Modulation of Gene Expression in the Digestive Tract of the Tropical Gar (Atractosteus tropicus) in Response to Cricket Meal (Acheta domesticus)
Abstract
1. Introduction
2. Materials and Methods
2.1. Organisms and Experimental Design
2.2. Biometric Parameters
2.3. Primers Design
2.4. Quantitative Polymerase Chain Reaction (qPCR)
2.5. Statistical Analysis
3. Results
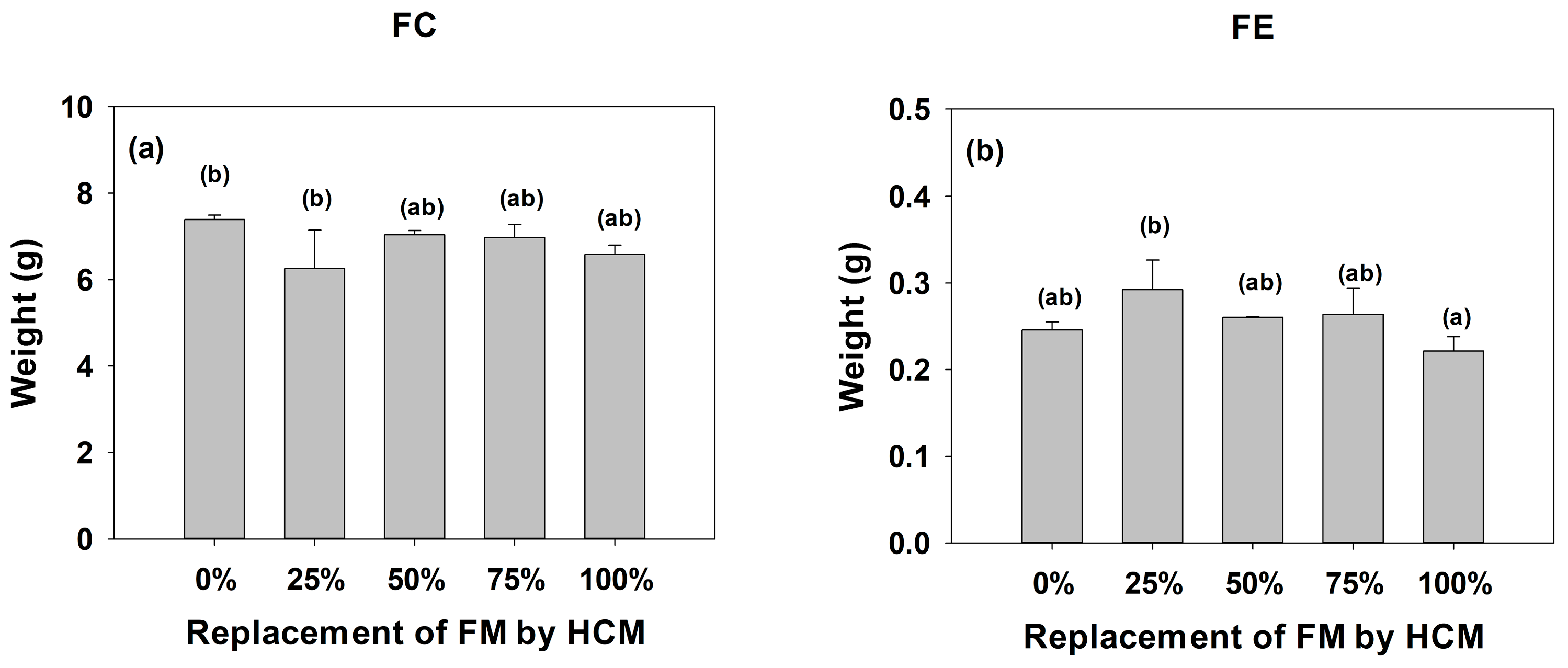
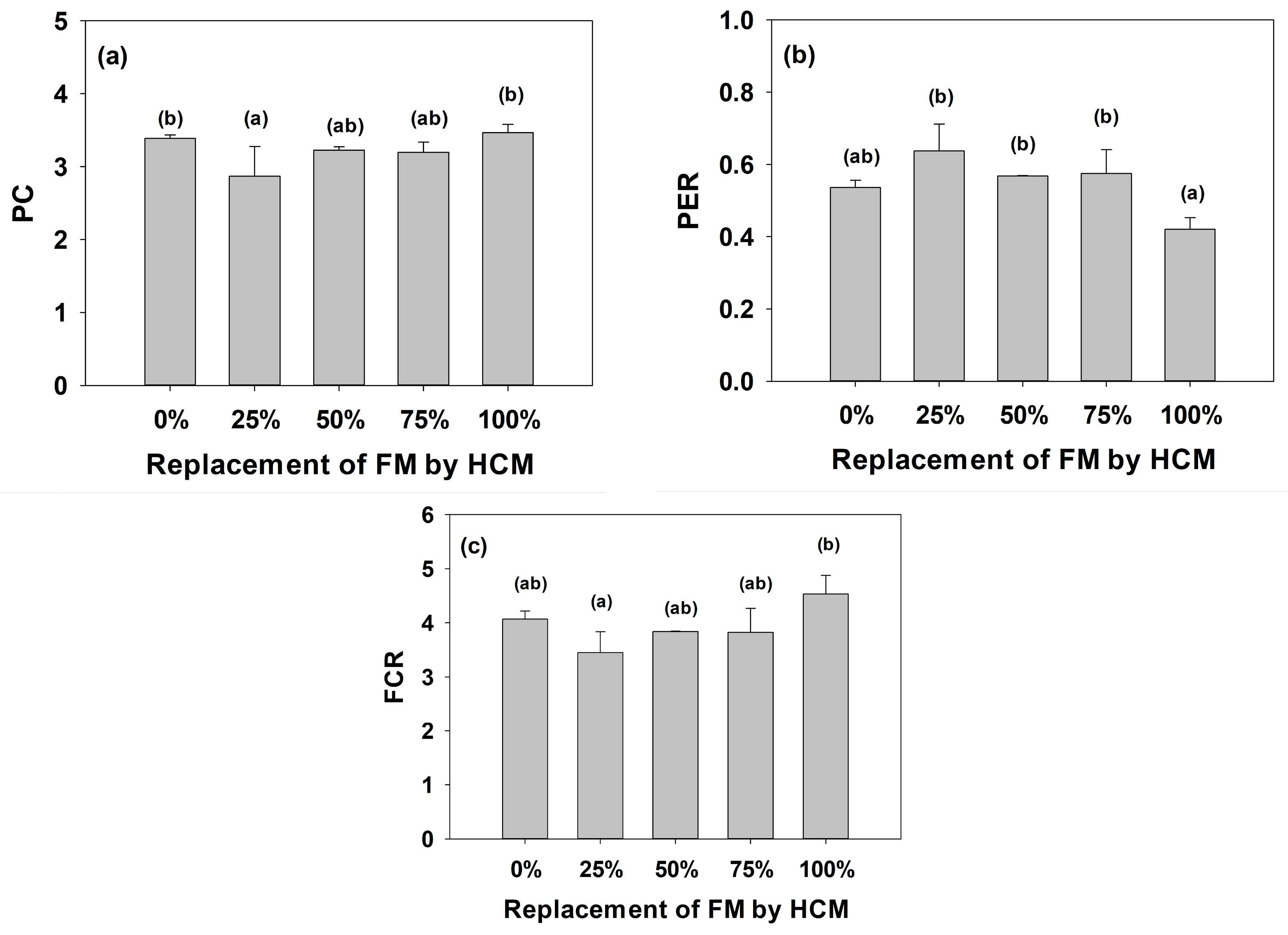
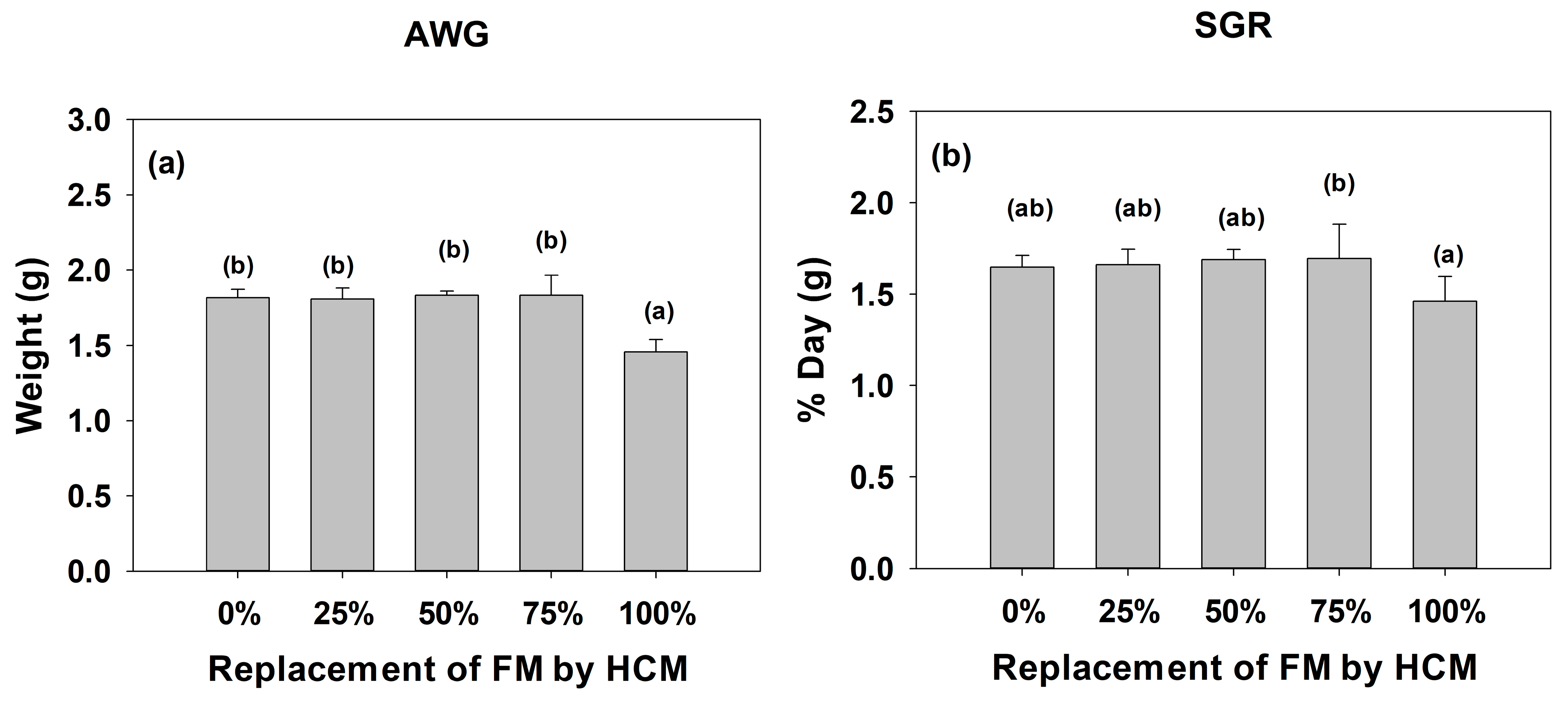
3.1. Growth and Biometric Parameters
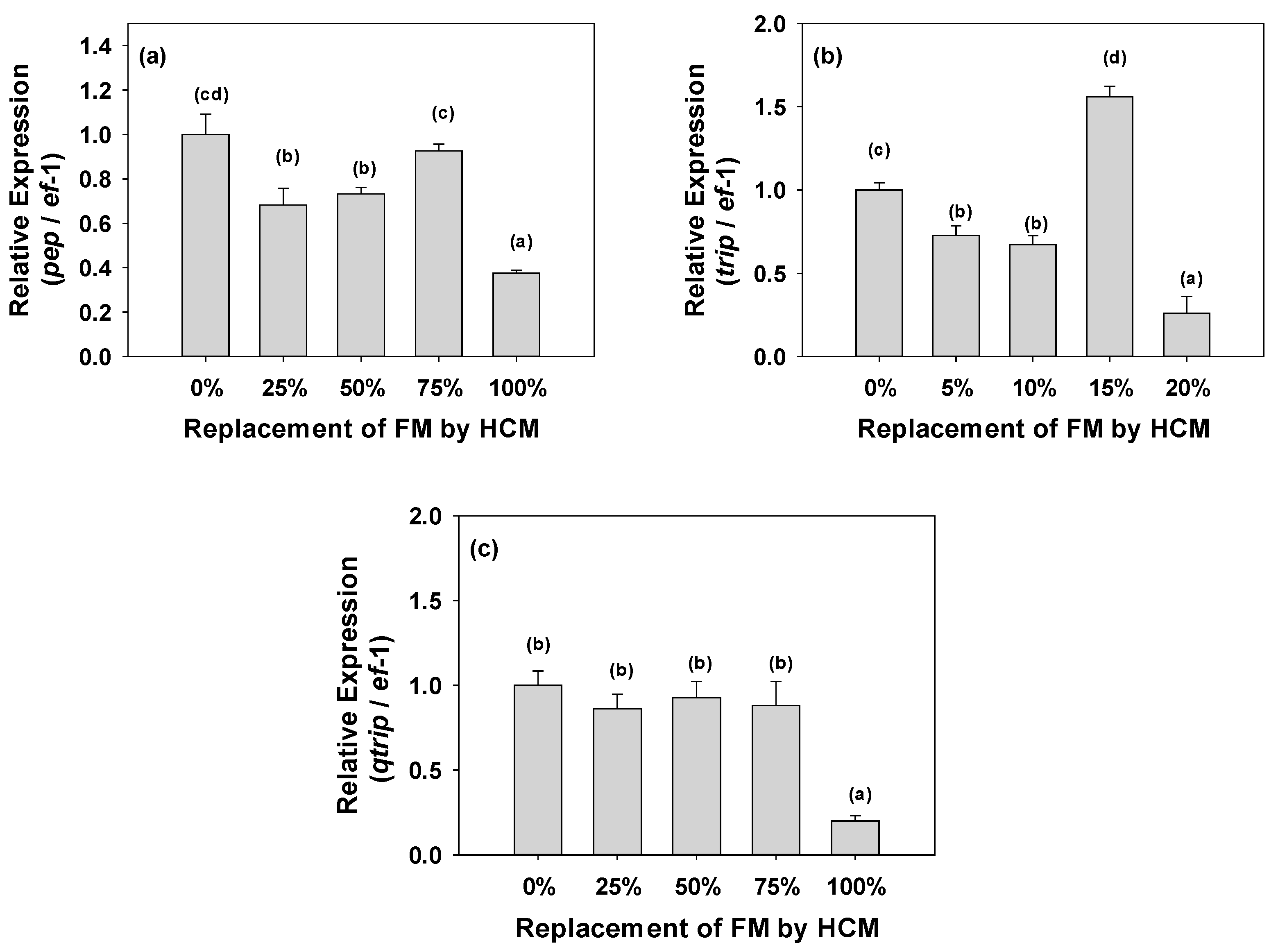
3.2. Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Official Journal of the Federation. National Aquaculture Charter 2021. National Fisheries