Anti-Osteogenic Activity of Cadmium in Zebrafish
Abstract
1. Introduction
2. Results
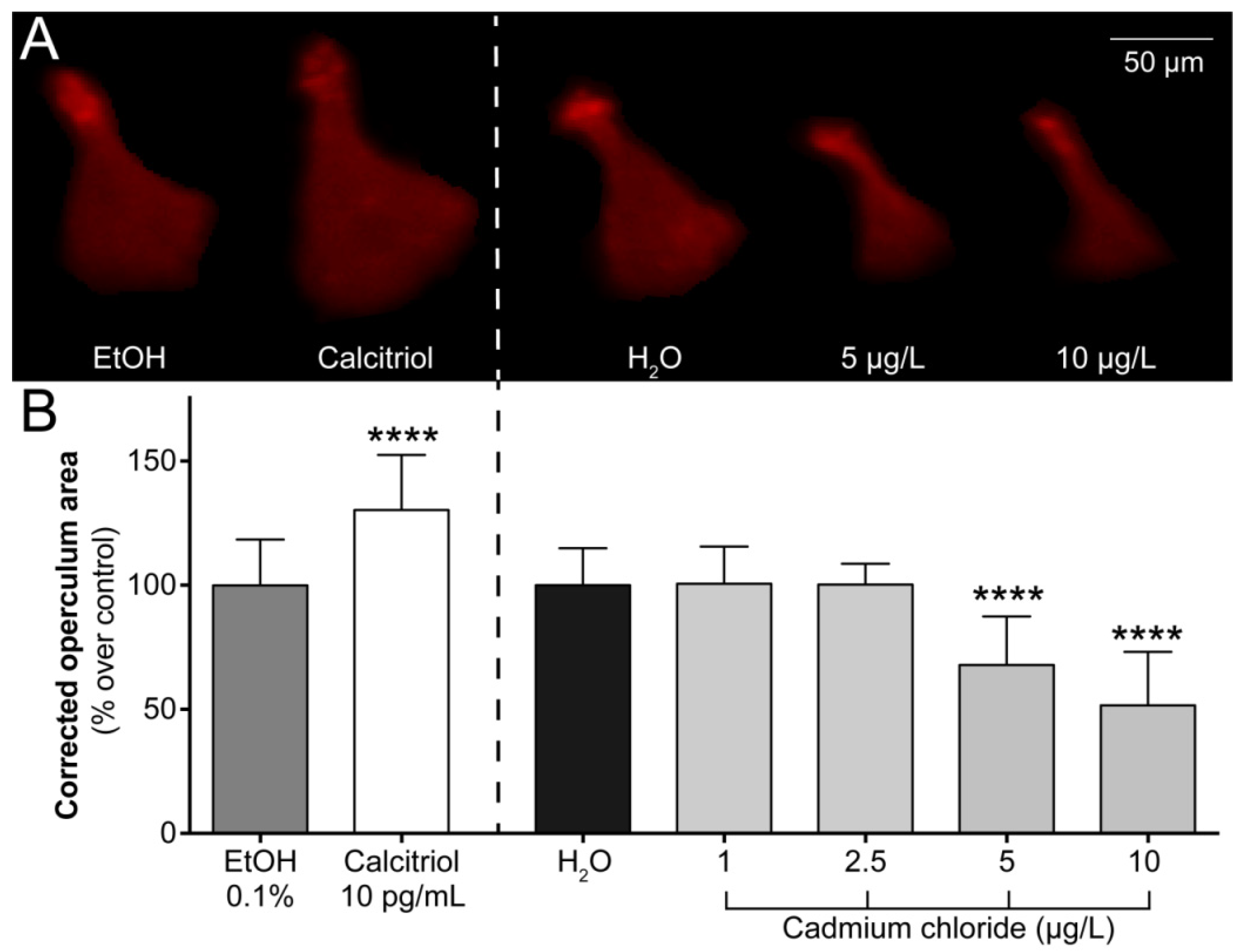
2.1. Operculum Growth is Impaired in 6-dpf Larvae Exposed to Cadmium
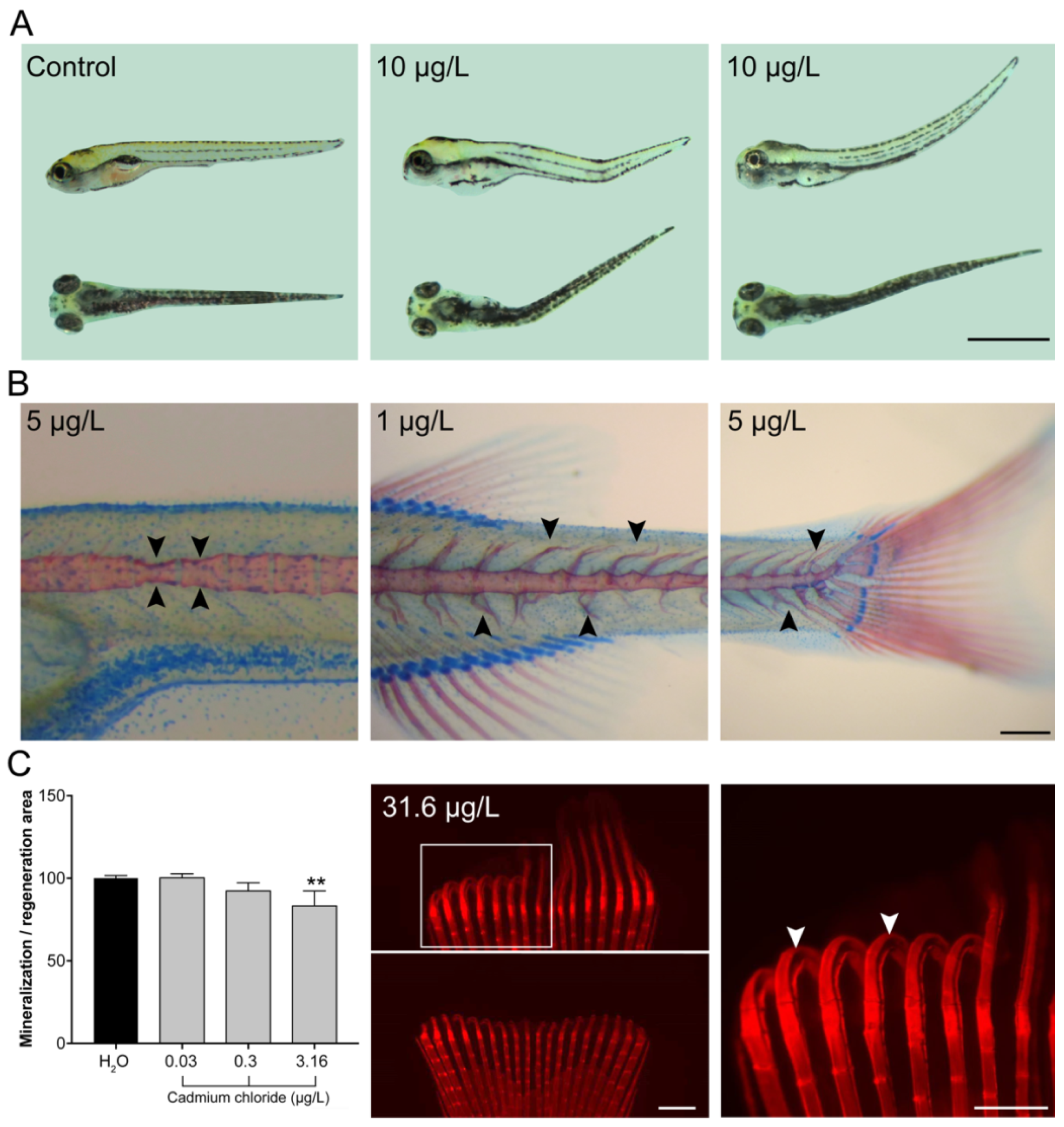
2.2. Larvae Exposed to Cadmium Exhibit More Skeletal Deformities
2.3. De Novo Bone Formation is Impaired During Caudal Fin Regeneration
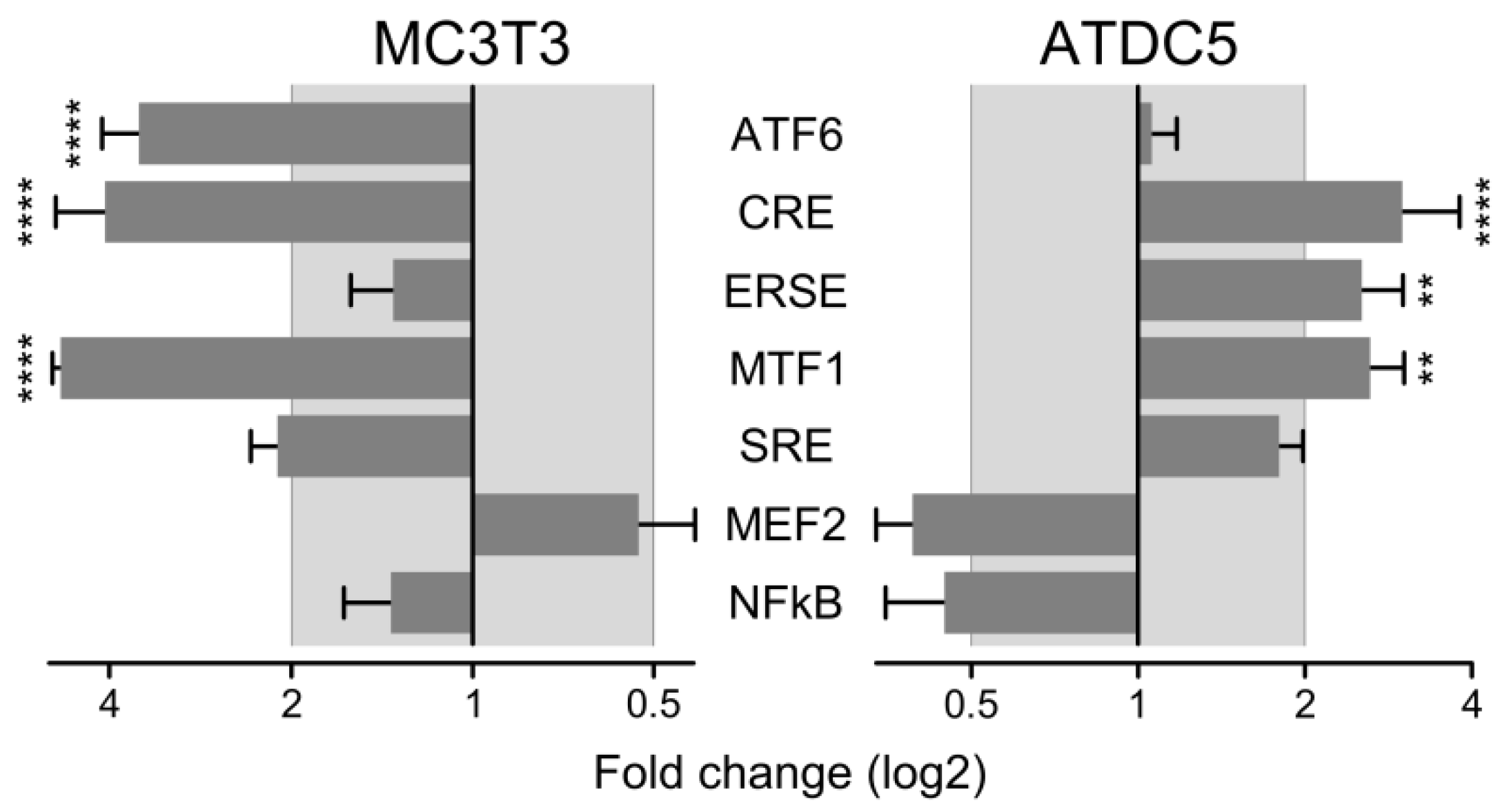
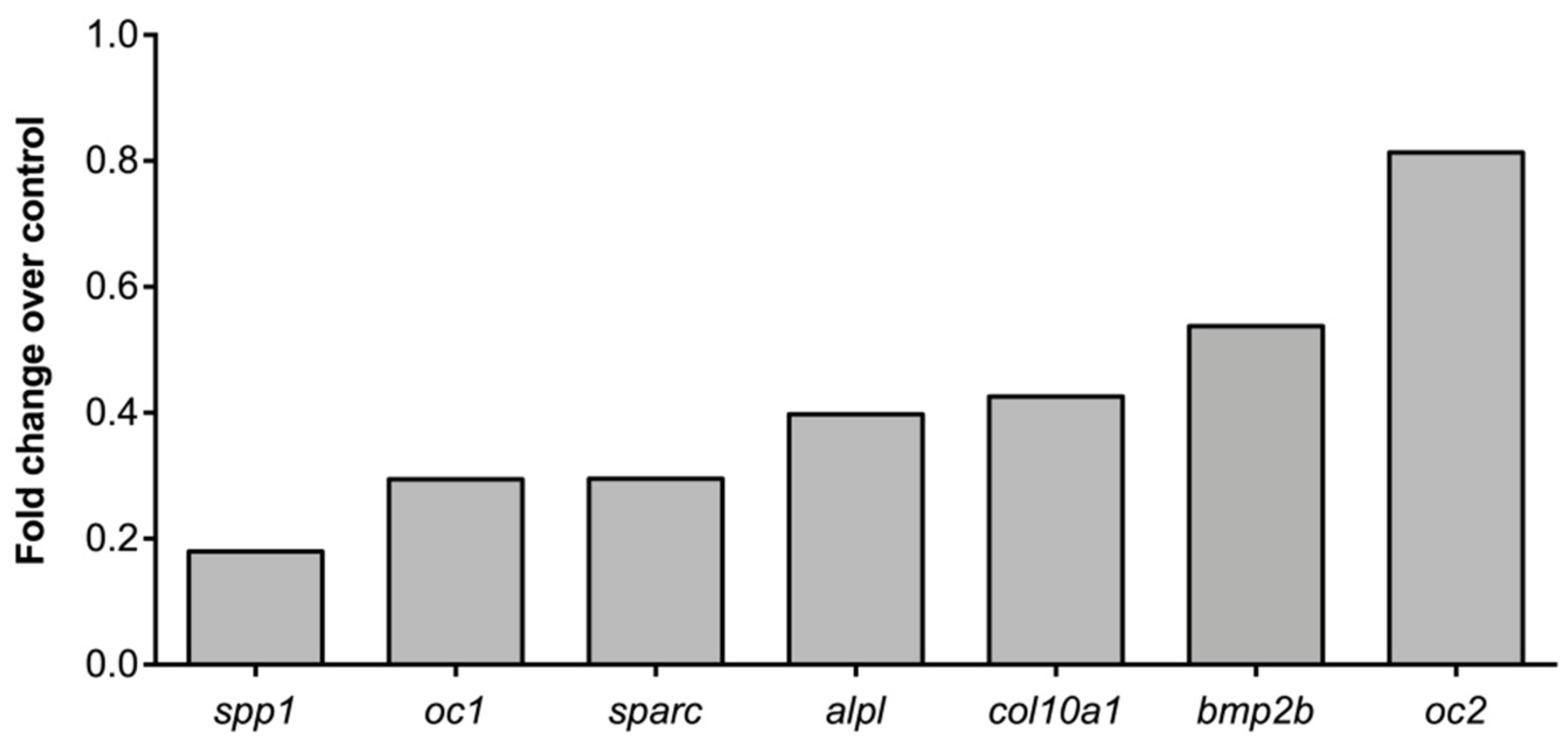
2.4. Molecular Pathways and Genes Targeted by Cadmium
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Chemical Solutions
4.3. Zebrafish Rearing and Breeding
4.4. Assessment of Operculum Growth
4.5. Assessment of Skeletal Deformities
4.6. Assessment of Caudal Fin Regeneration
4.7. Activity of Signal Pathways
4.8. RNA Extraction and Gene Expression by qPCR
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bosch, A.C.; O’Neill, B.; Sigge, G.O.; Kerwath, S.E.; Hoffman, L.C. Heavy metals in marine fish meat and consumer health: A review. J. Sci. Food Agric. 2016, 96, 32–48. [Google Scholar] [CrossRef] [PubMed]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Exp. Suppl. 2012, 101, 133–164. [Google Scholar] [PubMed]
- Singh, R.; Gautam, N.; Mishra, A.; Gupta, R. Heavy metals and living systems: An overview. Indian J. Pharmacol. 2011, 43, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Ravindran, G.; Krishnamurthy, V. A brief review on the effect of cadmium toxicity: From cellular to organ level. Int. J. Biotechnol. Res. 2013, 3, 17–36. [Google Scholar]
- Nordberg, G.F.; Fowler, B.A.; Nordberg, M.; Friberg, L.T. Handbook on the Toxicology of Metals, 3rd ed.; Academic Press: Cambridge, MA, USA, 2007. [Google Scholar]
- Hartwig, A. Cadmium and cancer. In Cadmium: From Toxicity to Essentiality; Sigel, A., Sigel, H., Sigel, R.K.O., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 491–507. [Google Scholar]
- Johri, N.; Jacquillet, G.; Unwin, R. Heavy metal poisoning: The effects of cadmium on the kidney. BioMetals 2010, 23, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Wallin, M.; Sallsten, G.; Lundh, T.; Barregard, L. Low-level cadmium exposure and effects on kidney function. Occup. Environ. Med. 2014, 71, 848–854. [Google Scholar] [CrossRef]
- Youness, E.R.; Mohammed, N.A.; Morsy, F.A. Cadmium impact and osteoporosis: Mechanism of action. Toxicol. Mech. Methods 2012, 22, 560–567. [Google Scholar] [CrossRef]
- Åkesson, A.; Bjellerup, P.; Lundh, T.; Lidfeldt, J.; Nerbrand, C.; Samsioe, G.; Skerfving, S.; Vahter, M. Cadmium-induced effects on bone in a population-based study of women. Environ. Health Perspect. 2006, 114, 830–834. [Google Scholar] [CrossRef]
- Kennedy, C.J. TOXICOLOGY | The Toxicology of Metals in Fishes. In Encyclopedia of Fish Physiology, 1st ed.; Farrell, A.P., Ed.; Academic Press: Cambridge, MA, USA, 2011; Volume 3, pp. 2061–2068. [Google Scholar]
- Levit, S.M. A Literature Review of Effects of Cadmium on Fish; The Nature Conservancy: Arlington, MA, USA, 2010. [Google Scholar]
- Kumar, P.; Singh, A. Cadmium toxicity in fish: An overview. GERF Bull. Biosci. 2010, 1, 41–47. [Google Scholar]
