From Gut to Fillet: Comprehensive Effects of Tenebrio molitor in Fish Nutrition
Abstract
1. Introduction
2. Materials and Methods
3. Growth Performance
Feed Digestibility
4. Fish Meat Composition
5. Fish Reproduction
6. Blood Biomarkers
7. Histopathological Changes
7.1. Intestine Histopathology
7.2. Liver Histopathology
7.3. Kidney Histopathology
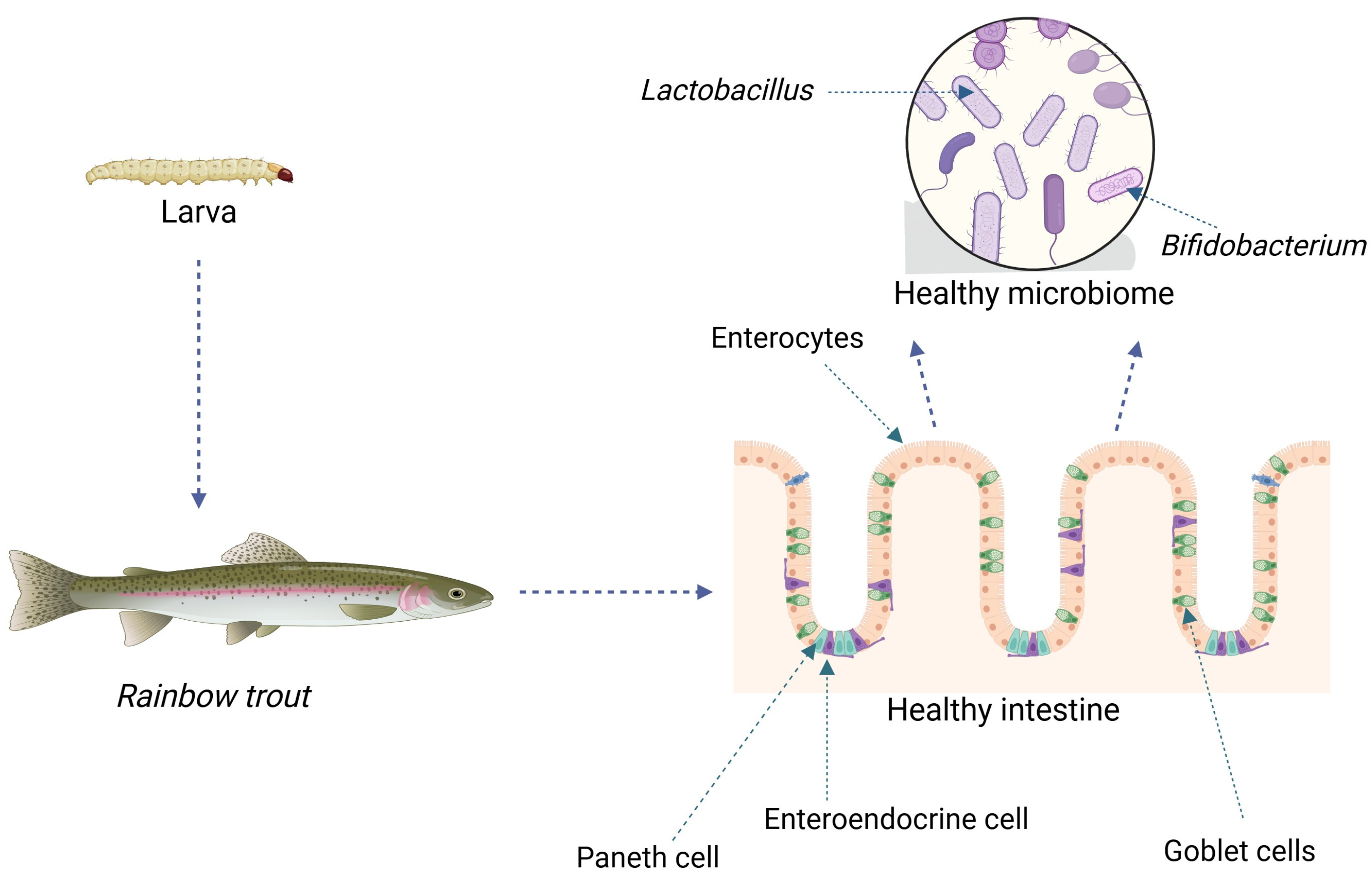
8. Intestinal Microbiota
9. Recommendations and Mitigation Strategies
9.1. Processing of Tenebrio molitor Meal and Nutrient Supplementation
9.2. Osmoregulation
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TM | Tenebrio molitor |
| FM | fishmeal |
| EU | European Union |
| PAP | processed animal proteins |
| ANF | anti-nutritional factors |
| DMW | defatted mealworm |
| LP | lamina propria |
| RBC | red blood cells |
| WBC | white blood cells |
| Hb | hemoglobin |
| Hct | hematocrit |
| MCV | mean corpuscular volume |
| MCH | mean corpuscular hemoglobin |
| MCHC | mean corpuscular hemoglobin concentration |
| SR | survival rates |
| TP | total protein |
| ALB | albumin |
| MDA | malondialdehyde |
| PC | proteic carbonyl |
| 8-OHdG | 8-Hydroxy-2-Deoxyguanosine |
| AST | aspartate aminotransferase |
| ALT | alanine aminotransferase |
| SBM | soybean meal |
| AAE | essential amino acids |
| AANE | non-essential amino acids |
| ROS | reactive oxygen species |
| GPx | glutathione peroxidase |
| CAT | catalase |
| n-3 PUFA | omega-3 polyunsaturated fatty acids |
| DHA | docosahexaenoic acid |
| SOD | superoxide dismutase |
| HSI | hepatosomatic index |
| CHOL | cholesterol |
| TG | triglyceride |
| EPA | eicosapentaenoic acid |
| ALP | alkaline phosphatase |
| IgM | immunoglobulin M |
| RAS | recirculating aquaculture systems |
| Glu | glucose |
| ACP | acid phosphatase |
| SR | survival rate |
| PLT | platelets |
| SB | sodium butyrate |
| DAO | diamine oxidase |
| OTUs | operational taxonomic units |
| ADC | apparent digestibility coefficients |
References
- Fletcher, A.J.; Lozano, R.; McNabb, W.C. Analysis of global nutrient gaps and their potential to be closed through redistribution and increased supply. Front. Nutr. 2024, 11, 1396549. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) 2017/893 of 24 May 2017; European Commission: Brussels, Belgium, 2017. [Google Scholar]
- European Commission. Amending Annexes I and IV to Regulation (EC) No 999/2001 of the European Parliament and of the Council and Annexes X, XIV and XV to Commission Regulation (EU) No 142/2011 as Regards the Provisions on Processed Animal Protein. Available online: https://eur-lex.europa.eu/eli/reg/2017/893/oj/eng (accessed on 21 July 2025).