Institute. Mexico, 15 April 2021. Available online: https://www.gob.mx/inapesca/documentos/carta-nacional-acuicola-2021 (accessed on 1 May 2024).
- Márquez-Couturier, G.; Vázquez-Navarrete, C.J.; Contreras-Sánchez, W.M.; Álvarez-González, C.A. Sustainable Tropical Aquaculture: A Strategy for the Production and Conservation of Pejelagarto (Atractosteus tropicus) in Tabasco, Mexico, 2nd ed.; Juárez Autonomous University of Tabasco: Villahermosa, Mexico, 2015. [Google Scholar]
- Gougbedji, A.; Detilleux, J.; Lalèyè, P.A.; Francis, F.; Caparros Megido, R. Can Insect Meal Replace Fishmeal? A Meta-Analysis of the Effects of Black Soldier Fly on Fish Growth Performances and Nutritional Values. Animals 2022, 12, 1700. [Google Scholar] [CrossRef] [PubMed]
- De la Cruz-Alvarado, F.J.; Álvarez-González, C.A.; Nolasco-Soria, H.; Martínez-García, R.; Piña-Gutierrez, J.M.; Concha-Frías, B.; Frás-Quintana, C.A.; Peña, E. Characterization and improvement of Cannavalia ensiformis flour as a balanced feed for Oreochromis niloticus. Hidrobiológica 2019, 29, 163–170. [Google Scholar] [CrossRef]
- Commission Regulation (EU) 2017/893 of 24 May 2017 Amending Annexes I and IV to Regulation (EC) No 999/2001 of the European Parliament and of the Council and Annexes X, XIV and XV to Commission Regulation (EU) No 142/2011 as Regards the Provisions on Processing. 2017. Available online: http://data.europa.eu/eli/reg/2017/893/oj (accessed on 18 September 2025).
