The Efficacy of Nile Tilapia (Oreochromis niloticus) Broodstock and Larval Immunization against Streptococcus agalactiae and Aeromonas hydrophila
Abstract
1. Introduction
2. Results
2.1. The First-Stage
2.1.1. Efficacy of Broodstock Immunization
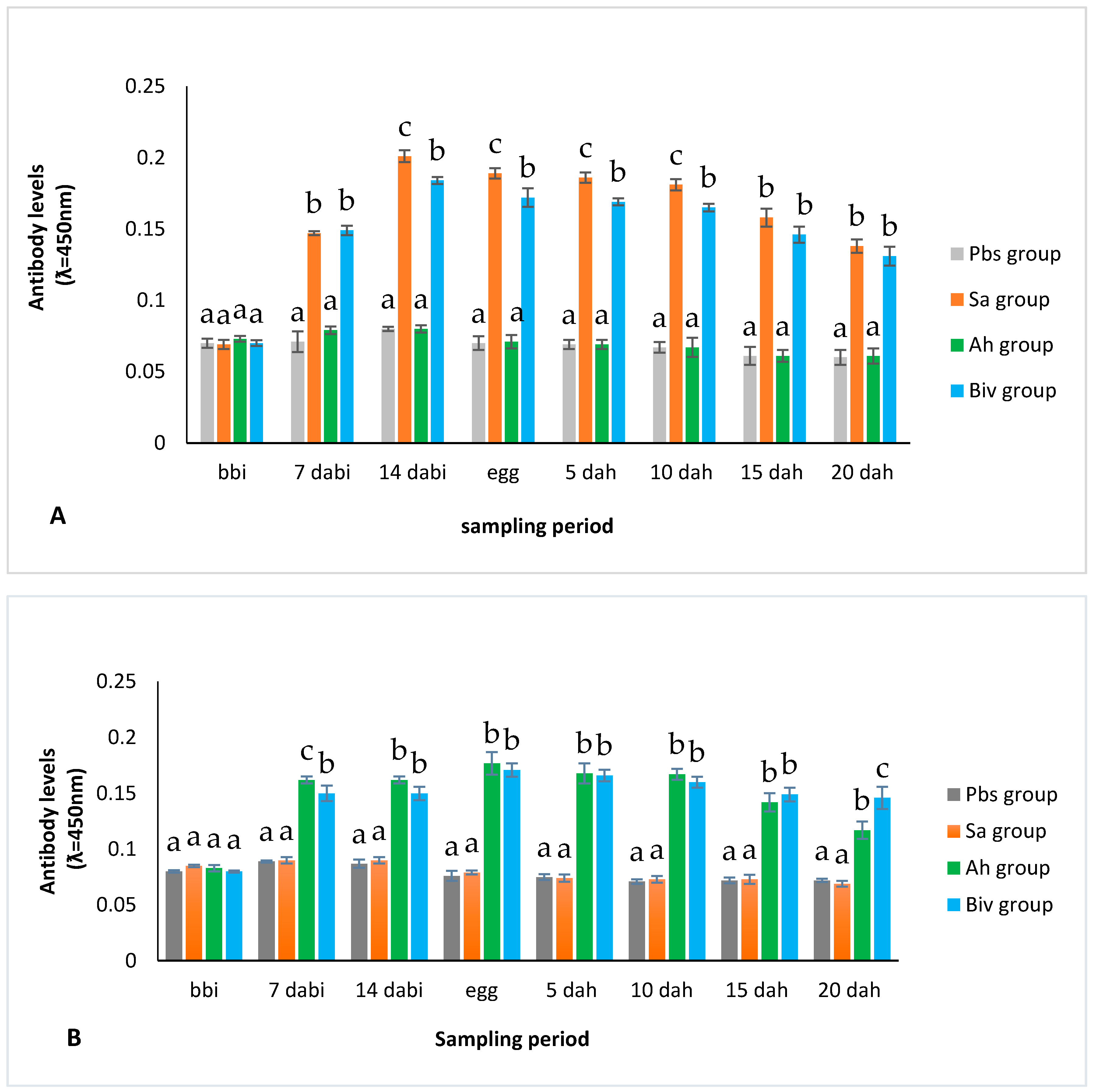
2.1.2. Antibody Titer of Immunized Broodstock
2.2. Second Stage
2.2.1. Efficacy of Immunization of Larvae Production from Immunization Broodstock
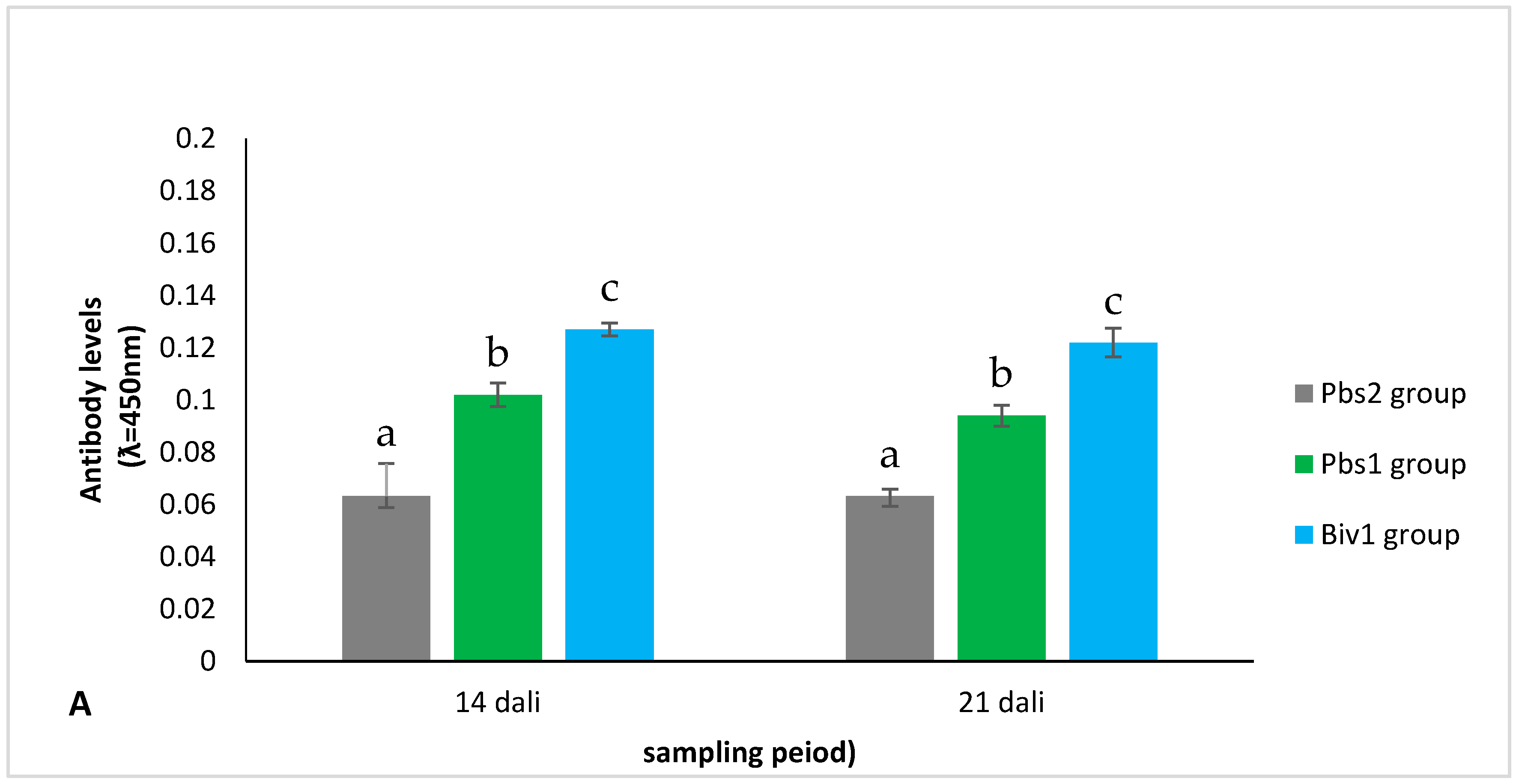
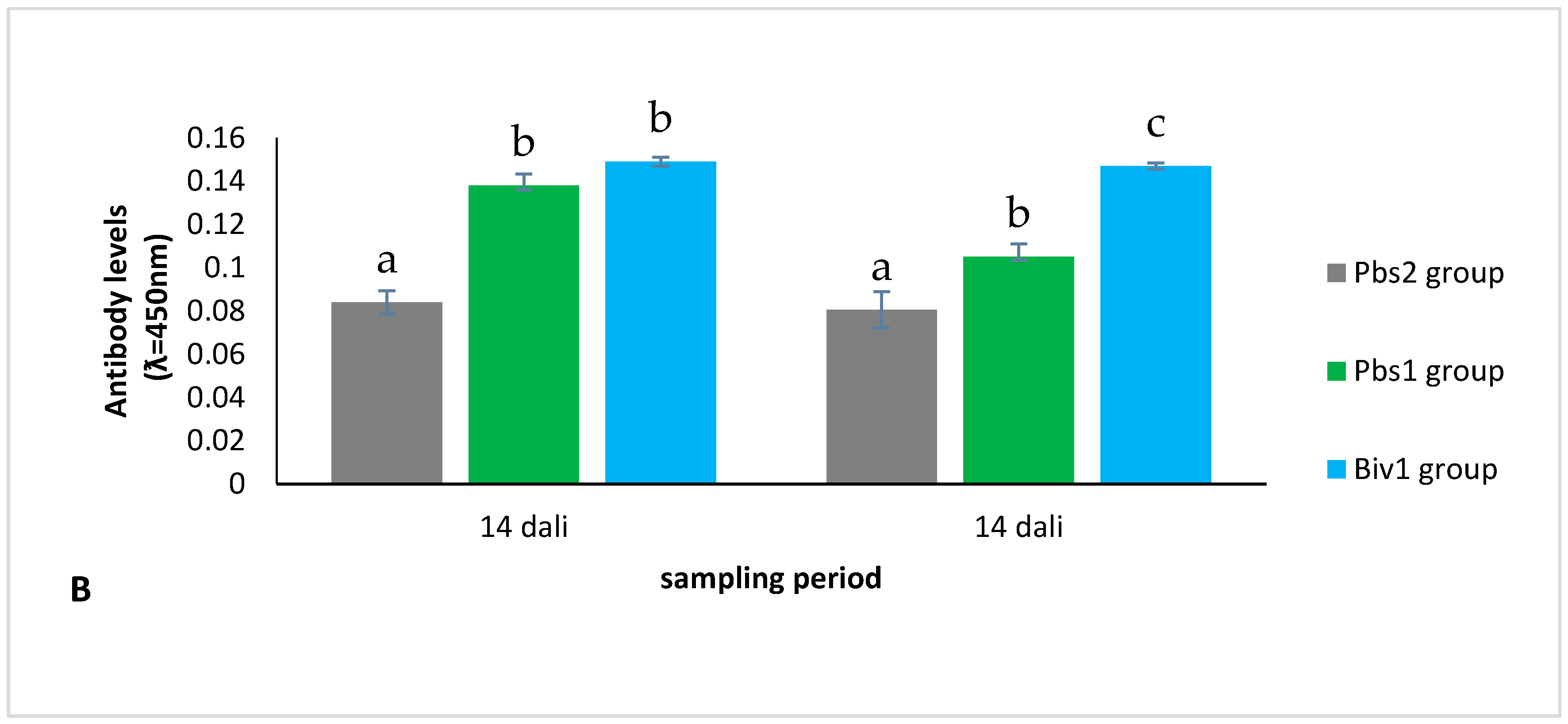
2.2.2. Antibody Level of Immunization of Larvae Production from Immunization Broodstock
3. Discussion
4. Materials and Methods
4.1. Broodstock
4.2. Vaccine Preparation
4.3. First Stage. Immunization of Broodstock
4.4. Challenge Experiment
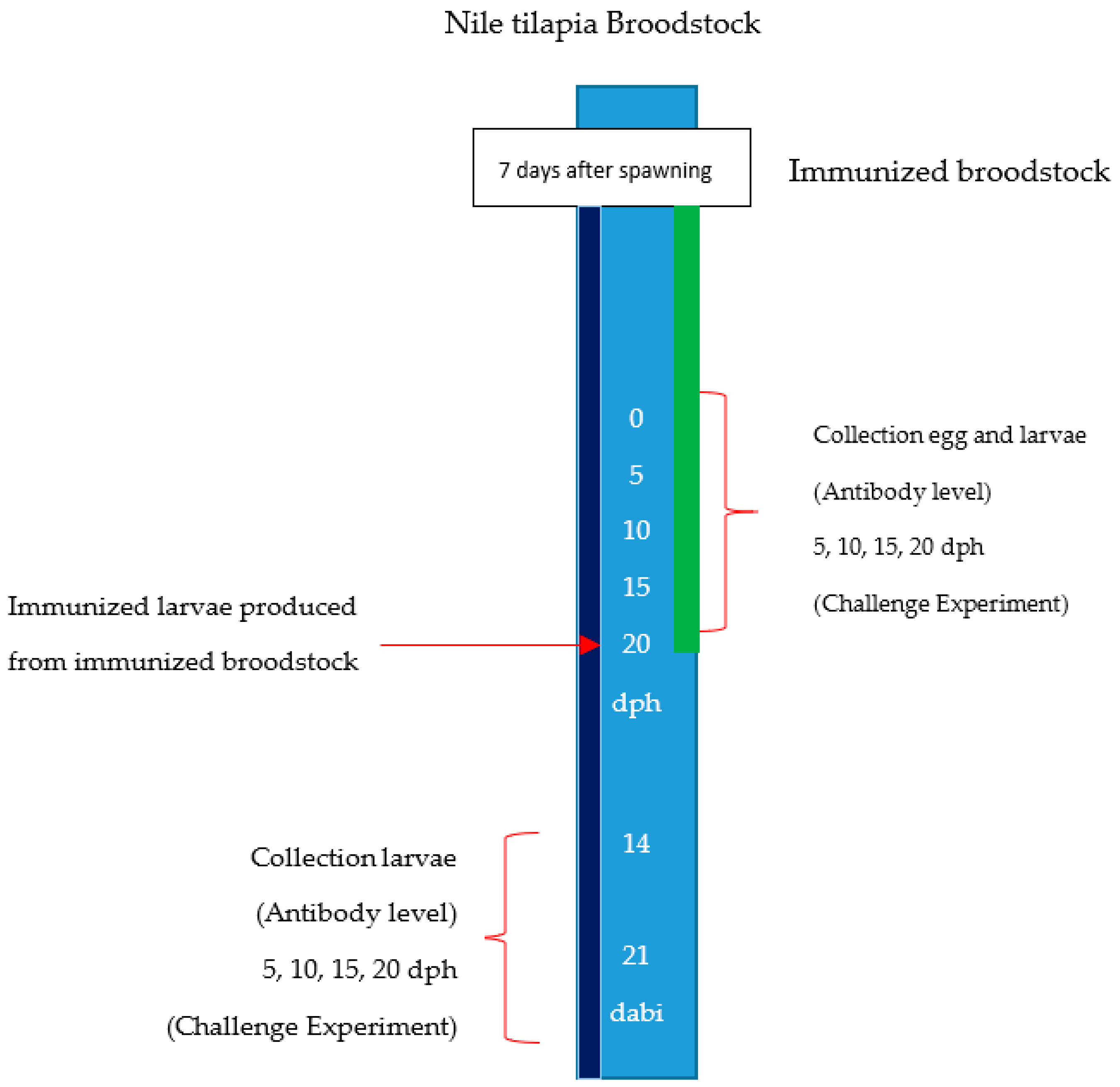
4.5. Second Stage. Immunization of Larvae from Immunized Broodstock.
4.6. Serum Preparation
4.7. Preparation of Eggs and Larvae Homogenates
4.8. Antibody Level
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations (FAO). Fisheries and Aquaculture Statistics 2015; FAO: Rome, Italy, 2015. [Google Scholar]
- Pridgeon, J.W.; Klesius, K. Major bacterial diseases in aquaculture and their vaccine development. CAB Rev. 2012, 7, 1–16. [Google Scholar] [CrossRef]
- Hardi, E.H.; Sukenda, S.; Harris, E.; Lusiastuti, A.M. Characterization and pathogenicity of β and non haemolytic Streptococcus agalactiae in cultured Nila tilapia. J. Vet. 2011, 12, 152–164. [Google Scholar]
- Buller, N.B. Bacteria and Fungi from Fish and Other Aquatic Animals: A Practical Identification Manual, 2nd ed.; CAB Internasional: Oxford, UK, 2014; p. 920. [Google Scholar]
