Compound Inhibitors Mitigate Skin Ulceration Induced by UVA and Vibrio splendidus in the Sea Cucumber Apostichopus japonicus
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Experimental Design
2.2.1. UVA Induction
2.2.2. Vibrio Splendidus Infection
2.3. Evaluation of Skin Ulceration in A. japonicus
2.3.1. Analysis of UVA-Induced Skin Ulceration Area
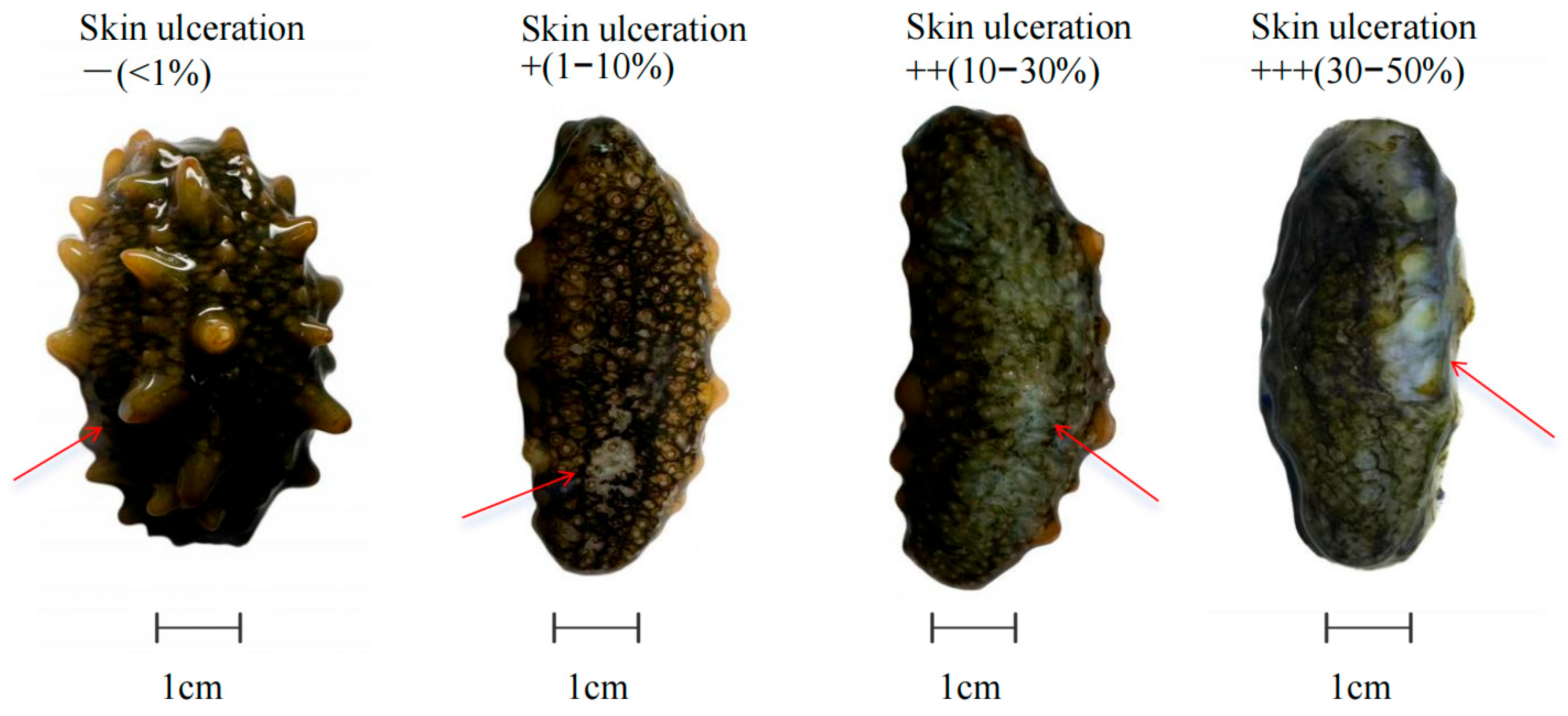
2.3.2. Vibrio splendidus Infection-Induced Skin Ulceration Grading Criteria
2.4. Measurement of Autolysis-Related Enzyme and Antioxidant Enzyme Activities
2.5. Real-Time Quantitative PCR
2.6. Data Analysis
3. Results
3.1. Degree of Skin Ulceration in A. japonicus
3.1.1. UVA-Induced Skin Ulceration Area Ratio
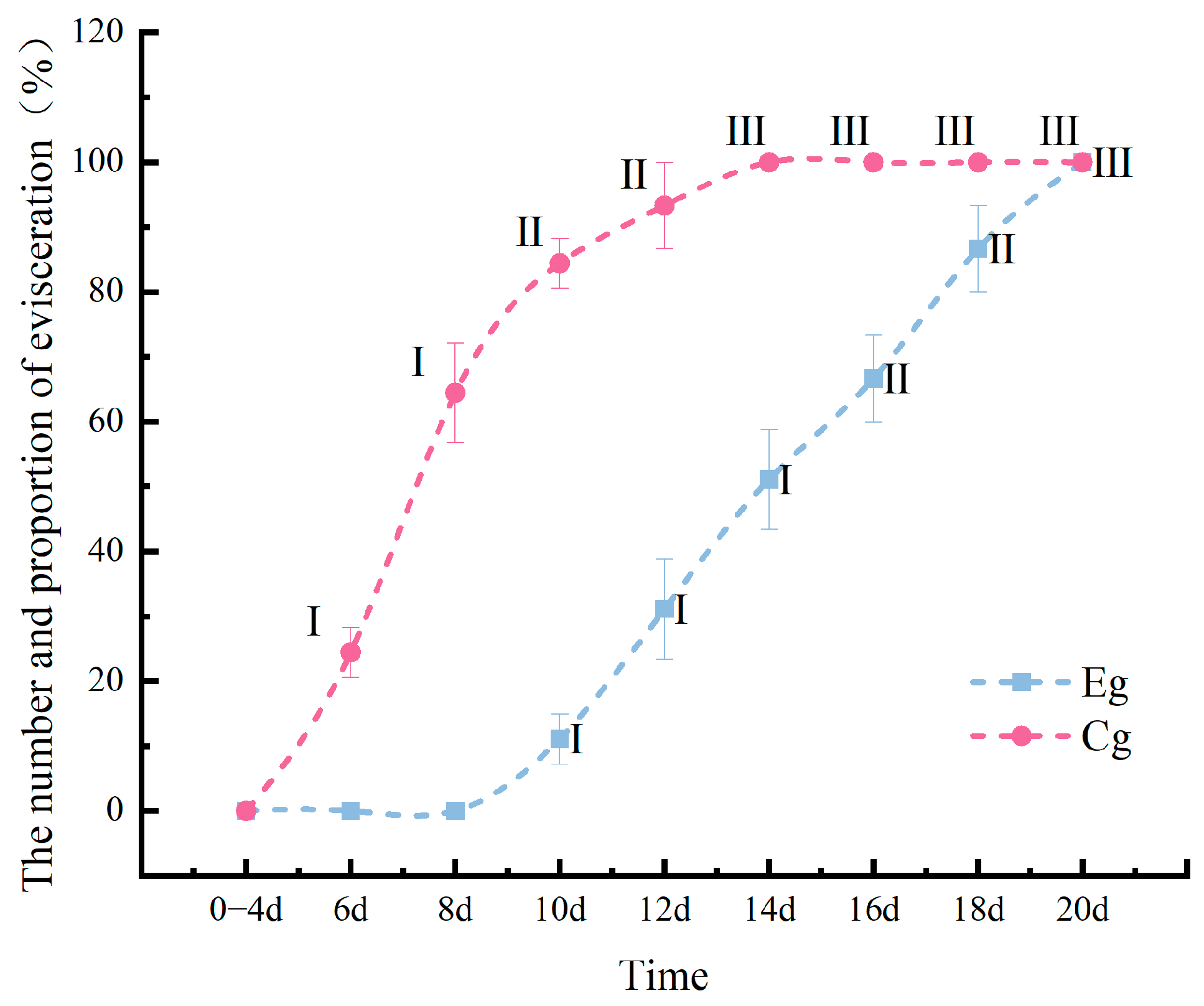
3.1.2. Comparison of Skin Ulceration Induced by Vibrio splendidus
3.2. Enzyme Activity Measurement
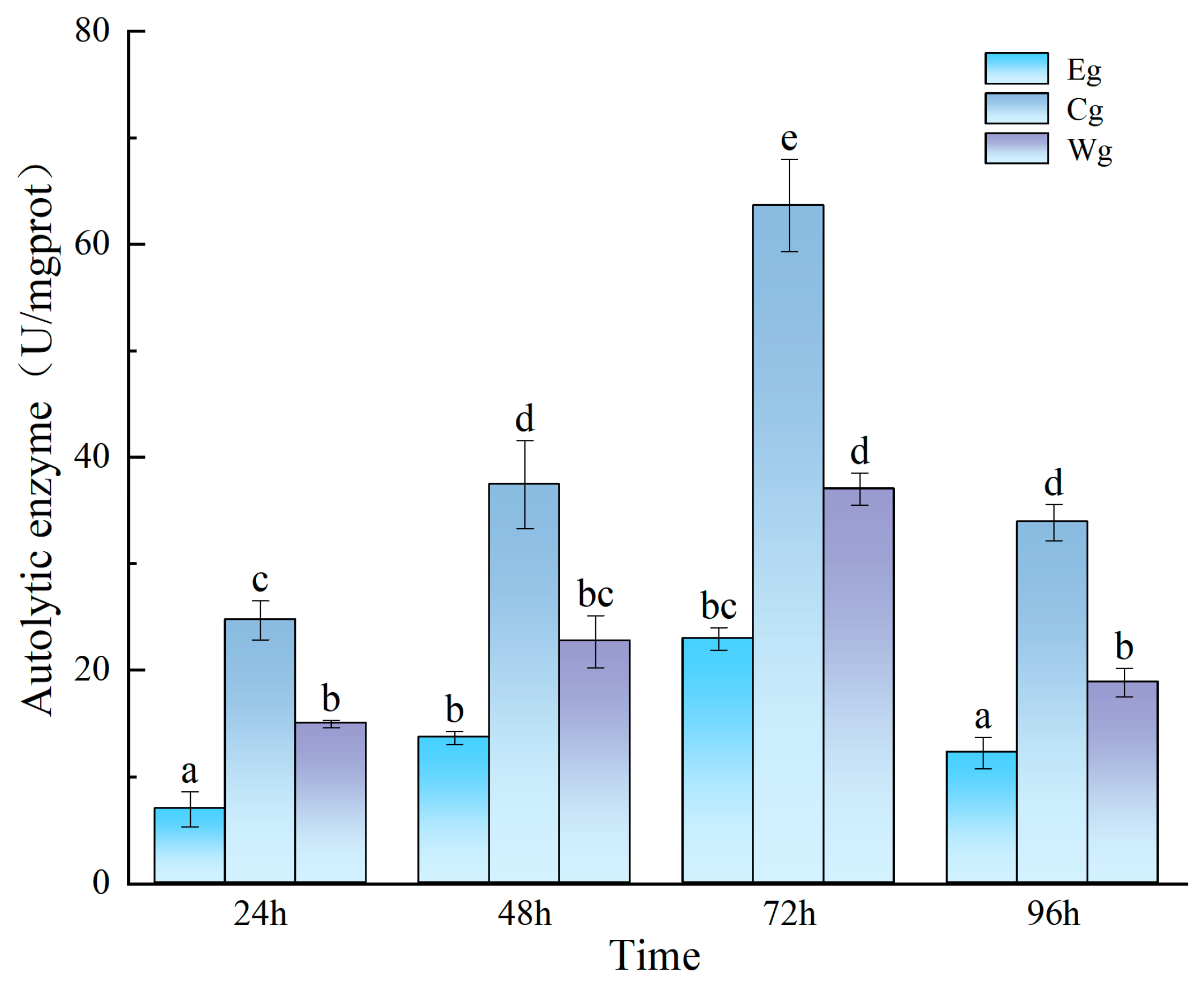
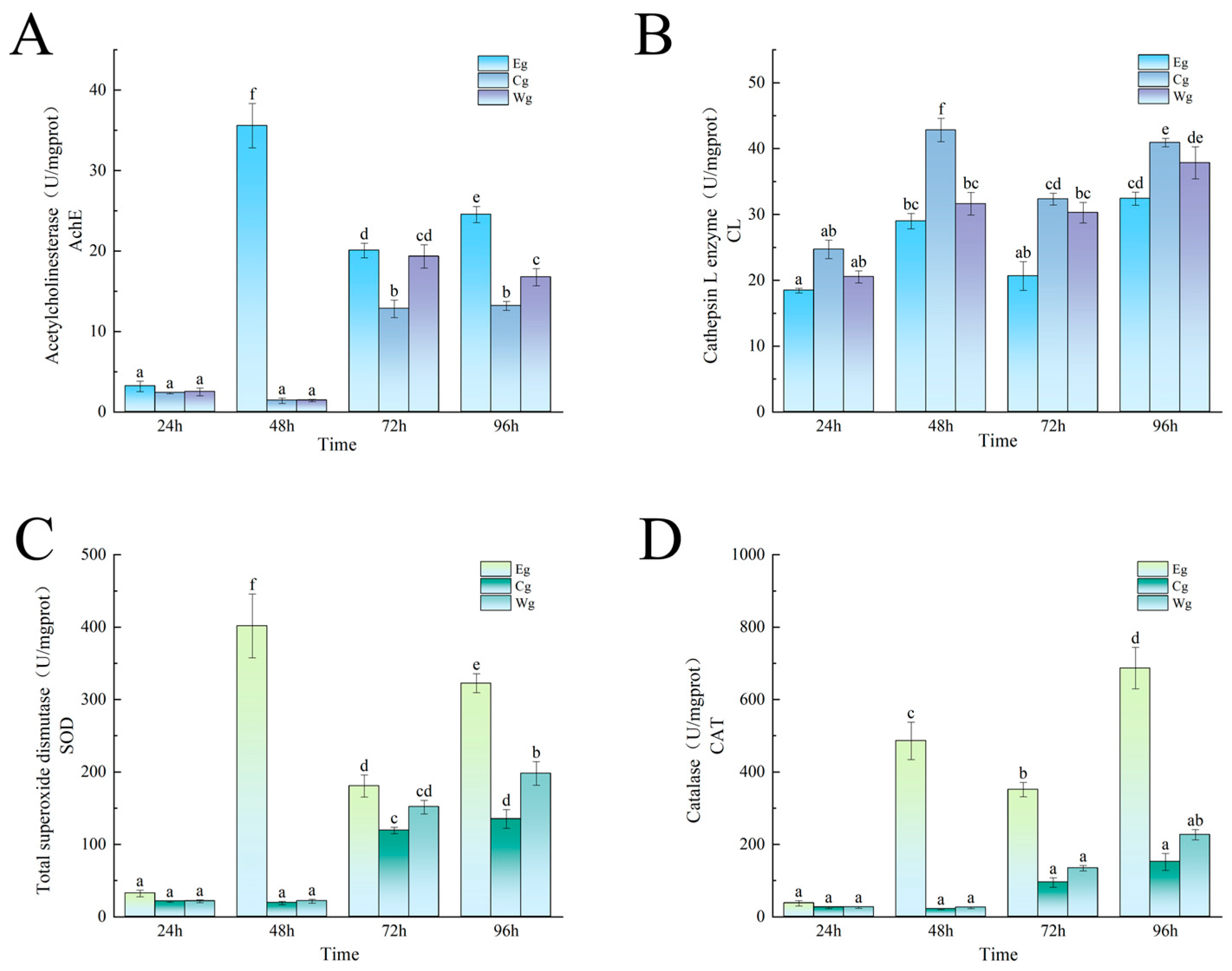
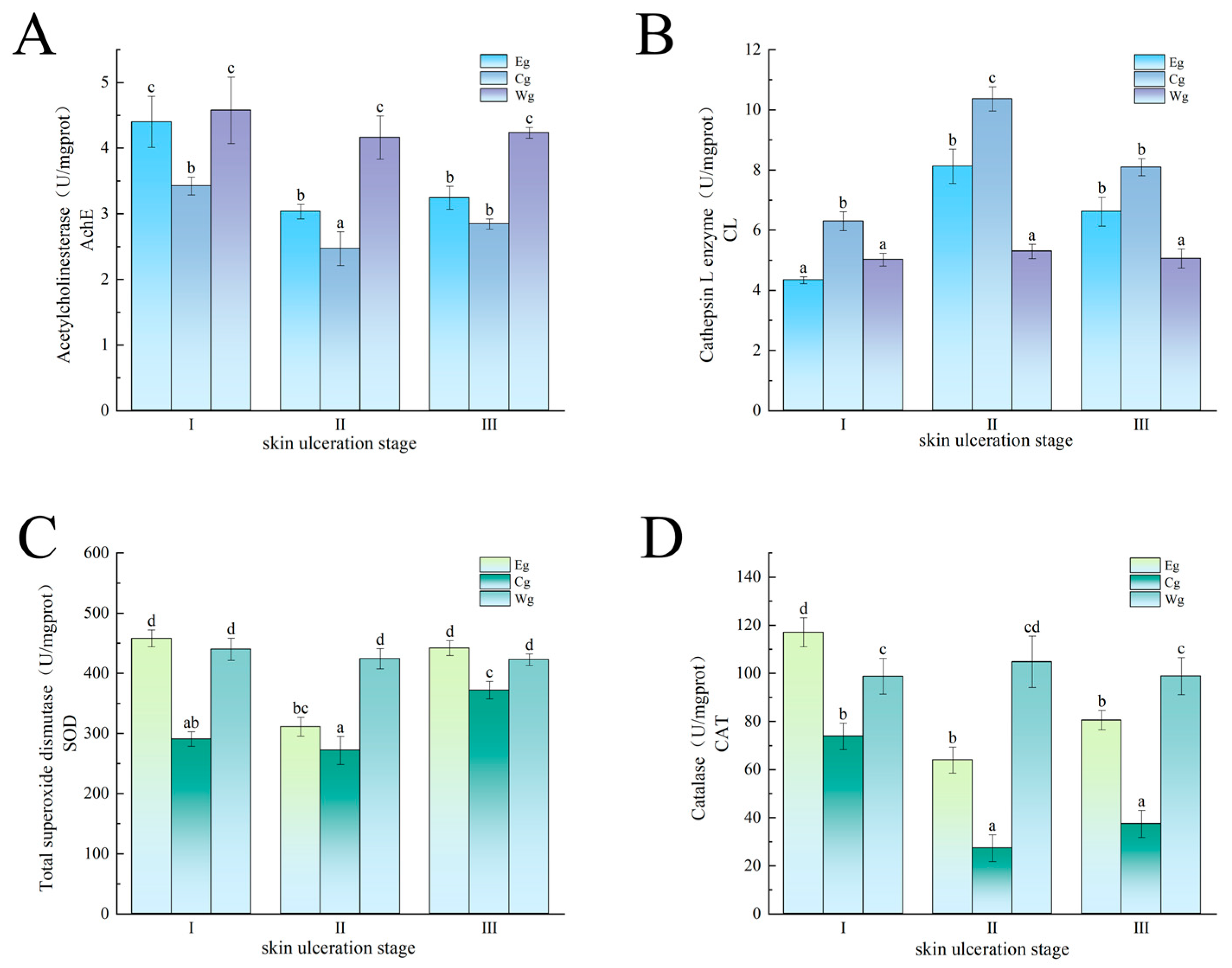
3.2.1. Effect of Inhibitor Composition on the Enzyme Activity in the Sea Cucumber Body Wall Induced by UVA Irradiation
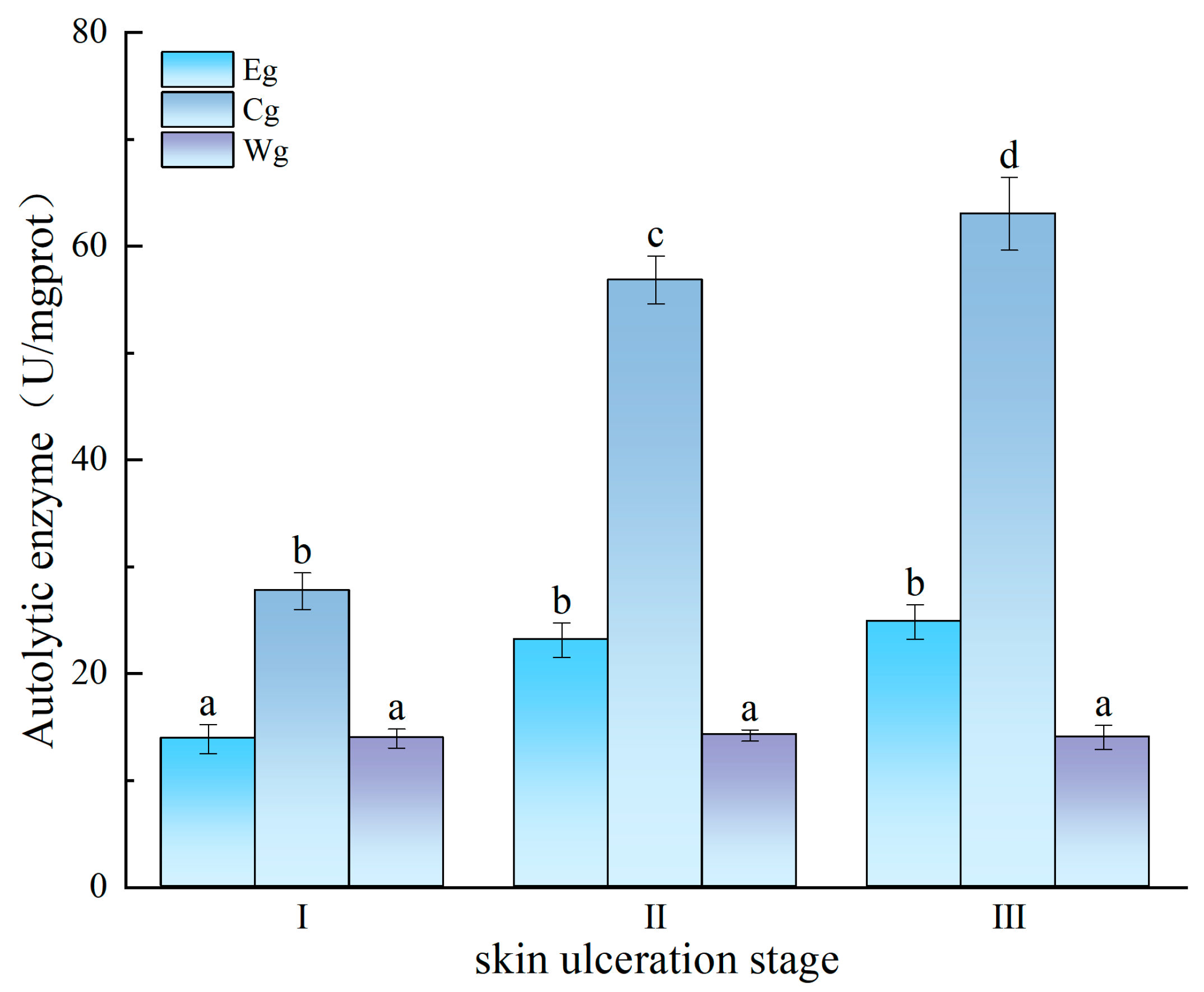
3.2.2. Effect of Inhibitor Composition on Enzyme Activity Changes Induced by Vibrio splendidus Infection
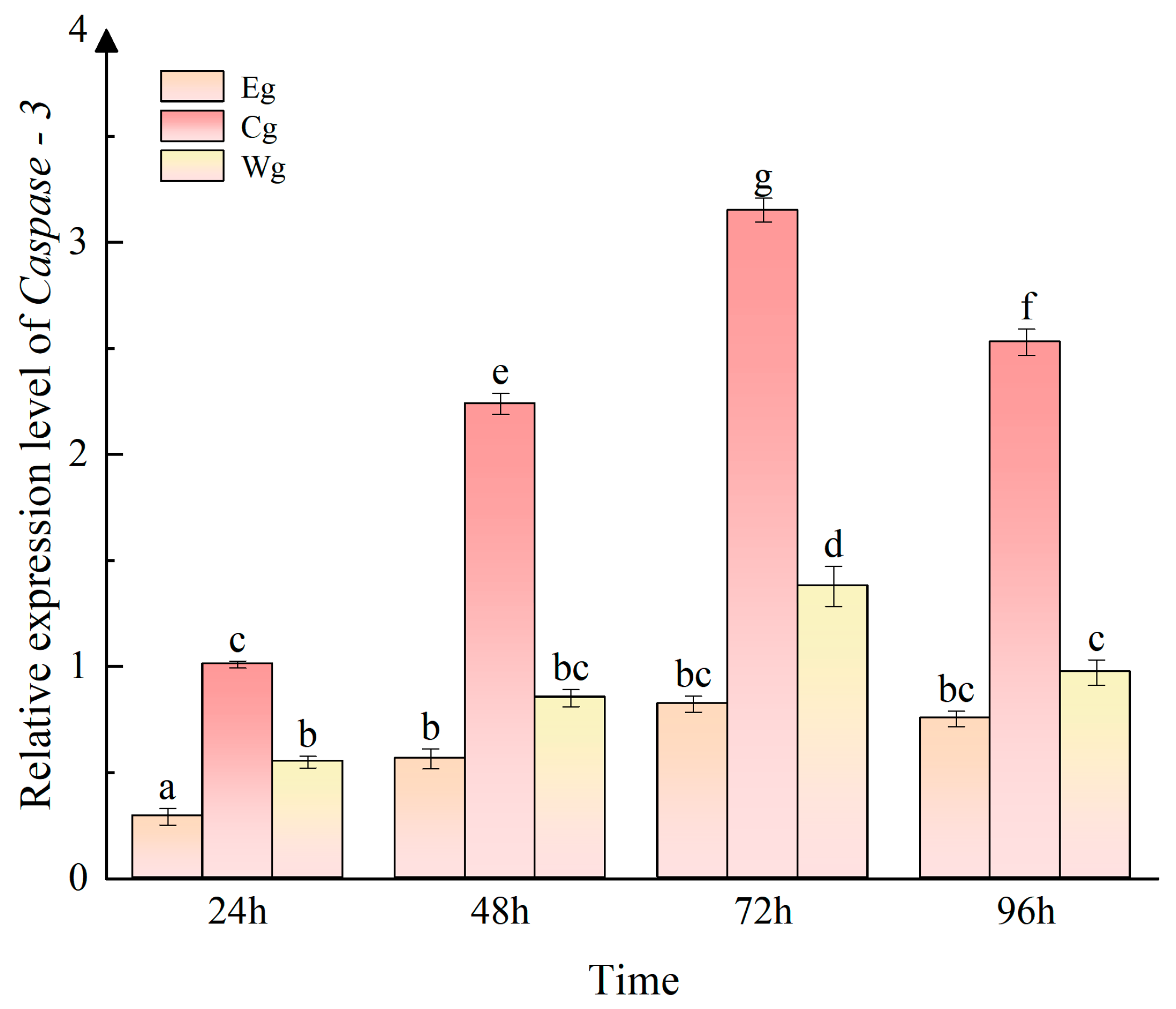
3.3. Effect of UVA on the Relative Expression of Caspase-3 in the Sea Cucumber Body Wall
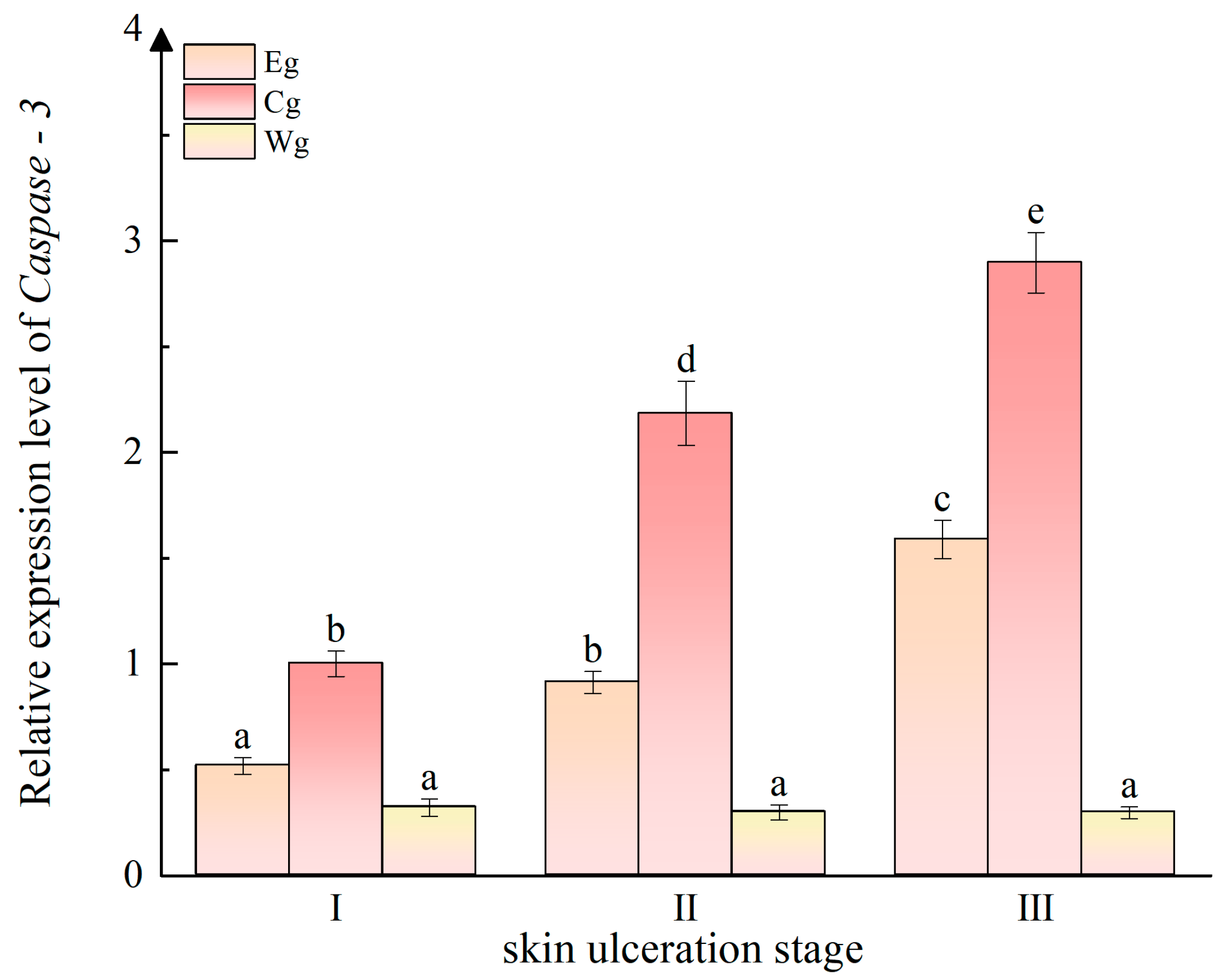
3.4. Effect of Vibrio splendidus on the Relative Expression of Caspase-3 in the Sea Cucumber Body Wall
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xia, S.; Wang, X. Nutritional and medicinal value. In Developments in Aquaculture and Fisheries Science; Elsevier: Amsterdam, The Netherlands, 2015; Volume 39, pp. 353–365. [Google Scholar] [CrossRef]