- Ferrer-Llagostera, P.F.; Kallas, Z.; Reig, L.; De Gea, D.A. The use of insect meal as a sustainable feeding alternative in aquaculture: Current situation, Spanish consumers’ perceptions and willingness to pay. J. Clean. Prod. 2019, 229, 10–21. [Google Scholar] [CrossRef]
- Sawant, S.S.; Salin, K.R. Use of insect-based meals in aquaculture. J. Exp. Zool. India 2020, 23, 715–724. [Google Scholar]
- Siddiqui, S.A.; Zhao, T.; Fitriani, A.; Rahmadhia, S.N.; Alirezalu, K.; Ito, F. Acheta domesticus (house cricket) as human foods-An approval of the European Commission-A systematic review. Food Front. 2024, 5, 435–473. [Google Scholar] [CrossRef]
- Gasco, L.; Henry, M.; Piccolo, G.; Marono, S.; Gai, F.; Renna, M.; Lussiana, C.; Antonopoulou, E.; Mola, P.; Chatzifotis, S. Tenebrio molitor meal in diets for European sea bass (Dicentrarchus labrax L.) juveniles: Growth performance, whole body composition and in vivo apparent digestibility. Anim. Feed Sci. Technol. 2016, 220, 34–45. [Google Scholar] [CrossRef]
- Rawski, M.; Mazurkiewicz, J.; Kierończyk, B.; Józefiak, D. Black Soldier Fly Full-Fat Larvae Meal as an Alternative to Fish Meal and Fish Oil in Siberian Sturgeon Nutrition: The Effects on Physical Properties of the Feed, Animal Growth Performance, and Feed Acceptance and Utilization. Animals 2020, 10, 2119. [Google Scholar] [CrossRef] [PubMed]
- Rawski, M.; Mazurkiewicz, J.; Kierończyk, B.; Józefiak, D. Black soldier fly full-fat larvae meal is more profitable than fish meal and fish oil in Siberian sturgeon farming: The effects on aquaculture sustainability, economy and fish growth development. Animals 2021, 11, 604. [Google Scholar] [CrossRef]
- Sándor, Z.J.; Banjac, V.; Vidosavljević, S.; Káldy, J.; Egessa, R.; Lengyel-Kónya, É.; Tömösközi-Farkas, R.; Zalán, Z.; Adányi, N.; Libisch, B.; et al. Apparent Digestibility Coefficients of Black Soldier Fly (Hermetia illucens), Yellow Mealworm (Tenebrio molitor), and Blue Bottle Fly (Calliphora vicina) Insects for Juvenile African Catfish Hybrids (Clarias gariepinus × Heterobranchus longifilis). Aquac. Nutr. 2022, 2022, 4717014. [Google Scholar] [CrossRef] [PubMed]
- Perera, G.S.C.; Bhujel, R.C. Replacement of fishmeal by house cricket (Acheta domesticus) and field cricket (Gryllus bimaculatus) meals: Effect for growth, pigmentation, and breeding performances of guppy (Poecilia reticulata). Aquac. Rep. 2022, 25, 101260. [Google Scholar] [CrossRef]
- Cadena-Cadena, F.; Cuevas-Acuña, D.A.; Concha-Frias, B.; Hernández, R.C.; Nuñez, J.C.G.; Martinez, B.A.; Arias-Moscoso, J.L. Replacement of fishmeal by common cricket (Acheta domesticus) meal in diets for juvenile tilapia (Oreochromis niloticus). Isr. J. Aquac.-Bamidgeh 2023, 75, 1–12. [Google Scholar] [CrossRef]
- Tran, G.; Heuzé, V.; Makkar, H.P.S. Insects in fish diets. Anim. Front. 2015, 5, 37–44. [Google Scholar]
- Homska, N.; Kowalska, J.; Bogucka, J.; Ziółkowska, E.; Rawski, M.; Kierónczyk, B.; Mazurkiewicz, J. Dietary Fish Meal Replacement with Hermetia illucens and Tenebrio molitor Larval Meals Improves the Growth Performance and Nutriphysiological Status of Ide (Leuciscus idus) Juveniles. Animals 2022, 12, 1227. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.A.; Hanboonsong, Y.; Totakul, P.; Matra, M.; Cherdthong, A.; Wanapat, M. Nutritional composition of various insects and potential uses as alternative protein sources in animal diets. Anim. Biosci. 2022, 35, 317–331. [Google Scholar] [CrossRef]
- Sun, Y.; Huang, H.; Li, B.; Su, L.; Deng, J.; Cao, Z. Effects of dietary lysine level on the growth performance, protein metabolism, and antioxidant status in Hemibagrus wyckioides juveniles. J. World Aquac. Soc. 2023, 54, 1317–1336. [Google Scholar] [CrossRef]
- Liaqat, R.; Fatima, S.; Komal, W.; Minahal, Q.; Hussain, A.S. Dietary supplementation of methionine, lysine, and tryptophan as possible modulators of growth, immune response, and disease resistance in striped catfish (Pangasius hypophthalmus). PLoS ONE 2024, 19, e0301205. [Google Scholar] [CrossRef]
- Gutiérrez-Vela, M. Effect of Feeding Sea Bream (Sparus aurata L.) with Fishmeal-Free Feed on Digestive Enzyme Activity. Ph.D. Dissertation, Polytechnic University of Valencia, Valencia, Spain, 2017. [Google Scholar]
- Sunde, J. Digestive Protease Activities, Growth and Feed Utilisation in Atlantic Salmon (Salmo salar L.). Ph.D. Dissertation, University of Bergen, Bergen, Norway, 2006. [Google Scholar]
- De Melo-Oliveira, V.; de Souza Bezerra, R.; Assis, C.R.D. Fish pepsin: Basic characteristics, extraction, determination and biotechnological applications. Nat. Resour. 2014, 4, 6–14. [Google Scholar]
- Hassaan, M.S.; Mohammady, E.Y.; Adnan, A.M.; Abd Elnabi, H.E.; Ayman, M.F.; Soltan, M.A.; El-Haroun, E.R. Effect of dietary protease at different levels of malic acid on growth, digestive enzymes and haemato-immunological responses of Nile tilapia, fed fish meal free diets. Aquaculture 2020, 522, 735124. [Google Scholar] [CrossRef]
