Population Structure of the European Seabass (Dicentrarchus labrax) in the Atlantic Iberian Coastal Waters Inferred from Body Morphometrics and Otolith Shape Analyses
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish Sampling
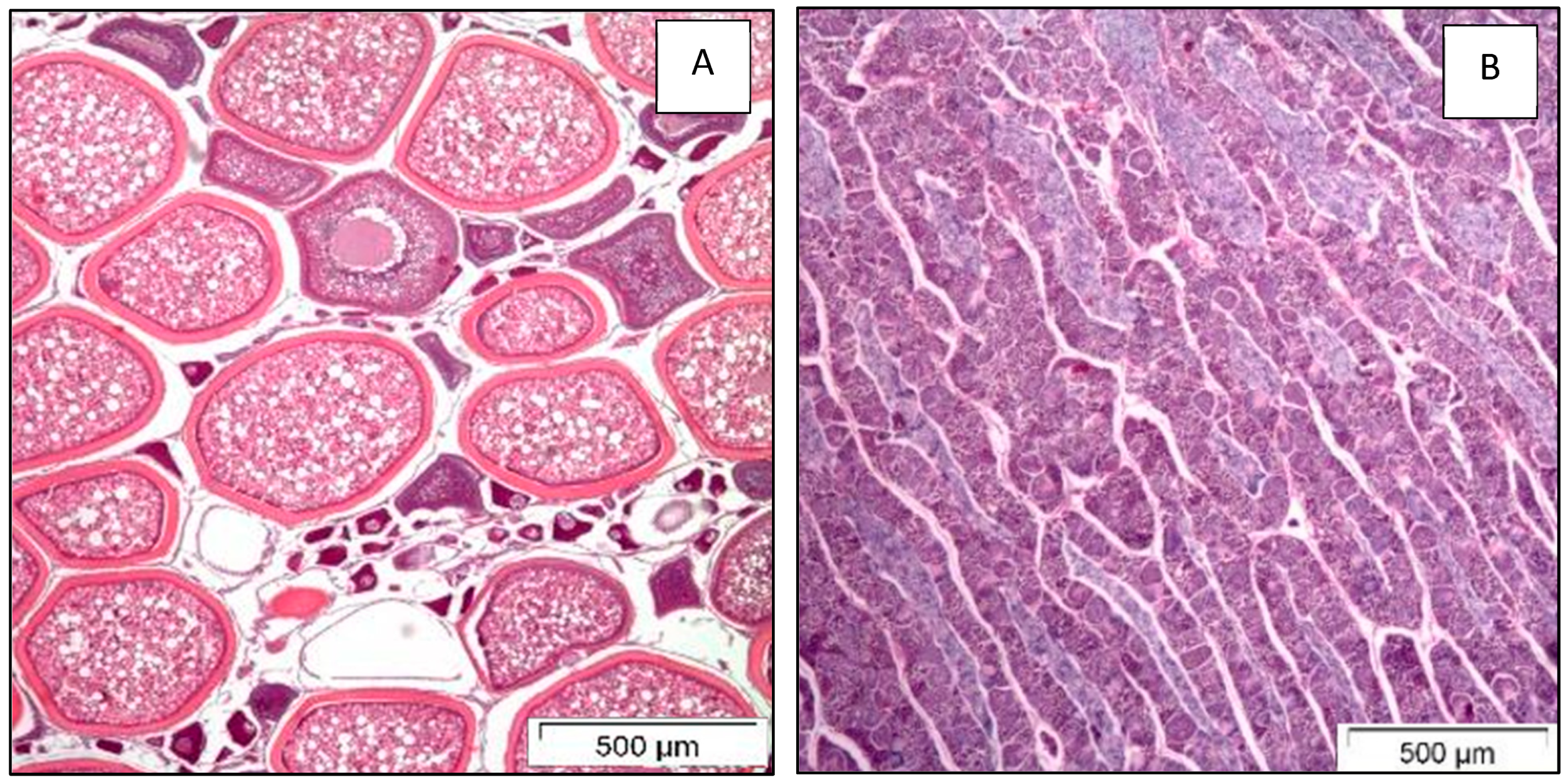
2.2. Sex Determination
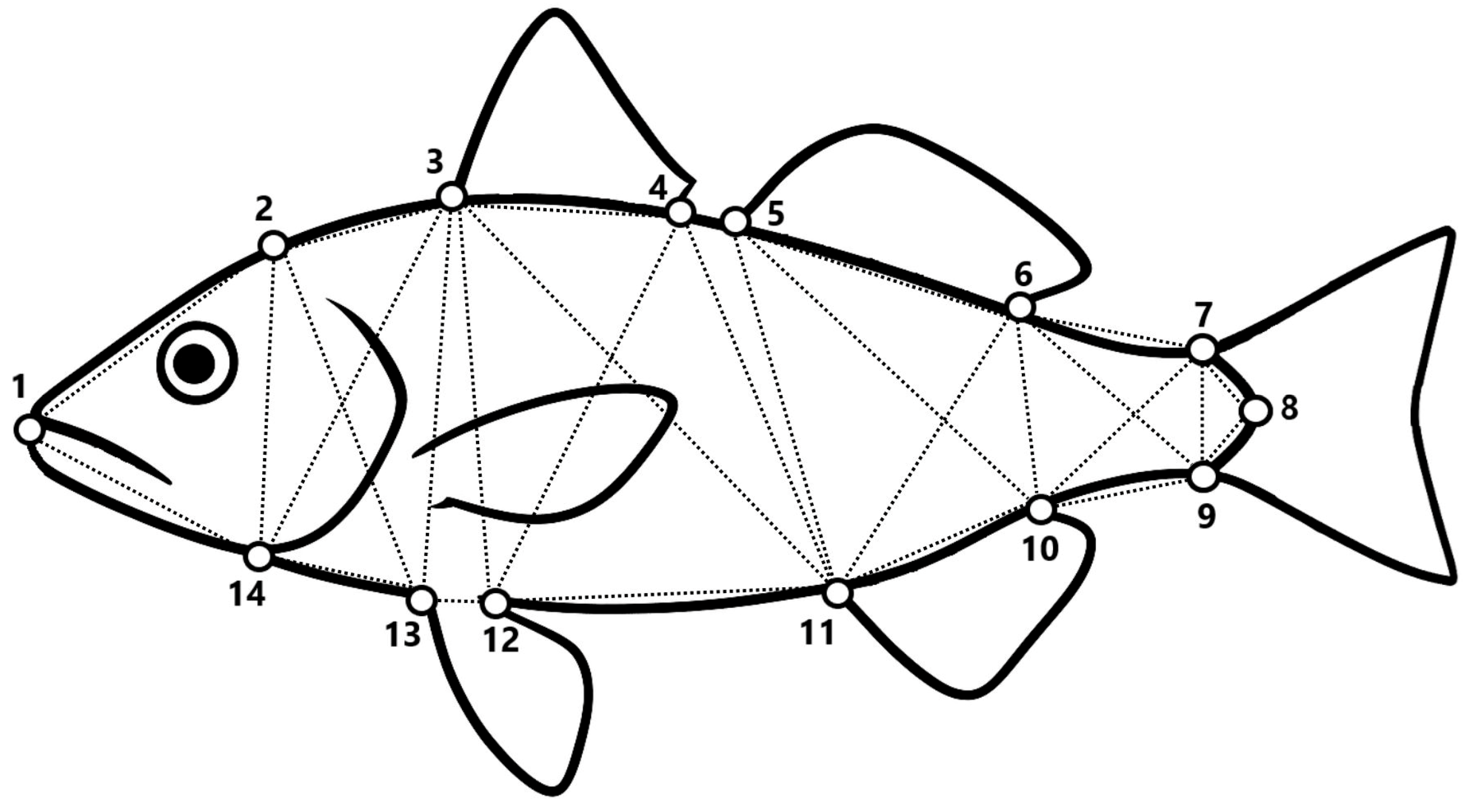
2.3. Body Geometric Morphometrics
2.4. Otolith Shape Contour
2.5. Statistical Analyses
3. Results
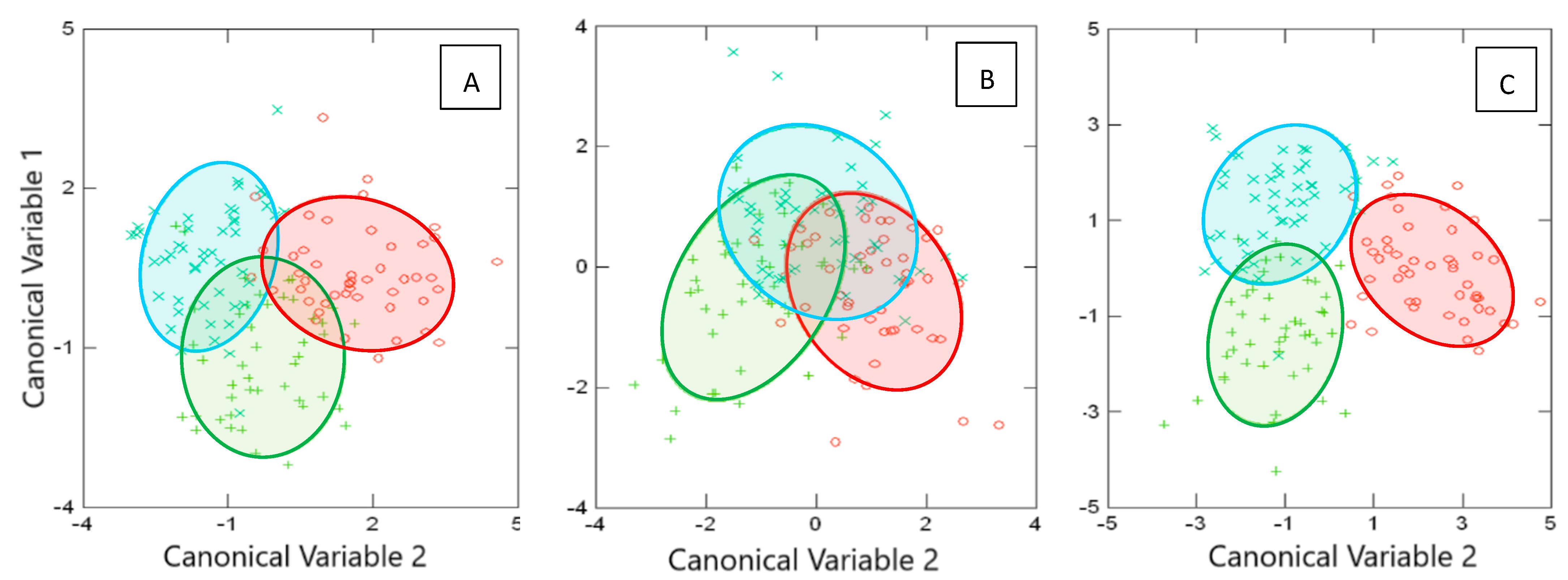
3.1. Body Geometric Morphometrics
3.2. Otolith Shape Contour
3.3. Combination of Both Approaches
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANCOVA | One-way Analysis of Covariance |
| ANOVA | One-way Analysis of Variance |
| D | Distance |
| DNA | Deoxyribonucleic Acid |
| EFD | Elliptical Fourier Descriptor |
| ICES | International Council for the Exploration of the Sea |
| LDFA | Linear Discriminant Function Analysis |
| MANOVA | Multivariate Analysis of Variance |
| MP | Megapixels |
| N | Sample Size |
| RFID | Radio-Frequency Identification |
| SNP | Single Nucleotide Polymorphism |
| TL | Total Length |
| TD | Transformed Distance |
| UK | United Kingdom |
References
- López, R.; De Pontual, H.; Bertignac, M.; Mahévas, S. What can exploratory modelling tell us about the ecobiology of European sea bass (Dicentrarchus labrax): A comprehensive overview. Aquat. Living Resour. 2015, 28, 61–79. [Google Scholar] [CrossRef]
- Bagdonas, K.; Nika, N.; Bristow, G.; Jankauskiene, R.; Salyte, A.; Kontautas, A. First record of Dicentrarchus labrax (Linnaeus, 1758) from the southeastern Baltic Sea (Lithuania). J. Appl. Ichthyol. 2011, 27, 1390–1391. [Google Scholar] [CrossRef]
- Quéré, N.; Desmarais, E.; Tsigenopoulos, C.S.; Belkhir, K.; Bonhomme, F.; Guinand, B. Gene flow at major transitional areas in sea bass (Dicentrarchus labrax) and the possible emergence of a hybrid swarm. Ecol. Evol. 2012, 2, 3061–3078. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.; Tanner, S.E.; Mateus, C.S.; Ribeiro, F.; Quintella, B.R. Not so much a sea bass: Divergent European Sea bass (Dicentrarchus labrax L.) freshwater incursions. J. Fish Biol. 2024, 104, 1241–1246. [Google Scholar] [CrossRef]
- Antunes, C.; Antunes, C.M.; Fernandes, M.; Dias, E. Prolonged use of freshwater habitats by the European sea bass revealed by otolith chemical analysis. J. Fish Biol. 2025, 107, 1201–1212. [Google Scholar] [CrossRef]
- Froese, R.; Zeller, D.; Kleisner, K.; Pauly, D. What catch data can tell us about the status of global fisheries. Mar. Biol. 2012, 159, 1283–1292. [Google Scholar] [CrossRef]
- De Pontual, H.; Lalire, M.; Fablet, R.; Laspougeas, C.; Garren, F.; Martin, S.; Drogou, M.; Woillez, M. New insights into behavioural ecology of European seabass off the West Coast of France: Implications at local and population scales. ICES J. Mar. Sci. 2019, 76, 501–515. [Google Scholar] [CrossRef]
- Bagni, M. Dicentrarchus labrax. In Cultured Aquatic Species Fact Sheets; Crespi, V., New, M., Eds.; FAO: Rome, Italy, 2009. [Google Scholar]
- Vázquez, F.J.S.; Munoz-Cueto, J.A. Biology of European Sea Bass; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar] [CrossRef]
- Pickett, G.D.; Pawson, M.G. Sea Bass: Biology, Exploitation and Conservation; Chapman & Hall: London, UK, 1994; Volume 12. [Google Scholar] [CrossRef]
- Spitz, J.; Chouvelon, T.; Cardinaud, M.; Kostecki, C.; Lorance, P. Prey preferences of adult sea bass Dicentrarchus labrax in the northeastern Atlantic: Implications for bycatch of common dolphin Delphinus delphis. ICES J. Mar. Sci. 2013, 70, 452–461. [Google Scholar] [CrossRef]
