Feed Sources for Sustainable Aquaculture: Black Soldier Fly Larvae (BSFL)
Abstract
1. Introduction
2. Materials and Methods
3. Hermetia illucens: Biology and Nutrient Composition
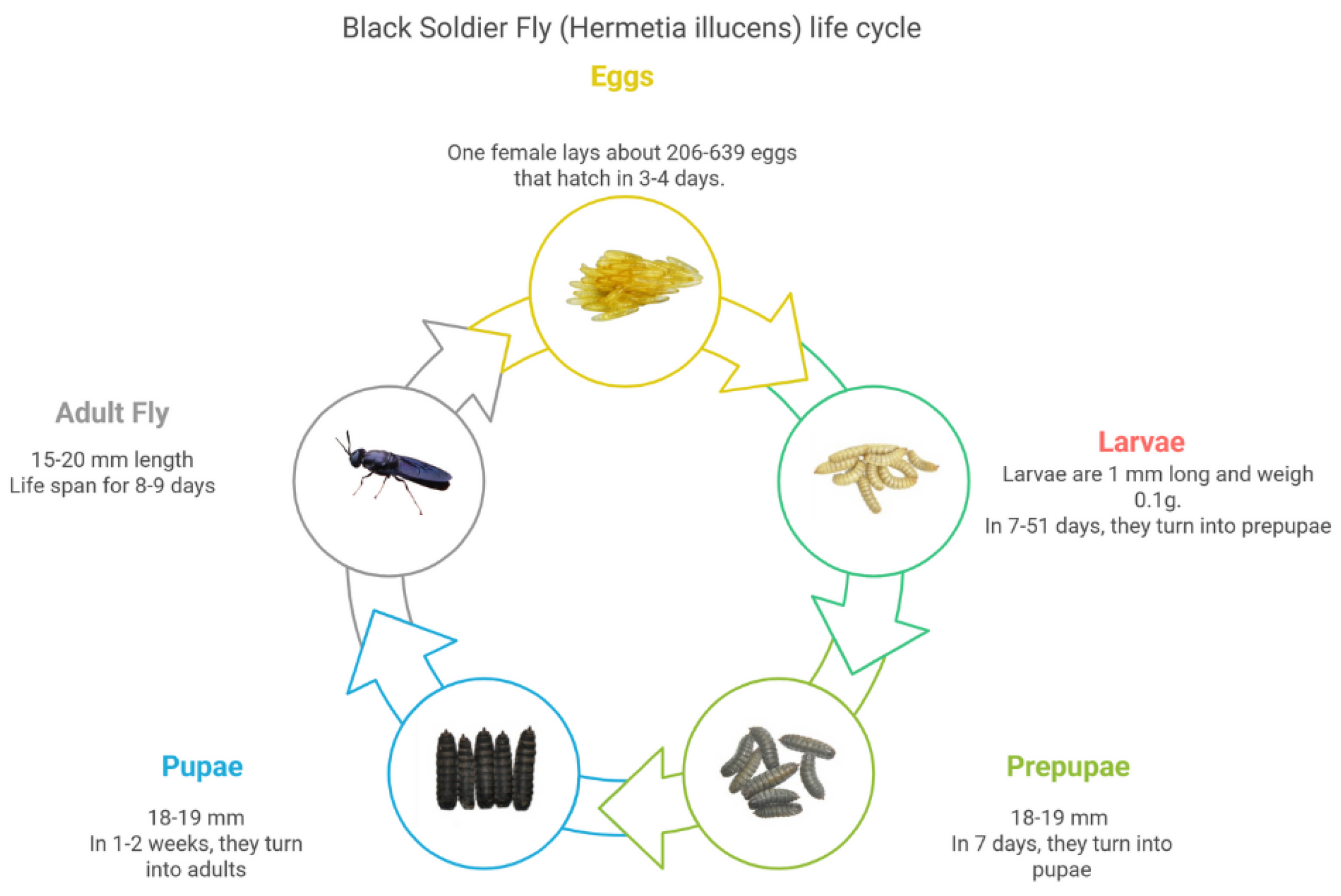
3.1. Biology of Species
3.2. Nutrient Composition of Insect Meal
3.2.1. Amino Acid Composition of BSFLM
3.2.2. Methionine and Lysine
3.2.3. Fatty Acids Composition of BSFL
3.2.4. Lauric Acid in BSFL
3.2.5. Chitin and Chitosan in BSFL
4. Impact of BSFL Meal in Aquaculture
| Species | Period | BSFL Level (%) | Results Obtained | References |
|---|---|---|---|---|
| Cyprinus carpio (carp)—fingerlings | - | 0, 12.5, 25, and 37.5% (fermented BSFL) | The 37.5% BSFL diet had the best results in terms of feed utilization efficiency, protein efficiency ratio, specific growth rate, survival, and linolenic acid levels. | [139] |
| Ctenopharyngodon idellus (grass carp)—juveniles | 8 weeks | 0, 25, 50, 75, and 100% (defatted BSFL) | There were no significant differences related to growth, feed efficiency, and proximal muscle composition. Malondialdehyde ↓. Gut microbiota analysis did not reveal significant changes. Aeromonas and Shewanella decreased significantly. BSFL 100% did not affect growth performance and carcass composition. | [140] |
| Oreochromis niloticus (Nile tilapia)—juveniles | 12 weeks | 0, 10, 20, 40, 60, 80, and 100% | Somatic indices, feed utilization efficiency, survival rate, and hematological parameters did not show significant changes. Lysozyme and peroxidase activities in skin mucus increased. | [141] |
| Hypsibarbus wetmorei (lemon fin barb)—fingerlings | 8 weeks | 0, 25, 50, 75, and 100% | Growth performance improved with 75% BSFL and above this percentage decreased. Protein retention ↓ and lipid retention ↑. No significant differences in FCR. No pathological changes. Up to 75% showed no negative effects on fish growth and health. | [142] |
| Argyrosomus regius (meagre)—juveniles | 9 weeks | 0, 10, 20, and 30% | No significant histomorphologic changes were observed between treatments. No significant differences in gut bacterial profiles. No significant differences in protease, peroxidase, lysozyme activities, nitric oxide production, and total immunoglobulin levels. A level of 10% BSF is recommended to avoid pathologic changes in the intestine. | [143] |
| Clarias gariepinus (catfish)—fingerlings | 16 weeks | 25, 50, and 75% | The 50% BSFL diet achieved a better FCR. Growth performance and survival demonstrated that BSFL has the potential to replace FM by up to 75%. Fish productivity and feed cost can be reduced. | [144] |
| Oncorhynchus mykiss (rainbow trout)—juveniles | 90 days | Dried BSFL prepupae (1, 2, or 3 times/day) | Reduced growth and feed intake, and chitin accumulation in the intestine led to constipation (2–3 meals of dry BSFL/day) and anus necrosis. Inclusion of one meal of dry BSF resulted in increased PUFAn-3, PUFAn-6, and DPA acids in fish. | [145] |
| Oncorhynchus mykiss (rainbow trout) and Clarias gariepinus (catfish)—fry | 4 weeks | 0, 33, 66, and 100% | BSFL could replace up to 66% of the diet of catfish and rainbow trout fry without negatively affecting growth performance. | [146] |
| Channa striata (snakehead) —juveniles | 9 weeks | 0, 25, 50, 75, and 100% | In over 50%, growth was negatively affected. SOD, catalase, and GPx activity was improved. Blood biochemistry and plasma metabolites were not altered. Reduced appetite on the 100 HM diet. | [147] |
| Oncorhynchus mykiss (rainbow trout)—juveniles | - | 30:70 diet, BSFL and standard feed | The diets tested had a good digestibility between 82.6 and 100%. | [148] |
| Sparus aurata (gilthead seabream)—juveniles | 67 days | 0, 15, 30, and 45% (defatted BSFL) | Growth-related gene expression and plasma metabolite profiles were not significantly affected. ALT and GDH ↑. Increased digestive enzyme activity in the hindgut of fish fed BSFL 15% diet. Enrichment of the gut microbiota | [149] |
| Oreochromis niloticus (Nile tilapia)—larvae | 4 weeks | 0, 40, 50, and 60% BSFLM | Good survival, specific growth rate varied significantly, and FCR decreased to 1.08 (60%). PER varied from 0.81 (40%) to 2.34 (imported feed). Better survival on experimental diets than on control. For economic profitability, 50 and 60% of BSFL mass is recommended. | [64] |
| Clarias gariepinus (catfish)—fingerlings | 8 weeks | 0, 33, 66, and 100% BSFL | Showed 100% survival. Using 100% replacement results in good growth rate. Protein and lipids ↑. Higher profit at 100% BSFL replacement. | [150] |
| Salmo salar (Atlantic salmon)—juveniles | 60 days | 0, 5, 10, and 20% full- fat BSFL | Up to 20% can improve the intestinal microbiota due to lauric acid, chitin, and peptides in BSFL. | [151] |
| Oncorhynchus mykiss (rainbow trout)—adults | 8 weeks | 0, 15, 30, 45, 60, and 75% BSFLM defatted using butane extraction | Using 45% BSF stimulated autophagy and gut health. Replacing over 60% of FM with BSF would reduce the growth rate. | [152] |