- European Commission. Commission Regulation (EU) 2021/1372 of 17 August 2021 Amending Annex IV to Regulation (EC) No 999/2001 of the European Parliament and of the Council as Regards the Prohibition to Feed Non-Ruminant Farmed Animals, Other than fur Animals, with Protein Derived from Animals; European Commission: Brussels, Belgium, 2021. [Google Scholar]
- European Commission. Amending Certain Annexes to Regulation (EU) No 142/2011 as Regards the Requirements for Placing on the Market of Certain Insect Products and the Adaptation of a Containment Method; European Commission: Brussels, Belgium, 2021. [Google Scholar]
- European Commission. On Novel Foods, Amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council and Repealing Regulation (EC) No 258/97 of the European Parliament and of the Council and Commission Regulation (EC) No 1852/2001; European Commission: Brussels, Belgium, 2015. [Google Scholar]
- European Commission. Authorising the Placing on the Market of dried Tenebrio Molitor Larva as a Novel Food Under Regulation (EU) 2015/2283 of the European Parliament and of the Council, and Amending Commission Implementing Regulation (EU) 2017/2470; European Commission: Brussels, Belgium, 2021. [Google Scholar]
- European Commission. Authorising the Placing on the Market of Frozen, Dried and Powder Forms of Locusta Migratoria as a Novel Food Under Regulation (EU) 2015/2283 of the European Parliament and of the Council and Amending Commission Implementing Regulation (EU) 2017/2470; European Commission: Brussels, Belgium, 2021. [Google Scholar]
- European Commission. Authorising the Placing on the Market of Frozen, Dried and Powder Forms of Acheta Domesticus as a Novel Food Under Regulation (EU) 2015/2283 of the European Parliament and of the Council, and Amending Commission Implementing Regulation (EU) 2017/2470; European Commission: Brussels, Belgium, 2022. [Google Scholar]
- European Commission. Authorising the Placing on the Market of the Frozen, Paste, Dried and Powder forms of Alphitobius Diaperinus Larvae (Lesser mealworm) as a Novel Food and Amending Implementing Regulation (EU) 2017/2470; European Commission: Brussels, Belgium, 2023. [Google Scholar]
- Sangiorgio, P.; Verardi, A.; Dimatteo, S.; Spagnoletta, A.; Moliterni, S.; Errico, S. Valorisation of agri-food waste and mealworms rearing residues for improving the sustainability ofTenebrio molitor industrial production. J. Insects Food Feed 2022, 8, 509–524. [Google Scholar] [CrossRef]
- Mboya, B.J. Enhancing Aquaculture Sustainability: The Use of Insect Meals in Fish Feed. Anim. Sci. Cases 2025, 2025, ascs20250015. [Google Scholar] [CrossRef]
- Fuertes-Mendizábal, T.; Salcedo, I.; Huérfano, X.; Riga, P.; Estavillo, J.M.; Ávila Blanco, D.; Duñabeitia, M.K. Mealworm frass as a potential organic fertilizer in synergy with PGP-based biostimulant for lettuce plants. Agronomy 2023, 13, 1258. [Google Scholar] [CrossRef]
- Peng, B.-Y.; Xu, Y.; Zhou, X.; Wu, W.-M.; Zhang, Y. Generation and fate of nanoplastics in the intestine of plastic-degrading insect (Tenebrio molitor larvae) during polystyrene microplastic biodegradation. Environ. Sci. Technol. 2024, 58, 10368–10377. [Google Scholar] [CrossRef] [PubMed]
- Boonthong, S.; Nuntapong, N.; Hahor, W.; Waeowannajit, S.; Thongprajukaew, K. Mealworm (Tenebrio molitor) feed substrate waste: An alternative protein source for aquafeed production. J. Insects Food Feed 2025, 1, 1–15. [Google Scholar] [CrossRef]
- Morales-Ramos, J.A.; Tomberlin, J.K.; Miranda, C.; Rojas, M.G. Rearing methods of four insect species intended as feed, food, and food ingredients: A review. J. Econ. Entomol. 2024, 117, 1210–1224. [Google Scholar] [CrossRef] [PubMed]
- Adamaki-Sotiraki, C.; Rumbos, C.I.; Athanassiou, C.G. From a stored-product pest to a promising protein source: A U-turn of human perspective for the yellow mealworm Tenebrio molitor. J. Pest Sci. 2025, 98, 113–129. [Google Scholar] [CrossRef]
- Oliveira, J.S.; Ribeiro, C.D.F.; de Souza, C.O.; Ribeiro, C.V.D.M. Mapping the use of insects in animal feed: Scientific and technological data of Tenebrio molitor, Hermetia illucens, and Zophobas morio. Anim. Prod. Sci. 2025, 65, AN24415. [Google Scholar] [CrossRef]
- Maphios, M.; Ndlovu, R.; Sisito, G.; Rukuni, T.; Mare, M.; Dominic Matekenya, T. Effect of Replacing Blood Meal for Mealworm Larvae (Tenebrio molitor) Meal in Broiler Starter Diets on Growth Performance, Carcass Traits and Meat Sensory Test. Int. J. Innov. Sci. Res. Technol. 2025, 10, 2466–2470. [Google Scholar] [CrossRef]
- Răducu, C.; Cocan, D.; Constantinescu, R.; Mireșan, V.; Huluba, C.; Munteanu, C.; Ihuț, A. Study on the growth performance of rainbow trout Oncorhynchus mykiss fed with Tenebrio molitor larvae. ABAH Bioflux 2024, 16, 32–37. [Google Scholar]
- Gkinali, A.-A.; Matsakidou, A.; Vasileiou, E.; Paraskevopoulou, A. Potentiality of Tenebrio molitor larva-based ingredients for the food industry: A review. Trends Food Sci. Technol. 2022, 119, 495–507. [Google Scholar] [CrossRef]
- Robles-Jimenez, L.E.; Angeles, S.; Ramirez-Perez, A.; Fuente, B.; Velazquez-Ordonez, V.; Cardoso-Gutierrez, E.; Renna, M.; Rastello, L.; Capucchio, M.; Hassan, T. In Vitro and in vivo investigations on the use of yellow mealworm (Tenebrio molitor) as a novel protein feed ingredient for fattening lambs. Anim. Feed Sci. Technol. 2025, 320, 116224. [Google Scholar] [CrossRef]
- Aragao, C.; Gonçalves, A.T.; Costas, B.; Azeredo, R.; Xavier, M.J.; Engrola, S. Alternative Proteins for Fish Diets: Implications beyond Growth. Animals 2022, 12, 1211. [Google Scholar] [CrossRef]
- Urán, P.; Gonçalves, A.; Taverne-Thiele, J.; Schrama, J.; Verreth, J.; Rombout, J. Soybean meal induces intestinal inflammation in common carp (Cyprinus carpio L.). Fish Shellfish Immunol. 2008, 25, 751–760. [Google Scholar] [CrossRef]
- Peng, K.-S.; Wu, N.; Cui, Z.-W.; Zhang, X.-Y.; Lu, X.-B.; Wang, Z.-X.; Zhang, Y.-A. Effect of the complete replacement of dietary fish meal by soybean meal on histopathology and immune response of the hindgut in grass carp (Ctenopharyngodon idellus). Vet. Immunol. Immunopathol. 2020, 221, 110009. [Google Scholar] [CrossRef]
- Gu, M.; Jia, Q.; Zhang, Z.; Bai, N.; Xu, X.; Xu, B. Soya-saponins induce intestinal inflammation and barrier dysfunction in juvenile turbot (Scophthalmus maximus). Fish Shellfish Immunol. 2018, 77, 264–272. [Google Scholar] [CrossRef]
- Krogdahl, Å.; Gajardo, K.; Kortner, T.M.; Penn, M.; Gu, M.; Berge, G.M.; Bakke, A.M. Soya saponins induce enteritis in Atlantic salmon (Salmo salar L.). J. Agric. Food Chem. 2015, 63, 3887–3902. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, C.; Zhao, Z.; Luo, L. Effects of replacement of fish meal by soy protein isolate on the growth, digestive enzyme activity and serum biochemical parameters for juvenile Amur sturgeon (Acipenser schrenckii). Asian-Australas. J. Anim. Sci. 2012, 25, 1588. [Google Scholar] [CrossRef]
- Aidos, L.; Mirra, G.; Pallaoro, M.; Herrera Millar, V.R.; Radaelli, G.; Bazzocchi, C.; Modina, S.C.; Di Giancamillo, A. How Do Alternative Protein Resources Affect the Intestine Morphology and Microbiota of Atlantic Salmon? Animals 2023, 13, 1922. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.-M.; Khosravi, S.; Mauliasari, I.R.; Lee, S.-M. Dietary inclusion of mealworm (Tenebrio molitor) meal as an alternative protein source in practical diets for rainbow trout (Oncorhynchus mykiss) fry. Fish. Aquat. Sci. 2020, 23, 12. [Google Scholar] [CrossRef]
- Shafique, L.; Abdel-Latif, H.M.; Hassan, F.-u.; Alagawany, M.; Naiel, M.A.; Dawood, M.A.; Yilmaz, S.; Liu, Q. The feasibility of using yellow mealworms (Tenebrio molitor): Towards a sustainable aquafeed industry. Animals 2021, 11, 811. [Google Scholar] [CrossRef]
- Flores-Moreno, S.; Alarcón-López, F.J.; Coronel-Domínguez, A.J.; Zuasti, E.; Hachero-Cruzado, I. A Comparative Study of the Effect of Including Full-Fat Tenebrio molitor for Replacing Conventional Ingredients in Practical Diets for Dicentrarchus labrax Juveniles. Animals 2025, 15, 131. [Google Scholar] [CrossRef]
- Rema, P.; Saravanan, S.; Armenjon, B.; Motte, C.; Dias, J. Graded incorporation of defatted yellow mealworm (Tenebrio molitor) in rainbow trout (Oncorhynchus mykiss) diet improves growth performance and nutrient retention. Animals 2019, 9, 187. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ellis, J.; Huyben, D. Examining the dietary effect of insect meals on the innate immune response of fish: A meta-analysis. Comp. Immunol. Rep. 2024, 7, 200169. [Google Scholar] [CrossRef]
- Hossain, M.S.; Hamidoghli, A.; Hong, J.; Sealey, W.; Small, B.C. Effects of High Dietary Inclusion of Defatted Mealworm (Tenebrio molitor) Meal as a Fish Meal Substitute on Growth, Histological Traits, and Health Performances of Rainbow Trout (Oncorhynchus mykiss). Aquac. Nutr. 2025, 2025, 5568058. [Google Scholar] [CrossRef] [PubMed]
- Mente, E.; Bousdras, T.; Feidantsis, K.; Panteli, N.; Mastoraki, M.; Kormas, K.A.; Chatzifotis, S.; Piccolo, G.; Gasco, L.; Gai, F. Tenebrio molitor larvae meal inclusion affects hepatic proteome and apoptosis and/or autophagy of three farmed fish species. Sci. Rep. 2022, 12, 121. [Google Scholar] [CrossRef]
- Chatha, A.M.M.; Naz, S.; Danabas, D. Effect of Insect Feed on Fish Growth: A Review. Asian Fish. Sci. 2024, 37, 52–68. [Google Scholar] [CrossRef]
- Bousdras, T.; Feidantsis, K.; Panteli, N.; Chatzifotis, S.; Piccolo, G.; Gasco, L.; Gai, F.; Antonopoulou, E. Dietary Tenebrio molitor larvae meal inclusion exerts tissue-specific effects on cellular, metabolic, and antioxidant status in European sea bass (Dicentrarchus labrax) and gilthead seabream (Sparus aurata). Aquac. Nutr. 2022, 2022, 9858983. [Google Scholar] [CrossRef]
- Costa, R.S.; Basto, A.; Monteiro, M.; Pinho, B.; Sá, T.; Santos, M.V.; Murta, D.; Schrama, J.W.; Valente, L.M. Combining Hermetia illucens and Tenebrio molitor meals in diets for European seabass: Effects on growth, nutrient utilisation, intestinal morphology and muscle quality. Aquaculture 2025, 610, 742899. [Google Scholar] [CrossRef]
- Camelia, M. The Relationship Between TM Food and Health of Intestine. 2025. Available online: https://BioRender.com/e6ydvkk (accessed on 19 September 2025).