- Vaidya, H.; Cheema, S.K. Sea cucumber and blue mussel: New sources of phospholipid enriched omega-3 fatty acids with a potential role in 3T3-L1 adipocyte metabolism. Food Funct. 2014, 5, 3287–3295. [Google Scholar] [CrossRef]
- Ru, X.; Feng, Q.; Zhang, S.; Liu, S.; Zhang, L.; Yang, H. Eco-friendly method for rearing sea cucumber (Apostichopus japonicus) larvae. Aquac. Res. 2022, 53, 3759–3766. [Google Scholar] [CrossRef]
- Guo, M.; Gui, M.; Xu, X.; Duan, X.; Zhao, X.; Zhang, W.; Shao, Y.; Wang, B.; Diao, J.; Li, C. BAG2 mediates coelomocyte apoptosis in Vibrio splendidus challenged sea cucumber Apostichopus japonicus. Int. J. Biol. Macromol. 2021, 189, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Huo, D.; Sun, L.; Zhang, L.; Ru, X.; Liu, S.; Yang, H. Metabolome responses of the sea cucumber Apostichopus japonicus to multiple environmental stresses: Heat and hypoxia. Mar. Pollut. Bull. 2019, 138, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Fu, H.; Dong, X.; Feng, D.; Li, N.; Wen, C.; Nakamura, Y.; Zhu, B. Apoptosis induction is involved in UVA-induced autolysis in sea cucumber Stichopus japonicus. J. Photochem. Photobiol. B Biol. 2016, 158, 130–135. [Google Scholar] [CrossRef]
- Zhang, C.; Liang, W.; Zhang, W.; Li, C. Characterization of a metalloprotease involved in Vibrio splendidus infection in the sea cucumber, Apostichopus japonicus. Microb. Pathog. 2016, 101, 96–103. [Google Scholar] [CrossRef]
- Ju, Z.; Liao, G.; Zhang, Y.; Li, N.; Li, X.; Zou, Y.; Yang, W.; Xiong, D. Oxidative stress responses in the respiratory tree and the body wall of sea cucumber Apostichopus japonicus (Selenka) to high temperature. Environ. Sci. Pollut. Res. 2023, 30, 21288–21298. [Google Scholar] [CrossRef]