| Oreochromis niloticus (Nile tilapia)—juveniles | 5 weeks | 0, 20, and 40% defatted BSF larvae meal | Improved FCR, SGR, and PER. BSF did not affect gene expression of proinflammatory cytokines. Improved intestinal health in juvenile Nile tilapia. | [153] |
5. Impact of BSFL Meal on Fish Meat Quality and Sensory Attributes
6. Consumer Attitudes and Acceptance
7. Advantages and Disadvantage of Using Insect Meal
7.1. Advantages
| Benefits | References |
|---|---|
| Using insects as a source of protein can help protect marine resources and reduce overfishing. | [183] |
| BSFL has high nutritional value, as insects are rich in protein, essential amino acids, and other nutrients necessary for optimal fish growth. | [184] |
| Insect meal, in particular from the larvae of Hermetia illucens, contains high amounts of lauric acid, which is why it is increasingly used in fish feed, stimulating both growth and the immune system. It is a quick source of energy that is easily metabolized, which can help convert feed more efficiently. It also has antimicrobial properties, contributing to the intestinal health of the fish and reducing the risk of infection, which is a plus in aquaculture, where high stocking densities are practiced and disease prevention is essential. | [185,186] |
| Studies show that it has high digestibility, over 92%. | [187] |
| BSFL ensures efficient feed conversion, with insects efficiently converting consumed feed into biomass, thus contributing to environmental protection. | [188] |
| BSF larvae consume organic waste from the food industry as well as from agriculture, thus reducing its volume by up to 80%. This can be an effective solution for reducing organic waste, especially in countries with lower living standards. | [189] |
| Insects can be grown on various organic wastes, turning them into valuable resources, reducing the need for land, water, and energy. | [183,188] |
| Insect production has a lower carbon footprint compared to traditional livestock farming. | [190] |
| Insect meal can be cheaper due to their short life cycle and their ability to utilize accessible and inexpensive resources. | [191] |
| The nutritional composition of insect meal can be adjusted by changing the insect diet during a growing cycle. Also, chitin is a constituent of the exoskeleton of insects, and studies show that it can stimulate the immune system in animals consuming feed containing insect meal. Chitin increases the body’s resistance to the attack of pathogens and can increase immunity. | [192,193,194] |
7.2. Disadvantage
8. Regulation
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tidwell, J.H.; Allan, G.L. Fish as Food: Aquaculture’s Contribution. EMBO Rep. 2001, 2, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Simeanu, C.; Simeanu, D.; Popa, A.; Usturoi, A.; Bodescu, D.; Dolis, M.G. Research Regarding Content in Amino-Acids and Biological Value of Proteins from Polyodon Spathula Sturgeon Meat. Rev. Chim. 2017, 68, 1063–1069. [Google Scholar] [CrossRef]
- FAO Report: Global Fisheries and Aquaculture Production Reaches a New Record High. Available online: https://www.fao.org/newsroom/detail/fao-report-global-fisheries-and-aquaculture-production-reaches-a-new-record-high/en (accessed on 5 June 2025).
- Kumar, L.; Chhogyel, N.; Gopalakrishnan, T.; Hasan, M.K.; Jayasinghe, S.L.; Kariyawasam, C.S.; Kogo, B.K.; Ratnayake, S. Chapter 4—Climate Change and Future of Agri-Food Production. In Future Foods; Bhat, R., Ed.; Academic Press: San Diego, CA, USA, 2022; pp. 49–79. ISBN 978-0-323-91001-9. [Google Scholar]
- Gil, M.; Rudy, M.; Duma-Kocan, P.; Stanisławczyk, R.; Krajewska, A.; Dziki, D.; Hassoon, W.H. Sustainability of Alternatives to Animal Protein Sources, a Comprehensive Review. Sustainability 2024, 16, 7701. [Google Scholar] [CrossRef]
- Henchion, M.; Hayes, M.; Mullen, A.M.; Fenelon, M.; Tiwari, B. Future Protein Supply and Demand: Strategies and Factors Influencing a Sustainable Equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef]
- Péron, G.; Mittaine, J.F.; Gallic, B.L. Where Do Fishmeal and Fish Oil Products Come from? An Analysis of the Conversion Ratios in the Global Fishmeal Industry. Mar. Policy 2010, 34, 815–820. [Google Scholar] [CrossRef]
- Hussain, S.M.; Bano, A.A.; Ali, S.; Rizwan, M.; Adrees, M.; Zahoor, A.F.; Sarker, P.K.; Hussain, M.; Arsalan, M.Z.-H.; Yong, J.W.H.; et al. Substitution of Fishmeal: Highlights of Potential Plant Protein Sources for Aquaculture Sustainability. Heliyon 2024, 10, e26573. [Google Scholar] [CrossRef]
- Majluf, P.; Matthews, K.; Pauly, D.; Skerritt, D.J.; Palomares, M.L.D. A Review of the Global Use of Fishmeal and Fish Oil and the Fish In: Fish Out Metric. Sci. Adv. 2024, 10, eadn5650. [Google Scholar] [CrossRef]
- Pauly, D.; Zeller, D. Catch Reconstructions Reveal That Global Marine Fisheries Catches Are Higher than Reported and Declining. Nat. Commun. 2016, 7, 10244. [Google Scholar] [CrossRef]
- IFFO 2024. Available online: www.iffo.com/iffos-estimates-2023-fishmeal-and-fish-oil-production-and-insights-2024 (accessed on 4 June 2025).