- Camelia, M. Graphical Abstract. 2025. Available online: https://BioRender.com/fporeml (accessed on 10 September 2025).
- Qu, P.; Yuan, J.; Wu, Y.; Tian, S.; Wu, Z.; Chen, P.; Pan, M.; Weng, H.; Mai, K.; Zhang, W. Yellow mealworm (Tenebrio molitor) meal replacing dietary fishmeal alters the intestinal microbiota, anti-oxidation and immunity of large yellow croaker (Larimichthys crocea). Fish Shellfish Immunol. 2025, 161, 110272. [Google Scholar] [CrossRef]
- Lebria, A.; Langroudi, H.E.; Sajjadi, M.; Pajand, Z.O. Evaluating full-fat mealworm (Tenebrio molitor) meal as a fishmeal alternative: Impacts on growth, physiology, and enzyme activity in stellate sturgeon (Acipenser stellatus). Aquac. Int. 2025, 33, 436. [Google Scholar] [CrossRef]
- Yang, L.; Cai, M.; Zhong, L.; Yin, Y.; Xie, Y.; Xie, S.; Hu, Y.; Zhang, J. Yellow mealworm (Tenebrio molitor) meal in diets of grass carp (Ctenopharyngodon idellus): Effects on growth performance, antioxidant capacity, immunity, intestinal morphology, and intestinal microbiota. Anim. Nutr. 2025, 21, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Gwaza, T.; Kang’ombe, J.; Sikawa, D.; Simfukwe, K.; Singoyi, P.; Mphande, J.; Kaunda, E. Growth, feed utilization and digestibility of Oreochromis shiranus (Boulenger 1905) fed dietary yellow mealworm larvae (Tenebrio molitor) meal raised in earthen ponds. Cogent Food Agric. 2025, 11, 2519813. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Huang, Y.; Wang, C. Triggering compensatory growth by completely replacing fishmeal with novel protein sources in the diets of juvenile largemouth bass (Micropterus salmoides): Effects on growth performance and liver health. Aquac. Fish. 2025, in press. [Google Scholar] [CrossRef]
- Amenyogbe, E.; Droepenu, E.K.; Ayisi, C.L.; Boamah, G.A.; Duker, R.Q.; Abarike, E.D.; Huang, J.-S. Impact of probiotics, prebiotics, and synbiotics on digestive enzymes, oxidative stress, and antioxidant defense in fish farming: Current insights and future perspectives. Front. Mar. Sci. 2024, 11, 1368436. [Google Scholar] [CrossRef]
- Abenaim, L.; Conti, B. Harnessing Chitin from Edible Insects for Livestock Nutrition. Insects 2025, 16, 799. [Google Scholar] [CrossRef]
- Shin, C.-S.; Kim, D.-Y.; Shin, W.-S. Characterization of chitosan extracted from Mealworm Beetle (Tenebrio molitor, Zophobas morio) and Rhinoceros Beetle (Allomyrina dichotoma) and their antibacterial activities. Int. J. Biol. Macromol. 2019, 125, 72–77. [Google Scholar] [CrossRef]
- Hasan, I.; Gai, F.; Cirrincione, S.; Rimoldi, S.; Saroglia, G.; Terova, G. Chitinase and insect meal in aquaculture nutrition: A comprehensive overview of the latest achievements. Fishes 2023, 8, 607. [Google Scholar] [CrossRef]
- Mengkrog Holen, M.; Tuveng, T.R.; Kent, M.P.; Vaaje-Kolstad, G. The gastric mucosa of Atlantic salmon (Salmo salar) is abundant in highly active chitinases. FEBS Open Bio 2024, 14, 23–36. [Google Scholar] [CrossRef]
- Eggink, K.M.; Pedersen, P.B.; Lund, I.; Dalsgaard, J. Chitin digestibility and intestinal exochitinase activity in Nile tilapia and rainbow trout fed different black soldier fly larvae meal size fractions. Aquac. Res. 2022, 53, 5536–5546. [Google Scholar] [CrossRef]
- Coutinho, F.; Castro, C.; Guerreiro, I.; Rangel, F.; Couto, A.; Serra, C.R.; Peres, H.; Pousão-Ferreira, P.; Rawski, M.; Oliva-Teles, A. Mealworm larvae meal in diets for meagre juveniles: Growth, nutrient digestibility and digestive enzymes activity. Aquaculture 2021, 535, 736362. [Google Scholar] [CrossRef]
- Liu, Q.; Li, J.; Liu, J.; Cai, Y.; Luo, Y.; Sangzhu, T.; Ye, B.; Yang, M. Enhanced Nile tilapia meat quality by the metabolomic effects of Tenebrio molitor larval meal dietary supplement. Appl. Food Res. 2025, 5, 101117. [Google Scholar] [CrossRef]
- Tubin, J.S.B.; Paiano, D.; de Oliveira Hashimoto, G.S.; Furtado, W.E.; Martins, M.L.; Durigon, E.; Emerenciano, M.G.C. Tenebrio molitor meal in diets for Nile tilapia juveniles reared in biofloc system. Aquaculture 2020, 519, 734763. [Google Scholar] [CrossRef]
- Chen, H.; Yu, J.; Ran, X.; Wu, J.; Chen, Y.; Tan, B.; Lin, S. Effects of yellow mealworm (Tenebrio molitor) on growth performance, hepatic health and digestibility in juvenile largemouth bass (Micropterus salmoides). Animals 2023, 13, 1389. [Google Scholar] [CrossRef]
- Iaconisi, V.; Marono, S.; Parisi, G.; Gasco, L.; Genovese, L.; Maricchiolo, G.; Bovera, F.; Piccolo, G. Dietary inclusion of Tenebrio molitor larvae meal: Effects on growth performance and final quality treats of blackspot sea bream (Pagellus bogaraveo). Aquaculture 2017, 476, 49–58. [Google Scholar] [CrossRef]
- Khosravi, S.; Kim, E.; Lee, Y.S.; Lee, S.M. Dietary inclusion of mealworm (Tenebrio molitor) meal as an alternative protein source in practical diets for juvenile rockfish (Sebastes schlegeli). Entomol. Res. 2018, 48, 214–221. [Google Scholar] [CrossRef]
- Hoffmann, L.; Rawski, M.; Nogales-Merida, S.; Mazurkiewicz, J. Dietary inclusion of Tenebrio molitor meal in sea trout larvae rearing: Effects on fish growth performance, survival, condition, and GIT and liver enzymatic activity. Ann. Anim. Sci. 2020, 20, 579–598. [Google Scholar] [CrossRef]
- Gebremichael, A.; Sándor, Z.; Kucska, B. Does dietary inclusion of defatted yellow mealworm (Tenebrio molitor) affect growth and body composition of juvenile common carp (Cyprinus carpio)? South Afr. J. Anim. Sci. 2022, 52, 444–451. [Google Scholar] [CrossRef]
- Belforti, M.; Gai, F.; Lussiana, C.; Renna, M.; Malfatto, V.; Rotolo, L.; De Marco, M.; Dabbou, S.; Schiavone, A.; Zoccarato, I. Tenebrio molitor meal in rainbow trout (Oncorhynchus mykiss) diets: Effects on animal performance, nutrient digestibility and chemical composition of fillets. Ital. J. Anim. Sci. 2015, 14, 4170. [Google Scholar] [CrossRef]