- Leis, J.M.; Balma, P.; Ricoux, R.; Galzin, R. Ontogeny of swimming ability in the European Sea Bass, Dicentrarchus labrax (L.) (Teleostei: Moronidae). Mar. Biol. Res. 2012, 8, 265–272. [Google Scholar] [CrossRef]
- Dawson, J.; Lincoln, H.; Sturrock, A.M.; Martinho, F.; McCarthy, I.D. Recruitment of European sea bass (Dicentrarchus labrax) in northerly UK estuaries indicates a mismatch between spawning and fisheries closure periods. J. Fish Biol. 2024, 105, 564–576. [Google Scholar] [CrossRef]
- EUMOFA. Commercial and Recreational Fisheries for Wild Seabass in the Atlantic. In Economic and Market Study; Office of the European Union: Luxembourg, 2021. [Google Scholar] [CrossRef]
- ICES. Report of the Working Group on Assessment of New MoU Species (WGNEW), 5–9 March 2012. In ICES Expert Group Reports (Until 2018); ICES: Copenhagen, Denmark, 2012. [Google Scholar] [CrossRef]
- ICES. ICES Advice 2025. In ICES Advice; ICES: Copenhagen, Denmark, 2025. [Google Scholar] [CrossRef]
- Grilli, G.; Curtis, J.; Hynes, S.; O’Reilly, P. Anglers’ views on stock conservation: Sea bass angling in Ireland. Mar. Policy 2019, 99, 34–41. [Google Scholar] [CrossRef]
- ICES. Stock Identification Methods Working Group (SIMWG). In ICES Scientific Reports; Report; ICES: Copenhagen, Denmark, 2021. [Google Scholar] [CrossRef]
- ICES. Sea bass (Dicentrarchus labrax) in divisions 8.c and 9.a (southern Bay of Biscay and Atlantic Iberian waters). In ICES Advice: Recurrent Advice; ICES: Copenhagen, Denmark, 2025. [Google Scholar] [CrossRef]
- Kerr, L.A.; Hintzen, N.T.; Cadrin, S.X.; Clausen, L.W.; Dickey-Collas, M.; Goethel, D.R.; Hatfield, E.M.C.; Kritzer, J.P.; Nash, R.D.M. Lessons learned from practical approaches to reconcile mismatches between biological population structure and stock units of marine fish. ICES J. Mar. Sci. 2017, 74, 1708–1722. [Google Scholar] [CrossRef]
- Cadrin, S.X. Defining spatial structure for fishery stock assessment. Fish. Res. 2020, 221, 105397. [Google Scholar] [CrossRef]
- Sarakinis, K.G.; Reis-Santos, P.; Ye, Q.; Earl, J.; Gillanders, B.M. Combining natural markers to investigate fish population structure and connectivity. Estuar. Coast. Shelf Sci. 2024, 308, 108920. [Google Scholar] [CrossRef]
- da Fonseca, R.R.; Campos, P.F.; Rey-Iglesia, A.; Barroso, G.V.; Bergeron, L.A.; Nande, M.; Tuya, F.; Abidli, S.; Pérez, M.; Riveiro, I.; et al. Population Genomics Reveals the Underlying Structure of the Small Pelagic European Sardine and Suggests Low Connectivity within Macaronesia. Genes 2024, 15, 170. [Google Scholar] [CrossRef]
- Andersson, L.; Bekkevold, D.; Berg, F.; Farrell, E.D.; Felkel, S.; Ferreira, M.S.; Fuentes-Pardo, A.P.; Goodall, J.; Pettersson, M. How Fish Population Genomics Can Promote Sustainable Fisheries: A Road Map. Annu. Rev. Anim. Biosci. 2024, 12, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Elsdon, T.; Wells, B.; Campana, S.; Gillanders, B.; Jones, C.; Limburg, K.; Secor, D.; Thorrold, S.; Walther, B. Otolith chemistry to describe movements and life-history parameters of fishes: Hypotheses, assumptions, limitations and inferences. Oceanogr. Mar. Biol. Annu. Rev. 2008, 46, 297–330. [Google Scholar] [CrossRef]
- Le Luherne, E.; Daverat, F.; Woillez, M.; Pécheyran, C.; de Pontual, H. Coupling natural and electronic tags to explore spawning site fidelity and natal homing in northeast Atlantic European seabass. Estuar. Coast. Shelf Sci. 2022, 278, 108118. [Google Scholar] [CrossRef]
- Muniz, A.A.; Moura, A.; Triay-Portella, R.; Moreira, C.; Santos, P.T.; Correia, A.T. Population structure of the chub mackerel (Scomber colias) in the North-east Atlantic inferred from otolith shape and body morphometrics. Mar. Freshw. Res. 2021, 72, 341–352. [Google Scholar] [CrossRef]