- Naylor, R.L.; Hardy, R.W.; Bureau, D.P.; Chiu, A.; Elliott, M.; Farrell, A.P.; Forster, I.; Gatlin, D.M.; Goldburg, R.J.; Hua, K.; et al. Feeding Aquaculture in an Era of Finite Resources. Proc. Natl. Acad. Sci. USA 2009, 106, 15103–15110. [Google Scholar] [CrossRef]
- Daniel, N. A Review on Replacing Fish Meal in Aqua Feeds Using Plant Protein Sources. Int. J. Fish. Aquat. Stud. 2018, 6, 164–179. [Google Scholar]
- Abd El-Hack, M.E.; Shafi, M.E.; Alghamdi, W.Y.; Abdelnour, S.A.; Shehata, A.M.; Noreldin, A.E.; Ashour, E.A.; Swelum, A.A.; Al-Sagan, A.A.; Alkhateeb, M.; et al. Black Soldier Fly (Hermetia illucens) Meal as a Promising Feed Ingredient for Poultry: A Comprehensive Review. Agriculture 2020, 10, 339. [Google Scholar] [CrossRef]
- Tacon, A.G.J.; Metian, M. Feed Matters: Satisfying the Feed Demand of Aquaculture. Rev. Fish. Sci. Aquac. 2015, 23, 1–10. [Google Scholar] [CrossRef]
- Kenny, T.A.; Woodside, J.V.; Perry, I.J.; Harrington, J.M. Consumer Attitudes and Behaviors toward More Sustainable Diets: A Scoping Review. Nutr. Rev. 2023, 81, 1665–1679. [Google Scholar] [CrossRef] [PubMed]
- Serra, V.; Pastorelli, G.; Tedesco, D.E.A.; Turin, L.; Guerrini, A. Alternative Protein Sources in Aquafeed: Current Scenario and Future Perspectives. Vet. Anim. Sci. 2024, 25, 100381. [Google Scholar] [CrossRef] [PubMed]
- Desouky, A.; Hwihy, H.; Shaban, W.; Azab, A. Evaluating of Pea Peels Meal as a Fishmeal Alternative in Formulated Diet Ingredients of Oreochromis Niloticus. Egypt. J. Aquat. Biol. Fish. 2023, 27, 725–737. [Google Scholar] [CrossRef]
- Hernández, C.; Lizárraga-Velázquez, C.E.; Contreras-Rojas, D.; Sánchez-Gutiérrez, E.Y.; Martínez-Montaño, E.; Ibarra-Castro, L.; Peña-Marín, E.S. Fish Meal Replacement by Corn Gluten in Feeds for Juvenile Spotted Rose Snapper (Lutjanus guttatus): Effect on Growth Performance, Feed Efficiency, Hematological Parameters, Protease Activity, Body Composition, and Nutrient Digestibility. Aquaculture 2021, 531, 735896. [Google Scholar] [CrossRef]
- Howlader, S.; Sumi, K.R.; Sarkar, S.; Billah, S.M.; Ali, M.L.; Howlader, J.; Shahjahan, M. Effects of Dietary Replacement of Fish Meal by Soybean Meal on Growth, Feed Utilization, and Health Condition of Stinging Catfish, Heteropneustes Fossilis. Saudi J. Biol. Sci. 2023, 30, 103601. [Google Scholar] [CrossRef]
- Kaiser, F.; Harbach, H.; Schulz, C. Rapeseed Proteins as Fishmeal Alternatives: A Review. Rev. Aquac. 2022, 14, 1887–1911. [Google Scholar] [CrossRef]
- Tan, B.; Mai, K.; Zheng, S.; Zhou, Q.; Liu, L.; Yu, Y. Replacement of Fish Meal by Meat and Bone Meal in Practical Diets for the White Shrimp Litopenaeus vannamai (Boone). Aquac. Res. 2005, 36, 439–444. [Google Scholar] [CrossRef]
- Barbacariu, C.-A.; Dîrvariu, L.; Șerban, D.A.; Rîmbu, C.M.; Horhogea, C.E.; Dumitru, G.; Todirașcu-Ciornea, E.; Lungoci, C.; Burducea, M. Evaluating the Use of Grape Pomace in Cyprinus carpio Nutrition: Effects on Growth, Biochemistry, Meat Quality, Microbiota, and Oxidative Status. Fishes 2024, 9, 219. [Google Scholar] [CrossRef]
- Barbacariu, C.-A.; Rimbu, C.M.; Dirvariu, L.; Burducea, M.; Boiangiu, R.S.; Todirascu-Ciornea, E.; Dumitru, G. Evaluation of DDGS as a Low-Cost Feed Ingredient for Common Carp (Cyprinus Carpio Linneus) Cultivated in a Semi-Intensive System. Life 2022, 12, 1609. [Google Scholar] [CrossRef]
- Glencross, B.; Huyben, D.; Schrama, J. The Application of Single-Cell Ingredients in Aquaculture Feeds—A Review. Fishes 2020, 5, 22. [Google Scholar] [CrossRef]
- Kumar, V.; Lee, S.; Cleveland, B.M.; Romano, N.; Lalgudi, R.S.; Benito, M.R.; McGraw, B.; Hardy, R.W. Comparative Evaluation of Processed Soybean Meal (EnzoMealTM) vs. Regular Soybean Meal as a Fishmeal Replacement in Diets of Rainbow Trout (Oncorhynchus Mykiss): Effects on Growth Performance and Growth-Related Genes. Aquaculture 2020, 516, 734652. [Google Scholar] [CrossRef]
- Oteri, M.; Di Rosa, A.R.; Lo Presti, V.; Giarratana, F.; Toscano, G.; Chiofalo, B. Black Soldier Fly Larvae Meal as Alternative to Fish Meal for Aquaculture Feed. Sustainability 2021, 13, 5447. [Google Scholar] [CrossRef]
- Rahimnejad, S.; Hu, S.; Song, K.; Wang, L.; Lu, K.; Wu, R.; Zhang, C. Replacement of Fish Meal with Defatted Silkworm (Bombyx Mori L.) Pupae Meal in Diets for Pacific White Shrimp (Litopenaeus Vannamei). Aquaculture 2019, 510, 150–159. [Google Scholar] [CrossRef]
- Gu, J.; Liang, H.; Ge, X.; Xia, D.; Pan, L.; Mi, H.; Ren, M. A Study of the Potential Effect of Yellow Mealworm (Tenebrio Molitor) Substitution for Fish Meal on Growth, Immune and Antioxidant Capacity in Juvenile Largemouth Bass (Micropterus Salmoides). Fish Shellfish Immunol. 2022, 120, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Okoye, F.C.; Nnaji, J. Effects of Substituting Fish Meal with Grasshopper Meal on Growth and Food Utilization of the Nile Tilapia, Oreochromis Niloticus Fingerlings. 2005. Available online: https://www.researchgate.net/publication/277161891 (accessed on 1 August 2025).
- Perera, G.S.C.; Bhujel, R.C. Replacement of Fishmeal by House Cricket (Acheta Domesticus) and Field Cricket (Gryllus Bimaculatus) Meals: Effect for Growth, Pigmentation, and Breeding Performances of Guppy (Poecilia Reticulata). Aquac. Rep. 2022, 25, 101260. [Google Scholar] [CrossRef]
- Saleh, H. Review on Using of Housefly Maggots (Musca Domestica) in Fish Diets. J. Zool. Res. 2020, 2, 39–46. [Google Scholar] [CrossRef]
- Arru, B.; Furesi, R.; Gasco, L.; Madau, F.A.; Pulina, P. The Introduction of Insect Meal into Fish Diet: The First Economic Analysis on European Sea Bass Farming. Sustainability 2019, 11, 1697. [Google Scholar] [CrossRef]
- Huis, A. Potential of Insects as Food and Feed in Assuring Food Security. Annu. Rev. Entomol. 2012, 58, 563–583. [Google Scholar] [CrossRef]
- Chia, S.Y. Black Soldier Fly Larvae as a Sustainable Animal Feed Ingredient in Kenya. Ph.D. Thesis, Wageningen University and Research, Wageningen, The Netherlands, 2021. [Google Scholar]
- Shumo, M.; Khamis, F.; Subramanian, S.; Ekesi, S.; Fiaboe, K.; Borgemeister, C.; Huis, A.; Osuga, I. The Nutritive Value of Black Soldier Fly Larvae Reared on Common Organic Waste Streams in Kenya. Sci. Rep. 2019, 9, 10110. [Google Scholar] [CrossRef]
- Hamid, N.; Zakaria, N.; Ali, N. Study on Utilization of Black Soldier Fly Larvae (Hermetia illucens) as Protein Substitute in the Pellet Diet of Clarias Gariepenus Fingerling. Adv. Agric. Food Res. J. 2021, 3, 1–6. [Google Scholar] [CrossRef]
- Fahmi, M.; Nurjanah, N.; Sudadi, A.; Adanitri, G.; Melisza, N. The Nutrient Content of Nile Tilapia Fed with Black Soldier Fly (BSF) Larvae. Aquac. Aquar. Conserv. Legis. 2021, 14, 2718–2727. [Google Scholar]
- Lin, F.Y.; Li, A. A Primer on the Black Soldier Fly Market in Asia. 2021. Available online: https://www.manaimpact.com/post/a-primer-on-the-black-soldier-fly-market-in-asia (accessed on 2 June 2025).