- Owens, C.E.; Powell, M.S.; Gaylord, T.G.; Conley, Z.B.; Sealey, W.M. Investigation of the suitability of 3 insect meals as protein sources for rainbow trout (Oncorhynchus mykiss). J. Econ. Entomol. 2024, 117, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- Fontes, T.V.; de Oliveira, K.R.B.; Gomes Almeida, I.L.; Orlando, T.M.; Rodrigues, P.B.; da Costa, D.V.; Rosa, P.V.E. Digestibility of insect meals for Nile tilapia fingerlings. Animals 2019, 9, 181. [Google Scholar] [CrossRef]
- Sándor, Z.J.; Banjac, V.; Vidosavljević, S.; Káldy, J.; Egessa, R.; Lengyel-Kónya, É.; Tömösközi-Farkas, R.; Zalán, Z.; Adányi, N.; Libisch, B. Apparent digestibility coefficients of black soldier fly (Hermetia illucens), yellow mealworm (Tenebrio molitor), and blue bottle fly (Calliphora vicina) insects for juvenile African catfish hybrids (Clarias gariepinus × Heterobranchus longifilis). Aquac. Nutr. 2022, 2022, 4717014. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, G.; Iaconisi, V.; Marono, S.; Gasco, L.; Loponte, R.; Nizza, S.; Bovera, F.; Parisi, G. Effect of Tenebrio molitor larvae meal on growth performance, in vivo nutrients digestibility, somatic and marketable indexes of gilthead sea bream (Sparus aurata). Anim. Feed Sci. Technol. 2017, 226, 12–20. [Google Scholar] [CrossRef]
- Bagheri, K.S.A.A.; Esmaeili, F.A.; Yeganeh, S.; Oraji, H. Effects of dietary replacement of fish meal by yellow larval mealworm (Tenebrio molitor) on growth performance, some blood and liver parameters in Beluga (Huso huso). Aquat. Anim. Nutr. 2025, 11, 67–88. [Google Scholar]
- Habte-Tsion, H.M.; Hawkyard, M.; Sealey, W.M.; Bradshaw, D.; Meesala, K.M.; Bouchard, D.A. Effects of fishmeal substitution with mealworm meals (Tenebrio molitor and Alphitobius diaperinus) on the growth, physiobiochemical response, digesta microbiome, and immune genes expression of Atlantic salmon (Salmo salar). Aquac. Nutr. 2024, 2024, 6618117. [Google Scholar] [CrossRef]
- Zhang, J.; Dong, Y.; Song, K.; Wang, L.; Li, X.; Tan, B.; Lu, K.; Zhang, C. Effects of the replacement of dietary fish meal with defatted yellow mealworm (Tenebrio molitor) on juvenile large yellow croakers (Larimichthys crocea) growth and gut health. Animals 2022, 12, 2659. [Google Scholar] [CrossRef]
- Basto, A.; Calduch-Giner, J.; Oliveira, B.; Petit, L.; Sá, T.; Maia, M.; Fonseca, S.; Matos, E.; Pérez-Sánchez, J.; Valente, L. The use of defatted Tenebrio molitor larvae meal as a main protein source is supported in European sea bass (Dicentrarchus labrax) by data on growth performance, lipid metabolism and flesh quality. Front. Physiol. 2021, 12, 659567. [Google Scholar] [CrossRef]
- Sharifinia, M.; Dashtiannasab, A.; Mobaraki, S.; Pazir, M. Effects of dietary inclusion of mealworm (Tenebrio molitor) on the fatty acid compositions of Pacific white shrimp (Litopenaeus vannamei). Iran. J. Fish. Sci. 2025, 24, 983–999. [Google Scholar]
- Abdolmanafi, M.; Safari, R.; Hosseinifar, S.H.; Gasco, L.; Yazici, M. Mealworm extract (Tenebrio molitor) affects gonad histology, reproduction performance and related genes expression in female zebra fish (Danio rerio). J. Insects Food Feed 2025, 1, 1–21. [Google Scholar] [CrossRef]
- Gelinçek, İ.; Yamaner, G. An investigation on the gamete quality of Black Sea trout (Salmo trutta labrax) broodstock fed with mealworm (Tenebrio molitor). Aquac. Res. 2020, 51, 2379–2388. [Google Scholar] [CrossRef]
- Valipour, M.; Oujifard, A.; Hosseini, A.; Sotoudeh, E.; Bagheri, D. Effects of dietary replacement of fishmeal by yellow mealworm (Tenebrio molitor) larvae meal on growth performance, hematological indices and some of non-specific immune responses of juvenile rainbow trout (Oncorhynchus mykiss). ISFJ 2019, 28, 13–26. [Google Scholar]
- Hilaj, N.; Zimmermann, M.B.; Galetti, V.; Zeder, C.; Lima, R.M.; Hammer, L.; Krzystek, A.; Andlauer, W.; Moretti, D. The effect of dechitinization on iron absorption from mealworm larvae (Tenebrio molitor) flour added to maize meals: Stable-isotope studies in young females with low iron stores. Am. J. Clin. Nutr. 2022, 116, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- Stanek, M.; Mazurkiewicz, J.; Rawski, M.; Bogucka, J.; Ziółkowska, E.; Dankowiakowska, A.; Kierończyk, B. Effect of chitosan on common carp (Cyprinus carpio) fry growth performance, feed utilization and nutriphysiological status. Aquac. Rep. 2023, 30, 101622. [Google Scholar] [CrossRef]
- Anany, E.M.; Ibrahim, M.A.; El-Razek, I.M.A.; El-Nabawy, E.-S.M.; Amer, A.A.; Zaineldin, A.I.; Gewaily, M.S.; Dawood, M.A. Combined effects of yellow mealworm (Tenebrio molitor) and Saccharomyces cerevisiae on the growth performance, feed utilization intestinal health, and blood biomarkers of Nile tilapia (Oreochromis niloticus) fed fish meal-free diets. Probiot. Antimicrob. Proteins 2023, 17, 1387–1398. [Google Scholar] [CrossRef] [PubMed]
- Elieh Ali Komi, D.; Sharma, L.; Dela Cruz, C.S. Chitin and its effects on inflammatory and immune responses. Clin. Rev. Allergy Immunol. 2018, 54, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Henry, M.; Gasco, L.; Chatzifotis, S.; Piccolo, G. Does dietary insect meal affect the fish immune system? The case of mealworm, Tenebrio molitor on European sea bass, Dicentrarchus labrax. Dev. Comp. Immunol. 2018, 81, 204–209. [Google Scholar] [CrossRef]
- Salman, N.A. Effect of dietary salt on feeding, digestion, growth and osmoregulation in teleost fish. Essent. Rev. Exp. Biol. 2009, 1, 109–150. [Google Scholar]
- Pietsch, C.; Schulz, C.; Rovira, P.; Kloas, W.; Burkhardt-Holm, P. Organ damage and hepatic lipid accumulation in carp (Cyprinus carpio L.) after feed-borne exposure to the mycotoxin, deoxynivalenol (DON). Toxins 2014, 6, 756–778. [Google Scholar] [CrossRef]
- Mohamed, A.S.; Gad, N.S.; El Desoky, M.A. Liver Enzyme Activity of Tilapia zillii and Mugil capito Collected Seasonally from Qarun Lake, Egypt. Fish. Aquac. J. 2019, 10, 1–5. [Google Scholar] [CrossRef]