- Amal, M.N.A.; Zamri-Saad, M.; Siti-Zahrah, A.; Zulkafli, A.R. Transmission of Streptococcus agalactiae from a hatchery into a newly established red hybrid tilapia, Oreochromis niloticus (L.) × Oreochromis mossambicus (Peters), farm. J. Fish Dis. 2013, 36, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, A.; Guo, Y.; Zhang, Q.; Chen, X.; Gong, X. Molecular characterization of Streptococcus agalactiae in diseased farmed tilapia in China. Aquaculture 2013, 412–413, 64–69. [Google Scholar] [CrossRef]
- Jantrakajorn, S.; Maisak, H.; Wongtavatchai, J. Comprehensive investigation of streptococcosis outbreaks in cultured Nile tilapia, Oreochromis niloticus, and Red Tilapia, Oreochromis sp., of Thailand. J. World Aquac. Soc. 2014, 45, 392–402. [Google Scholar] [CrossRef]
- Lusiastuti, A.M.; Textor, M.; Seeger, H.; Akineden, Ö.; Zschöck, M. The occurrence of Streptococcus agalactiae sequence type 261 from fish disease outbreaks of tilapia Oreochromis niloticus in Indonesia. Aquac. Res. 2014, 45, 260–1263. [Google Scholar] [CrossRef]
- Barato, P.; Martins, E.R.; Melo-Christino, J.; Iregui, C.A.; Ramirez, M. Persistence of a single clone of Streptococcus agalactiae causing disease in tilapia (Oreochromis sp.) cultured in Colombia over 8 years. J. Fish Dis. 2015, 38, 1083–1087. [Google Scholar] [CrossRef] [PubMed]
- Al-Harbi, A.H. Phenotypic and genotypic characterization of Streptococcus agalactiae isolated from hybrid tilapia (Oreochromis niloticus × O. aureus). Aquaculture 2016, 464, 515–520. [Google Scholar] [CrossRef]
- Asencios, Y.O.; Sánchez, F.B.; Mendizábal, H.B.; Pusari, K.H.; Alfonso, H.O.; Sayán, A.M. First report of Streptococcus agalactiae isolated from Oreochromis niloticus in Piura, Peru: Molecular identification and histopathological lesions. Aquac. Rep. 2016, 4, 74–79. [Google Scholar] [CrossRef]
- Mangunwardoyo, W.; Ismayasari, R.; Riani, E. Pathogenicity and virulency of Aeromonas hydrophila stainer on nila fish (Oreochromis niloticus L.) using Koch postulate. J. Riset Akuakultur 2010, 5, 145–225. [Google Scholar] [CrossRef]
