Seafood-Borne Parasitic Diseases: A “One-Health” Approach Is Needed
Abstract
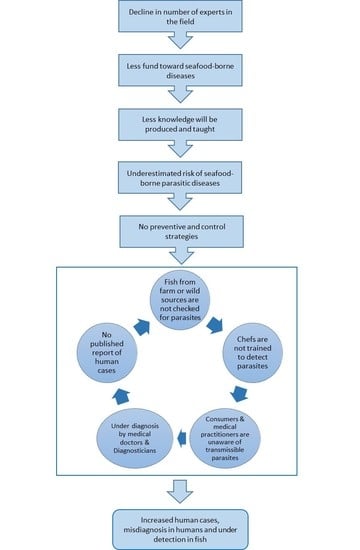
:1. Introduction
2. Seafood-Borne Parasitic Diseases
2.1. Protozoa
2.2. Cnidaria
2.3. Cestoda
2.4. Trematoda
2.5. Nematoda
2.6. Acanthocephala
3. Local-Global Interconnectedness
3.1. Role of Stakeholders and Researchers in Seafood Safety
3.2. Fish Producers and Chefs and Cooks
3.3. Consumers
3.4. Medical Doctors, Diagnosticians, and Publishing Relevant Cases
4. Seafood-Borne Parasites as Indicators of Environmental Change
A “One-Health” Approach Is Needed
5. Conclusions
Funding
Conflicts of Interest
References
- Gutierrez, N.L.; Hilborn, R.; Defeo, O. Leadership, social capital and incentives promote successful fisheries. Nature 2011, 470, 386–389. [Google Scholar] [CrossRef] [PubMed]
- Rohrmann, S.; Overvad, K.; Bueno-de-Mesquita, H.B.; Jakobsen, M.U.; Egeberg, R.; Tjønneland, A.; Nailler, L.; Boutron-Ruault, M.-C.; Clavel-Chapelon, F.; Krogh, V.; et al. Meat consumption and mortality—Results from the european prospective investigation into cancer and nutrition. BMC Med. 2013, 11, 63. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Positive Outlook for Global Seafood as Demand Surges for Multiple Species in Markets across the World. GLOBEFISH—Analysis and Information on World Fish Trade. 2018. Available online: http://www.fao.org/in-action/globefish/market-reports/resource-detail/en/c/1109513/ (accessed on 19 March 2018).
- FAO. The State of World Fisheries and Aquaculture-Opportunities and Challenges; Food and Agriculture Organisationof the United Nations: Rome, Italy, 2014. [Google Scholar]
- Deardorff, T.L.; Overstreet, R.M. Seafood-transmitted zoonoses in the united states: The fishes, the dishes, and the worms. In Microbiology of Marine Food Products; Ward, D.R., Hackney, C., Eds.; Springer: Boston, MA, USA, 1991; pp. 211–265. [Google Scholar]
- Shamsi, S. Seafood-borne parasitic diseases in Australia: How much do we know about them? Microbiol. Aust. 2016, 37, 27–29. [Google Scholar] [CrossRef]
- Chai, J.-Y.; Darwin Murrell, K.; Lymbery, A.J. Fish-borne parasitic zoonoses: Status and issues. Int. J. Parasitol. 2005, 35, 1233–1254. [Google Scholar] [CrossRef] [PubMed]
- Bethony, J.; Brooker, S.; Albonico, M.; Geiger, S.M.; Loukas, A.; Diemert, D.; Hotez, P.J. Soil-transmitted helminth infections: Ascariasis, trichuriasis, and hookworm. Lancet 2006, 367, 1521–1532. [Google Scholar] [CrossRef]
- Shamsi, S.; Shorey, H. Seafood-borne parasitic diseases in Australia: Are they rare or underdiagnosed? Intern. J. Med. 2018, 48, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Sandeman, M.; Warner, L. An Investment in Human and Animal Health: Parasitology in Australia; FASTS Occasional Paper Series, No. 4; FASTS: Deakin West, Australia, 2002. [Google Scholar]
- Gasser, R. Special issue: Learning and teaching of veterinary parasitology. Vet. Parasitol. 2018. [Google Scholar] [CrossRef]