- Authman, M.M.N.; Zaki, M.S.; Khallaf, E.A.; Abbas, H.H. Use of fish as bio-indicator of the effects of heavy metals pollution. J. Aquac. Res. Dev. 2015, 6, 328. [Google Scholar] [CrossRef]
- El-Ebiary, E.H.; Wahbi, O.M.; El-Greisy, Z.A. Influence of dietary cadmium on sexual maturity and reproduction of red tilapia. Egypt. J. Aquat. Res. 2013, 39, 313–317. [Google Scholar] [CrossRef]
- Final Review of Scientific Information on Cadmium; Chemical Branch, United Nations Environment Programme: New York, NY, USA, 2010.
- Niyogi, S.; Wood, C.M. Interaction between dietary calcium supplementation and chronic waterborne zinc exposure in juvenile rainbow trout, Oncorhynchus mykiss. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2006, 143, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Christoffersen, J.; Christoffersen, M.R.; Larsen, R.; Rostrup, E.; Tingsgaard, P.; Andersen, O.; Grandjean, P. Interaction of cadmium ions with calcium hydroxyapatite crystals: A possible mechanism contributing to the pathogenesis of cadmium-induced bone diseases. Calcif. Tissue Int. 1988, 42, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Duranova, H.; Martiniakova, M.; Omelka, R.; Grosskopf, B.; Bobonova, I.; Toman, R. Changes in compact bone microstructure of rats subchronically exposed to cadmium. Acta Vet. Scand. 2014, 56, 64. [Google Scholar] [CrossRef] [PubMed]
- Ha, T.T.; Burwell, S.T.; Goodwin, M.L.; Noeker, J.A.; Heggland, S.J. Pleiotropic roles of Ca+2/calmodulin-dependent pathways in regulating cadmium-induced toxicity in human osteoblast-like cell lines. Toxicol. Lett. 2016, 260, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.; Mandalunis, P.M. Effect of cadmium on bone tissue in growing animals. Exp. Toxicol. Pathol. 2016, 68, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Papa, V.; Bimonte, V.M.; Wannenes, F.; D’Abusco, A.S.; Fittipaldi, S.; Scandurra, R.; Politi, L.; Crescioli, C.; Lenzi, A.; Di Luigi, L.; et al. The endocrine disruptor cadmium alters human osteoblast-like Saos-2 cells homeostasis in vitro by alteration of Wnt/β-catenin pathway and activation of caspases. J. Endocrinol. Investig. 2015, 38, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhu, G.; Jin, T.; Zhou, Z.; Gu, S.; Qiu, J.; Xiao, H. Cadmium stimulates the osteoclastic differentiation of RAW264.7 cells in presence of osteoblasts. Biol. Trace Elem. Res. 2012, 146, 349–353. [Google Scholar] [CrossRef]
- Brzóska, M.M.; Moniuszko-Jakoniuk, J. Low-level lifetime exposure to cadmium decreases skeletal mineralization and enhances bone loss in aged rats. Bone 2004, 35, 1180–1191. [Google Scholar] [CrossRef]
- Bhattacharyya, M.H. Cadmium osteotoxicity in experimental animals: Mechanisms and relationship to human exposures. Toxicol. Appl. Pharmacol. 2009, 238, 258–265. [Google Scholar] [CrossRef]