- Aboyadak, I.M.; Ali, N.G.M.; Goda, A.M.A.S.; Aboelgalagel, W.H.; Salam, A.M.E. Molecular detection of Aeromonas hydrophila as the main cause of outbreak in tilapia farms in Egypt. J. Aquac. Mar. Biol. 2015, 2, 5. [Google Scholar] [CrossRef]
- Harikrishnan, R.; Balasundaram, C. Modern trends in Aeromonas hydrophila disease, management with fish. Rev. Fish Sci. 2005, 13, 281–320. [Google Scholar] [CrossRef]
- Dong, H.T.; Nguyen, V.V.; Le, H.D.; Sangsuriya, P.; Jitrakorn, S.; Saksmerprome, V. Naturally concurrent infections of bacterial and viral pathogens in disease outbreaks in cultured Nile tilapia (Oreochromis niloticus) farms. Aquaculture 2015, 448, 427–435. [Google Scholar] [CrossRef]
- Assis, G.B.N.; Tavares, G.C.; Pereira, F.L.; Figueiredo, H.C.P.; Leal, C.A.G. Natural coinfection by Streptococcus agalactiae and Francisella noatunensis subsp. orientalis in farmed Nile tilapia (Oreochromis niloticus L.). J. Fish Dis. 2017, 40, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, P.J.; Suebsing, R.; Sirithammajak, S.; Kampeera, J.; Turner, W.; Jeffs, A.; Withyachumanarnkul, B. Vertical transmission and concurrent infection of multiple bacterial pathogens in naturally infected red tilapia (Oreochromis spp.). Aquac. Res. 2016, 48, 2706–2717. [Google Scholar] [CrossRef]
- Sugiani, D.; Sukenda, S.; Harris, E.; Lusiastuti, A.M. Haemato responses and histopathology of tilapia (Oreochromis niloticus) to co-infection Streptococcus agalactiae and Aeromonas hydrophila. J. Riset Akuakultur 2012, 7, 85–91. [Google Scholar] [CrossRef]
- Sumiati, T.; Sukenda, S.; Nuryati, S.; Lusiastuti, A.M. Development of ELISA method to detect specific immune response in Nile tilapia (O. niloticus) vaccinated against A. hydrophila and S. agalactiae. J. Riset Akuakultur 2015, 10, 243–250. [Google Scholar] [CrossRef][Green Version]