- Ju, Z.; Li, X.; Yang, W.; Xiong, D. Immune responses of sea cucumber (Apostichopus japonicus) to combined environmental stress from high temperature and oil pollution. Mar. Freshw. Res. 2024, 75, MF23161. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, C.; Shen, L.; Ding, L.; Guo, H. Role of non-coding RNAs in UV-induced radiation effects. Exp. Ther. Med. 2024, 27, 262. [Google Scholar] [CrossRef] [PubMed]
- Yehia, M.A.-H.; Fawzy, M.A.; Elbashar, Y.H.; Abdelmigid, B.A.; ElGhnam, S.M. Histomorphometry, immunohistochemistry, and fine structure of Rats skin exposed to Ultraviolet-A radiation. J. Opt. 2021, 51, 574–584. [Google Scholar] [CrossRef]
- Yang, T.T.; Lan, C.C.E. Photocarcinogenesis of the skin: Current status and future trends. Kaohsiung J. Med. Sci. 2025, 41, e12946. [Google Scholar] [CrossRef] [PubMed]
- Umam, N.I.; Sulaiman, A.; Wong, Y.F.; Jaya-Ram, A.; Woo, S.P.; Zulkurnain, M. UV-Assisted Autolysis for Nutrient Bioconversion of Sea Cucumber (Stichopus horrens) Body Wall. Food Bioprocess Technol. 2024, 17, 1637–1657. [Google Scholar] [CrossRef]
- Dong, X.; Qi, H.; Feng, D.; He, B.; Nakamura, Y.; Yu, C.; Zhu, B. Oxidative stress involved in textural changes of sea cucumber Stichopus japonicus body wall during low-temperature treatment. Int. J. Food Prop. 2018, 21, 2646–2659. [Google Scholar] [CrossRef]
- Yang, J.-F.; Gao, R.-C.; Wu, H.-T.; Li, P.-F.; Hu, X.-S.; Zhou, D.-Y.; Zhu, B.-W.; Su, Y.-C. Analysis of apoptosis in ultraviolet-induced sea cucumber (Stichopus japonicus) melting using terminal deoxynucleotidyl-transferase-mediated dUTP nick end-labeling assay and cleaved Caspase-3 immunohistochemistry. J. Agric. Food Chem. 2015, 63, 9601–9608. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Liao, M.; Wang, Y.; Li, B.; Zhang, Z.; Rong, X.; Chen, G.; Wang, L. Transcriptome analysis and discovery of genes involved in immune pathways from coelomocytes of sea cucumber (Apostichopus japonicus) after Vibrio splendidus challenge. Int. J. Mol. Sci. 2015, 16, 16347–16377. [Google Scholar] [CrossRef] [PubMed]