- Smith, S.S.; Reyes, J.R.; Arbon, K.S.; Harvey, W.A.; Hunt, L.M.; Heggland, S.J. Cadmium-induced decrease in RUNX2 mRNA expression and recovery by the antioxidant N-acetylcysteine (NAC) in the human osteoblast-like cell line, Saos-2. Toxicol. In Vitro 2009, 23, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liu, W.; Wang, Y.; Dai, N.; Gu, J.; Yuan, Y.; Liu, X.; Bian, J.; Liu, Z.-P. Cadmium induces apoptosis in primary rat osteoblasts through caspase and mitogen-activated protein kinase pathways. J. Vet. Sci. 2015, 16, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Wallin, M.; Sallsten, G.; Fabricius-Lagging, E.; Öhrn, C.; Lundh, T.; Barregard, L. Kidney cadmium levels and associations with urinary calcium and bone mineral density: A cross-sectional study in Sweden. Environ. Health 2013, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Sfakianakis, D.G.; Renieri, E.; Kentouri, M.; Tsatsakis, A.M. Effect of heavy metals on fish larvae deformities: A review. Environ. Res. 2015, 137, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Laizé, V.; Gavaia, P.J.; Tarasco, M.; Viegas, M.N.; Caria, J.; Luis, N.; Cancela, M.L. Osteotoxicity of 3-methylcholanthrene in fish. Ecotoxicol. Environ. Saf. 2018, 161, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, Y.; Jafarzadeh, S.; Moradi, B.; Zandsalimi, Y.; Langarizadeh, G.; Amirhajeloo, L.; Mirzaei, M. The non-carcinogenic risk of cadmium in bottled water in different age groups humans: Bandar Abbas City, Iran. Mater. Sociomed. 2015, 27, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Idrees, N.; Tabassum, B.; Abd_Allah, E.F.; Hashem, A.; Sarah, R.; Hashim, M. Groundwater contamination with cadmium concentrations in some West U.P. Regions, India. Saudi J. Biol. Sci. 2018, 25, 1365–1368. [Google Scholar] [CrossRef] [PubMed]
- Tarasco, M.; Laizé, V.; Cardeira, J.; Cancela, M.L.; Gavaia, P.J. The zebrafish operculum: A powerful system to assess osteogenic bioactivities of molecules with pharmacological and toxicological relevance. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 197, 45–52. [Google Scholar] [CrossRef]
- Miyahara, T.; Yamada, H.; Takeuchi, M.; Kozuka, H.; Kato, T.; Sudo, H. Inhibitory effects of cadmium on in vitro calcification of a clonal osteogenic cell, MC3T3-E1. Toxicol. Appl. Pharmacol. 1988, 96, 52–59. [Google Scholar] [CrossRef]
- Roberto, V.P.; Martins, G.; Pereira, A.; Rodrigues, S.; Grenha, A.; Pinto, W.; Cancela, M.L.; Dias, J.; Gavaia, P.J. Insights from dietary supplementation with zinc and strontium on the skeleton of zebrafish, Danio rerio (Hamilton, 1822) larvae: From morphological analysis to osteogenic markers. J. Appl. Ichthyol. 2018, 34, 512–523. [Google Scholar] [CrossRef]
- Fazenda, C.; Martins, G.; Gavaia, P.J.; Cancela, M.L.; Conceição, N. Generation of zebrafish Danio rerio (Hamilton, 1822) transgenic lines overexpressing a heat-shock mediated Gla-rich protein. J. Appl. Ichthyol. 2018, 34, 472–480. [Google Scholar] [CrossRef]
- Martins, G.; Diogo, P.; Pinto, W.; Gavaia, P.J. Early transition to microdiets improves growth, reproductive performance and reduces skeletal anomalies in zebrafish (Danio rerio). Zebrafish 2018. [Google Scholar] [CrossRef] [PubMed]