- Shah, S.Z.H.; Fatima, M.; Afzal, M.; Bilal, M. Interactive effect of citric acid, phytase and chelated mineral on growth performance, nutrient digestibility and whole-body composition of Labeo rohita fingerlings. Aquac. Res. 2021, 52, 842–858. [Google Scholar] [CrossRef]
- Brilianto-Muttaqin, R.A.; Surya, A.; Istiarini, I.; Duanassurya, M.; Samosir, W.; Makatita, S.; Nurhayati, T. Extraction and characterization of pepsin enzyme from the skipjack vicera (Katsuwonus pelamis). BIO Web Conf. 2024, 147, 01018. [Google Scholar] [CrossRef]
- Kurniawan, R.; Nurul-Izzati, S.A.; Nurhayati, T.; Jacoeb, A.M. Application of pepsin enzyme from yellowfin tuna gastrics on the physical and histological characteristics of beef. BIO Web Conf. 2024, 147, 01026. [Google Scholar] [CrossRef]
- Maryam; Shah, S.Z.H.; Fatima, M.; Nadeem, H.; Ashraf, S.; Hussain, M. Roles of dietary supplementation of exogenous protease in low fishmeal aquafeed—A mini review. Ann. Anim. Sci. 2024, 24, 27–39. [Google Scholar] [CrossRef]
- Solovyev, M.; Kashinskaya, E.; Gisbert, E. A meta-analysis for assessing the contributions of trypsin and chymotrypsin as the two major endoproteases in protein hydrolysis in fish intestine. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2023, 278, 111372. [Google Scholar] [CrossRef] [PubMed]
- Zamani, A.; Khajavi, M.; Abedian Kenari, A.; Haghbin Nazarpak, M.; Solouk, A.; Esmaeili, M.; Gisbert, E. Physicochemical and Biochemical Properties of Trypsin-like Enzyme from Two Sturgeon Species. Animals 2023, 13, 853. [Google Scholar] [CrossRef]
- Whitaker, J.R. Principles of Enzymology for the Food Sciences, 2nd ed.; Dekker, M., Ed.; INC: New York, NY, USA, 1994. [Google Scholar]
- Hofer, R.; Schiemer, F. Proteolytic activity in the digestive tract of several species of fish with different feeding habits. Oecologia 1981, 48, 342–345. [Google Scholar] [CrossRef]
- Moyano, F.J.; Diaz, M.; Alarcón, F.J.; Sarasquete, M.C. Characterization of digestive enzyme activity during larval development of gilthead seabream (Sparus aurata). Fish Physiol. Biochem. 1996, 15, 121–130. [Google Scholar] [CrossRef]
- Concha-Frías, B.; González, C.A.A.; Gaxiola, G.; Chiappa, X.; Sánchez-Zamora, A.; Martínez-García, R.; Camarillo-Coop, S.; Peña, E.; Jiménez-Martínez, L.D.; De la Cruz-Alvarado, F.J. Dietary protein requirement in common snook (Centropomus undecimalis) juveniles reared in marine and brackish water. Ecosistemas Recur. Agropecu. 2018, 5, 45–54. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Jimenez-Martinez, L.D.; Morales-Garcia, V.; Frías-Quintana, C.A.; Castillo-Collado, A.C.; Asencio-Alcudia, G.G.; Álvarez-Villagomez, C.S.; Peña, E.; Concha-Frías, B.; Álvarez-González, C.A. Quality evaluation of reference gene expression on different tissues in adults of tropical gar Atractostreus tropicus. Pak. J. Zool. 2021, 54, 363–372. [Google Scholar] [CrossRef]
- Molinari, G.S.; Wojno, M.; Kwasek, K. Effects of dietary indispensable amino acid deficiencies on feed intake in stomachless fish. Comp. Biochem. Physiol. Part A 2024, 298, 111742. [Google Scholar] [CrossRef]
- Makinde, O.J. Maggot meal: A sustainable protein source for livestock production—A Review. Adv. Life Sci. Technol. 2015, 31, 35–42. [Google Scholar]
- Chaalala, S.; Leplat, A.; Makkar, H. Importance of insects for use as animal feed in low-income countries. In Edible Insects in Sustainable Food Systems; Springer: Cham, Switzerland, 2018; pp. 303–319. [Google Scholar]
- Mikołajczak, Z.; Rawski, M.; Mazurkiewicz, J.; Kierończyk, B.; Józefiak, D. The effect of hydrolyzed insect meals in sea trout fingerling (Salmo trutta m. trutta) diets on growth performance, microbiota and biochemical blood parameters. Animals 2020, 10, 1031. [Google Scholar] [CrossRef]
- Bosi, A.; Bani, D.; Moroni, F.; Ceccotti, C.; Giron, M.C.; Antonini, M.; Giaroni, C.; Terova, G. Effect of partial substitution of ishmeal with insect meal (Hermetia illucens) on gut neuromuscular function in Gilthead sea bream (Sparus aurata). Sci. Rep. 2021, 11, 21788. [Google Scholar] [CrossRef]
- Peng, K.; Mo, W.; Xiao, H.; Hu, J.; Zhu, X.; Huang, Y.; Wang, G. Dietary black soldier fly pulp affects growth, antioxidant and immune capacity of Micropterus salmoides. J. Insects Food Feed 2021, 8, 1197–1203. [Google Scholar] [CrossRef]
- Terova, G.; Gini, E.; Gasco, L.; Moroni, F.; Antonini, M.; Rimoldi, S. Effects of full replacement of dietary fishmeal with insect meal from Tenebrio molitor on rainbow trout gut and skin microbiota. J. Anim. Sci. Biotechnol. 2021, 12, 30. [Google Scholar] [CrossRef]