- Swain, P.; Dash, S.; Bal, J.; Routray, P.; Sahoo, P.K.; Sahoo, S.K.; Meher, P.K. Passive transfer of maternal antibodies and their existence in eggs, larvae and fry of Indian major carp, Labeo rohita (Ham.). Fish Shellfish Immunol. 2006, 20, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Seppola, M.; Johnsen, H.; Mennen, S.; Myrnes, B.; Tveiten, H. Maternal transfer and transcriptional onset of immune genes during ontogenesis in Atlantic cod. Dev. Comp. Immunol. 2009, 33, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, Z.; Wang, H. Maternal immunity in fish: A review. Dev. Comp. Immunol. 2013, 39, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Ming, H.; Fu, D.; Zhen, M.; Ji, L. The effect of vaccinating turbot broodstocks on the maternal immunity transfer to offspring immunity. Fish Shellfish Immunol. 2014, 39, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Chen, Q.; Lu, M.; Hu, X.; Wang, M. Histology and ultrastructure of the tymus during development in tilapia Oreochromis niloticus. J. Anat. 2017, 230, 720–733. [Google Scholar] [CrossRef] [PubMed]
- Takemura, A. Changes in an immunoglobulin M (IgM)-like protein during larval stage in tilapia, Oreochromis mosambicus. Aquculture 1993, 115, 233–241. [Google Scholar] [CrossRef]
- Evans, J.J.; Klesius, P.H.; Shoemaker, C.A. Efficacy of Streptococcus agalactiae (group B) vaccine in tilapia (Oreocromis niloticus) by intraperitoneal and bath immersion administration. Vaccine 2004, 22, 3769–3773. [Google Scholar] [CrossRef] [PubMed]
- Swain, P.; Nayak, S.K. Role of maternally derived immunity in fish: A review. Fish Shellfish Immunol. 2009, 27, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Hanif, A.; Bakopoulos, V.; Dimitriadis, G.J. Maternal transfer of humoral specific and non-specific immune parameters to sea bream (Sparus aurata) larvae. Fish Shellfish Immunol. 2004, 17, 411–435. [Google Scholar] [CrossRef] [PubMed]