- Fraysse, B.; Mons, R.; Garric, J. Development of a zebrafish 4-day embryo-larval bioassay to assess toxicity of chemicals. Ecotoxicol. Environ. Saf. 2006, 63, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Witeska, M.; Jezierska, B.; Chaber, J. The influence of cadmium on common carp embryos and larvae. Aquaculture 1995, 129, 129–132. [Google Scholar] [CrossRef]
- Williams, N.D.; Holdway, D.A. The effects of pulse-exposed cadmium and zinc on embryo hatchability, larval development, and survival of Australian crimson spotted rainbow fish (Melanotaenia fluviatilis). Environ. Toxicol. 2000, 15, 165–173. [Google Scholar] [CrossRef]
- Cao, L.; Huang, W.; Shan, X.; Xiao, Z.; Wang, Q.; Dou, S. Cadmium toxicity to embryonic–larval development and survival in red sea bream Pagrus major. Ecotoxicol. Environ. Saf. 2009, 72, 1966–1974. [Google Scholar] [CrossRef] [PubMed]
- Barjhoux, I.; Baudrimont, M.; Morin, B.; Landi, L.; Gonzalez, P.; Cachot, J. Effects of copper and cadmium spiked-sediments on embryonic development of Japanese medaka (Oryzias latipes). Ecotoxicol. Environ. Saf. 2012, 79, 272–282. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, H.; Meng, Y.; Jin, G.; Zhu, M. The toxicity of cadmium (Cd2+) towards embryos and pro-larva of soldatov’s catfish (Silurus soldatovi). Ecotoxicol. Environ. Saf. 2012, 80, 258–265. [Google Scholar] [CrossRef]
- Sassi, A.; Annabi, A.; Kessabi, K.; Kerkeni, A.; Saïd, K.; Messaoudi, I. Influence of high temperature on cadmium-induced skeletal deformities in juvenile mosquitofish (Gambusia affinis). Fish Physiol. Biochem. 2010, 36, 403–409. [Google Scholar] [CrossRef]
- Bengtsson, B.-E. Biological variables, especially skeletal deformities in fish, for monitoring marine pollution. Philos. Trans. R. Soc. Lond. B 1979, 286, 457–464. [Google Scholar] [CrossRef]
- Cheng, S.H.; Wai, A.W.K.; So, C.H.; Wu, R.S.S. Cellular and molecular basis of cadmium-induced deformities in zebrafish embryos. Environ. Toxicol. Chem. 2000, 19, 3024–3031. [Google Scholar] [CrossRef]
- Rishi, K.K.; Jain, M. Effect of toxicity of cadmium on scale morphology in Cyprinus carpio (Cyprinidae). Bull. Environ. Contam. Toxicol. 1998, 60, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Yoshitomi, T.; Koyama, J.; Iida, A.; Okamoto, N.; Ikeda, Y. Cadmium-induced scale deformation in carp (Cyprinus carpio). Bull. Environ. Contam. Toxicol. 1998, 60, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Yamamoto, M.; Watanabe, K.; Kambegawa, A.; Hattori, A. Both mercury and cadmium directly influence calcium homeostasis resulting from the suppression of scale bone cells: The scale is a good model for the evaluation of heavy metals in bone metabolism. J. Bone Miner. Metab. 2004, 22, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Nogawa, K.; Yamada, Y.; Honda, R.; Ishizaki, M.; Tsuritani, I.; Kawano, S.; Kato, T. The relationship between itai-itai disease among inhabitants of the Jinzu river basin and cadmium in rice. Toxicol. Lett. 1983, 17, 263–266. [Google Scholar] [CrossRef]