- Tomberlin, J.K.; Van Huis, A. Black Soldier Fly from Pest to ‘Crown Jewel’ of the Insects as Feed Industry: An Historical Perspective. J. Insects Food Feed. 2020, 6, 1–4. [Google Scholar] [CrossRef]
- Raghuvaran, N.; Varghese, T.; Jana, P.; Angela Brighty, R.J.; Sethupathy, A.M.; Sudarshan, S.; Alrashdi, Y.B.A.; Ibrahim, A.E.; El Deeb, S. Current Status and Global Research Trend Patterns of Insect Meal in Aquaculture from Scientometric Perspective: (2013–2022). Aquac. Nutr. 2024, 2024, 5466604. [Google Scholar] [CrossRef] [PubMed]
- Spranghers, T.; Ottoboni, M.; Klootwijk, C.; Ovyn, A.; Deboosere, S.; Meulenaer, B.; Michiels, J.; Eeckhout, M.; De Clercq, P.; De Smet, S. Nutritional Composition of Black Soldier Fly (Hermetia illucens) Prepupae Reared on Different Organic Waste Substrates. J. Sci. Food Agric. 2017, 97, 2594–2600. [Google Scholar] [CrossRef]
- Moyo, S.; Moyo, B. Potential Utilization of Insect Meal as Livestock Feed. In Animal Feed Science and Nutrition: Production, Health and Environment; IntechOpen: London, UK, 2022; ISBN 978-1-83969-860-6. [Google Scholar]
- Caruso, D.; Devic, E.; Subamia, I.; Talamond, P.; Baras, E. Technical Handbook of Domestication and Production of Diptera Black Soldier Fly (BSF) Hermetia illucens, Stratiomyidae; IPB Press: Bogor, Indonesia, 2013; ISBN 978-979-493-610-8. [Google Scholar]
- Jeyaprakashsabari, S.; Aanand, S. Black Soldier Fly Larvae Meal in Fish Culture. AgriCos E-Newsl. 2022, 2, 52–56. [Google Scholar] [CrossRef]
- Diener, S.; Zurbrügg, C.; Tockner, K. Conversion of Organic Material by Black Soldier Fly Larvae: Establishing Optimal Feeding Rates. Waste Manag. Res. J. Int. Solid Wastes Public Clean. Assoc. ISWA 2009, 27, 603–610. [Google Scholar] [CrossRef]
- Loho, L.; Lo, D. Proximate and Fatty Acid Analysis of Black Soldier Fly Larvae (Hermetia illucens). IOP Conf. Ser. Earth Environ. Sci. 2023, 1169, 012082. [Google Scholar] [CrossRef]
- Lalander, C.; Fidjeland, J.; Diener, S.; Eriksson, S.; Vinnerås, B. High Waste-to-Biomass Conversion and Efficient Salmonella Spp. Reduction Using Black Soldier Fly for Waste Recycling. Agron. Sustain. Dev. 2014, 35, 261–271. [Google Scholar] [CrossRef]
- Veldkamp, T.; Van Duinkerken, G.; Van Huis, A.; Lakemond, C.M.M.; Ottevanger, E.; Bosch, G.; Van Boekel, T. Insects as a Sustainable Feed Ingredient in Pig and Poultry Diets: A Feasibility Study = Insecten Als Duurzame Diervoedergrondstof in Varkens-En Pluimveevoeders: Een Haalbaarheidsstudie; Report 638; Wageningen UR Livestock Research, Part of Stichting Dienst Landbouwkundig Onderzoek (DLO Foundation): Wageningen, The Netherlands, 2012; ISSN 1570–8616. [Google Scholar]
- Williams, J.P.; Williams, J.R.; Kirabo, A.; Chester, D.; Peterson, M. Nutrient Content and Health Benefits of Insects. In Insects as Sustainable Food Ingredients; Academic Press: San Diego, CA, USA, 2016; pp. 61–84. ISBN 978-0-12-802856-8. [Google Scholar]
- Schiavone, A.; Marco, M.; Martinez, S.; Dabbou, S.; Renna, M.; Madrid, J.; Hernandez, F.; Rotolo, L.; Costa, P.; Gai, F.; et al. Nutritional Value of a Partially Defatted and a Highly Defatted Black Soldier Fly Larvae (Hermetia illucens L.) Meal for Broiler Chickens: Apparent Nutrient Digestibility, Apparent Metabolizable Energy and Apparent Ileal Amino Acid Digestibility. J. Anim. Sci. Biotechnol. 2017, 8, 51. [Google Scholar] [CrossRef]
- Halloran, A.; Flore, R.; Vantomme, P.; Roos, N. Edible Insects in Sustainable Food Systems; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar] [CrossRef]
- Fish Meal, Protein 65%|Tables of Composition and Nutritional Values of Feed Materials INRA CIRAD AFZ. Available online: https://www.feedtables.com/content/fish-meal-protein-65 (accessed on 4 June 2025).
- Black Soldier Fly Larvae, Fat > 20%, Dried|Tables of Composition and Nutritional Values of Feed Materials INRA CIRAD AFZ. Available online: https://www.feedtables.com/content/black-soldier-fly-larvae-fat-20-dried-0 (accessed on 4 June 2025).
- Mahmoud, A.E.; Morel, P.C.H.; Potter, M.A.; Ravindran, V. The Apparent Metabolisable Energy and Ileal Amino Digestibility of Black Soldier Fly (Hermetia illucens) Larvae Meal for Broiler Chickens. Br. Poult. Sci. 2023, 64, 377–383. [Google Scholar] [CrossRef]
- Alfiko, Y.; Xie, D.; Astuti, R.T.; Wong, J.; Wang, L. Insects as a Feed Ingredient for Fish Culture: Status and Trends. Aquac. Fish. 2022, 7, 166–178. [Google Scholar] [CrossRef]
- Zulkifli, N.F.N.M.; Seok-Kian, A.Y.; Seng, L.L.; Mustafa, S.; Kim, Y.-S.; Shapawi, R. Nutritional Value of Black Soldier Fly (Hermetia illucens) Larvae Processed by Different Methods. PLoS ONE 2022, 17, e0263924. [Google Scholar] [CrossRef] [PubMed]
- Sándor, Z.J.; Banjac, V.; Vidosavljević, S.; Káldy, J.; Egessa, R.; Lengyel-Kónya, É.; Tömösközi-Farkas, R.; Zalán, Z.; Adányi, N.; Libisch, B.; et al. Apparent Digestibility Coefficients of Black Soldier Fly (Hermetia illucens), Yellow Mealworm (Tenebrio Molitor), and Blue Bottle Fly (Calliphora Vicina) Insects for Juvenile African Catfish Hybrids (Clarias Gariepinus × Heterobranchus Longifilis). Aquac. Nutr. 2022, 2022, 4717014. [Google Scholar] [CrossRef]
- Riekkinen, K.; Väkeväinen, K.; Korhonen, J. The Effect of Substrate on the Nutrient Content and Fatty Acid Composition of Edible Insects. Insects 2022, 13, 590. [Google Scholar] [CrossRef] [PubMed]
- Laganaro, M.; Bahrndorff, S.; Eriksen, N.T. Growth and Metabolic Performance of Black Soldier Fly Larvae Grown on Low and High-Quality Substrates. Waste Manag. 2021, 121, 198–205. [Google Scholar] [CrossRef]
- Wamboga, M.; Musinguzi, S.P.; Tinzaara, W.; Echaku, S. Effect of Types and Quantities of Substrates on Growth Performance of Black Soldier Fly Larvae (Hermetia illucens). J. Exp. Agric. Int. 2025, 47, 263–270. [Google Scholar] [CrossRef]
- Holeh, G.; Opiyo, M.; Brown, C.; Sumbule, E.; Gatagwu, J.; Oje, E.; Munyi, F. Effect of Different Waste Substrates on the Growth, Development and Proximate Composition of Black Soldier Fly (Hermetia illucens) Larvae. Livest. Res. Rural. Dev. 2022, 34, 1–11. [Google Scholar]
- Hosseindoust, A.; Ha, S.H.; Mun, J.Y.; Kim, J.S. A Metanalysis to Evaluate the Effects of Substrate Sources on the Nutritional Performance of Black Soldier Fly Larvae: Implications for Sustainable Poultry Feed. Poult. Sci. 2024, 103, 103299. [Google Scholar] [CrossRef]
- Vodounnou, J.V.; Iko, R.; Okou, G.; Kpogue, D.; Montcho, S.A.; Micha, J.-C. Complete Substitution of Fish Meal with Black Soldier Flies Hermetia illucens (L. 1758) Larvae Meal at Varying Incorporation Rates for Feeding Oreochromis Niloticus Raised in Captivity. Aquac. Sci. Manag. 2025, 2, 1. [Google Scholar] [CrossRef]