- Sindhu Priya, A.; Suriya Prabha, V.; Jayasree, R.; Parthasarathy, N.; Rajkumar, J.; Pazhanivel, N.; Balakrishnan, A. Enzymatic studies in the liver and muscle of freshwater fish, Pangasius hypophthalmus exposed to tannery effluent. Int. J. Biosci. Technol. 2012, 5, 98–111. [Google Scholar]
- Morales, A.E.; Pérez-Jiménez, A.; Hidalgo, M.C.; Abellán, E.; Cardenete, G. Oxidative stress and antioxidant defenses after prolonged starvation in Dentex dentex liver. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2004, 139, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Suryadi, I.B.B.; Ali, M.F.Z.; Nishiguchi, H.; Akanuma, S.; Miura, C.; Miura, T. Bioactive Substance Derived from Mealworm Larvae (Tenebrio molitor) Potentially Induces Immune Performance of Zebrafish (Danio rerio). Fishes 2025, 10, 285. [Google Scholar] [CrossRef]
- Alagawany, M.; Farag, M.R.; Salah, A.S.; Mahmoud, M.A. The role of oregano herb and its derivatives as immunomodulators in fish. Rev. Aquac. 2020, 12, 2481–2492. [Google Scholar] [CrossRef]
- Özel, O.T.; Çimagil, R.; Gürkan, S.E.; Coşkun, İ.; Türe, M.; Kutlu, İ. The effects of Fennel (Foeniculum vulgare) Essential Oils on Growth Performance and Digestive Physiological Traits in Black Sea Salmon (Salmo labrax PALLAS 1814) Juveniles. J. Agric. Sci. 2023, 29, 362–370. [Google Scholar]
- Munglue, P.; Rattana, K.; Sangchanjiradet, S.; Dasri, K. Effect of dietary lasia (Lasia spinosa (L.) Thwaites) extract on growth performance and intestinal histology in hybrid catfish (Clarias macrocephalus × Clarias gariepinus). Chiang Mai Univ. J. Nat. Sci. 2019, 18, 226–249. [Google Scholar] [CrossRef]
- El-Desouky, F.F.; Ibrahim, M.A.; Abd El-Razek, I.M.; El-Nabawy, E.-S.M.; Amer, A.A.; Zaineldin, A.I.; Gewaily, M.S.; Dawood, M.A. Improving yellow mealworm (Tenebrio molitor) utilization with sodium butyrate in Nile tilapia diets: Effects on growth performance, intestinal histology, antioxidative response, and blood biomarkers. Aquac. Nutr. 2024, 2024, 2442308. [Google Scholar] [CrossRef]
- Kalemi, V.; Rimoldi, S.; Costa, R.S.; Basto, A.; Monteiro, M.; Terova, G.; Valente, L.M. Replacing fishmeal with an insect meal blend: Implications for intestinal microbiota in European seabass. Aquac. Rep. 2025, 43, 102939. [Google Scholar] [CrossRef]
- Antonopoulou, E.; Nikouli, E.; Piccolo, G.; Gasco, L.; Gai, F.; Chatzifotis, S.; Mente, E.; Kormas, K.A. Reshaping gut bacterial communities after dietary Tenebrio molitor larvae meal supplementation in three fish species. Aquaculture 2019, 503, 628–635. [Google Scholar] [CrossRef]
- Terova, G.; Gini, E.; Gasco, L.; Moroni, F.; Antonini, M.; Rimoldi, S. Effects of full replacement of dietary fishmeal with insect meal from Tenebrio molitor on rainbow trout gut and skin microbiota. J. Anim. Sci. Biotechnol. 2021, 12, 30. [Google Scholar] [CrossRef]
- Ge, C.; Liang, X.; Wu, X.; Wang, J.; Wang, H.; Qin, Y.; Xue, M. Yellow mealworm (Tenebrio Molitor) enhances intestinal immunity in largemouth bass (Micropterus salmoides) via the NFκB/survivin signaling pathway. Fish Shellfish Immunol. 2023, 136, 108736. [Google Scholar] [CrossRef]
- Chewaka, L.S.; Park, C.S.; Cha, Y.-S.; Desta, K.T.; Park, B.-R. Enzymatic hydrolysis of Tenebrio molitor (Mealworm) using nuruk extract concentrate and an evaluation of its nutritional, functional, and sensory properties. Foods 2023, 12, 2188. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Jang, J.S.; Kim, E.H.; Lee, W.H.; Yu, Y.H.; Kim, H.J.; Huh, C.K. Physicochemical fermentation characteristics and changes in antioxidant activity of mealworms (Tenebrio molitor) during fermentation with lactic acid bacteria: Application and selection of commercial lactic acid bacteria starters. Appl. Food Res. 2025, 5, 100811. [Google Scholar] [CrossRef]
- Ge, C.; Cheng, H.; Li, J.; Wang, H.; Ma, S.; Qin, Y.; Xue, M. Effects of defatted yellow mealworm (Tenebrio molitor) on the feed qualities and the growth performance of largemouth bass (Micropterus salmoides). J. Insects Food Feed 2022, 8, 1265–1280. [Google Scholar] [CrossRef]
- Yoon, Y.; Oh, E.; Park, W.-J.; Kim, H.S.; Kim, Y. Effects of enzymatic hydrolysis and size fractionation of mealworm (Tenebrio molitor larvae) protein on antioxidant and anti-inflammatory activities and inhibition of muscle loss. ACS Food Sci. Technol. 2023, 3, 850–857. [Google Scholar] [CrossRef]
- Munoz-Seijas, N.; Fernandes, H.; Domínguez, J.M.; Salgado, J.M. Recent Advances in Biorefinery of Tenebrio molitor Adopting Green Technologies. Food Bioprocess Technol. 2025, 18, 1061–1078. [Google Scholar] [CrossRef]
- Copelotti, E.; De Schutter, K.; Tzompa-Sosa, D.A.; Coudron, C.; Deruytter, D.; Mancini, S. Saturated fatty acid-enriched diets in Tenebrio molitor larvae: Effects on growth performances and nutritional composition. J. Sci. Food Agric. 2025. online ahead of print. [Google Scholar] [CrossRef]
- Morales, M.L.; Segura-Borrego, M.P.; Aguilera-Velázquez, J.R.; Callejón, R.M.; Gutiérrez-Praena, D.; Ubeda, C. Insight into the chemical and nutritional fat profile of Tenebrio molitor larvae reared on different Agri-food by-products. Food Res. Int. 2025, 209, 116223. [Google Scholar] [CrossRef]
- Saravanan, S.; Geurden, I.; Orozco, Z.; Kaushik, S.; Verreth, J.; Schrama, J. Dietary electrolyte balance affects the nutrient digestibility and maintenance energy expenditure of Nile tilapia. Br. J. Nutr. 2013, 110, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
| Species and References | Fish Feeding Habits | Aquaculture System | Initial Weight | Culture Period/Photoperiod | Experimental Period Days | Types of Meal Used | Substitution of Fish Meal | Substitution of Vegetal Protein | Tenebrio molitor Inclusion |
|---|---|---|---|---|---|---|---|---|---|
| Stellate sturgeon Acipenser stellatus [43] | Carnivorous | flow-through | 28.08 ± 0.13 g | 12 h light/ 12 h dark | 56 | FFMW | - | - | 10% |