- Brzóska, M.M.; Moniuszko-Jakoniuk, J. Low-level exposure to cadmium during the lifetime increases the risk of osteoporosis and fractures of the lumbar spine in the elderly: Studies on a rat model of human environmental exposure. Toxicol. Sci. 2004, 82, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Scimeca, M.; Feola, M.; Romano, L.; Rao, C.; Gasbarra, E.; Bonanno, E.; Brandi, M.L.; Tarantino, U. Heavy metals accumulation affects bone microarchitecture in osteoporotic patients. Environ. Toxicol. 2017, 32, 1333–1342. [Google Scholar] [CrossRef] [PubMed]
- Galicka, A.; Brzóska, M.M.; Sredzińska, K.; Gindzienski, A. Effect of cadmium on collagen content and solubility in rat bone. Acta Biochim. Pol. 2004, 51, 825–829. [Google Scholar]
- Miyahara, T.; Tsukada, M.; Mori, M.-A.; Kozuka, H. The effect of cadmium on the collagen solubility of embryonic chick bone in tissue culture. Toxicol. Lett. 1984, 22, 89–92. [Google Scholar] [CrossRef]
- Kazantzis, G. Cadmium, osteoporosis and calcium metabolism. BioMetals 2004, 17, 493–498. [Google Scholar] [CrossRef]
- Martelli, A.; Rousselet, E.; Dycke, C.; Bouron, A.; Moulis, J.-M. Cadmium toxicity in animal cells by interference with essential metals. Biochimie 2006, 88, 1807–1814. [Google Scholar] [CrossRef] [PubMed]
- Prigodich, R.V.; O’Connor, T.; Coleman, J.E. 1H, 113Cd, and 31P NMR of osteocalcin (bovine γ-carboxyglutamic acid containing protein). Biochemistry 1985, 24, 6291–6298. [Google Scholar] [CrossRef] [PubMed]
- Poteat, M.D.; Buchwalter, D.B. Calcium uptake in aquatic insects: Influences of phylogeny and metals (Cd and Zn). J. Exp. Biol. 2014, 217, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, N.C.; Cosma, V.; Skyler, D.; LeGeros, J.; Walters, M. The effect of cadmium on the formation and properties of hydroxyapatite in vitro and its relation to cadmium toxicity in the skeletal system. Calcif. Tissue Int. 1995, 56, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Stohs, S.J.; Bagchi, D.; Hassoun, E.; Bagchi, M. Oxidative mechanisms in the toxicity of chromium and cadmium ions. J. Environ. Pathol. Toxicol. Oncol. 2000, 19, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Bodo, M.; Balloni, S.; Lumare, E.; Bacci, M.; Calvitti, M.; Dell’Omo, M.; Murgia, N.; Marinucci, L. Effects of sub-toxic cadmium concentrations on bone gene expression program: Results of an in vitro study. Toxicol. In Vitro 2010, 24, 1670–1680. [Google Scholar] [CrossRef] [PubMed]
- Pierron, F.; Baillon, L.; Sow, M.; Gotreau, S.; Gonzalez, P. Effect of low-dose cadmium exposure on DNA methylation in the endangered European eel. Environ. Sci. Technol. 2014, 48, 797–803. [Google Scholar] [CrossRef]
- Zheng, J.-L.; Guo, S.-N.; Yuan, S.-S.; Xia, H.; Zhu, Q.-L.; Lv, Z.-M. Preheating mitigates cadmium toxicity in zebrafish livers: Evidence from promoter demethylation, gene transcription to biochemical levels. Aquat. Toxicol. 2017, 190, 104–111. [Google Scholar] [CrossRef]
- Hontela, A.; Daniel, C.; Ricard, A.C. Effects of acute and subacute exposures to cadmium on the interrenal and thyroid function in rainbow trout, Oncorhynchus mykiss. Aquat. Toxicol. 1996, 35, 171–182. [Google Scholar] [CrossRef]
