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Poss, K.D.; Keating, M.T.; Nechiporuk, A. Tales of regeneration in zebrafish. Dev. Dyn. 2003, 226, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, Y.; Kawakami, A.; Kudo, A. Cellular and molecular processes of regeneration, with special emphasis on fish fins. Dev. Growth Differ. 2007, 49, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Yoshinari, N.; Kawakami, A. Mature and juvenile tissue models of regeneration in small fish species. Biol. Bull. 2011, 221, 62–78. [Google Scholar] [CrossRef] [PubMed]
- Gemberling, M.; Bailey, T.J.; Hyde, D.R.; Poss, K.D. The zebrafish as a model for complex tissue regeneration. Trends Genet. 2013, 29, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Akimenko, M.-A.; Smith, A. Paired fin repair and regeneration. In Fins into Limbs: Evolution, Development, and Transformation; Hall, B.K., Ed.; University of Chicago Press: Chicago, IL, USA, 2007; pp. 152–162. [Google Scholar]
- Knof, F.; Hammond, C.; Chekuru, A.; Kurth, T.; Hans, S.; Weber, C.W.; Mahatama, G.; Fisher, S.; Brand, M.; Schulte-Merker, S.; et al. Bone regenerates via dedifferentiation of osteoblasts in the zebrafish fin. Dev. Cell 2011, 20, 713–724. [Google Scholar] [CrossRef]
- Sousa, S.; Afonso, N.; Bensimon-Brito, A.; Fonseca, M.; Simões, M.; Leon, J.; Roehl, H.; Cancela, M.L.; Jacinto, A. Differentiated skeletal cells contribute to blastema formation during zebrafish fin regeneration. Development 2011, 138, 3897–3905. [Google Scholar] [CrossRef]
- Tu, S.; Johnson, S.L. Fate restriction in the growing and regenerating zebrafish fin. Dev. Cell 2011, 20, 725–732. [Google Scholar] [CrossRef]
- Stoick-Cooper, C.L.; Moon, R.T.; Weidinger, G. Advances in signaling in vertebrate regeneration as a prelude to regenerative medicine. Genes Dev. 2007, 21, 1292–1315. [Google Scholar] [CrossRef]
- Wehner, D.; Weidinger, G. Signaling networks organizing regenerative growth of the zebrafish fin. Trends Genet. 2015, 31, 336–343. [Google Scholar] [CrossRef]
- Pfefferli, C.; Jaźwińska, A. The art of fin regeneration in zebrafish. Regeneration 2015, 19, 72–83. [Google Scholar] [CrossRef]
- Bergen, D.J.M.; Kague, E.; Hammond, C.L. Zebrafish as an emerging model for osteoporosis: A primary testing platform for screening new osteo-active compounds. Front. Endocrinol. 2019, 10, 6. [Google Scholar] [CrossRef]
- Whitehead, G.G.; Makino, S.; Lien, C.L.; Keating, M.T. fgf20 is essential for initiating zebrafish fin regeneration. Science 2005, 310, 1957–1960. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Hami, D.; De Val, S.; Kagermeier-Schenk, B.; Wills, A.A.; Black, B.L.; Weidinger, G.; Poss, K.D. Maintenance of blastemal proliferation by functionally diverse epidermis in regenerating zebrafish fins. Dev. Biol. 2009, 331, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Yoshinari, N.; Ishida, T.; Kudo, A.; Kawakami, A. Gene expression and functional analysis of zebrafish larval fin fold regeneration. Dev. Biol. 2009, 325, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Holdway, J.E.; Poss, K.D. Regeneration of amputated zebrafish fin rays from de novo osteoblasts. Dev. Cell 2012, 22, 879–886. [Google Scholar] [CrossRef]