- Li, C. Research progress on molecular regulation mechanism ofskin ulcer syndrome in sea cucumber Apostichopus japonicus :A review. J. Dalian Ocean Univ. 2021, 36, 355–373. [Google Scholar] [CrossRef]
- Zhang, W.; Li, C. Virulence mechanisms of vibrios belonging to the Splendidus clade as aquaculture pathogens, from case studies and genome data. Rev. Aquac. 2021, 13, 2004–2026. [Google Scholar] [CrossRef]
- Yang, J.; Liu, H.; Zheng, G.; Xiang, X.; Lv, Z.; Wang, T. Cathepsin L of the sea cucumber Apostichopus japonicus-molecular characterization and transcriptional response to Vibrio splendidus infection. Fish Shellfish Immunol. 2016, 49, 387–395. [Google Scholar] [CrossRef]
- Li, Y.; Yang, H.; Zhang, J.; Shi, W.; Li, W.; Zhang, W. VspC from Vibrio splendidus is responsible for collagen degradation in Apostichopus japonicus. Aquaculture 2023, 571, 739489. [Google Scholar] [CrossRef]
- Shao, Y.; Li, C.; Zhang, W.; Duan, X.; Li, Y.; Jin, C.; Xiong, J.; Qiu, Q. Molecular cloning and characterization of four Caspase members in Apostichopus japonicus. Fish Shellfish Immunol. 2016, 55, 203–211. [Google Scholar] [CrossRef]
- Ding, J.; Chang, Y.; Guo, C.; Wang, L.; Yin, D. Composition and Application for Inhibiting Sea Cucumber Skin Degeneration. CN113826567B, 19 August 2022. [Google Scholar]
- Delroisse, J.; Van Wayneberghe, K.; Flammang, P.; Gillan, D.; Gerbaux, P.; Opina, N.; Todinanahary, G.G.B.; Eeckhaut, I. Epidemiology of a SKin Ulceration Disease (SKUD) in the sea cucumber Holothuria scabra with a review on the SKUDs in Holothuroidea (Echinodermata). Sci. Rep. 2020, 10, 22150. [Google Scholar] [CrossRef]