- Kanjanarat, K.; Laksanawimol, P.; Lersawhanwaree, J.; Khan, S.; Thancharoen, A. Utilizing Spent Mushroom Substrate for Rearing Black Soldier Fly (Hermetia illucens) Larvae: Enhancing Fertilizer Efficiency and Improving Animal Feed Quality for Sustainable Agriculture. PeerJ 2025, 13, e19590. [Google Scholar] [CrossRef] [PubMed]
- Weko, M.; Bao, F.; Ega, M.; Mia, H.; Una, K.; Viana, M.; Wale, L.; Nalle, C.; Burithnaban, Y.; Lema, A.; et al. Nutrient Profile Black Soldier Fly Larvae (Hermetia Illucens): Effect of Feeding Substrate and Harvested Time. Biotropia 2023, 30, 297–307. [Google Scholar] [CrossRef]
- Rossi, G.; Ojha, S.; Müller-Belecke, A.; Schlüter, O. Fresh Aquaculture Sludge Management with Black Soldier Fly (Hermetia illucens L.) Larvae: Investigation on Bioconversion Performances. Sci. Rep. 2023, 13, 20982. [Google Scholar] [CrossRef] [PubMed]
- Abduh, M.Y.; Perdana, M.P.; Bara, M.A.; Anggraeni, L.W.; Putra, R.E. Effects of Aeration Rate and Feed on Growth, Productivity and Nutrient Composition of Black Soldier Fly (Hermetia illucens L.) Larvae. J. Asia-Pac. Entomol. 2022, 25, 101902. [Google Scholar] [CrossRef]
- Somarny, W.W.M.Z.; Kuppusamy, G.; Samat, N.; Azam Ali, S. Performance of Black Soldier Fly Larvae (Hermetia illucens L.) on Different Feed Substrates. J. Insects Food Feed. 2023, 10, 345–358. [Google Scholar] [CrossRef]
- Barragán-Fonseca, K.; Dicke, M.; van Loon, J. Nutritional Value of the Black Soldier Fly (Hermetia illucens L.) and Its Suitability as Animal Feed. J. Insects Food Feed 2017, 3, 105–120. [Google Scholar] [CrossRef]
- Oonincx, D.; Broekhoven, S.; Huis, A.; van Loon, J. Feed Conversion, Survival and Development, and Composition of Four Insect Species on Diets Composed of Food By-Products. PLoS ONE 2015, 10, e0144601. [Google Scholar] [CrossRef]
- IJdema, F.; Lievens, S.; Smets, R.; Poma, G.; Borght, M.V.D.; Lievens, B.; Smet, J.D. Modulating the Fatty Acid Composition of Black Soldier Fly Larvae via Substrate Fermentation. Animal 2025, 19, 101383. [Google Scholar] [CrossRef]
- Boafo, H.; Gbemavo, C.; Timpong-Jones, E.; Eziah, V.; Billah, M.; Chia, S.Y.; Aidoo, O.; Clottey, V.; Kenis, M. Substrates Most Preferred for Black Soldier Fly Hermetia illucens (Linnaeus, 1758) (Diptera: Stratiomyidae) Oviposition Are Not the Most Suitable for Their Larval Development. J. Insects Food Feed 2022, 9, 183–192. [Google Scholar] [CrossRef]
- Jucker, C.; Erba, D.; Leonardi, M.G.; Lupi, D.; Savoldelli, S. Assessment of Vegetable and Fruit Substrates as Potential Rearing Media for Hermetia illucens (Diptera: Stratiomyidae) Larvae. Environ. Entomol. 2017, 46, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Mohamed, Z.; Amrul, N.; Quan, C.; Jalil, N.; Basri, N.; Azmi, M.R. Composting Fruit and Vegetable Waste Using Black Soldier Fly Larvae. J. Kejuruter. 2021, 33, 837–843. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Ankers, P. State-of-the-Art on Use of Insects as Animal Feed. Anim. Feed. Sci. Technol. 2014, 197, 1–33. [Google Scholar] [CrossRef]
- Lu, S.; Taethaisong, N.; Meethip, W.; Surakhunthod, J.; Sinpru, B.; Sroichak, T.; Archa, P.; Thongpea, S.; Paengkoum, S.; Purba, R.A.P.; et al. Nutritional Composition of Black Soldier Fly Larvae (Hermetia illucens L.) and Its Potential Uses as Alternative Protein Sources in Animal Diets: A Review. Insects 2022, 13, 831. [Google Scholar] [CrossRef]
- Kawasaki, K.; Hashimoto, Y.; Hori, A.; Kawasaki, T.; Hirayasu, H.; Iwase, S.; Hashizume, A.; Ido, A.; Miura, C.; Miura, T.; et al. Evaluation of Black Soldier Fly (Hermetia illucens) Larvae and Pre-Pupae Raised on Household Organic Waste, as Potential Ingredients for Poultry Feed. Animals 2019, 9, 98. [Google Scholar] [CrossRef]
- Espe, M.; Adam, A.C.; Saito, T.; Skjærven, K.H. Methionine: An Indispensable Amino Acid in Cellular Metabolism and Health of Atlantic Salmon. Aquac. Nutr. 2023, 2023, 5706177. [Google Scholar] [CrossRef]
- Wang, W.; Yang, P.; He, C.; Chi, S.; Li, S.; Mai, K.; Song, F. Effects of Dietary Methionine on Growth Performance and Metabolism through Modulating Nutrient-Related Pathways in Largemouth Bass (Micropterus Salmoides). Aquac. Rep. 2021, 20, 100642. [Google Scholar] [CrossRef]
- Wang, L.; Gao, C.; Wang, B.; Wang, C.; Sagada, G.; Yan, Y. Methionine in Fish Health and Nutrition: Potential Mechanisms, Affecting Factors, and Future Perspectives. Aquaculture 2023, 568, 739310. [Google Scholar] [CrossRef]
- Elesho, F.E.; Sutter, D.A.H.; Swinkels, M.A.C.; Verreth, J.A.J.; Kröckel, S.; Schrama, J.W. Quantifying Methionine Requirement of Juvenile African Catfish (Clarias Gariepinus). Aquaculture 2021, 532, 736020. [Google Scholar] [CrossRef]
- Schwarz, F.J.; Kirchgessner, M.; Deuringer, U. Studies on the Methionine Requirement of Carp (Cyprinus carpio L.). Aquaculture 1998, 161, 121–129. [Google Scholar] [CrossRef]
- Kim, K.-I.; Kayes, T.B.; Amundson, C.H. Requirements for Sulfur Amino Acids and Utilization of D-Methionine by Rainbow Trout (Oncorhynchus Mykiss). Aquaculture 1992, 101, 95–103. [Google Scholar] [CrossRef]
- Liaqat, I.; Mukhtar, B.; Malik, M.; Shah, S.H.; Azzam, A.; Slahuddin, S. Lysine Supplementation in Fish Feed. Int. J. Appl. Biol. Forensics 2017, 1, 26–31. [Google Scholar]
- Huang, D.; Liang, H.; Ren, M.; Ge, X.; Ji, K.; Yu, H.; Maulu, S. Effects of Dietary Lysine Levels on Growth Performance, Whole Body Composition and Gene Expression Related to Glycometabolism and Lipid Metabolism in Grass Carp, Ctenopharyngodon Idellus Fry. Aquaculture 2021, 530, 735806. [Google Scholar] [CrossRef]
- Kim, K.-I.; Kayes, T.B.; Amundson, C.H. Requirements for Lysine and Arginine by Rainbow Trout (Oncorhynchus Mykiss). Aquaculture 1992, 106, 333–344. [Google Scholar] [CrossRef]
- Ng, W.; Hung, S. Estimating the Ideal Dietary Essential Amino Acid Pattern for Growth of White Sturgeon, Acipenser Transmontanus (Richardson). Aquac. Nutr. 2006, 1, 85–94. [Google Scholar] [CrossRef]
- Santiago, C.B.; Lovell, R.T. Amino Acid Requirements for Growth of Nile Tilapia. J. Nutr. 1988, 118, 1540–1546. [Google Scholar] [CrossRef]
- Lin, X.; Du, Y.; de Cruz, C.; Zhao, J.; Shao, X.; Xu, Q. Effects of Supplementation with Crystalline or Coated Methionine and Lysine in Low Protein Diet on Growth Performance, Intestinal Health and Muscle Quality of Gibel Carp, Carassius Auratus Gibelio. Aquac. Rep. 2024, 36, 102084. [Google Scholar] [CrossRef]
- Liu, C.-M.; Uehara, T.; Shimoda, M. Enhancing the Nutritional Value of Black Soldier Fly Larvae through the Nutrient Amino Acid Transporter Suppression in the Excretion System. J. Insects Food Feed. 2024, 11, 497–507. [Google Scholar] [CrossRef]