- Le Guével, R.; Petit, F.G.; Le Goff, P.; Métivier, R.; Valotaire, Y.; Pakdel, F. Inhibition of rainbow trout (Oncorhynchus mykiss) estrogen receptor activity by cadmium. Biol. Reprod. 2000, 63, 259–266. [Google Scholar] [CrossRef]
- Pratap, H.B.; Wendelaar Bonga, S.E. Effect of ambient and dietary cadmium on pavement cells, chloride cells, and Na+/K+-ATPase activity in the gills of the freshwater teleost Oreochromis mossambicus at normal and high calcium levels in the ambient water. Aquat. Toxicol. 1993, 26, 133–149. [Google Scholar] [CrossRef]
- Chen, W.-Y.; John, J.A.C.; Lin, C.-H.; Chang, C.-Y. Expression pattern of metallothionein, MTF-1 nuclear translocation, and its DNA-binding activity in zebrafish (Danio rerio) induced by zinc and cadmium. Environ. Toxicol. Chem. 2007, 26, 110–117. [Google Scholar] [CrossRef] [PubMed]
- O’Shields, B.; McArthur, A.G.; Holowiecki, A.; Kamper, M.; Tapley, J.; Jenny, M.J. Inhibition of endogenous MTF-1 signaling in zebrafish embryos identifies novel roles for MTF-1 in development. Biochim. Biophys. Acta Mol. Cell Res. 2014, 1843, 1818–1833. [Google Scholar] [CrossRef] [PubMed]
- Grider, A.; Bakre, A.A.; Laing, E.M.; Lewis, R.D. In silico analysis of microRNA regulation of bone development: Metal Response Element-Binding Transcription Factor 1 and bone signaling pathways. Austin J. Nutr. Metab. 2017, 4, 1042. [Google Scholar]
- Park, S.-S.; Gomes, C.; Oh, S.-D.; Soh, J. Cadmium up-regulates transcription of the steroidogenic acute regulatory protein (StAR) gene through phosphorylated CREB rather than SF-1 in K28 cells. J. Toxicol. Sci. 2015, 40, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Tchounwou, P.B.; Ishaque, A.B.; Schneider, J. Cytotoxicity and transcriptional activation of stress genes in human liver carcinoma cells (HepG2) exposed to cadmium chloride. In Molecular Mechanisms of Metal Toxicity and Carcinogenesis. Developments in Molecular and Cellular Biochemistry; Shi, X., Castranova, V., Vallyathan, V., Perry, W.G., Eds.; Springer: Boston, MA, USA, 2001; Volume 34, pp. 21–28. [Google Scholar]
- Epstein, P.M. Bone and the cAMP signaling pathway: Emerging therapeutics. In Bone Metabolic Functions and Modulators; Bronner, F., Farach-Carson, M.C., Roach, H.I., Eds.; Springer: London, UK, 2012; pp. 271–287. [Google Scholar]
- Zhang, R.; Edwards, J.R.; Ko, S.-Y.; Dong, S.; Liu, H.; Oyajobi, B.O.; Papasian, C.; Deng, H.-W.; Zhao, M. Transcriptional regulation of BMP2 expression by the PTH-CREB signaling pathway in osteoblasts. PLoS ONE 2011, 6, e20780. [Google Scholar] [CrossRef]
- Huang, W.-C.; Xie, Z.; Konaka, H.; Sodek, J.; Zhau, H.E.; Chung, L.W.K. Human osteocalcin and bone sialoprotein mediating osteomimicry of prostate cancer cells: Role of cAMP-dependent protein kinase A signaling pathway. Cancer Res. 2005, 65, 2303–2313. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.J.; Umayahara, Y.; Shu, H.; Centrella, M.; Rotwein, P.; McCarthy, T.L. Identification of the cAMP response element that controls transcriptional activation of the insulin-like growth factor-I gene by prostaglandin E2 in osteoblasts. J. Biol. Chem. 1996, 271, 21835–21841. [Google Scholar] [CrossRef] [PubMed]