- Ferreira, I.; Schroeder, R.; Mugerza, E.; Oyarzabal, I.; McCarthy, I.D.; Correia, A.T. Chelidonichthys lucerna (Linnaeus, 1758) Population Structure in the Northeast Atlantic Inferred from Landmark-Based Body Morphometry. Biology 2024, 13, 17. [Google Scholar] [CrossRef]
- Freitas, F.L.; Pereira, N.S.; Pinheiro, P.B.; Schroeder, R.; Correia, A.T. Assessing the population structure of Plagioscion squamosissimus (Teleostei, Perciformes, Sciaenidae) from the São Francisco River, Bahia, Brazil, using body morphology and otolith shape signatures. J. Fish Biol. 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.; Santos, D.; Moreira, C.; Feijó, D.; Rocha, A.; Correia, A.T. Population structure of Chelidonichthys lucerna in Portugal mainland using otolith shape and elemental signatures. Mar. Biol. Res. 2019, 15, 500–512. [Google Scholar] [CrossRef]
- Vasconcelos, J.; Cirera, M.; Vieira, A.R.; Otero-Ferrer, J.L.; Tuset, V.M. Application of shape analysis for the identification of pelagic fish stocks (Trachurus trachurus): Body and otolith shape using geometric morphometrics and wavelet functions. Hydrobiologia 2025, 852, 2847–2869. [Google Scholar] [CrossRef]
- McQuinn, I.H. Metapopulations and the Atlantic herring. Rev. Fish Biol. Fish. 1997, 7, 297–329. [Google Scholar] [CrossRef]
- Tuset, V.M.; Lozano, I.J.; González, J.A.; Pertusa, J.F.; García-Díaz, M.M. Shape indices to identify regional differences in otolith morphology of comber, Serranus cabrilla (L., 1758). J. Appl. Ichthyol. 2003, 19, 88–93. [Google Scholar] [CrossRef]
- Heimbrand, Y.; Limburg, K.; Hüssy, K.; Næraa, T.; Casini, M. Cod otoliths document accelerating climate impacts in the Baltic Sea. Sci. Rep. 2024, 14, 16750. [Google Scholar] [CrossRef] [PubMed]
- Hoff, N.T.; Dias, J.F.; Zani-Teixeira, M.L.; Soeth, M.; Correia, A.T. Population structure of the bigtooth corvina Isopisthus parvipinnis from the Southwest Atlantic Ocean as determined by whole-body morphology. Reg. Stud. Mar. Sci. 2020, 39, 101379. [Google Scholar] [CrossRef]
- Moreira, C.; Froufe, E.; Vaz-Pires, P.; Correia, A.T. Otolith shape analysis as a tool to infer the population structure of the blue jack mackerel, Trachurus picturatus, in the NE Atlantic. Fish. Res. 2019, 209, 40–48. [Google Scholar] [CrossRef]
- Schroeder, R.; Schwingel, P.R.; Correia, A.T. Population structure of the Brazilian sardine (Sardinella brasiliensis) in the South-West Atlantic inferred from body morphology and otolith shape signatures. Hydrobiologia 2022, 849, 1367–1381. [Google Scholar] [CrossRef]
- Souche, E.L.; Hellemans, B.; Babbucci, M.; Macaoidh, E.; Guinand, B.; Bargelloni, L.; Chistiakov, D.A.; Patarnello, T.; Bonhomme, F.; Martinsohn, J.T.; et al. Range-wide population structure of European sea bass Dicentrarchus labrax. Biol. J. Linn. Soc. 2015, 116, 86–105. [Google Scholar] [CrossRef]
- Robinet, T.; Roussel, V.; Cheze, K.; Gagnaire, P.A. Spatial gradients of introgressed ancestry reveal cryptic connectivity patterns in a high gene flow marine fish. Mol. Ecol. 2020, 20, 3857–3871. [Google Scholar] [CrossRef]
- Taylor, M.I.; Lamb, P.D.; Coscia, I.; Murray, D.S.; Brown, M.; Cameron, T.C.; Davison, P.I.; Freeman, H.A.; Georgiou, K.; Grati, F.; et al. High-density SNP panel provides little evidence for population structure in European sea bass (Dicentrarchus labrax) in waters surrounding the UK. ICES J. Mar. Sci. 2025, 82, fsaf064. [Google Scholar] [CrossRef]
- Castilho, R.; McAndrew, B.J. Population structure of seabass in Portugal: Evidence from allozymes. J. Fish Biol. 1998, 53, 1038–1049. [Google Scholar] [CrossRef]
- Jónsdóttir, I.G.; Campana, S.E.; Marteinsdóttir, G. Otolith shape and temporal stability of spawning groups of Icelandic cod (Gadus morhua L.). ICES J. Mar. Sci. 2006, 63, 1501–1512. [Google Scholar] [CrossRef]