- Koethe, M.; Taubert, J.; Vervuert, I. Impact of Lysine Supplementation on Growth and Development of Hermetia illucens Larvae. J. Insects Food Feed. 2021, 8, 35–44. [Google Scholar] [CrossRef]
- Sprague, M.; Dick, J.; Tocher, D. Impact of Sustainable Feeds on Omega-3 Long-Chain Fatty Acid Levels in Farmed Atlantic Salmon, 2006–2015. Sci. Rep. 2016, 6, 21892. [Google Scholar] [CrossRef]
- Tocher, D. Omega-3 Long-Chain Polyunsaturated Fatty Acids and Aquaculture in Perspective. Aquaculture 2015, 449, 94–107. [Google Scholar] [CrossRef]
- Turchini, G.; Torstensen, B.; Ng, W.-K. Fish Oil Replacement in Finfish Nutrition. Rev. Aquac. 2009, 1, 10–57. [Google Scholar] [CrossRef]
- Sprague, M.; Betancor, M.; Tocher, D. Microbial and Genetically Engineered Oils as Replacements for Fish Oil in Aquaculture Feeds. Biotechnol. Lett. 2017, 39, 1599–1609. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ji, H.; Zhang, B.; Tian, J.; Zhou, J.; Yu, H. Influence of Black Soldier Fly (Hermetia illucens) Larvae Oil on Growth Performance, Body Composition, Tissue Fatty Acid Composition and Lipid Deposition in Juvenile Jian Carp (Cyprinus carpio Var. Jian). Aquaculture 2016, 465, 43–52. [Google Scholar] [CrossRef]
- Almeida, C.; Murta, D.; Nunes, R.P.; Baby, A.; Fernandes, A.; Barros, L.; Rijo, P.; Rosado, C. Characterization of Lipid Extracts from the Hermetia illucens Larvae and Their Bioactivities for Potential Use as Pharmaceutical and Cosmetic Ingredients. Heliyon 2022, 8, e09455. [Google Scholar] [CrossRef]
- Borrelli, L.; Varriale, L.; Dipineto, L.; Pace, A.; Menna, L.; Fioretti, A. Insect Derived Lauric Acid as Promising Alternative Strategy to Antibiotics in the Antimicrobial Resistance Scenario. Front. Microbiol. 2021, 12, 620798. [Google Scholar] [CrossRef]
- Daszkiewicz, T.; Murawska, D.; Kubiak, D.; Han, J. Chemical Composition and Fatty Acid Profile of the Pectoralis Major Muscle in Broiler Chickens Fed Diets with Full-Fat Black Soldier Fly (Hermetia illucens) Larvae Meal. Animals 2022, 12, 464. [Google Scholar] [CrossRef]
- Suryati, T.; Julaeha, E.; Farabi, K.; Ambarsari, H.; Hidayat, A.T. Lauric Acid from the Black Soldier Fly (Hermetia illucens) and Its Potential Applications. Sustainability 2023, 15, 10383. [Google Scholar] [CrossRef]
- Kabara, J.J.; Swieczkowski, D.M.; Conley, A.J.; Truant, J.P. Fatty Acids and Derivatives as Antimicrobial Agents. Antimicrob. Agents Chemother. 1972, 2, 23–28. [Google Scholar] [CrossRef]
- Thormar, H.; Isaacs, C.; Brown, H.R.; Barshatzky, M.R.; Pessolano, T. Inactivation of Enveloped Viruses and Killing of Cells by Fatty Acids and Monoglycerides. Antimicrob. Agents Chemother. 1987, 31, 27–31. [Google Scholar] [CrossRef]
- Akula, S.T.; Nagaraja, A.; Ravikanth, M.; Kumar, N.G.R.; Kalyan, Y.; Divya, D. Antifungal Efficacy of Lauric Acid and Caprylic Acid—Derivatives of Virgin Coconut Oil against Candida Albicans. Biomed. Biotechnol. Res. J. BBRJ 2021, 5, 229. [Google Scholar] [CrossRef]
- Lappano, R.; Sebastiani, A.; Cirillo, F.; Rigiracciolo, D.; Galli, G.; Curcio, R.; Malaguarnera, R.; Belfiore, A.; Cappello, A.; Maggiolini, M. The Lauric Acid-Activated Signaling Prompts Apoptosis in Cancer Cells. Cell Death Discov. 2017, 3, 17063. [Google Scholar] [CrossRef] [PubMed]
- Belghit, I.; Liland, N.S.; Gjesdal, P.; Biancarosa, I.; Menchetti, E.; Li, Y.; Waagbø, R.; Krogdahl, Å.; Lock, E.-J. Black Soldier Fly Larvae Meal Can Replace Fish Meal in Diets of Sea-Water Phase Atlantic Salmon (Salmo Salar). Aquaculture 2019, 503, 609–619. [Google Scholar] [CrossRef]
- Fontinha, F.; Martins, N.; Magalhães, R.; Peres, H.; Oliva-Teles, A. Dietary Lauric Acid Supplementation Positively Affects Growth Performance, Oxidative and Immune Status of European Seabass Juveniles. Fishes 2025, 10, 190. [Google Scholar] [CrossRef]
- Elieh-Ali-Komi, D.; Sharma, L.; Dela Cruz, C. Chitin and Its Effects on Inflammatory and Immune Responses. Clin. Rev. Allergy Immunol. 2018, 54, 213. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Mohan, K.; Ganesan, A.R.; Muralisankar, T.; Jayakumar, R.; Sathishkumar, P.; Uthayakumar, V.; Chandirasekar, R.; Revathi, N. Recent Insights into the Extraction, Characterization, and Bioactivities of Chitin and Chitosan from Insects. Trends Food Sci. Technol. 2020, 105, 17–42. [Google Scholar] [CrossRef]
- Khatami, N.; Guerrero, P.; Martín, P.; Quintela, E.; Ramos, V.; Saa, L.; Cortajarena, A.L.; de la Caba, K.; Camarero-Espinosa, S.; Abarrategi, A. Valorization of Biological Waste from Insect-Based Food Industry: Assessment of Chitin and Chitosan Potential. Carbohydr. Polym. 2024, 324, 121529. [Google Scholar] [CrossRef]
- Triunfo, M.; Tafi, E.; Guarnieri, A.; Salvia, R.; Scieuzo, C.; Hahn, T.; Zibek, S.; Gagliardini, A.; Panariello, L.; Coltelli, M.; et al. Characterization of Chitin and Chitosan Derived from Hermetia illucens, a Further Step in a Circular Economy Process. Sci. Rep. 2022, 12, 6613. [Google Scholar] [CrossRef]
- Marino-Perez, R. Edible Orthopteroids: The Mexican Case. Metaleptea 2015, 35, 7–8. [Google Scholar]
- Gopalakannan, A.; Arul, V. Immunomodulatory Effects of Dietary Intake of Chitin, Chitosan and Levamisole on the Immune System of Cyprinus Carpio and Control of Aeromonas Hydrophila Infection in Ponds. Aquaculture 2006, 255, 179–187. [Google Scholar] [CrossRef]
- Mari, L.S.S.; Jagruthi, C.; Anbazahan, S.M.; Yogeshwari, G.; Thirumurugan, R.; Arockiaraj, J.; Mariappan, P.; Balasundaram, C.; Harikrishnan, R. Protective Effect of Chitin and Chitosan Enriched Diets on Immunity and Disease Resistance in Cirrhina Mrigala against Aphanomyces Invadans. Fish Shellfish Immunol. 2014, 39, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Elserafy, S.S.; Abdel-Hameid, N.H.; Abdel-Salam, H.A.; Dakrouni, A.M. Effect of shrimp waste extracted chitin on growth and some biochemical parameters of the Nile tilapia. Egypt. J. Aquat. Biol. Fish. 2021, 25, 313–329. [Google Scholar] [CrossRef]
- Harikrishnan, R.; Kim, J.-U.; Balasundaram, C.; Heo, S.-M. Dietary Supplementation with Chitin and Chitosan on Haematology and Innate Immune Response in Epinephelus Bruneus against Philasterides Dicentrarchi. Exp. Parasitol. 2012, 131, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Hasan, I.; Gai, F.; Cirrincione, S.; Rimoldi, S.; Saroglia, G.; Terova, G. Chitinase and Insect Meal in Aquaculture Nutrition: A Comprehensive Overview of the Latest Achievements. Fishes 2023, 8, 607. [Google Scholar] [CrossRef]