- Sander, V.; Suñe, G.; Jopling, C.; Morera, C.; Izpisua Belmonte, J.C. Isolation and in vitro culture of primary cardiomyocytes from adult zebrafish hearts. Nat. Protoc. 2013, 8, 800–809. [Google Scholar] [CrossRef]
- Tapanes-Castillo, A.; Shabazz, F.S.; Mboge, M.Y.; Vajn, K.; Oudega, M.; Plunkett, J.A. Characterization of a novel primary culture system of adult zebrafish brain stem cells. J. Neurosci. Methods 2014, 223, 11–19. [Google Scholar] [CrossRef]
- Chen, Z.; Lee, H.; Henle, S.J.; Cheever, T.R.; Ekker, S.C.; Henley, J.R. Primary neuron culture for nerve growth and axon guidance studies in zebrafish (Danio rerio). PLoS ONE 2013, 8, e57539. [Google Scholar] [CrossRef]
- Yan, C.H.; Chan, K.M. Characterization of zebrafish metallothionein gene promoter in a zebrafish caudal fin cell-line, SJD. 1. Mar. Environ. Res. 2002, 54, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Kalaiselvi Sivalingam, N.N.; Seepoo, A.M.; Gani, T.; Selvam, S.; AzeezSait, S.H. Zebrafish fin-derived fibroblast cell line: A model for in vitro wound healing. J. Fish Dis. 2019, 42, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Yadav, K.; Lakra, W.S.; Sharma, J.; Goswami, M.; Singh, A. Development and characterization of a cell line TTCF from endangered mahseer Tor tor (Ham.). Fish Physiol. Biochem. 2012, 38, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Lakra, W.S.; Goswami, M. Development and characterization of a continuous cell line PSCF from Puntius sophore. J. Fish Biol. 2011, 78, 987–1001. [Google Scholar] [CrossRef]
- Kumar, R.; Ravi, C.; Das, S.; Dharmaratnam, A.; Basheer, V.S.; Swaminathan, T.R. Establishment and characterization of a caudal fin-derived cell line, AOF, from the Oscar, Astronotus ocellatus. Fish Physiol. Biochem. 2019, 45, 123–131. [Google Scholar] [CrossRef]
- Lakra, W.S.; Goswami, M.; Yadav, K.; Gopalakrishnan, A.; Patiyal, R.S.; Singh, M. Development and characterization of two cell lines PDF and PDH from Puntius denisonii (Day 1865). In Vitro Cell. Dev. Biol. Anim. 2011, 47, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Godwin, J.W. Preparation and culture of limb blastema stem cells from regenerating larval and adult salamanders. Cold Spring Harb. Protoc. 2010, 2010, pdb–prot5367. [Google Scholar] [CrossRef]
- Rosa, J.; Tiago, D.M.; Dias, J.; Cancela, M.L.; Laizé, V. Serum-specific stimulation of proliferation and mineralization of fish bone-derived cells. J. Appl. Ichthyol. 2010, 26, 251–256. [Google Scholar] [CrossRef]
- Collodi, P.; Kamei, Y.; Ernst, T.; Miranda, C.; Buhler, D.R.; Barnes, D.W. Culture of cells from zebrafish (Brachydanio rerio) embryo and adult tissues. Cell Biol. Toxicol. 1992, 8, 43–61. [Google Scholar] [CrossRef]
- Van Camp, J.K.; Beckers, S.; Zegers, D.; Van Hul, W. Wnt signaling and the control of human stem cell fate. Stem Cell Rev. 2014, 10, 207–229. [Google Scholar] [CrossRef]
- Itoh, F.; Watabe, T.; Miyazono, K. Roles of TGF-β family signals in the fate determination of pluripotent stem cells. Semin. Cell Dev. Biol. 2014, 32, 98–106. [Google Scholar] [CrossRef]
- Wang, L.; Schulz, T.C.; Sherrer, E.S.; Dauphin, D.S.; Shin, S.; Nelson, A.M.; Ware, C.B.; Zhan, M.; Song, C.Z.; Chen, X.; et al. Self-renewal of human embryonic stem cells requires insulin-like growth factor-1 receptor and ERBB2 receptor signaling. Blood 2007, 110, 4111–4119. [Google Scholar] [CrossRef] [PubMed]