- Hanif, A.; Bakopoulos, V.; Leonardos, I.; Dimitriadis, G.J. The effect of sea bream (Sparus aurata) broodstock and larval vaccination on the susceptibility by Photobacterium damsela subsp. piscicida and on the humoral immune parameters. Fish Shellfish Immunol. 2005, 19, 345–361. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ji, D.; Shao, J.; Zhang, S. Maternal transfer and protective role of antibodies in zebrafish Danio rerio. Mol. Immunol. 2012, 51, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Ye, N.; Wu, H.; Zhang, Y. Maternal transfer and protection role in zebrafish (Danio rerio) offspring following vaccination of the brood stock with a live attenuated Vibrio anguillarum vaccine. Aquac. Res. 2016, 47, 3667–3678. [Google Scholar] [CrossRef]
- Sukenda, S.; Pratiwi, K.A.; Rahman, R.; Hidayatullah, D. Efficacy of whole cell vaccine Aeromonas hydrophila on catfish broodstock and its offspring resistance againt motile aeromonad septicemia (MAS). J. Akuakultur Indones. 2017, 16, 92–100. [Google Scholar] [CrossRef]
- Nur, I.; Sukenda, S.; Dana, D. Resistance of fry from vaccinated mother of Gift tilapia (Oreochromis niloticus Linn.) to artificial infection of Streptococcus iniae. J. Akuakultur Indones. 2004, 3, 37–43. [Google Scholar] [CrossRef]
- Nisaa, K.; Sukenda, S.; Junior, M.Z.; Lusiastuti, A.M.; Nuryati, S. Resistance of tilapia (Oreochromis niloticus) fry vaccinated at different gonadal developmental stages toward Streptococcus agalactiae infection. J. Vet. 2016, 17, 355–364. [Google Scholar]