- Renna, M.; Schiavone, A.; Gai, F.; Dabbou, S.; Lussiana, C.; Malfatto, V.; Prearo, M.; Capucchio, M.; Biasato, I.; Biasibetti, E.; et al. Evaluation of the Suitability of a Partially Defatted Black Soldier Fly (Hermetia illucens L.) Larvae Meal as Ingredient for Rainbow Trout (Oncorhynchus Mykiss Walbaum) Diets. J. Anim. Sci. Biotechnol. 2017, 8, 57. [Google Scholar] [CrossRef]
- Pascon, G.; Cardinaletti, G.; Daniso, E.; Bruni, L.; Messina, M.; Parisi, G.; Tulli, F. Effect of Dietary Chitin on Growth Performance, Nutrient Utilization, and Metabolic Response in Rainbow Trout (Oncorhynchus Mykiss). Aquac. Rep. 2024, 37, 102244. [Google Scholar] [CrossRef]
- Eggink, K.; Pedersen, P.; Lund, I.; Dalsgaard, J. Chitin Digestibility and Intestinal Exochitinase Activity in Nile Tilapia and Rainbow Trout Fed Different Black Soldier Fly Larvae Meal Size Fractions. Aquac. Res. 2022, 53, 5536–5546. [Google Scholar] [CrossRef]
- Pascon, G.; Opere Akinyi, R.; Cardinaletti, G.; Daniso, E.; Messina, M. Chitin and Its Effects When Included in Aquafeed. Aquac. Int. 2025, 33, 202–224. [Google Scholar] [CrossRef]
- Kroeckel, S.; Harjes, A.-G.E.; Roth, I.; Katz, H.; Wuertz, S.; Susenbeth, A.; Schulz, C. When a Turbot Catches a Fly: Evaluation of a Pre-Pupae Meal of the Black Soldier Fly (Hermetia illucens) as Fish Meal Substitute—Growth Performance and Chitin Degradation in Juvenile Turbot (Psetta Maxima). Aquaculture 2012, 364, 345–352. [Google Scholar] [CrossRef]
- Karlsen, Ø.; Amlund, H.; Berg, A.; Olsen, R. The Effect of Dietary Chitin on Growth and Nutrient Digestibility in Farmed Atlantic Cod, Atlantic Salmon and Atlantic Halibut. Aquac. Res. 2015, 48, 123–133. [Google Scholar] [CrossRef]
- Dumas, A.; Raggi, T.; Barkhouse, J.; Lewis, E.; Weltzien, E. The Oil Fraction and Partially Defatted Meal of Black Soldier Fly Larvae (Hermetia illucens) Affect Differently Growth Performance, Feed Efficiency, Nutrient Deposition, Blood Glucose and Lipid Digestibility of Rainbow Trout (Oncorhynchus Mykiss). Aquaculture 2018, 492, 24–34. [Google Scholar] [CrossRef]
- Ratti, S.; Zarantoniello, M.; Chemello, G.; Giammarino, M.; Palermo, F.; Cocci, P.; Mosconi, G.; Tignani, M.; Pascon, G.; Cardinaletti, G.; et al. Spirulina-Enriched Substrate to Rear Black Soldier Fly (Hermetia illucens) Prepupae as Alternative Aquafeed Ingredient for Rainbow Trout (Oncorhynchus Mykiss) Diets: Possible Effects on Zootechnical Performances, Gut and Liver Health Status, and Fillet Quality. Animals 2023, 13, 173. [Google Scholar] [CrossRef]
- Katya, K.; Borsra, M.Z.S.; Ganesan, D.; Kuppusamy, G.; Herriman, M.; Salter, A.; Ali, S.A. Efficacy of Insect Larval Meal to Replace Fish Meal in Juvenile Barramundi, Lates Calcarifer Reared in Freshwater. Int. Aquat. Res. 2017, 9, 303–312. [Google Scholar] [CrossRef]
- Magalhães, R.; Sánchez-López, A.; Leal, R.S.; Martínez-Llorens, S.; Oliva-Teles, A.; Peres, H. Black Soldier Fly (Hermetia illucens) Pre-Pupae Meal as a Fish Meal Replacement in Diets for European Seabass (Dicentrarchus Labrax). Aquaculture 2017, 476, 79–85. [Google Scholar] [CrossRef]
- Li, S.; Ji, H.; Zhang, B.; Zhou, J.; Yu, H. Defatted Black Soldier Fly (Hermetia illucens) Larvae Meal in Diets for Juvenile Jian Carp (Cyprinus carpio Var. Jian): Growth Performance, Antioxidant Enzyme Activities, Digestive Enzyme Activities, Intestine and Hepatopancreas Histological Structure. Aquaculture 2017, 477, 62–70. [Google Scholar] [CrossRef]
- Zhou, J.S.; Liu, S.S.; Ji, H.; Yu, H.B. Effect of Replacing Dietary Fish Meal with Black Soldier Fly Larvae Meal on Growth and Fatty Acid Composition of Jian Carp (Cyprinus carpio Var. Jian). Aquac. Nutr. 2018, 24, 424–433. [Google Scholar] [CrossRef]
- Wang, G.; Peng, K.; Hu, J.; Yi, C.; Chen, X.; Wu, H.; Huang, Y. Evaluation of Defatted Black Soldier Fly (Hermetia illucens L.) Larvae Meal as an Alternative Protein Ingredient for Juvenile Japanese Seabass (Lateolabrax Japonicus) Diets. Aquaculture 2019, 507, 144–154. [Google Scholar] [CrossRef]
- Rawski, M.; Mazurkiewicz, J.; Kierończyk, B.; Józefiak, D. Black Soldier Fly Full-Fat Larvae Meal as an Alternative to Fish Meal and Fish Oil in Siberian Sturgeon Nutrition: The Effects on Physical Properties of the Feed, Animal Growth Performance, and Feed Acceptance and Utilization. Animals 2020, 10, 2119. [Google Scholar] [CrossRef]
- Kumar, V.; Fawole, F.J.; Romano, N.; Hossain, M.S.; Labh, S.N.; Overturf, K.; Small, B.C. Insect (Black Soldier Fly, Hermetia illucens) Meal Supplementation Prevents the Soybean Meal-Induced Intestinal Enteritis in Rainbow Trout and Health Benefits of Using Insect Oil. Fish Shellfish Immunol. 2021, 109, 116–124. [Google Scholar] [CrossRef]
- Khieokhajonkhet, A.; Uanlam, P.; Ruttarattanamongkol, K.; Aeksiri, N.; Tatsapong, P.; Kaneko, G. Replacement of Fish Meal by Black Soldier Fly Larvae Meal in Diet for Goldfish Carassius Auratus: Growth Performance, Hematology, Histology, Total Carotenoids, and Coloration. Aquaculture 2022, 561, 738618. [Google Scholar] [CrossRef]
- Carral, J.M.; Sáez-Royuela, M. Replacement of Dietary Fishmeal by Black Soldier Fly Larvae (Hermetia illucens) Meal in Practical Diets for Juvenile Tench (Tinca Tinca). Fishes 2022, 7, 390. [Google Scholar] [CrossRef]
- Kari, Z.A.; Téllez-Isaías, G.; Hamid, N.K.A.; Rusli, N.D.; Mat, K.; Sukri, S.A.M.; Kabir, M.A.; Ishak, A.R.; Dom, N.C.; Abdel-Warith, A.-W.A.; et al. Effect of Fish Meal Substitution with Black Soldier Fly (Hermetia illucens) on Growth Performance, Feed Stability, Blood Biochemistry, and Liver and Gut Morphology of Siamese Fighting Fish (Betta Splendens). Aquac. Nutr. 2023, 2023, 6676953. [Google Scholar] [CrossRef] [PubMed]
- Linh, N.V.; Wannavijit, S.; Tayyamath, K.; Dinh-Hung, N.; Nititanarapee, T.; Sumon, M.A.A.; Srinual, O.; Permpoonpattana, P.; Doan, H.V.; Brown, C.L. Black Soldier Fly (Hermetia illucens) Larvae Meal: A Sustainable Alternative to Fish Meal Proven to Promote Growth and Immunity in Koi Carp (Cyprinus carpio Var. Koi). Fishes 2024, 9, 53. [Google Scholar] [CrossRef]
- Ouko, K.O.; Mboya, J.B.; Mukhebi, A.W.; Obiero, K.O.; Ogello, E.O.; Munguti, J.M.; Tanga, C.M. Effect of Replacing Fish Meal with Black Soldier Fly Larvae Meal on Growth Performance and Economic Efficiency of Nile Tilapia. Fundam. Appl. Agric. 2024, 9, 1–9. [Google Scholar] [CrossRef]
- Herawati, V.E.; Pinandoyo; Darmanto, Y.; Hutabarat, J.; Seto; Windarto; Rismaningsih, N.; Radjasa, O.K. Fermented Black Soldier Fly (Hermetia illucens) Meal Utilization in Artificial Feed for Carp (Cyprinus Carpio). AACL Bioflux 2020, 13, 1038–1047. Available online: http://www.bioflux.com.ro/aacl (accessed on 4 June 2025).