- Lotz, S.; Goderie, S.; Tokas, N.; Hirsch, S.E.; Ahmad, F.; Corneo, B.; Le, S.; Banerjee, A.; Kane, R.S.; Stern, J.H.; et al. Sustained levels of FGF2 maintain undifferentiated stem cell cultures with biweekly feeding. PLoS ONE 2013, 8, e56289. [Google Scholar] [CrossRef] [PubMed]
- Pease, S.; Braghetta, P.; Gearing, D.; Grail, D.; Williams, R.L. Isolation of embryonic stem (ES) cells in media supplemented with recombinant leukemia inhibitory factor (LIF). Dev. Biol. 1990, 141, 344–352. [Google Scholar] [CrossRef]
- Shibata, E.; Yokota, Y.; Horita, N.; Kudo, A.; Abe, G.; Kawakami, K.; Kawakami, A. Fgf signalling controls diverse aspects of fin regeneration. Development 2016, 143, 2920–2929. [Google Scholar] [CrossRef] [PubMed]
- Lehrberg, J.; Gardiner, D.M. Regulation of axolotl (Ambystoma mexicanum) limb blastema cell proliferation by nerves and BMP2 in organotypic slice culture. PLoS ONE 2015, 10, e0123186. [Google Scholar] [CrossRef]
- Suemori, H.; Nakatsuji, N. Establishment of the embryo-derived stem (ES) cell lines from mouse blastocysts: Effects of the feeder cell layer. Dev. Growth Differ. 1987, 29, 133–139. [Google Scholar] [CrossRef]
- Adams, J.C.; Watt, F.M. Regulation of development and differentiation by the extracellular matrix. Development 1993, 117, 1183–1198. [Google Scholar]
- Robertson, E.J. Embryo-derived stem cell lines. In Teratocarcinomas and Embryonic Stem Cells: A Practical Approach; Robertson, E.J., Ed.; IRL Press: Oxford, UK, 1987; pp. 71–112. [Google Scholar]
- Saeinasab, M.; Matin, M.M.; Rassouli, F.B.; Bahrami, A.R. Blastema cells derived from New Zealand white rabbit’s pinna carry stemness properties as shown by differentiation into insulin producing, neural, and osteogenic lineages representing three embryonic germ layers. Cytotechnology 2016, 68, 497–507. [Google Scholar] [CrossRef]
- Andrews, P.W. Retinoic acid induces neuronal differentiation of a cloned human embryonal carcinoma cell line in vitro. Dev. Biol. 1984, 103, 285–293. [Google Scholar] [CrossRef]
- Wakamatsu, Y.; Ozato, K.; Sasado, T. Establishment of a pluripotent cell line derived from a medaka (Oryzias latipes) blastula embryo. Mol. Mar. Biol. Biotechnol. 1994, 3, 185–191. [Google Scholar]
- Parameswaran, V.; Laizé, V.; Gavaia, P.J.; Cancela, M.L. ESSA1 embryonic stem like cells from gilthead seabream: A new tool to study mesenchymal cell lineage differentiation in fish. Differentiation 2012, 84, 240–251. [Google Scholar] [CrossRef] [PubMed]
- White, J.A.; Boffa, M.B.; Jones, B.; Petkovich, M. A zebrafish retinoic acid receptor expressed in the regenerating caudal fin. Development 1994, 120, 1861–1872. [Google Scholar] [PubMed]
- Means, A.L.; Gudas, L.J. The roles of retinoids in vertebrate development. Annu. Rev. Biochem. 1995, 64, 201–233. [Google Scholar] [CrossRef] [PubMed]
- Mongan, N.P.; Gudas, L.J. Diverse actions of retinoid receptors in cancer prevention and treatment. Differentiation 2007, 75, 853–870. [Google Scholar] [CrossRef] [PubMed]
- Soprano, D.R.; Teets, B.W.; Soprano, K.J. Role of retinoic acid in the differentiation of embryonal carcinoma and embryonic stem cells. Vitam. Horm. 2007, 75, 69–95. [Google Scholar] [CrossRef] [PubMed]