- de Pontual, H.; Heerah, K.; Goossens, J.; Garren, F.; Martin, S.; Le Ru, L.; Le Roy, D.; Woillez, M.; Edwards, J.E.; De Putter, G.; et al. Seasonal migration, site fidelity, and population structure of European seabass (Dicentrarchus labrax). ICES J. Mar. Sci. 2023, 80, 1606–1618. [Google Scholar] [CrossRef]
- Goossens, J.; Woillez, M.; Wright, S.; Edwards, J.E.; De Putter, G.; Torreele, E.; Verhelst, P.; Sheehan, E.; Moens, T.; Reubens, J. Elucidating the migrations of European seabass from the southern north sea using mark-recapture data, acoustic telemetry and data storage tags. Sci. Rep. 2024, 14, 13180. [Google Scholar] [CrossRef] [PubMed]
- Secor, D.H.; Dean, J.M.; Laban, E.H. Otolith removal and preparation for microstructural examination. In Otolith Microstructure Examination and Analysis; Stevenson, D.K., Campana, S.E., Eds.; Canadian Special Publication of Fisheries and Aquatic Sciences: Ottawa, ON, Canada, 1992; Volume 117, pp. 19–57. [Google Scholar]
- Suvarna, S.K.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques, 8th ed.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- OECD. Guidance Document on the Diagnosis of Endocrine-Related Histopathology in Fish Gonads; OECD Publishing: Paris, France, 2010. [Google Scholar] [CrossRef]
- Strauss, R.E.; Bookstein, F.L. The Truss: Body Form Reconstructions in Morphometrics. Syst. Biol. 1982, 31, 113–135. [Google Scholar] [CrossRef]
- Mahé, K.; Clota, F.; Blanc, M.O.; Defruit, G.B.; Chatain, B.; de Pontual, H.; Amara, R.; Ernande, B. Otolith morphogenesis during the early life stages of fish is temperature-dependent: Validation by experimental approach applied to European seabass (Dicentrarchus labrax). J. Fish. Biol. 2024, 104, 2032–2043. [Google Scholar] [CrossRef] [PubMed]
- Rodgveller, C.J.; Hutchinson, C.E.; Harris, J.P.; Vulstek, S.C.; Guthrie, C.M. Otolith shape variability and associated body growth differences in giant grenadier, Albatrossia pectoralis. PLoS ONE 2017, 12, e0180020. [Google Scholar] [CrossRef]
- Campana, S.E.; Casselman, J.M. Stock discrimination using otolith shape analysis. Can. J. Fish. Aquat. Sci. 1993, 50, 1062–1083. [Google Scholar] [CrossRef]
- Quinn, G.P.; Keough, M.J. Experimental Design and Data Analysis for Biologists; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar] [CrossRef]
- Reist, J. An empirical evaluation of several univariate methods that adjust for size variation in morphometric data. Can. J. Zool. 2011, 63, 1429–1439. [Google Scholar] [CrossRef]
- Moreira, C.; Froufe, E.; Vaz-Pires, P.; Triay-Portella, R.; Correia, A.T. Landmark based geometric morphometrics analysis of body shape variation among populations of the blue jack mackerel, Trachurus picturatus, from the North-East Atlantic. J. Sea Res. 2020, 163, 101926. [Google Scholar] [CrossRef]
- Federica, S.; Silvio, K.; Umberto, S.; Andrea, D.G.; Massimiliano, S. Population-level shape variation and otolith asymmetry in Diplodus annularis. Sci. Rep. 2025, 15, 87096. [Google Scholar] [CrossRef]
- Fritsch, M.; Morizur, Y.; Lambert, E.; Bonhomme, F.; Guinand, B. Assessment of sea bass (Dicentrarchus labrax, L.) stock delimitation in the Bay of Biscay and the English Channel based on mark-recapture and genetic data. Fish. Res. 2007, 83, 123–132. [Google Scholar] [CrossRef]
- Doyle, T.K.; Haberlin, D.; Clohessy, J.; Bennison, A.; Jessopp, M. Localised residency and inter-annual fidelity to coastal foraging areas may place sea bass at risk to local depletion. Sci. Rep. 2017, 7, 45841. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, R.; Ó Maoiléidigh, N.; McGinnity, P.; Bond, N.; Culloty, S. The novel use of pop-off satellite tags (PSATs) to investigate the migratory behaviour of European sea bass Dicentrarchus labrax. J. Fish Biol. 2018, 92, 1404–1421. [Google Scholar] [CrossRef] [PubMed]