- Gasco, L.; Schiavone, A.; Caimi, C.; Trocino, A.; Lussiana, C.; Bellezza-Oddon, S.; Malfatto, V.; Anedda, R.; Serra, G.; Biasato, I.; et al. Digestibility of defatted insect meals for rainbow trout aquafeeds. J. Insects Food Feed 2022, 8, 1385–1399. [Google Scholar] [CrossRef]
- Sajid, Q.U.A.; Asghar, M.U.; Tariq, H.; Wilk, M.; Płatek, A. Insect Meal as an Alternative to Protein Concentrates in Poultry Nutrition with Future Perspectives (An Updated Review). Agriculture 2023, 13, 1239. [Google Scholar] [CrossRef]
- Mikołajczak, Z.; Mazurkiewicz, J.; Rawski, M.; Kierończyk, B.; Józefiak, A.; Świątkiewicz, S.; Józefiak, D. Black soldier fly full-fat meal in Atlantic salmon nutrition–Part A: Effects on growth performance, feed utilization, selected nutriphysiological traits and production sustainability in fries. Ann. Anim. Sci. 2022, 23, 225–238. [Google Scholar] [CrossRef]
- Ahmed, I.; Ahmad, I. Effect of dietary protein levels on growth performance, hematological profile and biochemical composition of fingerlings rainbow trout, Oncorhynchus mykiss reared in Indian Himalayan region. Aquac. Rep. 2020, 16, 100268. [Google Scholar] [CrossRef]
- Adewumi, A.A. The impact of nutrition on fish development, growth and health. Int. J. Sci. Res. Publ. 2018, 8, 147–153. [Google Scholar]
- Susanto, A.; Hutabarat, J.; Anggoro, S.; Subandiyono. The effects of dietary carbohydrate level on the growth performance, body composition and feed utilization of juvenile Kelabau (Osteochilus melanopleurus). AACL Bioflux 2020, 13, 261–270. [Google Scholar]
- Ghosi-Mobaraki, M.R.; Abedian-Kenari, A.; Bahrami-Gorji, S.; Esmaeili, M. Effect of dietary fish and vegetable oil on the growth performance, body composition, fatty acids profile, reproductive performance and larval resistance in pearl gourami (Trichogaster leeri). Aquac. Nutr. 2020, 26, 894–907. [Google Scholar] [CrossRef]
- Odu-Onikosi, S.G.; Babalola, O.A.; Ogunbanwo, O. Effect of alternate feeding of normal and low protein diet on the growth performance and biochemical composition of Clarias gariepinus (Burchell, 1822) fingerlings. Int. J. Fish. Aquat. Stud. 2021, 9, 63–67. [Google Scholar] [CrossRef]
- Wang, J.; Liu, T.; Zheng, P.; Xu, H.; Su, H.; Han, T.; Yang, Y. Effect of dietary lipid levels on growth performance, body composition, and feed utilization of juvenile spotted knifejaw Oplegnathus punctatus. Aquac. Rep. 2021, 21, 100797. [Google Scholar] [CrossRef]
- Hamdy, A.M.S.; Emad, E.I.A.M.; Faiza, M.S.; Ahmed, E.A.B.; Alaa, G.M.O. Fish growth performance, body composition and water quality in integrated system producing Grass carp (Ctenopharyngodon idella) in the Eastern Desert. Sohag J. Sci. 2022, 7, 67–75. [Google Scholar] [CrossRef]
- Baek, S.I.; Cho, S.H. Dietary Replacement Effect of Fish Meal by Tuna By-Product Meal on Growth and Feed Availability of Red Sea Bream (Pagrus major). Animals 2024, 14, 688. [Google Scholar] [CrossRef]
- Qu, H.; Chen, L.; Yang, J.; Liao, J.; Wei, D.; Lu, X. Effects of feeding strategies on growth, body composition, intestine digestive enzymes activities and intestine histology of Megalobrama pellegrini (Tchang, 1930) early juveniles raised in flow-through system. Isr. J. Aquac.-Bamidgeh 2020, 72, 1–9. [Google Scholar] [CrossRef]
- Ndione, A.; Fall, J.; Mbaye, S.; Ndour, P.M.; Khady, L.F.S.; Jatta, S.; Loum, A.; Sagne, M.; Diouf, A.; Ndong, D.; et al. Effect of replacing fishmeal with caterpillar (Cirina butyrospermi) meal on the growth performance, feed efficiency, survival and body composition of Nile tilapia (Oreochromis niloticus) fry. J. Fish. Soc. Taiwan 2022, 49, 193–199. [Google Scholar] [CrossRef]
- Alfiko, Y.; Xie, D.; Tri Astuti, R.; Wong, J.; Wan, L. Insects as a feed ingredient for fish culture: Status and trends. Aquac. Fish. 2022, 7, 166–178. [Google Scholar] [CrossRef]
- Manan, M.C.A.; Naz, S.; Danabas, D. Effect of Insect Feed on Fish Growth: A Review. Asian Fish. Sci. 2024, 37, 52–68. [Google Scholar] [CrossRef]
- Reyes, M.; Rodríguez, M.; Montes, J.; Barroso, M.G.; Fabrikov, D.; Morote, E.; Sánchez-Muros, M.J. Nutritional and Growth Effect of Insect Meal Inclusion on Seabass (Dicentrarchuss labrax) Feeds. Fishes 2020, 5, 16. [Google Scholar] [CrossRef]
- Hua, K. A meta-analysis of the effects of replacing fish meals with insect meals on growth performance of fish. Aquaculture 2021, 530, 735732. [Google Scholar] [CrossRef]
- Liland, N.; Araujo, P.; Xu, X.; Lock, E.-J.; Radhakrishnan, G.; Prabhu, A.; Belghit, I. A meta-analysis on the nutritional value of insects in aquafeeds. J. Insects Food Feed 2021, 7, 743–760. [Google Scholar] [CrossRef]
- Ndione, A.; Fall, J.; Mbaye, S.; Ndour, P.M.; Khady, L.F.S.; Jatta, S.; Loum, A.; Sagne, M.; Diouf, A.; Ndong, D.; et al. Effects of replacement of fishmeal by cricket meal on the growth performance, feed efficiency, survival and body composition of Nile Tilapia (Oreochromis niloticus) fry. J. Fish. Soc. Taiwan 2022, 49, 183–191. [Google Scholar] [CrossRef]