- Ferreira, T.; Rasband, W. Section 28: Plugins. In The ImageJ User Guide, 2nd ed.; Ferreira, T., Rasband, W., Eds.; National Institutes of Health: Bethesda, MD, USA, 2011; Volume 1, pp. 155–161. [Google Scholar]
- Zhu, B.; Han, B. Characterization and Purification of Holothurians Autoerzyme. Food Ferment. Ind. 2004, 30, 132. (In Chinese) [Google Scholar]
- Wang, Z.; Xu, R.; Yang, H.; Li, R.; Ding, J.; Chang, Y.; Zuo, R. Vitamin E Regulates the Collagen Contents in the Body Wall of Sea Cucumber (Apostichopus japonicus) via Its Antioxidant Effects and the TGF-β/Smads Pathway. Antioxidants 2024, 13, 847. [Google Scholar] [CrossRef]
- Liu, Z.-q.; Zhou, D.-y.; Liu, Y.-x.; Yu, M.-m.; Liu, B.; Song, L.; Dong, X.-p.; Qi, H.; Shahidi, F. Inhibitory effect of natural metal ion chelators on the autolysis of sea cucumber (Stichopus japonicus) and its mechanism. Food Res. Int. 2020, 133, 109205. [Google Scholar] [CrossRef]
- Souza, M.S.; Balseiro, E.; Laspoumaderes, C.; Modenutti, B. Effect of ultraviolet radiation on acetylcholinesterase activity in freshwater copepods. Photochem. Photobiol. 2010, 86, 367–373. [Google Scholar] [CrossRef]
- Liu, Y.-X.; Zhou, D.-Y.; Ma, D.-D.; Liu, Y.-F.; Li, D.-M.; Dong, X.-P.; Tan, M.-Q.; Du, M.; Zhu, B.-W. Changes in collagenous tissue microstructures and distributions of cathepsin L in body wall of autolytic sea cucumber (Stichopus japonicus). Food Chem. 2016, 212, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Heck, D.E.; Vetrano, A.M.; Mariano, T.M.; Laskin, J.D. UVB light stimulates production of reactive oxygen species: Unexpected role for catalase. J. Biol. Chem. 2003, 278, 22432–22436. [Google Scholar] [CrossRef]