| Rainbow Trout Oncorhynchus mykiss [35] | Carnivorous | flow-through | 23 ± 0.4 g | 12 h light/ 10 h dark | 91 | DMW | 50–75% | - | 20–30% |
| Rainbow trout Oncorhynchus mykiss [30] | Carnivorous | RAS | 1.11 ± 0.01 g | natural conditions | 56 | TM | 28% | - | - |
| European seabass Dicentrarchus labrax [32] | Carnivorous | RAS | 21.1 ± 4.9 g | 12 h light/ 12 h dark | 49 | FFMW | - | 20% | - |
| Large yellow croaker (Larimichthys crocea) [42] | Carnivorous | marine floating net cages | 189.18 ± 0.13 g | natural conditions | 80 | FFMW | 45% | - | 25.57% |
| Grass carp Ctenopharyngodon idellus [44] | Herbivorous | nylon net pond cages | 20.57 ± 0.34 g | natural conditions | 56 | FFMW | - | 25% | - |
| Nile tilapia (Oreochromis niloticus) [47] | Omnivorous | flow-through | 60.00 ± 5.35 g | natural conditions | 30 | TM | - | 15–60% | - |
| Nile tilapia (Oreochromis niloticus) [48] | Omnivorous | biofloc system | 2.08 ± 0.19 g | not specified | 42 | TM | - | - | 10% |
| Shire tilapia Oreochromis shiranus [45] | Omnivorous | concrete earthen ponds | 15.02 ± 1.23 g | natural conditions | 126 | FFMW | 50–75% | - | - |
| Largemouth Bass juvenile (Micropterus salmoides) [49] | Carnivorous | RAS | 158.9 ± 1.7 g | 12 h light/ 12 h dark | 77 | TM | 19.52% | - | - |
| Blackspot sea bream (Pagellus bogaraveo) [50] | Omnivorous | flow-through | 110.67–246.36 g | natural conditions | 131 | FFMW | 25% | - | - |
| Rockfish (Sebastes schlegeli) [51] | Carnivorous | flow-through | 3.11 ± 0.01 g | natural conditions | 56 | TM | 32% | - | 16% |
| Sea trout— (Salmo trutta) [52] | Carnivorous | flow-through | 0.14 g | 16 h light/ 8 h dark | 60 | TM | - | - | 20% |
| Common carp (Cyprinus carpio) [53] | Omnivorous | RAS | 97.54 g ± 51.0 g | not specified | 21 | DMW | 50% | - | - |
| Species and References | Diet | Fish Feeding Habits | Aquaculture System/Period Days | Initial Weight g | Final Weight g | PER | SR% | Chitin Content % | Effect on Digestibility |
|---|---|---|---|---|---|---|---|---|---|
| Rainbow Trout Oncorhynchus mykiss [61] | FM | Carnivorous | flow-through 90 days | 116.5 ± 0.40 | 313.8 ± 2.51 | 1.69 | 86.7 | - | ADC of CP 92.2% |
| 25% TM | 115.2 ± 0.40 | 316.6 ± 2.51 | 2.06 | 96.7 | - | ADC of CP 91.5% | |||
| 50% TM | 115.9 ± 0.40 | 308.2 ± 2.51 | 2.04 | 97.5 | - | ↓ ADC of CP 90.1% | |||
| Rainbow Trout Oncorhynchus mykiss [62] | 30% TM | Carnivorous | RAS 14 days | 370 ± 23 | - | - | - | - | ↑ good ↑ ADC of CP. No differences in the ADC |
| Nile tilapia (Oreochromis niloticus) [63] | 20% TM | Omnivorous | RAS 15 days | 3.0 ± 0.2 | - | - | - | 3.87 | ↑ good ↑ ADC of moisture 95.8; ↑ ADC of CP 85.4% |
| Meagre (Argyrosomus regius) [60] | FM | Carnivorous | RAS with flow of seawater 48 days | 18.0 ± 0.02 | 80.5 ± 8.9 | 2.49 | 0 | 0 | ↑ ADC of CP 94.1% |
| 10% TM | 66.1 ± 4.2 | 2.22 | 0 | 0.74 | ADC of CP 92.9% | ||||
| 20% TM | 53.2 ± 3.1 | 2.07 | 0 | 0.97 | ADC of CP 92.5% | ||||
| 30% TM | 40.0 ± 1.0 | 1.67 | 3.7 | 1.47 | ↓ ADC of CP 91.9%; ↓ ADC of AA, | ||||
| African Catfish Hybrids (Clarias gariepinus × Heterobranchus longifilis) [64] | 30% TM | Omnivorous | RAS 18 days | 217.4 ± 9.5 | 346 ± 35.8 | - | - | 5.81 | ↓ ADC of CP 72.07% ↓ ADC of CF 81.24% ↓ ADC of AA |
| Gilthead sea bream (Sparus aurata) [65] | FM | mainly Carnivorous | RAS 163 days | 105.2 ± 0.17 | 239.6 | 1.74 | - | 1.15–2.31 | ADC of CP 89.97% ADC of CF 91.12% |
| 20% TM | 294.6 | 2.26 | - | ADC of CP 87.26% ADC of CF 89.93% | |||||
| 50% TM | 238.9 | 1.79 | - | ↓ ADC of CF 82.39%; ↓ ADC of CP 79.19% |
| Species and References | Fish Feeding Habits | Aquaculture System/Period Days | Culture Period/Photoperiod | Types of Meal Used | Composition of Mealworm % | FM Substitution/Inclusion TM | Initial Weight (g) | Final Weight (g) | SGR %/Day | FCR | PER | SR% | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Moisture | Crude Protein | Crude Fat | Ash | Energy (MJ/Kg) | ||||||||||||
| Rainbow Trout Oncorhynchus mykiss [35] | Carnivorous | flow-through 91 days | 12 h light/ 10 h dark | FM | 4.94 | 47.02 | 16.81 | 5.48 | 22.23 | 0% | 23.1 | 264.4 | 2.68 | 0.72 | 3.00 | 94.4 |
| DMW | 4.78 | 47.11 | 16.01 | 5.56 | 22.21 | 25% | 23.5 | 242.4 | 2.56 | 0.76 | 2.80 | 86.9 | ||||
| 4.83 | 46.48 | 17.42 | 5.65 | 22.35 | 50% | 23.4 | 268.3 | 2.68 | 0.76 | 2.82 | 91.9 | |||||
| 5.07 | 47.42 | 16.02 | 5.71 | 22.27 | 75% | 23.0 | 264.6 | 2.68 | 0.79 | 2.68 | 87.5 | |||||
| 4.88 | 47.10 | 16.06 | 5.71 | 22.67 | 100% | 23.2 | 264.9 | 2.68 | 0.77 | 2.75 | 96.9 | |||||
| Stellate sturgeon Acipenser stellatus [43] | Carnivorous | flow-through 56 days | 12 h light/ 12 h dark | FM | 6.25 | 45.27 | 25.15 | 8.57 | 19.31 | 0% | 28.20 ± 3.68 | 71.40 ± 1.90 | 1.76 | 1.98 | 1.98 | 100 |
| FFMW | 6.13 | 3.15 | 7.75 | 19.59 | 10% | 27.90 ± 2.12 | 77.25 ± 2.04 | 1.87 | 2.02 | 2.82 | 100 | |||||
| 6.19 | 15.62 | 8.16 | 19.48 | 20% | 28.10 ± 2.97 | 63.12 ± 4.32 | 1.52 | 3.45 | 1.81 | 90 | ||||||
| 6.25 | 15.25 | 8.57 | 19.31 | 30% | 28.10 ± 0.42 | 62.49 ± 2.21 | 1.50 | 3.76 | 1.78 | 85 | ||||||
| European sea bass Dicentrarchus labrax [32] | Carnivorous | RAS 49 days | 12 h light/ 12 h dark | FM | 8.35 | 46.7 | 21.8 | 5.52 | - | 0% | 21.16 ± 4.89 | 48.45 ± 8.39 | - | 1.11 | - | - |
| FFMW | 9.07 | 47.0 | 21.1 | 5.98 | - | 5% | 21.61 ± 4.64 | 47.89 ± 8.61 | - | 1.08 | - | - | ||||
| 9.49 | 47.2 | 21.5 | 6.16 | - | 10% | 21.23 ± 4.90 | 46.61 ± 8.35 | - | 1.11 | - | - | |||||
| Grass carp Ctenopharyngodon idellus [44] | Herbivorous | nylon net pond cages 56 days | natural conditions | SBM | - | 30.56 | 5.64 | 10.29 | - | 0% | 20.73 ± 0.534 | 60.11 ± 0.669 | 0.94 | 1.71 | 1.98 | 87.33 |
| FFMW | - | 30.62 | 5.67 | 10.07 | - | 25% | 20.27 ± 0.500 | 64.16 ± 0.557 | 0.96 | 1.62 | 2.29 | 90 | ||||
| - | 30.65 | 5.69 | 10.04 | - | 50% | 20.24 ± 0.334 | 58.56 ± 1.011 | 0.87 | 1.90 | 2.15 | 88.67 | |||||
| - | 30.67 | 5.61 | 10.15 | - | 75% | 20.60 ± 0.193 | 58.20 ± 0.367 | 0.92 | 1.90 | 2.04 | 90.00 | |||||