- Lee, S.W.; Tey, H.C.; Wendy, W.; Wan Zahari, M. The effect of house cricket (Acheta domesticus) meal on growth performance of red hybrid tilapia (Oreochromis sp.). Int. J. Aquat. Sci. 2017, 8, 78–82. [Google Scholar]
- Irungu, F.G.; Mutungi, C.M.; Faraj, A.K.; Affognon, H.; Tanga, C.; Ekesi, S.; Nakimbugwe, D.; Fiaboe, K.K.M. Mineral content of extruded fish feeds containing cricket (Acheta domesticus) and black soldier fly (Hermetia illucens) fractions. Int. Aquat. Res. 2018, 10, 101–113. [Google Scholar] [CrossRef]
- Perera, G.S.C.; Perera, D.A.; Piyavorasakul, C.; Pumpuang, S. Fishmeal Replacement by House cricket (Acheta domesticus) and Field cricket (Gryllus bimaculatus) Meals in Nile Tilapia (Oreochromis niloticus) Fingerling Feed. Aquac. Stud. 2023, 23, AQUAST1187. [Google Scholar] [CrossRef]
- Tilami, S.K.; Turek, J.; Červený, D.; Lepič, P.; Kozák, P.; Burkina, V.; Sakalli, S.; Tomčala, A.; Sampels, S.; Mráz, J. Insect Meal as a Partial Replacement for Fish Meal in a Formulated Diet for Perch Perca fluviatilis. Turk. J. Fish. Aquat. Sci. 2020, 20, 867–878. [Google Scholar] [CrossRef]
- Silva, A. Marine Fish Farming; Catholic University of the North, Faculty of Marine Sciences: Santiago, Chile, 2005; p. 262. [Google Scholar]
- Baldisserotto, B.; Mancera, J.M.; Kapoor, B.G. Fish Osmoregulation; Science Publishers: Enfield, NH, USA, 2007; p. 527. [Google Scholar] [CrossRef]
- Navarro-Guillén, N.C.; Yúfera, M.; Perera, E. Biochemical features and modulation of digestive enzymes by environmental temperature in the greater amberjack, Seriola dumerili. Front. Mar. Sci. 2022, 9, 960746. [Google Scholar] [CrossRef]
- Lal, V.; Naeem, M.; Asad, M.; Tanveer, K.; Zulfiqar, A.; Kausar, S. Study of digestive enzymes in marine fish, Terapon jarbua, from Pakistan. Braz. J. Biol. 2024, 84, e267508. [Google Scholar] [CrossRef]
- Carginale, V.; Trinchella, F.; Capasso, C.; Scudiero, R.; Parisi, E. Gene amplification and cold adaptation of pepsin in Antarctic fish. A possible strategy for food digestion at low temperature. Gene 2004, 336, 195–205. [Google Scholar] [CrossRef]
- Silva, W.S.; Ribeiro, P.A.P.; Costa, L.S.; López-Olmeda, J.F.; Costa, N.C.S.; Santos, W.M.; Luz, R.K. Gene expression, enzyme activity and performance of Nile tilapia larvae fed with diets of different CP levels. Animal 2019, 13, 1376–1384. [Google Scholar] [CrossRef]
- Syed-Makhdoom, H.; Muniba, J.; Shafaqat, A.; Muhammad, M.S.; Majid, H.; Nisar, A.; Danish, R. Gene Expression, Growth Performance and Body Composition of Labeo rohita Fingerlings Fed on Polyphenols Supplement. Sains Malays. 2023, 52, 3357–3370. [Google Scholar] [CrossRef]
- Eggink, K.M.; Pedersen, P.B.; Lund, I.; Dalsgaard, J. Chitin digestibility and intestinal exochitinase activity in Nile tilapia and rainbow trout fed different black soldier fly larvae meal size fractions. Aquac. Res. 2022, 53, 5536–5546. [Google Scholar] [CrossRef]
- Gutowska, M.A.; Drazen, J.C.; Robison, B.H. Digestive chitinolytic activity in marine fishes of Monterey Bay, California. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2004, 139, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Jonás, E.; Rágyanszki, M.; Oláh, J.; Boross, L. Proteolytic digestive enzymes of carnivorous (Silurus glanis L.), herbivorous (Hypophthalmichthys molitrix Val.) and omnivorous (Cyprinus carpio L.) fishes. Aquaculture 1983, 30, 145–154. [Google Scholar] [CrossRef]
- Kanno, G.; Klomklao, S.; Kumagai, Y.; Kishimura, H. A thermostable trypsin from freshwater fish Japanese dace (Tribolodon hakonensis): A comparison of the primary structures among fish trypsins. Fish Physiol. Biochem. 2018, 45, 561–571. [Google Scholar] [CrossRef]
- Krebs, J.E.; Goldstein, E.S.; Kilpatrick, S.T. Lewin’s Genes XII; Jones & Bartlett Learning, LLC.: Burlington, MA, USA, 2018; p. 3194. [Google Scholar]
- Kroeckel, S.; Harjes, A.G.; Roth, I.; Katz, H.; Wuertz, S.; Susenbeth, A.; Schulz, C. When a turbot catches a fly: Evaluation of a pre-pupae meal of the Black Soldier Fly (Hermetia illucens) as fish meal substitute—Growth performance and chitin degradation in juvenile turbot (Psetta maxima). Aquaculture 2012, 364, 345–352. [Google Scholar] [CrossRef]
- Steinberg, C.E. Diets and digestive tracts–‘Your food determines your intestine’. In Aquatic Animal Nutrition: A Mechanistic Perspective from Individuals to Generations; Springer: Cham, Switzerland, 2018; pp. 9–59. [Google Scholar]
- Méndez, E. Digestive and Metabolic Biochemical Physiology of Decapod Crustaceans and Teleost Fish of Regional, Ecological, and/or Economic Interest: Characterization and Modulation of Key Enzymes in the Digestive Tract. Ph.D. Thesis, National University of Mar del Plata, Mar del Plata, Argentina, 2020. [Google Scholar]