- Mo, Q.; You, S.; Fu, H.; Wang, D.; Zhang, J.; Wang, C.; Li, M. Purification and identification of antioxidant peptides from rice fermentation of Lactobacillus plantarum and their protective effects on UVA–Induced oxidative stress in skin. Antioxidants 2022, 11, 2333. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Zang, Y.; Chen, J.; Shang, S.; Gao, L.; Tang, X. Ultraviolet-B radiation stress triggers reactive oxygen species and regulates the antioxidant defense and photosynthesis systems of intertidal red algae Neoporphyra haitanensis. Front. Mar. Sci. 2022, 9, 1043462. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Xiong, X.; Zhu, H.; Chen, R.; Zhang, S.; Chen, G.; Jian, Z. Nrf2 regulates oxidative stress and its role in cerebral ischemic stroke. Antioxidants 2022, 11, 2377. [Google Scholar] [CrossRef]
- Sample, A.; He, Y.Y. Autophagy in UV damage response. Photochem. Photobiol. 2017, 93, 943–955. [Google Scholar] [CrossRef]
- He, L.; Sui, C.; Li, J.; Yao, Y.; Li, M.; Wang, R.; Zhu, W. N-Terminal 5-Mer peptide analog P165 of amyloid precursor protein repairs skin photodamage induced by UVB through the Nrf2 signaling pathway. Indian J. Dermatol. 2021, 66, 574. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, C.-F.; Rossiter, H.; Eckhart, L.; König, U.; Karner, S.; Mildner, M.; Bochkov, V.N.; Tschachler, E.; Gruber, F. Autophagy is induced by UVA and promotes removal of oxidized phospholipids and protein aggregates in epidermal keratinocytes. J. Investig. Dermatol. 2013, 133, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Tamori, M.; Saha, A.K.; Matsuno, A.; Noskor, S.C.; Koizumi, O.; Kobayakawa, Y.; Nakajima, Y.; Motokawa, T. Stichopin-containing nerves and secretory cells specific to connective tissues of the sea cucumber. Proc. R. Soc. B Biol. Sci. 2007, 274, 2279–2285. [Google Scholar] [CrossRef] [PubMed]