| - | 30.73 | 5.66 | 10.25 | - | 100% | 21.02 ± 0.070 | 55.77 ± 0.670 | 0.89 | 1.80 | 1.83 | 86.00 | |||||
| Shire tilapia Oreochromis shiranus [45] | Omnivorous | concrete earthen ponds 126 days | natural conditions | FM | - | 30.45 | 8.34 | 7.14 | 22.03 | 0% | 15.19 ± 0.24 | 51.47 ± 1.97 | 3.18 | 2.93 | - | 70.56 |
| FFMW | - | 30.62 | 8.55 | 5.73 | 24.30 | 25% | 15.06 ± 0.34 | 49.63 ± 1.68 | 3.13 | 2.89 | - | 73.33 | ||||
| FFMW | - | 29.95 | 9.18 | 5.88 | 22.42 | 50% | 15.01 ± 0.55 | 48.62 ± 1.95 | 3.11 | 2.67 | - | 78.33 | ||||
| FFMW | - | 30.98 | 9.41 | 5.47 | 23.99 | 75% | 14.95 ± 0.23 | 52.86 ± 0.45 | 3.22 | 2.62 | - | 76.67 | ||||
| Blackspot sea bream (Pagellus bogaraveo) [50] | Omnivorous | flow-through 131 days | natural conditions | FM | 7.14 | 45.80 | 19.71 | 10.09 | - | 0% | 171.25 | 223.69 | 0.21 | 5.32 | - | - |
| FFMW | 7.35 | 45.90 | 19.82 | 8.77 | - | 25% | 171.32 | 218.81 | 0.20 | 5.87 | - | - | ||||
| FFMW | 7.03 | 45.93 | 20.80 | 7.42 | - | 50% | 174.15 | 218.20 | 0.19 | 5.52 | - | - | ||||
| Beluga (Huso huso) [66] | Carnivorous | RAS 56 days | not specified | FM | - | 40.26 | 21.55 | 10.64 | 19.01 | 0% | 121.66 ± 2.51 | 391.86 ± 24.53 | 2.07 | 1.60 | - | 100 |
| TM | - | 40.41 | 21.27 | 10.93 | 18.92 | 20% | 121.66 ± 3.78 | 451.80 ± 23.40 | 2.34 | 1.30 | - | 100 | ||||
| TM | - | 41.27 | 20.86 | 9.83 | 19.03 | 40% | 105.33 ± 10.15 | 424.53 ± 18.41 | 2.5 | 1.34 | - | 100 | ||||
| TM | - | 40.11 | 21.30 | 9.11 | 19.10 | 60% | 105.33 ± 13.32 | 416.80 ± 28.23 | 2.47 | 1.38 | - | 100 | ||||
| TM | - | 40.62 | 20.23 | 8.65 | 18.94 | 80% | 110.33 ± 8.57 | 409.8 ± 16.25 | 2.37 | 1.42 | - | 100 | ||||
| TM | - | 40.26 | 20.28 | 8.25 | 18.96 | 100% | 116.1 ± 6.2 | 415.26 ± 11.37 | 2.27 | 1.43 | - | 100 | ||||
| Nile tilapia (Oreochromis niloticus) [48] juveniles reared in biofloc system | Omnivorous | biofloc system 42 days | not specified | FM | - | 30.94 | 12.08 | 8.81 | - | 0% | 2.07 | 8.36 | 3.32 | 1.44 | - | 96.67 |
| TM | - | 34.13 | 11.53 | 8.76 | - | 5% | 2.08 | 9.41 | 3.60 | 1.34 | - | 83.33 | ||||
| TM | - | 33.01 | 11.99 | 8.31 | - | 10% | 2.1 | 9.44 | 3.57 | 1.28 | - | 90.00 | ||||
| TM | - | 30.95 | 13.58 | 7.78 | - | 15% | 2.07 | 9.44 | 3.61 | 1.32 | - | 73.33 | ||||
| TM | - | 33.65 | 15.35 | 8.74 | - | 20% | 2.1 | 10.37 | 3.80 | 1.21 | - | 53.33 | ||||
| Atlantic Salmon (Salmo salar) [67] | Carnivorous | RAS 84 days | 12 h light/ 12 h dark | FM | 4.00 | 43.8 | 22.5 | 5.28 | - | 0% | 38.5 ± 0.1 | 182.8 ± 5.5 | 1.53 | 1.23 | 2.64 | 100 |
| DMW | 3.50 | 43.7 | 22.5 | 5.02 | - | 50% | 38.7 ± 0.1 | 179.0 ± 2.6 | 1.50 | 1.20 | 2.54 | 100 | ||||
| DMW | 3.50 | 43.6 | 22.5 | 5.58 | - | 100% | 38.4 ± 0.1 | 191.4 ± 5.5 | 1.50 | 1.25 | 2.69 | 98.8 | ||||
| FFMW | 3.00 | 43.8 | 22.7 | 4.86 | - | 50% | 38.4 ± 0.2 | 191.5 ± 3.6 | 1.60 | 1.23 | 2.57 | 100 | ||||
| Large Yellow Croakers (Larimichthys crocea) [68] | Carnivorous | floating sea cages 56 days | natural conditions | FM | 1.98 | 47.98 | 8.25 | 11.12 | - | 0% | 11.76 ± 0.06 | 26.59 ± 0.27 | 2.12 | 0.72 | 1.50 | 84.22 |
| DMW | 1.51 | 48.30 | 8.63 | 10.70 | - | 15% | 11.78 ± 0.06 | 26.18 ± 0.31 | 2.00 | 0.70 | 1.44 | 89.33 | ||||
| DMW | 1.41 | 48.00 | 8.76 | 10.85 | - | 30% | 11.84 ± 0.02 | 24.89 ± 0.15 | 2.24 | 0.57 | 1.20 | 76.33 | ||||
| DMW | 1.15 | 48.10 | 8.79 | 10.50 | - | 45% | 11.84 ± 0.04 | 21.31 ± 1.02 | 2.36 | 0.47 | 0.98 | 76.67 | ||||
| Species and References | Diet | Fish Feeding Habits | Aquaculture System/Period Days | Culture Period/Photoperiod | Moisture% | CP% | CF% | Ash |
|---|---|---|---|---|---|---|---|---|
| Stellate sturgeon Acipenser stellatus [43] | FM | Carnivorous | flow-through 56 days | 12 h light/ 12 h dark | 71.63 ± 1.78 | 15.12 ± 1.63 | 11.84 ± 0.47 | 2.42 ± 0.14 |
| 10% FFMW | 69.73 ± 1.25 | 15.14 ± 0.46 | 11.88 ± 0.58 | 2.28 ± 0.27 | ||||
| 20% FFMW | 67.92 ± 1.89 | 14.82 ± 0.62 | 12.15 ± 0.44 | 1.97 ± 0.14 | ||||
| 30% FFMW | 68.12 ± 1.68 | 14.75 ± 1.12 | 14.52 ± 0.54 | 1.92 ± 0.44 | ||||
| Rainbow Trout Oncorhynchus mykiss [35] | FM | Carnivorous | flow-through 91 days | 12 h light/ 10 h dark | 73.37 | 20.33 | 5.16 | 2.07 |
| 25% DMW | 73.66 | 20.54 | 4.83 | 2.09 | ||||
| 50% DMW | 73.35 | 20.55 | 5.12 | 2.21 | ||||
| 75% DMW | 72.57 | 20.42 | 5.72 | 2.00 | ||||
| 100% DMW | 73.20 | 20.58 | 5.23 | 2.17 | ||||
| Grass carp Ctenopharyngodon idellus [44] | SBM | Herbivorous | nylon net pond cages 56 days | natural conditions | 68.61 ± 0.165 | 17.63 ± 0.414 | 8.27 ± 0.496 | 3.95 ± 0.260 |
| 25% FFMW | 70.99 ± 0.152 | 19.44 ± 0.311 | 5.54 ± 0.370 | 3.36 ± 0.122 | ||||
| 50% FFMW | 70.29 ± 0.261 | 19.43 ± 0.220 | 5.78 ± 0.393 | 4.51 ± 0.069 | ||||
| 75% FFMW | 70.52 ± 0.212 | 20.1 ± 0.578 | 5.97 ± 0.374 | 4.39 ± 0.114 | ||||
| 100% FFMW | 69.98 ± 0.795 | 19.45 ± 0.807 | 7.86 ± 0.411 | 3.44 ± 1.786 | ||||
| Shire tilapia Oreochromis shiranus [45] | FM | Omnivorous | concrete earthen ponds 126 days | natural conditions | 7.70 ± 0.11 | 51.04 ± 2.10 | 10.11 ± 0.08 | 11.13 ± 0.33 |
| 25% FFMW | 7.54 0.016 | 50.30 ± 0.29 | 10.07 ± 0.02 | 11.16 ± 0.09 | ||||
| 50% FFMW | 7.32 ± 0.02 | 50.35 ± 0.39 | 10.10 ± 0.45 | 11.20 ± 0.73 | ||||
| 75% FFMW | 7.20 ± 0.06 | 50.45 ± 0.70 | 10.23 ± 0.07 | 11.32 ± 1.46 | ||||
| Blackspot sea bream (Pagellus bogaraveo) [50] | FM | Omnivorous | flow-through 131 days | natural conditions | 70.60 | 20.45 | 6.75 | 1.51 |
| 25% TM | 69.65 | 20.79 | 7.31 | 1.52 | ||||
| 50% TM | 68.26 | 21.58 | 7.440 | 1.55 | ||||
| Nile tilapia (Oreochromis niloticus) [48] | FM | Omnivorous | biofloc system 42 days | not specified | 74.78 | 54.45 | 24.11 | 15.64 |
| 5% TM | 74.91 | 52.18 | 26.12 | 15.56 | ||||
| 10% TM | 74.39 | 56.92 | 25.55 | 15.31 | ||||
| 15% TM | 73.15 | 50.43 | 33.08 | 13.92 | ||||
| 20% TM | 72.3 | 52.31 | 33.95 | 14.38 | ||||
| Atlantic Salmon (Salmo salar) [67] | FM | Carnivorous | RAS 84 days | 12 h light/ 12 h dark | 4.27 ± 0.56 | 51.34 ± 0.76 | 39.27 ± 0.66 | 4.84 ± 0.18 |