- Palma, J.; Andrade, J.P. Morphological study of Diplodus sargus, Diplodus puntazzo, and Lithognathus mormyrus (Sparidae) in the Eastern Atlantic and Mediterranean Sea. Fish. Res. 2002, 57, 1–8. [Google Scholar] [CrossRef]
- Moreira, C.; Correia, A.T.; Vaz-Pires, P.; Froufe, E. Genetic diversity and population structure of the blue jack mackerel Trachurus picturatus across its western distribution. J. Fish Biol. 2019, 94, 725–731. [Google Scholar] [CrossRef]
- Alvarez, I.; Gomez-Gesteira, M.; de Castro, M.; Lorenzo, M.N.; Crespo, A.J.C.; Dias, J.M. Comparative analysis of upwelling influence between the western and northern coast of the Iberian Peninsula. Cont. Shelf Res. 2011, 31, 388–399. [Google Scholar] [CrossRef]
- del Rosario, J.J.A.; Gómez, E.B.; Pérez, J.M.V.; Rey, F.M.; Silva-Ramírez, E.L. A New Insight on the Upwelling along the Atlantic Iberian Coasts and Warm Water Outflow in the Gulf of Cadiz from Multiscale Ultrahigh Resolution Sea Surface Temperature Imagery. J. Mar. Sci. Eng. 2024, 12, 1580. [Google Scholar] [CrossRef]
- Geladakis, G.; Nikolioudakis, N.; Koumoundouros, G.; Somarakis, S. Morphometric discrimination of pelagic fish stocks challenged by variation in body condition. ICES J. Mar. Sci. 2018, 75, 711–718. [Google Scholar] [CrossRef]
- Imre, I.; McLaughlin, R.L.; Noakes, D.L.G. Phenotypic plasticity in brook charr: Changes in caudal fin induced by water flow. J. Fish Biol. 2002, 61, 1171–1181. [Google Scholar] [CrossRef]
- Lostrom, S.; Evans, J.P.; Grierson, P.F.; Collin, S.P.; Davies, P.M.; Kelley, J.L. Linking stream ecology with morphological variability in a native freshwater fish from semi arid Australia. Ecol. Evol. 2015, 5, 3272–3287. [Google Scholar] [CrossRef]
- Satterfield, D.R.; Claverie, T.; Wainwright, P.C. Body shape and mode of propulsion do not constrain routine swimming in coral reef fishes. Funct. Ecol. 2023, 37, 343–357. [Google Scholar] [CrossRef]
- Llope, M.; Anadón, R.; Viesca, L.; Quevedo, M.; González-Quirós, R.; Stenseth, N.C. Hydrography of the southern Bay of Biscay shelf-break region: Integrating the multiscale physical variability over the period 1993–2003. J. Geophys. Res. Oceans 2006, 111, C09020. [Google Scholar] [CrossRef]
- Maestro, A.; Gallastegui, A.; Moreta, M.; Llave, E.; Bohoyo, F.; Druet, M.; Navas, J.; Mink, S.; Fernández-Sáez, F.; Catalán, M.; et al. Echo-character distribution in the Cantabrian Margin and the Biscay Abyssal Plain. J. Maps 2021, 17, 533–542. [Google Scholar] [CrossRef]
- González-Pola, C.; Somavilla, R.; Graña, R.; Viloria, A.; Ibáñez-Tejero, L. A decade long flow reversal in the intergyre region of the eastern north Atlantic. Prog. Oceanogr. 2025, 231, 103406. [Google Scholar] [CrossRef]
- Capoccioni, F.; Costa, C.; Aguzzi, J.; Menesatti, P.; Lombarte, A.; Ciccotti, E. Ontogenetic and environmental effects on otolith shape variability in three Mediterranean European eel (Anguilla anguilla, L.) local stocks. J. Exp. Mar. Biol. Ecol. 2011, 397, 1–7. [Google Scholar] [CrossRef]
- Vaz, A.; Guerreiro, M.A.; Landa, J.; Hannipoula, O.; Thasitis, I.; Scarcella, G.; Sabatini, L.; Vitale, S.; Mugerza, E.; Mahé, K.; et al. Otolith shape analysis as a tool for stock identification of two commercially important marine fishes: Helicolenus dactylopterus and Merluccius merluccius. Estuarine Coastal Shelf Sci. 2023, 293, 108471. [Google Scholar] [CrossRef]
- NASA Ocean Biology Processing Group. Ocean Color Data (MODIS-Aqua). Available online: https://oceancolor.gsfc.nasa.gov/ (accessed on 1 July 2024).