- Sperandio, B.; Fischer, N.; Sansonetti, P.J. Mucosal physical and chemical innate barriers: Lessons from microbial evasion strategies. Semin. Immunol. 2015, 27, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, C.; Li, Y.; Zhang, P.; Shao, Y.; Jin, C.; Li, T. Proteomic identification of differentially expressed proteins in sea cucumber Apostichopus japonicus coelomocytes after Vibrio splendidus infection. Dev. Comp. Immunol. 2014, 44, 370–377. [Google Scholar] [CrossRef]
- Sun, Y.; Du, X.; Li, S.; Wen, Z.; Li, Y.; Li, X.; Meng, N.; Mi, R.; Ma, S.; Sun, A. Dietary Cordyceps militaris protects against Vibrio splendidus infection in sea cucumber Apostichopus japonicus. Fish Shellfish Immunol. 2015, 45, 964–971. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, H.; Zhang, W.; Ai, Q.; Mai, K.; Xu, W.; Wang, X.; Liufu, Z. Effects of dietary β-glucan on the growth, immune responses and resistance of sea cucumber, Apostichopus japonicus against Vibrio splendidus infection. Aquaculture 2011, 315, 269–274. [Google Scholar] [CrossRef]
- Song, H.; Xie, C.; Dong, M.; Zhang, Y.; Huang, H.; Han, Y.; Liu, Y.; Wei, L.; Wang, X. Effects of ambient UVB light on Pacific oyster Crassostrea gigas mantle tissue based on multivariate data. Ecotoxicol. Environ. Saf. 2024, 274, 116236. [Google Scholar] [CrossRef]
- Cailhier, J.-F.; Sirois, I.; Laplante, P.; Lepage, S.; Raymond, M.-A.; Brassard, N.; Prat, A.; Iozzo, R.V.; Pshezhetsky, A.V.; Hebert, M.-J. Caspase-3 activation triggers extracellular cathepsin L release and endorepellin proteolysis. J. Biol. Chem. 2008, 283, 27220–27229. [Google Scholar] [CrossRef]