- Sukenda, S.; Carman, O.; Rahman, R.; Hidayatullah, D.; Yumaidawati, N.S. Vaccination in tilapia with whole cell vaccine and disease resistance in its fry against Aeromona hyrophila. J. Akuakultur Indones. 2017, 16, 268–276. [Google Scholar] [CrossRef]
- Sukenda, S.; Sumiati, T.; Nuryati, S.; Lusiastuti, A.M.; Hidayatullah, D. Specific immune response kinetics and mortality patterns of tilapia Oreochromis niloticus on post-cocktail vaccination period against the infection of Aeromonas hydrophila and Streptococcus agalactiae. OmniAkuatika 2017, 13, 7–15. [Google Scholar] [CrossRef]
- Munang’andu, H.M.; Paul, J.; Evensen, Ø. An overview of vaccination strategies and antigen delivery systems for Streptococcus agalactiae vaccines in Nile tilapia (Oreochromis niloticus). Vaccines 2016, 4, 48. [Google Scholar] [CrossRef] [PubMed]
- Nisaa, K.; Sukenda, S.; Junior, M.Z.; Lusiastuti, A.M.; Nuryati, S. Fry tilapia (Oreochromis niloticus) antibody improvement against Streptococcus agalactiae through broodstock vaccination. Pak. J. Biotechnol. 2017, 14, 9–16. [Google Scholar]
- Shoemaker, C.A.; LaFrentz, B.R.; Klesius, P.H. Bivalent vaccination of sex reversed hybrid tilapia against Streptococcus iniae and Vibrio vulnificus. Aquaculture 2012, 354–355, 45–49. [Google Scholar] [CrossRef]
- Sukenda, S.; Febriansyah, T.R.; Nuryati, S. Whole-cell vaccine of Streptococcus agalactiae in Oreochromis sp. with immersion method. J. Akuakultur Indones. 2014, 13, 83–93. [Google Scholar] [CrossRef]
- Nisaa, K. Broodstock Vaccination of Tilapia (Oreochromis niloticus) and Resistance of Their Seeds against Streptococcus agalactiae Infection; Bogor Agricultural University: Bogor, Indonesia, 2016; Available online: http://repository.ipb.ac.id/handle/123456789/81514 (accessed on 24 July 2017).
- Firdausi, A.P.; Sukenda, S.; Nuryati, S. The efficacy of vaccination on tilapia seeds of (Oreochromis niloticus) using hyperosmotic infiltration method to prevent Streptococcus agalactiae infection. J. Vet. 2017, 18, 634–641. [Google Scholar]