- Yang, R.C.; Reid, A.; Lymbery, A.; Ryan, U. Identification of zoonotic Giardia genotypes in fish. Int. J. Parasitol. 2010, 40, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Reid, A.; Lymbery, A.; Ng, J.; Tweedle, S.; Ryan, U. Identification of novel and zoonotic Cryptosporidium species in marine fish. Vet. Parasitol. 2010, 168, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Heiniger, H.; Adlard, R.D. Host specificity and local infection dynamics of Kudoa leptacanthae n. Sp (Multivalvulida: Kudoidae) from the pericardial cavity of two Zoramia spp. (Perciformes: Apogonidae) at Lizard Island Lagoon, Queensland, Australia. Parasitol. Int. 2012, 61, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Heiniger, H.; Cribb, T.H.; Adlard, R.D. Intra-specific variation of Kudoa spp. (Myxosporea: Multivalvulida) from apogonid fishes (Perciformes), including the description of two new species, K. cheilodipteri n. Sp and k. cookii n. Sp., from Australian waters. Syst. Parasitol. 2013, 84, 193–215. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.L.; Adlard, R.D. Brain infecting kudoids of Australia’s coral reefs, including a description of Kudoa lemniscati n. Sp (Myxosporea: Kudoidae) from Lutjanus lemniscatus (Perciformes: Lutjanidae) off Ningaloo Reef, Western Australia. Parasitol. Int. 2012, 61, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.L.; Adlard, R.D. Unicapsula species (Myxosporea: Trilosporidae) of Australian marine fishes, including the description of Unicapsula andersenae n. Sp in five teleost families off Queensland, Australia. Parasitol. Res. 2013, 112, 2945–2957. [Google Scholar] [CrossRef] [PubMed]
- Sandars, D.F. Diphyllobothrium latum (linne) in Australia. Med. J. Aust. 1951, 2, 533–534. [Google Scholar] [PubMed]
- Munckhof, W.J.; Grayson, M.L.; Susil, B.J.; Pullar, M.J.; Turnidge, J. Cerebral sparganosis in an East Timorese refugee. Med. J. Aust. 1994, 161, 263–264. [Google Scholar] [PubMed]
- Hughes, A.J.; Biggs, B.A. Parasitic worms of the central nervous system: An australian perspective. Intern. Med. J. 2002, 32, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.V.; Thompson, R.C.A.; Jabbar, A.; Williams, J.; Rasiah, K.; Pallant, L.; Koehler, A.P.; Graham, C.; Weldhagen, G.F. Rare human infection with pacific broad tapeworm Adenocephalus pacificus, Australia. Emerg. Infect. Dis. 2016, 22, 1510–1512. [Google Scholar] [CrossRef] [PubMed]
- Mukerjee, C.M.; Simpson, S.E.; Bell, R.J.; Walker, J.C. Pleuropulmonary paragonimiasis in a Laotian immigrant to Australia. Chest 1992, 101, 849–851. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.W.; Clarke, R.J.; Denham, I.; Trembath, P.W. Pulmonary paragonimiasis in an immigrant from Laos. Med. J. Aust. 1983, 2, 668–669. [Google Scholar] [PubMed]
- Attwood, H.D.; Chou, S.T. Longevity of clonorchis sinensis. Pathology 1978, 10, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Ryan, N.; Plackett, M.; Dwyer, B. Parasitic infections of refugees. Med. J. Aust. 1988, 148, 491–494. [Google Scholar] [PubMed]
- De Silva, S.; Saykao, P.; Kelly, H.; MacIntyre, C.R.; Ryan, N.; Leydon, J.; Biggs, B.A. Chronic Strongyloides stercoralis infection in Laotian immigrants and refugees 7–20 years after resettlement in Australia. Epidemiol. Infect. 2002, 128, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Hostettler, R.; Cutmore, S.C.; Cribb, T.H. Two new species of Haplorchoides Chen, 1949 (Digenea: Heterophyidae) infecting an Australian siluriform fish, Neoarius graeffei Kner & Steindachner. Syst. Parasitol. 2018, 95, 201–211. [Google Scholar] [PubMed]