- Monian, P.; Jiang, X. The cellular apoptosis susceptibility protein (CAS) promotes tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis and cell proliferation. J. Biol. Chem. 2016, 291, 2379–2388. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Shao, Y.; Lv, Z.; Li, C. Serpin-type serine protease inhibitor mediates coelomocyte apoptosis in Apostichopus japonicus. Fish Shellfish Immunol. 2020, 104, 410–418. [Google Scholar] [CrossRef] [PubMed]

| Groups | 24 h | 48 h | 72 h | 96 h |
|---|---|---|---|---|
| Eg | 0 a | 0.03 ± 0.006 a | 0.25 ± 0.04 a | 0.55 ± 0.02 a |
| − | − | − | − | |
| Cg | 7.99 ± 0.91 b | 19.58 ± 2.06 c | 32.80 ± 1.26 d | 46.02 ± 1.97 e |
| + | ++ | +++ | +++ | |
| Wg | 4.94 ± 0.27 c | 14.44 ± 0.92 d | 23.25 ± 1.27 e | 28.36 ± 1.27 f |
| + | ++ | ++ | ++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Tian, Y.; Xiao, H.; Tian, F.; Han, L.; Zhao, C.; Wang, L.; Ding, J. Compound Inhibitors Mitigate Skin Ulceration Induced by UVA and Vibrio splendidus in the Sea Cucumber Apostichopus japonicus. Fishes 2025, 10, 470. https://doi.org/10.3390/fishes10090470
Li X, Tian Y, Xiao H, Tian F, Han L, Zhao C, Wang L, Ding J. Compound Inhibitors Mitigate Skin Ulceration Induced by UVA and Vibrio splendidus in the Sea Cucumber Apostichopus japonicus. Fishes. 2025; 10(9):470. https://doi.org/10.3390/fishes10090470
Chicago/Turabian StyleLi, Xiaonan, Ye Tian, Haoran Xiao, Fenglin Tian, Lingshu Han, Chong Zhao, Luo Wang, and Jun Ding. 2025. "Compound Inhibitors Mitigate Skin Ulceration Induced by UVA and Vibrio splendidus in the Sea Cucumber Apostichopus japonicus" Fishes 10, no. 9: 470. https://doi.org/10.3390/fishes10090470
APA StyleLi, X., Tian, Y., Xiao, H., Tian, F., Han, L., Zhao, C., Wang, L., & Ding, J. (2025). Compound Inhibitors Mitigate Skin Ulceration Induced by UVA and Vibrio splendidus in the Sea Cucumber Apostichopus japonicus. Fishes, 10(9), 470. https://doi.org/10.3390/fishes10090470