- Pretto-Giordano, L.G.; Müller, E.E.; Klesius, P.; Gomes da Silva, P. Efficacy of an experimentally inactivated Streptococcus agalactiae vaccine in Nile tilapia (Oreochromis niloticus) reared in Brazil. Aquac. Res. 2010, 41, 1539–1544. [Google Scholar]
- Chettri, J.K.; Jaafar, R.M.; Skov, J.; Kania, P.W.; Dalsgaard, I.; Buchmann, K. Booster immersion vaccination using diluted Yersinia ruckeri bacterin confers protection against ERM in rainbow trout. Aquaculture 2015, 440, 1–5. [Google Scholar] [CrossRef]
- Lillehaug, A. Vaccination strategies and procedures. In Fish Vaccination; Gudding, R., Lillehaug, A., Evensen, Ø., Eds.; John Wiley & Sons: Oxford, UK, 2014; pp. 140–152. [Google Scholar]
- Swain, P.; Nayak, S.K.; Sahu, A.; Mohapatra, B.C.; Meher, P.K. Bath immunisation of spawn, fry and fingerlings of Indian major carps using a particulate bacterial antigen. Fish Shellfish Immunol. 2002, 13, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.S.; Syafiq, M.R.; Siti-Zahrah, A.; Fahmi, S.; Shahidan, H.; Hanan, Y.; Saad, M.Z. The effect of feed-based vaccination on tilapia farm endemic for streptococcosis. Fish Shellfish Immunol. 2017, 60, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Hardi, E.H.; Sukenda, S.; Enang, H.; Lusiastuti, A.M. Potential vaccine candidate of Streptococcus agalactiae for prevent strepcococosis on Nila tilapia (Oreochromis niloticus). J. Vet. 2013, 14, 408–416. [Google Scholar]
- Sumiati, T.; Lusiastuti, A.M.; Taukhid, T. Pengembangan Vaksin Koktail (A. hydrophila-S. agalactiae) Melalui Rendaman dan pakan untuk Pencegahan Penyakit Potensial pada Budidaya ikan nila; Balai Penelitian dan Pengebangan Budidaya Air Tawar (BPPBAT): Bogor, Indonesia, 2012.