| 50% DMW | 4.48 ± 0.56 | 52.40 ± 0.37 | 37.66 ± 0.67 | 5.11 ± 0.26 | ||||
| 100% DMW | 3.89 ± 0.31 | 51.35 ± 0.44 | 39.12 ± 0.87 | 5.26 ± 0.09 | ||||
| 50% FFMW | 3.98 ± 0.54 | 51.91 ± 1.44 | 37.22 ± 0.78 | 5.60 ± 0.44 | ||||
| Sea Bass (Dicentrarchus labrax) [69] | FM | Carnivorous | RAS 70 days | not specified | 66.3 ± 0.2 | 16.9 ± 0.2 | 11.4 ± 0.6 | 4.2 ± 0.5 |
| 40% DMW | 66.6 ± 0.5 | 17.4 ± 0.3 | 11.6 ± 0.3 | 3.9 ± 0.2 | ||||
| 80% DMW | 66.3 ± 0.1 | 17.1 ± 0.03 | 12.2 ± 0.3 | 4.1 ± 0.3 | ||||
| 100% DMW | 63.9 ± 0.9 | 17.0 ± 0.2 | 14.1 ± 0.7 | 4.1 ± 0.1 | ||||
| Large Yellow Croakers (Larimichthys crocea) [68] | FM | Carnivorous | floating sea cages 56 days | natural conditions | 74.16 ± 0.18 | 13.65 ± 0.22 | 7.71 ± 0.26 | 3.18 ± 0.08 |
| 15% DMW | 74.68 ± 0.38 | 13.44 ± 0.14 | 7.34 ± 0.23 | 3.16 ± 0.01 | ||||
| 30% DMW | 75.71 ± 0.41 | 13.59 ± 0.25 | 7.17 ± 0.02 | 3.38 ± 0.02 | ||||
| 45% DMW | 75.91 ± 0.38 | 13.56 ± 0.06 | 7.19 ± 0.10 | 3.31 ± 0.02 |
| Species and References | Diet | Fish Feeding Habits | Aquaculture System/Period Days | Culture Period/Photoperiod | Hematological Profile | Plasma Biochemical Profile | Antioxidant/ Immunological Profile |
|---|---|---|---|---|---|---|---|
| Stellate sturgeon Acipenser stellatus [43] | FM | Carnivorous | flow-through 56 days | 12 h light/ 12 h dark | - | - | - |
| 10% FFMW | - | ↑ Glu; ↓ TP | |||||
| 20% FFMW | ↑ WBC; ↓ Hct; ↓ Hb | ↑ Glu; ↓ TP | |||||
| 30% FFMW | ↑ WBC; ↓ Hct; ↓ Hb | ↑ Glu ↑ TG; ↓ TP | |||||
| Rainbow Trout Oncorhynchus mykiss [35] | FM | Carnivorous | flow-through 91 days | 12 h light/ 10 h dark | - | - | - |
| 25% DMW | - | ||||||
| 50% DMW | ↑ Na | ||||||
| 75% DMW | - | ||||||
| 100% DMW | ↑ Na | ||||||
| Grass carp Ctenopharyngodon idellus [44] | SBM | Herbivorous | nylon net pond cages 56 days | natural conditions | - | ↑ MDA | |
| 25% FFMW | - | ↑ ALP; ↑ ACP | ↑ GPx, ↑ CAT, ↓ MDA, ↑ IgM | ||||
| 50% FFMW | - | ↑ ALP; ↑ ACP | ↑ CAT, ↓ MDA, ↑ ROS, ↑ IgM | ||||
| 75% FFMW | - | ↑ ALT; ↑ AST, ↑ ALP; ↑ ACP | ↑ ROS, ↑ MDA, ↑ IgM | ||||
| 100% FFMW | - | ↑ ALT; ↑ AST | ↑ ROS, ↑ MDA | ||||
| Nile tilapia (Oreochromis niloticus) [48] | FM | Omnivorous | biofloc system 42 days | not specified | ↓ RBC; | - | - |
| 5% TM | ↓ Hct; ↑ WBC | ↓ Glu; | - | ||||
| 10% TM | ↑ Hct; | - | - | ||||
| 15% TM | ↓ PLT | ↑ CHOL; ↓ Glu; | - | ||||
| 20% TM | ↓ Hct; ↓ RBC; ↑ WBC; ↓ PLT | ↑ TP; | - | ||||
| Atlantic Salmon (Salmo salar) [67] | FM | Carnivorous | RAS 84 days | 12 h light/ 12 h dark | -- | ↓ TP | ↓ IgM |
| 50% DMW | - | - | ↑ IgM; ↓ GPx; ↓ MDA | ||||
| 100% DMW | - | ↑ ALP; | ↓ SOD | ||||
| 50% FFMW | - | ↓ ALT; ↑ ALP; | ↑ GPx; ↓ SOD | ||||
| Beluga (Huso huso) [66] | FM | Carnivorous | RAS 56 days | not specified | ↓ Glu; ↓ ALB | ||
| 20% TM | ↓ RBC; | ↓ TP | |||||
| 40% TM | ↓ TG; ↑ LDH | ||||||
| 60% TM | ↑ CHOL; ↓ AST; ↓ ALT; ↓ ALP; ↑ LDH | ||||||
| 80% TM | ↓ ALB; ↑ CHOL; ↓ TG; ↑ LDH | ||||||
| 100% TM | ↓ RBC; ↓ Hb; ↓ Hct | ↓ ALP; ↑ LDH | |||||
| Sea Bass (Dicentrarchus labrax) [69] | FM | Carnivorous | RAS 70 days | not specified | - | ↓ TP | - |
| 40% DMW | ↑ Glu; ↑ TP; CHOL | ||||||
| 80% DMW | ↑ CHOL | ||||||
| 100% DMW | ↑ CHOL; ↑ TG |
| Species and References | Diet | Fish Feeding Habits | Aquaculture System/Period Days | Culture Period/Photoperiod | Histological Changes |
|---|---|---|---|---|---|
| Rainbow Trout Oncorhynchus mykiss [35] | FM | Carnivorous | flow-through 91 days | 12 h light/ 10 h dark | Liver—rare lymphocyte aggregates |
| 25% DMW | LP ↑—Distal intestinal inflammation | ||||
| 50% DMW | |||||
| 75% DMW | LP ↑—Distal intestinal inflammation Liver—sparse lymphocyte aggregates Kidney—tubular mineralization | ||||
| 100% DMW | LP ↑—Distal intestinal inflammation Liver—rare lymphocyte aggregates Kidney—tubular mineralization | ||||
| Grass carp Ctenopharyngodon idellus [44] | SBM | Herbivorous | nylon net pond cages 56 days | natural conditions | - |
| 25% FFMW | ↑ intestinal fold height | ||||
| 50% FFMW | |||||
| 75% FFMW | ↓ intestinal fold height, ↓ muscle thickness, ↓ intestinal goblet cell numbers; | ||||
| 100% FFMW | |||||
| Nile Tilapia (Oreochromis niloticus) [88] | FM+SB | Omnivorous | glass aquaria (100 L) 60 days | not specified | ↑ Villus height; ↑ Villus width; ↑ Crypt depth; ↑ Muscularis thickness; ↑ Goblet cell count |
| FM-SB | - | ||||
| TM+SB | ↑ Villus height; ↑ Villus width; ↑ Crypt depth; ↑ Muscularis thickness; ↑ Goblet cell count | ||||
| TM-SB | - | ||||
| Sea Bass (Dicentrarchus labrax) [69] | FM | Carnivorous | RAS 70 days | not specified | - |
| 40% DMW | ↑ submucosa thickness | ||||
| 80% DMW | ↑ submucosa thickness | ||||
| 100% DMW | ↑ submucosa thickness | ||||
| Large Yellow Croakers (Larimichthys crocea) [68] | FM | Carnivorous | floating sea cages 56 days | natural condition | |
| 15% DMW | ↑ Villus height; | ||||
| 30% DMW | ↑ Villus thickness; ↑ Muscularis thickness; ↓ DAO | ||||
| 45% DMW | ↑ Villus height; ↑ Villus thickness; ↑ Muscularis thickness; ↓ DAO |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ihuț, A.; Răducu, C.; Uiuiu, P.; Munteanu, C. From Gut to Fillet: Comprehensive Effects of Tenebrio molitor in Fish Nutrition. Fishes 2025, 10, 468. https://doi.org/10.3390/fishes10090468
Ihuț A, Răducu C, Uiuiu P, Munteanu C. From Gut to Fillet: Comprehensive Effects of Tenebrio molitor in Fish Nutrition. Fishes. 2025; 10(9):468. https://doi.org/10.3390/fishes10090468
Chicago/Turabian StyleIhuț, Andrada, Camelia Răducu, Paul Uiuiu, and Camelia Munteanu. 2025. "From Gut to Fillet: Comprehensive Effects of Tenebrio molitor in Fish Nutrition" Fishes 10, no. 9: 468. https://doi.org/10.3390/fishes10090468
APA StyleIhuț, A., Răducu, C., Uiuiu, P., & Munteanu, C. (2025). From Gut to Fillet: Comprehensive Effects of Tenebrio molitor in Fish Nutrition. Fishes, 10(9), 468. https://doi.org/10.3390/fishes10090468