- Marengo, M.; Baudouin, M.; Viret, A.; Laporte, M.; Berrebi, P.; Vignon, M.; Marchand, B.; Durieux, E.D.H. Combining microsatellite, otolith shape and parasites community analyses as a holistic approach to assess population structure of Dentex dentex. J. Sea Res. 2017, 128, 1–14. [Google Scholar] [CrossRef]
- Neves, J.; Veríssimo, A.; Santos, A.M.; Garrido, S. Comparing otolith shape descriptors for population structure inferences in a small pelagic fish, the European sardine Sardina pilchardus (Walbaum, 1792). J. Fish Biol. 2023, 102, 1219–1236. [Google Scholar] [CrossRef] [PubMed]
- Jurado-Ruzafa, A.; Vasconcelos, J.; Otero-Ferrer, J.L.; Navarro, M.R.; Massaro, A.; Hernández, C.; Tuset, V.M. Phenotypic response of a geographically expanding species, Scomber colias: Clues in the fish otolith shape. Estuar. Coast. Shelf Sci. 2024, 305, 108880. [Google Scholar] [CrossRef]
- Spreitzer, M.L.; Mautner, S.; Makasa, L.; Sturmbauer, C. Genetic and morphological population differentiation in the rock-dwelling and specialized shrimp-feeding cichlid fish species Altolamprologus compressiceps from Lake Tanganyika, East Africa. Hydrobiologia 2012, 682, 143–154. [Google Scholar] [CrossRef] [PubMed]
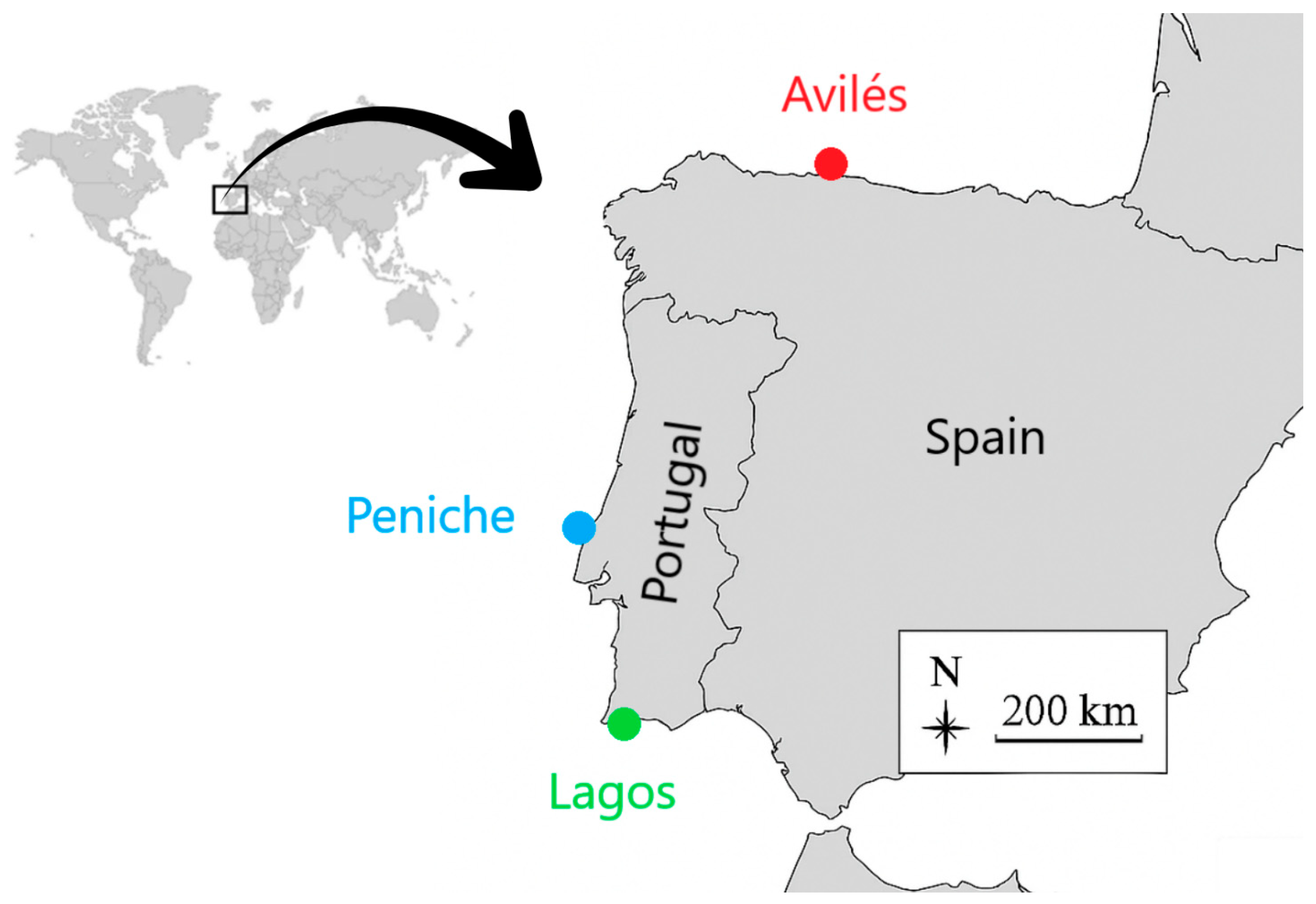

| Location | Date of Collection | n | TL (cm) | Age (Years) |
|---|---|---|---|---|
| Avilés | 14 January 2025 | 47 | 41.7 ± 0.3 | 3–5 |
| Peniche | 12 February 2025 | 48 | 44.3 ± 0.3 | 2–5 |
| Lagos | 25 March 2025 | 45 | 45.3 ± 0.5 | 2–4 |
| Body Landmarks | ||
| Number | Location | |
| 1 | Anterior tip of the mouth | |
| 2 | Most posterior aspect of neurocranium | |
| 3 | Anterior insertion of the 1st (spiny) dorsal fin | |
| 4 | Posterior insertion of the 1st (spiny) dorsal fin | |
| 5 | Anterior insertion of the 2nd (soft-rayed) dorsal fin | |
| 6 | Posterior insertion of the 2nd (soft-rayed) dorsal fin | |
| 7 | Dorsal insertion of the caudal fin | |
| 8 | Posterior margin of the caudal peduncle | |
| 9 | Ventral insertion of the caudal fin | |
| 10 | Posterior insertion of the anal fin | |
| 11 | Anterior insertion of the anal fin | |
| 12 | Posterior insertion of the pelvic fin | |
| 13 | Anterior insertion of the pelvic fin | |
| 14 | Ventral insertion of the operculum | |
| Morphometric Distances | ||
| Number | Landmarks | Description |
| D1 | 1 to 2 | Head length |
| D2 | 1 to 14 | Maxilla length |
| D3 | 2 to 3 | Distance from the most posterior aspect of neurocranium to the 1st dorsal fin |
| D4 | 2 to 13 | Posterior height of head |
| D5 | 2 to 14 | Anterior height of head |
| D6 | 3 to 13 | Anterior body height |
| D7 | 3 to 14 | Distance from the posterior tip of the mouth to the anterior insertion of the 1st dorsal fin |
| D8 | 13 to 12 | Length of pelvic fin |
| D9 | 13 to 14 | Distance from maxilla to pelvic fin |
| D10 | 3 to 4 | Length of 1st (spiny) dorsal fin |
| D11 | 3 to 11 | Distance between the origin of 1st dorsal fin and the origin of anal fin |
| D12 | 3 to 12 | Distance from the anterior insertion of the (spiny) dorsal fin to the posterior insertion of the pelvic fin |
| D13 | 4 to 11 | Posterior body height |