- Henderson, J.E.; Goltzman, D. The Osteoporosis Primer; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Dermience, M.; Lognay, G.; Mathieu, F.; Goyens, P. Effects of thirty elements on bone metabolism. J. Trace Elem. Med. Biol. 2015, 32, 86–106. [Google Scholar] [CrossRef] [PubMed]
- Sauk, J.J.; Smith, T.; Silbergeld, E.K.; Fowler, B.A.; Somerman, M.J. Lead inhibits secretion of osteonectin/SPARC without significantly altering collagen or Hsp47 production in osteoblast-like ROS 17/2.8 cells. Toxicol. Appl. Pharmacol. 1992, 116, 240–247. [Google Scholar] [CrossRef]
- Angle, C.R.; Thomas, D.J.; Swanson, S.A. Toxicity of cadmium to rat osteosarcoma cells (ROS 17/2.8): Protective effect of 1α,25-dihydroxyvitamin D3. Toxicol. Appl. Pharmacol. 1990, 103, 113–120. [Google Scholar] [CrossRef]
- Kaji, T.; Kawatani, R.; Takata, M.; Hoshino, T.; Miyahara, T.; Kozuka, H.; Koizumi, F. The effects of cadmium, copper or zinc on formation of embryonic chick bone in tissue culture. Toxicology 1988, 50, 303–316. [Google Scholar] [CrossRef]
- Miyahara, T.; Oh-e, Y.; Takaine, E.; Kozuka, H. Interaction between cadmium and zinc, copper, or lead in relation to the collagen and mineral content of embryonic chick bone in tissue culture. Toxicol. Appl. Pharmacol. 1983, 67, 41–48. [Google Scholar] [CrossRef]
- Westerfield, M. The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish Danio rerio, 5th ed.; University of Oregon Press: Eugene, OR, USA, 2007. [Google Scholar]
- Walker, M.B.; Kimmel, C.B. A two-color acid-free cartilage and bone stain for zebrafish larvae. Biotech. Histochem. 2007, 82, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Cardeira, J.; Gavaia, P.J.; Fernández, I.; Cengiz, I.F.; Moreira-Silva, J.; Oliveira, J.M.; Reis, R.L.; Cancela, M.L.; Laizé, V. Quantitative assessment of the regenerative and mineralogenic performances of the zebrafish caudal fin. Sci. Rep. 2016, 6, 39191. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.L.; Fernandez, I.; Viegas, M.N.; Cox, C.J.; Martel, P.; Rosa, J.; Cancela, M.L.; Laizé, V. Comparative analysis of zebrafish bone morphogenetic proteins 2, 4 and 16: Molecular and evolutionary perspectives. Cell. Mol. Life Sci. 2016, 73, 841–857. [Google Scholar] [CrossRef] [PubMed]
- Collet, B.; Collins, C.; Lester, K. Engineered cell lines for fish health research. Dev. Comp. Immunol. 2018, 80, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
| Total Length (mm) | n | N | D | %N | %D | No. Deformities | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | mean ± SD | 0 | 1 | 2 | ≥3 | ||||||
| Control | 52 | 7.64 ± 1.30 | 25 | 11 | 14 | 44.0 | 56.0 | 11 | 12 | 2 | 0 |
| 5 µg/L | 44 | 7.70 ± 1.30 | 25 | 4 | 21 | 16.0 | 84.0 * | 4 | 9 | 8 | 4 |
| 1 µg/L | 49 | 7.50 ± 1.43 | 25 | 1 | 24 | 4.0 | 96.0 * | 1 | 7 | 12 | 5 |
| 0.2 µg/L | 48 | 7.45 ± 1.28 | 23 | 5 | 18 | 21.7 | 78.3 * | 5 | 6 | 8 | 4 |
| 0.04 µg/L | 51 | 7.39 ± 1.24 | 24 | 4 | 20 | 16.7 | 83.3 * | 4 | 12 | 7 | 1 |
| 0.008 µg/L | 51 | 7.55 ± 1.17 | 22 | 5 | 17 | 22.7 | 77.3 * | 5 | 12 | 4 | 1 |