- Zhao, S.; Xu, W.; Jiang, W.; Yu, W.; Lin, Y.; Zhang, T.; Yao, J.; Zhou, L.; Zeng, Y.; Li, H.; et al. Regulation of cellular metabolism by protein lysine acetylation. Science 2010, 327, 1000–1004. [Google Scholar] [CrossRef]
- Bazer, F.W.; Johnson, G.A.; Wu, G. Amino acids and conceptus development during the peri-implantation period of pregnancy. In Cell Signaling During Mammalian Bendhack Fabiano Early Embryo Development; Springer: New York, NY, USA, 2015; pp. 23–52. [Google Scholar]
- Morin, G.; Pinel, K.; Dias, K.; Seiliez, I.; Beaumatin, F. RTH-149 Cell Line, a Useful Tool to Decipher Molecular Mechanisms Related to Fish Nutrition. Cells 2020, 9, 1754. [Google Scholar] [CrossRef]
- Machado, M.; Serra, C.R.; Oliva-Teles, A.; Costas, B. Methionine and Tryptophan Play Different Modulatory Roles in the European Seabass (Dicentrarchus labrax) Innate Immune Response and Apoptosis Signaling—An In Vitro Study. Front. Immunol. 2021, 12, 660448. [Google Scholar] [CrossRef] [PubMed]
- Rungruangsak-Torrissen, K.; Moss, R.; Andresen, L.H.; Berg, A.; Waagbø, R. Different expressions of trypsin and chymotrypsin in relation to growth in Atlantic salmon (Salmo salar L.). Fish Physiol. Biochem. 2006, 32, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Biteau, C.; Bry-Chevalier, T.; Crummett, D.; Ryba, R.; St. Jules, M. Insect-based livestock feeds are unlikely to become economically viable in the near future. Food Humanit. 2024, 3, 100383. [Google Scholar] [CrossRef]
- Auzins, A.; Leimane, I.; Reissaar, R.; Brobakk, J.; Sakelaite, I.; Grivins, M.; Zihare, L. Assessing the socio-economic benefits and cost of insect meal as a fishmeal substitute in livestock and aquaculture. Animals 2024, 14, 1461. [Google Scholar] [CrossRef] [PubMed]
| Ingredient | 0% HCM | 25% HCM | 50% HCM | 75% HCM | 100% HCM |
|---|---|---|---|---|---|
| Soft wheat grain | 43.44 | 42.56 | 42.74 | 41.86 | 41.6 |
| Fish, Herring meal | 26.01 | 20 | 12.83 | 5.72 | 0 |
| Soybean flour 44% | 20 | 18.9 | 18 | 18 | 17.03 |
| Cricket flour | 0 | 8 | 16 | 24 | 3 |
| Fish oil | 3.05 | 4 | 4 | 4 | 3.86 |
| Soy lecithin | 3 | 2.05 | 1.93 | 1.92 | 2 |
| Granadina | 2 | 2 | 2 | 2 | 2 |
| Vitamin premix | 1 | 1 | 1 | 1 | 1 |
| Fish solubles, dehy | 1 | 1 | 1 | 1 | 1 |
| Vitamin C | 0.5 | 0.5 | 0.5 | 0 | 0 |
| Nutrient | As food | As food | As food | As food | As food |
| Energy MEt (m) | 1355.36 | 1654.59 | 1987.15 | 2286.38 | 2564.29 |
| Protein | 35 | 35 | 35 | 35 | 35 |
| Fat | 10 | 10 | 10 | 10 | 10 |
| Fiber | 2.89 | 3.54 | 4.1 | 4.75 | 5.27 |
| Ash | 5.07 | 4.31 | 3.68 | 2.92 | 2.31 |
| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) | Size (bp) | Amplification Tion Efficiency (%) |
|---|---|---|---|---|
| pepsin | GGCTGCATGACTGGTTTTGG | TCTAGTGGATCTCTCAGTGGATA | 154 | 97.5 |
| trypsin (tryp) | GAACTCCGGCTACCACTTCT | TAGGAGCTGTAGTTGGGGTG | 183 | 97.20 |
| chymotrypsin- sina (qtryp) | AACGAGAACTGGGTGGTGAC | ATTGTGGGGGTTCCAGTA | 158 | 97.63 |
| ef-1 | GAGGAAATCACCAAGGAAGTGA | CTTGAACCAGCTCATCTTGGAG | 154 | 99.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz-Alvarado, F.J.D.l.; Concha Frías, B.; López-Cerino, M.G.; Álvarez-González, C.A.; Gaxiola-Cortés, G.; Arias-Moscoso, J.L.; Bautista-Ortega, J.; Hernández-García, S.; Palma-Cancino, D.J. Modulation of Gene Expression in the Digestive Tract of the Tropical Gar (Atractosteus tropicus) in Response to Cricket Meal (Acheta domesticus). Fishes 2025, 10, 469. https://doi.org/10.3390/fishes10090469
Cruz-Alvarado FJDl, Concha Frías B, López-Cerino MG, Álvarez-González CA, Gaxiola-Cortés G, Arias-Moscoso JL, Bautista-Ortega J, Hernández-García S, Palma-Cancino DJ. Modulation of Gene Expression in the Digestive Tract of the Tropical Gar (Atractosteus tropicus) in Response to Cricket Meal (Acheta domesticus). Fishes. 2025; 10(9):469. https://doi.org/10.3390/fishes10090469
Chicago/Turabian StyleCruz-Alvarado, Fanny Janet De la, Bartolo Concha Frías, María Guadalupe López-Cerino, Carlos Alfonso Álvarez-González, Gabriela Gaxiola-Cortés, Joe Luis Arias-Moscoso, Jaime Bautista-Ortega, Sergio Hernández-García, and David Julián Palma-Cancino. 2025. "Modulation of Gene Expression in the Digestive Tract of the Tropical Gar (Atractosteus tropicus) in Response to Cricket Meal (Acheta domesticus)" Fishes 10, no. 9: 469. https://doi.org/10.3390/fishes10090469
APA StyleCruz-Alvarado, F. J. D. l., Concha Frías, B., López-Cerino, M. G., Álvarez-González, C. A., Gaxiola-Cortés, G., Arias-Moscoso, J. L., Bautista-Ortega, J., Hernández-García, S., & Palma-Cancino, D. J. (2025). Modulation of Gene Expression in the Digestive Tract of the Tropical Gar (Atractosteus tropicus) in Response to Cricket Meal (Acheta domesticus). Fishes, 10(9), 469. https://doi.org/10.3390/fishes10090469