| D14 | 4 to 12 | Distance from the anterior insertion of the caudal fin to the anterior insertion of the 2nd dorsal fin |
| D15 | 11 to 12 | Distance between pelvic fin and anal fin |
| D16 | 4 to 5 | Distance between 1st and 2nd dorsal fins |
| D17 | 5 to 6 | Length of 2nd dorsal fin |
| D18 | 5 to 10 | Anterior diagonal height of posterior body |
| D19 | 5 to 11 | Distance from the anterior insertion of the anal fin to the anterior insertion of the 2nd dorsal fin |
| D20 | 6 to 10 | Anterior caudal peduncle height |
| D21 | 6 to 11 | Distance from the anterior insertion of the anal fin to the posterior insertion of the 2nd dorsal fin |
| D22 | 10 to 11 | Length of anal fin |
| D23 | 6 to 7 | Distance between 2nd dorsal fin and caudal fin |
| D24 | 6 to 9 | Anterior diagonal of caudal peduncle |
| D25 | 7 to 9 | Posterior caudal peduncle height |
| D26 | 7 to 10 | Posterior diagonal of caudal peduncle |
| D27 | 9 to 10 | Distance between anal fin and caudal fin |
| D28 | 7 to 8 | Distance between the dorsal insertion of caudal fin and the posterior end of vertebrate column |
| D29 | 8 to 9 | Distance between the ventral insertion of caudal fin and the posterior end of vertebrate column |
| Avilés | Peniche | Lagos | |
|---|---|---|---|
| Transformed Distances | |||
| TD1 | 9.68 ± 0.11 a | 8.86 ± 0.08 b | 9.23 ± 0.11 c |
| TD2 | 8.39 ± 0.11 a | 8.52 ± 0.08 ab | 8.82 ± 0.08 b |
| TD3 | 5.50 ± 0.07 a | 6.11 ± 0.05 b | 5.91 ± 0.05 c |
| TD4 | 9.56 ± 0.07 a | 9.63 ± 0.06 a | 9.64 ± 0.06 a |
| TD5 | 7.82 ± 0.06 a | 7.54 ± 0.04 b | 7.70 ± 0.05 ab |
| TD7 | 10.75 ± 0.08 a | 10.50 ± 0.05 b | 10.54 ± 0.06 ab |
| TD8 | 1.90 ± 0.03 a | 2.20 ± 0.05 b | 2.23 ± 0.04 b |
| TD9 | 4.85 ± 0.07 a | 4.35 ± 0.08 b | 4.47 ± 0.05 b |
| TD11 | 15.43 ± 0.10 a | 15.95 ± 0.13 a | 15.51 ± 0.08 a |
| TD13 | 9.41 ± 0.07 a | 9.74 ± 0.09 b | 9.62 ± 0.07 ab |
| TD15 | 12.37 ± 0.1 a | 12.19 ± 0.22 a | 11.99 ± 0.09 a |
| TD16 | 1.09 ± 0.04 a | 1.08 ± 0.06 a | 1.21 ± 0.06 a |
| TD17 | 6.91 ± 0.07 a | 7.26 ± 0.14 b | 7.22 ± 0.06 ab |
| TD18 | 10.23 ± 0.08 a | 10.46 ± 0.05 b | 10.30 ± 0.06 ab |
| TD19 | 8.99 ± 0.07 a | 9.41 ± 0.11 b | 9.21 ± 0.06 ab |
| TD20 | 5.26 ± 0.05 ab | 5.51 ± 0.14 a | 5.19 ± 0.04 b |
| TD21 | 7.82 ± 0.06 a | 8.45 ± 0.03 a | 8.10 ± 0.06 a |
| TD22 | 4.95 ± 0.05 a | 5.28 ± 0.16 a | 5.12 ± 0.06 a |
| TD23 | 6.66 ± 0.10 a | 6.40 ± 0.09 ab | 6.31 ± 0.07 a |
| TD24 | 8.19 ± 0.10 a | 7.87 ± 0.06 b | 7.80 ± 0.07 b |
| TD25 | 4.36 ± 0.05 a | 4.27 ± 0.07 a | 4.44 ± 0.04 a |
| TD26 | 7.40 ± 0.08 a | 7.42 ± 0.12 a | 7.37 ± 0.07 a |
| TD27 | 5.70 ± 0.07 a | 5.43 ± 0.05 b | 5.50 ± 0.08 ab |
| TD28 | 2.95 ± 0.03 a | 3.00 ± 0.02 a | 2.92 ± 0.03 a |
| TD29 | 2.95 ± 0.03 a | 3.13 ± 0.04 a | 3.00 ± 0.03 a |
| Elliptical Fourier Descriptors | |||
| D1 | 0.0014 ± 0.0036 a | 0.0020 ± 0.0036 a | −0.00262 ± 0.0042 a |
| A2 | 0.0019 ± 0.0039 a | 0.0033 ± 0.0033 a | −0.00548 ± 0.0032 a |
| B2 | −0.0057 ± 0.0039 a | 0.0009 ± 0.00211 ab | 0.0052 ± 0.0027 b |
| C2 | −0.0052 ± 0.0020 a | −0.0007 ± 0.0022 a | 0.0066 ± 0.0024 b |
| D2 | 0.0009 ± 0.0017 a | 0.0008 ± 0.0015 a | −0.002 ± 0.0021 a |
| A3 | 0.0030 ± 0.0012 a | −0.0031 ± 0.0013 b | 0.0003 ± 0.016 a |