- Amend, D.E. Potency testing of fish vaccines. Dev. Biol. Stand. 1981, 49, 447–454. [Google Scholar]
- Shelby, R.A.; Shoemaker, C.A.; Evans, J.J.; Klesius, P.H. Development of an Indirect ELISA to detect humoral response to Streptococcus iniae infection of Nile tilapia, Oreochromis niloticus. J. Appl. Aquac. 2001, 11, 35–44. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
| Treatments | Days After Hatch | ||||
|---|---|---|---|---|---|
| Group Vaccine | Challenging Bacteria | 5 | 10 | 15 | 20 |
| Sa | S. agalactiae | 87.22 ± 6.31 a | 85.35 ± 7.17 a | 77.03 ± 7.27 a | 68.52 ± 7.40 a |
| Ah | S. agalactiae | 43.89 ± 5.36 b | 31.50 ± 10.2 b | 24.54 ± 10.90 b | 22.96 ± 3.39 b |
| Biv | S. agalactiae | 84.44 ± 5.09 a | 82.97 ± 3.85 a | 68.67 ± 1.96 a | 62.59 ± 3.57 a |
| Sa | A. hydrophila | 53.00 ± 7.89 b | 35.84 ± 2.57 b | 31.94 ± 6.36 b | 26.76 ± 7.80 b |
| Ah | A. hydrophila | 89.93 ± 1.01 a | 87.15 ± 8.88 a | 72.36 ± 3.24 a | 58.48 ± 4.96 a |
| Biv | A. hydrophila | 89.93 ± 1.01 a | 84.55 ± 1.19 a | 65.97 ± 3.18 a | 58.36 ± 1.92 a |
| Sa | Co-infection | 45.94 ± 4.17 b | 38.10 ± 10.9 b | 23.40 ± 10.00 b | 16.40 ± 8.60 b |
| Ah | Co-infection | 45.94 ± 4.17 b | 33.93 ± 4.72 b | 21.55 ± 7.42 b | 16.60 ± 5.05 b |
| Biv | Co-infection | 81.78 ± 5.89 a | 79.46 ± 7.31 a | 63.42 ± 9.51 a | 57.11 ± 8.76 a |
| Broodstock Immunization | Larvae Immunized | Group | Challenging Bacteria | RPS (%) Days after Larvae Immunization | |
|---|---|---|---|---|---|
| 14 | 21 | ||||
| Pbs group | Biv | Pbs1 | S. agalactiae | 45.77 ± 3.75 a | 45.77 ± 3.75 a |
| Biv group | Biv | Biv1 | S. agalactiae | 71.03 ± 4.18 b | 74.74 ± 3.18 b |
| Pbs group | Biv | Pbs1 | A. hydrophila | 51.79 ± 9.94 a | 47.62 ± 4.12 a |
| Biv group | Biv | Biv1 | A. hydrophila | 76.59 ± 6.11 b | 73.81 ± 8.58 b |
| Pbs group | Biv | Pbs1 | Co-infection | 37.67 ± 8.19 a | 39.26 ± 5.59 a |
| Biv group | Biv | Biv1 | Co-infection | 69.37 ± 2.44 b | 71.48 ± 5.70 b |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasaribu, W.; Sukenda, S.; Nuryati, S. The Efficacy of Nile Tilapia (Oreochromis niloticus) Broodstock and Larval Immunization against Streptococcus agalactiae and Aeromonas hydrophila. Fishes 2018, 3, 16. https://doi.org/10.3390/fishes3010016
Pasaribu W, Sukenda S, Nuryati S. The Efficacy of Nile Tilapia (Oreochromis niloticus) Broodstock and Larval Immunization against Streptococcus agalactiae and Aeromonas hydrophila. Fishes. 2018; 3(1):16. https://doi.org/10.3390/fishes3010016
Chicago/Turabian StylePasaribu, Wesly, Sukenda Sukenda, and Sri Nuryati. 2018. "The Efficacy of Nile Tilapia (Oreochromis niloticus) Broodstock and Larval Immunization against Streptococcus agalactiae and Aeromonas hydrophila" Fishes 3, no. 1: 16. https://doi.org/10.3390/fishes3010016
APA StylePasaribu, W., Sukenda, S., & Nuryati, S. (2018). The Efficacy of Nile Tilapia (Oreochromis niloticus) Broodstock and Larval Immunization against Streptococcus agalactiae and Aeromonas hydrophila. Fishes, 3(1), 16. https://doi.org/10.3390/fishes3010016