- Snyder, S.D.; Tkach, V.V. Haplorchis popelkae n. Sp (digenea: Heterophyidae) from short necked turtles (Chelidae) in northern Australia. J. Parasitol. 2009, 95, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Evans, B.B.; Lester, R.J.G. Parasites of ornamental fish imported into Australia. Bull. Eur. Assoc. Fish Pathol. 2001, 21, 51–55. [Google Scholar]
- Yagi, K.; Nagasawa, K.; Ishikura, H.; Nakagawa, A.; Sato, N.; Kikuchi, K.; Ishikura, H. Female worm Hysterothylacium aduncum excreted from human: A case report. Jpn. J. Parasitol. 1996, 45, 12–23. [Google Scholar]
- Audicana, M.T.; Ansotegui, I.J.; Fernandez de Corres, L.; Kennedy, M.W. Anisakis simplex: Dangerous—Dead and alive? Trends Parasitol. 2002, 18, 20–25. [Google Scholar] [CrossRef]
- Van Thiel, P.H.; Kuipers, F.C.; Roskam, R.T. A nematode parasitic to herring, causing acute abdominal syndromes in man. Trop. Geogr. Med. 1960, 2, 97–113. [Google Scholar]
- Roser, D.; Stensvold, C.R. Anisakiasis mistaken for dientamoebiasis? Clin. Infect. Dis. 2013, 57, 1500. [Google Scholar] [CrossRef] [PubMed]
- Sakanari, J.A. Anisakis—From the platter to the microfuge. Parasitol. Today 1990, 6, 323–327. [Google Scholar] [CrossRef]
- McCarthy, J.; Moore, T.A. Emerging helminth zoonoses. Int. J. Parasitol. 2000, 30, 1351–1360. [Google Scholar] [CrossRef]
- Cross, J.H. Intestinal capillariasis. Clin. Microbiol. Rev. 1992, 5, 120–129. [Google Scholar] [CrossRef] [PubMed]
- El-Dib, N.A.; El-Badry, A.A.; Ta-Tang, T.-H.; Rubio, J.M. Molecular detection of Capillaria philippinensis: An emerging zoonosis in Egypt. Exp. Parasitol. 2015, 154, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Hooper, J.N.A. Parasites of estuarine and oceanic flathead fishes (family Platycephalidae) from northern New South Wales. Aust. J. Zool. 1983, 90, 1–69. [Google Scholar] [CrossRef]
- Habibi, F.; Shamsi, S. Preliminary report of occurrence of Corynosoma spp. (Acanthocephala: Polymorphidae) in Southern Caspian sprat (Clupeonella grimmi). Parasitol. Res. 2018, 117, 3327–3331. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Waga, E.; Kitaoka, K.; Imagawa, T.; Komatsu, Y.; Takanashi, K.; Anbo, F.; Anbo, T.; Katuki, S.; Ichihara, S.; et al. Human infection by acanthocephalan parasites belonging to the genus Corynosoma found from small bowel endoscopy. Parasitol. Int. 2016, 65, 491–493. [Google Scholar] [CrossRef] [PubMed]
- Bao, M.; Liu, G.H.; Zhou, D.H.; Song, H.Q.; Wang, S.D.; Tang, F.; Qu, L.D.; Zou, F.C. Prevalence of Clonorchis sinensis infection in market-sold freshwater fishes in Jinzhou city, northeastern China. Afr. J. Microbiol. Res. 2012, 6, 629–632. [Google Scholar]
- Choi, S.H.; Kim, J.; Jo, J.O.; Cho, M.K.; Yu, H.S.; Cha, H.J.; Ock, M.S. Anisakis simplex larvae: Infection status in marine fish and cephalopods purchased from the cooperative fish market in Busan, Korea. Korean J. Parasitol. 2011, 49, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, S.; Suthar, J. A revised method of examining fish for infection with zoonotic nematode larvae. Int. J. Food Microbiol. 2016, 227, 13–16. [Google Scholar] [CrossRef] [PubMed]
- McClelland, G. The trouble with sealworms (pseudoterranova decipiens species complex, Nematoda): A review. Parasitology 2002, 124, s183–s203. [Google Scholar] [CrossRef] [PubMed]