| B3 | 0.0007 ± 0.001 a | 0.0008 ± 0.0019 a | −0.0013 ± 0.0014 a |
| C3 | −0.0003 ± 0.0010 ab | −0.0023 ± 0.0009 a | 0.0028 ± 0.0013 b |
| D3 | −0.0012 ± 0.0009 a | 0.0010 ± 0.0012 a | 0.0001 ± 0.0009 a |
| A4 | −0.0018 ± 0.0009 a | 0.0009 ± 0.0008 a | 0.0009 ± 0.0010 a |
| B4 | −0.0002 ± 0.0009 a | 0.0004 ± 0.0010 a | 0.0003 ± 00008 a |
| C4 | 0.010 ± 0.0007 a | 0.0001 ± 0.0008 a | −0.0011 ± 0.0010 a |
| D4 | 0.0011 ± 0.0006 a | −0.0009 ± 0.0007 a | −0.0002 ± 0.0008 b |
| A5 | 0.0013 ± 0.0006 a | 0.0007 ± 0.007 a | −0.0021 ± 0.0007 b |
| B5 | −0.0001 ± 0.0008 a | −0.0002 ± 0.0006 a | 0.0002 ± 0.0006 a |
| C5 | 0.0014 ± 0.0006 a | −0.0007 ± 0.0006 b | −0.0008 ± 0.0007 b |
| D5 | 0.0002 ± 0.0005 a | 0.0001 ± 0.0005 a | −0.0004 ± 0.0007 a |
| A6 | 0.0006 ± 0.0006 a | −0.0004 ± 0.0006 a | −0.0002 ± 0.0005 a |
| B6 | −0.0003 ± 0.0005 a | −0.0001 ± 0.0005 a | 0.0004 ± 0.0006 a |
| C6 | 0.0004 ± 0.0004 a | −0.0007 ± 0.0006 a | 0.0003 ± 0.0006 a |
| Transformed Distances | Predicted Location | |||
| Original Location | Avilés | Peniche | Lagos | %Correct |
| Avilés | 36 | 4 | 7 | 77 |
| Peniche | 3 | 34 | 11 | 71 |
| Lagos | 11 | 9 | 25 | 56 |
| Total | 50 | 47 | 43 | 68 |
| Elliptical Fourier Descriptors | Predicted Location | |||
| Original Location | Avilés | Peniche | Lagos | %Correct |
| Avilés | 29 | 14 | 4 | 62 |
| Peniche | 13 | 17 | 18 | 35 |
| Lagos | 5 | 14 | 26 | 58 |
| Total | 47 | 45 | 48 | 51 |
| Both Approaches | Predicted Location | |||
| Original Location | Avilés | Peniche | Lagos | %Correct |
| Avilés | 34 | 9 | 4 | 72 |
| Peniche | 6 | 28 | 14 | 58 |
| Lagos | 5 | 11 | 29 | 64 |
| Total | 45 | 48 | 47 | 65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Kulzer, R.G.; Silva, R.M.; Rocha, A.F.; Carrola, J.S.; Seabra, R.C.; Rocha, E.; Erzini, K.; Correia, A.T. Population Structure of the European Seabass (Dicentrarchus labrax) in the Atlantic Iberian Coastal Waters Inferred from Body Morphometrics and Otolith Shape Analyses. Fishes 2026, 11, 16. https://doi.org/10.3390/fishes11010016
Kulzer RG, Silva RM, Rocha AF, Carrola JS, Seabra RC, Rocha E, Erzini K, Correia AT. Population Structure of the European Seabass (Dicentrarchus labrax) in the Atlantic Iberian Coastal Waters Inferred from Body Morphometrics and Otolith Shape Analyses. Fishes. 2026; 11(1):16. https://doi.org/10.3390/fishes11010016
Chicago/Turabian StyleKulzer, Rafael Gaio, Rodolfo Miguel Silva, Ana Filipa Rocha, João Soares Carrola, Rosária Catarino Seabra, Eduardo Rocha, Karim Erzini, and Alberto Teodorico Correia. 2026. "Population Structure of the European Seabass (Dicentrarchus labrax) in the Atlantic Iberian Coastal Waters Inferred from Body Morphometrics and Otolith Shape Analyses" Fishes 11, no. 1: 16. https://doi.org/10.3390/fishes11010016
APA StyleKulzer, R. G., Silva, R. M., Rocha, A. F., Carrola, J. S., Seabra, R. C., Rocha, E., Erzini, K., & Correia, A. T. (2026). Population Structure of the European Seabass (Dicentrarchus labrax) in the Atlantic Iberian Coastal Waters Inferred from Body Morphometrics and Otolith Shape Analyses. Fishes, 11(1), 16. https://doi.org/10.3390/fishes11010016