| Control (n = 25) | 5 µg/L (n = 25) | 1 µg/L (n = 25) | 0.2 µg/L (n = 23) | 0.04 µg/L (n = 24) | 0.008 µg/L (n = 22) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Structures | N | %D | N | %D | N | %D | N | %D | N | %D | N | %D |
| Cephalic area | 1 | 4.0 | 8 | 32.0 * | 14 | 56.0 * | 6 | 26.1 * | 12 | 50.0 * | 4 | 18.2 * |
| Abdominal vertebrae | 6 | 24.0 * | 2 | 8.0 * | 2 | 8.7 * | 1 | 4.2 * | 1 | 4.5 * | ||
| Caudal vertebrae | 2 | 8.0 | 4 | 16.0 | 5 | 20.0 * | 2 | 8.7 | 1 | 4.2 | 3 | 13.6 |
| Caudal fin vertebrae | 1 | 4.0 | 2 | 8.0 | ||||||||
| Vertebral arches | 14 | 56.0 | 21 | 84.0 * | 33 | 132.0 * | 23 | 100.0 * | 17 | 70.8 * | 15 | 68.2 |
| Scoliosis | 1 | 4.0 * | ||||||||||
| Notochord | 1 | 4.0 | 4.0 * | 2 | 8.7 | |||||||
| Pectoral fin + rays | 1 | |||||||||||
| Anal fin + rays | 1 | 4.0 * | 1 | 4.3 * | ||||||||
| Caudal fin + rays | 2 | 8.0 * | 1 | 4.2 * | ||||||||
| Epural | 1 | 4.0 * | 1 | 4.5 * | ||||||||
| Urostyle | 1 | 4.0 * | 1 | 4.5 * | ||||||||
| Parahypural + hypurals 1–5 | 2 | 8.0 * | 1 | 4.0 * | 1 | 4.2 * | ||||||
| Total N (N/n) | 19 (0.76) | 49 (1.96) | 56 (2.24) | 36 (1.57) | 33 (1.38) | 25 (1.14) | ||||||
| Gene Name (acronym) | Accession No. | Sequence (5’– 3’) |
|---|---|---|
| elongation factor 1 alpha (ef1a) | NM_131263 | Fw-AGCCCCTCCTGGCTTTCACCC |
| Rv-TGGGACGAAGGCAACACTGGC | ||
| osteonectin (sparc) | AY239014 | Fw-CTTCTTCCTGTTCTGCCTCGCTGG |
| Rv-TCTCAGCAATAACATCCTCCACGACCT | ||
| osteopontin (spp1) | AY651247 | Fw-GAACCTACACAGACCACGCCAACAG |
| Rv-GGTAGCCCAAACTGTCTCCCCG | ||
| tissue non-specific alkaline phosphatase (alpl) | NM_201007 | Fw-TTCCTCTGCGGTGTCAAAGCCAA |
| Rv-AAGCAGCACTCGGGGTGGCAT | ||
| collagen, type X, alpha 1 (col10a1) | NM_001083827 | Fw-AGAAGGTGATGAAGGCCCCGCAGTAC |
| Rv-CACCATCTTGTCCTGCAGGTCCAGGT | ||
| bone morphogenetic protein 2b (bmp2b) | NM_131360 | Fw GAGGAACTTAGGAGACGACGGGAACGC |
| Rv TCTCGGGAATGAGTCCAACGGCAC | ||
| osteocalcin 1 (oc1) | * | Fw-GAAGCGAACATGAAGAGTCTGACAGTCC |
| Rv-GGAATCATCGCCGCCTATAAA | ||
| osteocalcin 2 (oc2) | ** | Fw-CCAACTCCGCATCAGACTCCGCATCA |
| Rv-ATGTGCTGCTGAAGCGGAGTGTTGCT |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarasco, M.; Cardeira, J.; Viegas, M.N.; Caria, J.; Martins, G.; Gavaia, P.J.; Cancela, M.L.; Laizé, V. Anti-Osteogenic Activity of Cadmium in Zebrafish. Fishes 2019, 4, 11. https://doi.org/10.3390/fishes4010011
Tarasco M, Cardeira J, Viegas MN, Caria J, Martins G, Gavaia PJ, Cancela ML, Laizé V. Anti-Osteogenic Activity of Cadmium in Zebrafish. Fishes. 2019; 4(1):11. https://doi.org/10.3390/fishes4010011
Chicago/Turabian StyleTarasco, Marco, João Cardeira, Michael N. Viegas, Joana Caria, Gil Martins, Paulo J. Gavaia, M. Leonor Cancela, and Vincent Laizé. 2019. "Anti-Osteogenic Activity of Cadmium in Zebrafish" Fishes 4, no. 1: 11. https://doi.org/10.3390/fishes4010011
APA StyleTarasco, M., Cardeira, J., Viegas, M. N., Caria, J., Martins, G., Gavaia, P. J., Cancela, M. L., & Laizé, V. (2019). Anti-Osteogenic Activity of Cadmium in Zebrafish. Fishes, 4(1), 11. https://doi.org/10.3390/fishes4010011