- Gudas, L.J.; Wagner, J.A. Retinoids regulate stem cell differentiation. J. Cell. Physiol. 2011, 226, 322–330. [Google Scholar] [CrossRef]
- Gudas, L.J. Emerging roles for retinoids in regeneration and differentiation in normal and disease states. Biochim. Biophys. Acta 2012, 1821, 213–221. [Google Scholar] [CrossRef]
- Mathew, L.K.; Sengupta, S.; Franzosa, J.A.; Perry, J.; La Du, J.; Andreasen, E.A.; Tanguay, R.L. Comparative expression profiling reveals an essential role for Raldh2 in epimorphic regeneration. J. Biol. Chem. 2009, 284, 33642–33653. [Google Scholar] [CrossRef]
- Blum, N.; Begemann, G. Retinoic acid signaling controls the formation, proliferation and survival of the blastema during adult zebrafish fin regeneration. Development 2012, 139, 107–116. [Google Scholar] [CrossRef]
- Géraudie, J.; Monnot, M.J.; Brulfert, A.; Ferretti, P. Caudal fin regeneration in wild type and long-fin mutant zebrafish is affected by retinoic acid. Int. J. Dev. Biol. 1995, 39, 373–381. [Google Scholar]
- Cardeira, J.; Gavaia, P.J.; Fernández, I.; Cengiz, I.F.; Moreira-Silva, J.; Oliveira, J.M.; Reis, R.L.; Leonor Cancela, M.; Laizé, V. Quantitative assessment of the regenerative and mineralogenic performances of the zebrafish caudal fin. Sci. Rep. 2016, 6, 39191. [Google Scholar] [CrossRef] [PubMed]
- Tighe, A.P.; Gudas, L.J. Retinoic acid inhibits leukemia inhibitory factor signaling pathways in mouse embryonic stem cells. J. Cell. Physiol. 2004, 198, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.G.; Lee, L.E.; Fan, L.; Collodi, P.; Holt, S.E.; Bols, N.C. Initiation of a zebrafish blastula cell line on rainbow trout stromal cells and subsequent development under feeder-free conditions into a cell line, ZEB2J. Zebrafish 2008, 5, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Robles, V.; Marti, M.; Izpisua Belmonte, J.C. Study of pluripotency markers in zebrafish embryos and transient embryonic stem cell cultures. Zebrafish 2011, 8, 57–63. [Google Scholar] [CrossRef]
- Simes, D.C.; Williamson, M.K.; Schaff, B.J.; Gavaia, P.J.; Ingleton, P.M.; Price, P.A.; Cancela, M.L. Characterization of osteocalcin (BGP) and matrix gla protein (MGP) fish specific antibodies: Validation for immunodetection studies in lower vertebrates. Calcif. Tissue Int. 2004, 74, 170–180. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vijayakumar, P.; Cancela, M.L.; Laizé, V. Isolation, Culture, and Differentiation of Blastema Cells from the Regenerating Caudal Fin of Zebrafish. Fishes 2020, 5, 6. https://doi.org/10.3390/fishes5010006
Vijayakumar P, Cancela ML, Laizé V. Isolation, Culture, and Differentiation of Blastema Cells from the Regenerating Caudal Fin of Zebrafish. Fishes. 2020; 5(1):6. https://doi.org/10.3390/fishes5010006
Chicago/Turabian StyleVijayakumar, Parameswaran, M. Leonor Cancela, and Vincent Laizé. 2020. "Isolation, Culture, and Differentiation of Blastema Cells from the Regenerating Caudal Fin of Zebrafish" Fishes 5, no. 1: 6. https://doi.org/10.3390/fishes5010006
APA StyleVijayakumar, P., Cancela, M. L., & Laizé, V. (2020). Isolation, Culture, and Differentiation of Blastema Cells from the Regenerating Caudal Fin of Zebrafish. Fishes, 5(1), 6. https://doi.org/10.3390/fishes5010006