- Gray, T.J.; Kwan, Y.L.; Thuy, P.; Robertson, G.; Cheong, E.Y.L.; Gottlieb, T. Anisakiasis mistaken for dientamoebiasis? Clin. Infect. Dis. 2013, 57, 1500–1501. [Google Scholar] [CrossRef] [PubMed]
- Anantanawat, S.; Kiermeier, A.; McLeod, C.; Sumner, J. A Semi-Quantitative Risk Assessment of Harmful Parasites in Australian Finfish; South Australian Research & Development Institute: Adelaide, Australia, 2012. [Google Scholar]
- Sumner, J.; Anantanawat, S.; Kiermeier, A.; McLeod, C.; Shamsi, S. Raw fish consumption in Australia: How safe is it? Food Aust. 2015, 67, 24–26. [Google Scholar]
- Shamsi, S.; Butcher, A.R. First report of human anisakidosis in Australia. Med. J. Aust. 2011, 194, 199–200. [Google Scholar] [PubMed]
- Marušić, M. Why physicians should publish, how easy it is, and how important it is in clinical work. Arch. Oncol. 2003, 11, 59–64. [Google Scholar] [CrossRef]
- Shamsi, S. Parasite loss or parasite gain? Story of Contracaecum nematodes in antipodean waters. Parasite Epidemiol. Control 2019, e00087. [Google Scholar] [CrossRef] [PubMed]
- Zuo, S.; Kania, P.W.; Mehrdana, F.; Marana, M.H.; Buchmann, K. Contracaecum osculatum and other anisakid nematodes in grey seals and cod in the baltic sea: Molecular and ecological links. J. Helminthol. 2018, 92, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, S.; Steller, E.; Chen, Y. New and known zoonotic nematode larvae within selected fish species from Queensland waters in Australia. Int. J. Food Microbiol. 2018, 272, 73–82. [Google Scholar] [CrossRef] [PubMed]

| Protozoa | Cnidaria | Cestoda (Tapeworms) | Trematoda (Flukes) | Nematoda (Round Worms) | Acanthocephala (Thorny-Headed Worms) |
|---|---|---|---|---|---|
| Cryptospoidium | Kudoa | Adenocephalus | Appophalus | Angiostrongylus | Corynosoma |
| Enterocytozoon | Myxobolus | Diphyllobothrium | Centrocestus | Anisakis | Bolbosoma |
| Giardia | Toxoplasma | Diplogonoporus | Clinostomum | Capillaria | |
| Unicapsula | Ligula | Clonorchis | Contracaecum | ||
| Spirometra | Cryptocotyle | Dioctophyma | |||
| Diplostomum | Echinocephalus | ||||
| Echinostoma | Eustrongyloides | ||||
| Haplorchis | Filaria | ||||
| Heterophyes | Gnathostoma | ||||
| Heterophyopsis | Hysterothylaccium | ||||
| Metorchis | Philometra | ||||
| Metagonimus | Pseudoterranova | ||||
| Nanophyetus | |||||
| Opisthorchis | |||||
| Phagicola | |||||
| Procerovum | |||||
| Paragonimus | |||||
| Prohemistomum | |||||
| Pygidiopsis | |||||
| Schistosoma | |||||
| Stellantchasmus | |||||
| Stictodora |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shamsi, S. Seafood-Borne Parasitic Diseases: A “One-Health” Approach Is Needed. Fishes 2019, 4, 9. https://doi.org/10.3390/fishes4010009
Shamsi S. Seafood-Borne Parasitic Diseases: A “One-Health” Approach Is Needed. Fishes. 2019; 4(1):9. https://doi.org/10.3390/fishes4010009
Chicago/Turabian StyleShamsi, Shokoofeh. 2019. "Seafood-Borne Parasitic Diseases: A “One-Health” Approach Is Needed" Fishes 4, no. 1: 9. https://doi.org/10.3390/fishes4010009
APA StyleShamsi, S. (2019). Seafood-Borne Parasitic Diseases: A “One-Health” Approach Is Needed. Fishes, 4(1), 9. https://doi.org/10.3390/fishes4010009