- Lu, R.; Chen, Y.; Yu, W.; Lin, M.; Yang, G.; Qin, C.; Meng, X.; Zhang, Y.; Ji, H.; Nie, G. Defatted Black Soldier Fly (Hermetia illucens) Larvae Meal Can Replace Soybean Meal in Juvenile Grass Carp (Ctenopharyngodon Idellus) Diets. Aquac. Rep. 2020, 18, 100520. [Google Scholar] [CrossRef]
- Tippayadara, N.; Dawood, M.A.O.; Krutmuang, P.; Hoseinifar, S.H.; Doan, H.V.; Paolucci, M. Replacement of Fish Meal by Black Soldier Fly (Hermetia illucens) Larvae Meal: Effects on Growth, Haematology, and Skin Mucus Immunity of Nile Tilapia, Oreochromis Niloticus. Animals 2021, 11, 193. [Google Scholar] [CrossRef]
- Kamarudin, M.S.; Rosle, S.; Yasin, I.S.M. Performance of Defatted Black Soldier Fly Pre-Pupae Meal as Fishmeal Replacement in the Diet of Lemon Fin Barb Hybrid Fingerlings. Aquac. Rep. 2021, 21, 100775. [Google Scholar] [CrossRef]
- Couto, A.; Serra, C.R.; Guerreiro, I.; Coutinho, F.; Castro, C.; Rangel, F.; Lavrador, A.S.; Monteiro, M.; Santos, R.A.; Peres, H.; et al. Black Soldier Fly Meal Effects on Meagre Health Condition: Gut Morphology, Gut Microbiota and Humoral Immune Response. J. Insects Food Feed. 2022, 8, 1281–1295. [Google Scholar] [CrossRef]
- Maranga, B.; Kagali, R.; Mbogo, K.; Orina, S.; Munguti, J.; Ogello, E. Growth Performance of African Catfish (Clarias Gariepinus) Fed on Diets Containing Black Soldier Fly (Hermetia illucens) Larvae Under Aquaponic System. Aquac. Stud. 2022, 23, AQUAST910. [Google Scholar] [CrossRef]
- Öztürk, R.Ç.; Yandi, I.; Terzi, Y.; Altinok, I. Growth, Health and Fillet Quality of Rainbow Trout (Oncorhynchus Mykiss) Fed Directly with Black Soldier Fly (Hermetia illucens) Prepupae. Turk. J. Fish. Aquat. Sci. 2023, 23, 21683. [Google Scholar] [CrossRef]
- Bartucz, T.; Csókás, E.; Nagy, B.; Gyurcsák, M.P.; Bokor, Z.; Bernáth, G.; Molnár, J.; Urbányi, B.; Csorbai, B. Black Soldier Fly (Hermetia illucens) Meal as Direct Replacement of Complex Fish Feed for Rainbow Trout (Oncorhynchus Mykiss) and African Catfish (Clarias Gariepinus). Life 2023, 13, 1978. [Google Scholar] [CrossRef] [PubMed]
- Siddaiah, G.M.; Kumar, R.; Kumari, R.; Chandan, N.K.; Debbarma, J.; Damle, D.K.; Das, A.; Giri, S.S. Dietary Fishmeal Replacement with Hermetia illucens (Black Soldier Fly, BSF) Larvae Meal Affected Production Performance, Whole Body Composition, Antioxidant Status, and Health of Snakehead (Channa striata) Juveniles. Anim. Feed. Sci. Technol. 2023, 297, 115597. [Google Scholar] [CrossRef]
- Oddon, S.B.; Biasato, I.; Caimi, C.; Belghit, I.; Radhakrishnan, G.; Gasco, L. Batch-to Batch Variation in Nutrient Digestibility of Black Soldier Fly Larvae Meals in Rainbow Trout. J. Insects Food Feed. 2024, 11, 61–72. [Google Scholar] [CrossRef]
- Moutinho, S.; Peres, H.; Martins, N.; Serra, C.; Santos, R.A.; Monroig, Ó.; Oliva-Teles, A. Use of Black Soldier Fly (Hermetia illucens) Larvae Meal in Diets for Gilthead Seabream Juveniles: Effects on Growth-Related Gene Expression, Intermediary Metabolism, Digestive Enzymes, and Gut Microbiota Modulation. Aquaculture 2024, 580, 740357. [Google Scholar] [CrossRef]
- Gangbazo, D.N.S.K.; Adjahouinou, D.C.; Djissou, A.; Godome, T.; Tossavi, E.; Diegane, N.; Fall, J.; Aboh, A.B. Use of Black Soldier Fly (Hermetia illucens) Larvae Meal in the Diet of Clarias Gariepinus (Burchell, 1882) Fingerlings Reared in Controlled Environments. J. Anim. Health Prod. 2025, 13, 632–641. [Google Scholar] [CrossRef]
- Rawski, M.; Mazurkiewicz, J.; Mikołajczak, Z.; Kierończyk, B.; Skrzypczak, P.; Szymkowiak, P.; Józefiak, D. Black Soldier Fly Meal as a Gastrointestinal Tract Microbiota Remodelling Factor: A New Natural and Sustainable Source of Prebiotic Substances for Fish? Aquac. Res. 2025, 2025, 8852384. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Liao, Z.; Chen, X.; Gu, X.; Ye, T.; Pu, H.; Li, W.; Zhu, B.; Zhao, W.; et al. Effects of Butane-Defatted Black Soldier Fly Larvae Meal Replace Dietary Fishmeal on Growth, Antioxidant Capacity and Intestine Health of Rainbow Trout (Oncorhynchus Mykiss). Aquaculture 2025, 607, 742640. [Google Scholar] [CrossRef]
- Maulu, S.; Eynon, B.; Abarra, S.; Rawling, M.; Merrifield, D.L. Black Soldier Fly, Hermetia illucens, Larvae Meal Improves Intestinal Health and Growth Performance of Nile Tilapia, Oreochromis Niloticus, Juveniles. J. World Aquac. Soc. 2025, 56, e70035. [Google Scholar] [CrossRef]
- Chen, Y.; Chi, S.; Zhang, S.; Dong, X.; Yang, Q.; Liu, H.; Tan, B.; Xie, S. Effect of Black Soldier Fly (Hermetia illucens) Larvae Meal on Lipid and Glucose Metabolism of Pacific White Shrimp Litopenaeus vannamei. Br. J. Nutr. 2021, 128, 1674–1688. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.Y.; Gan, Z.R.; Huang, T.Y.; Xiao, Y.; Xu, W.Y.; Li, X.Q.; Leng, X.J. Replacement of Fish Meal with Defatted Black Soldier Fly (Hermetia illucens) in Diet of Pacific White Shrimp (Litopenaeus vannamei): Growth, Flesh Quality and Transcriptome. J. Insects Food Feed. 2024, 11, 873–893. [Google Scholar] [CrossRef]
- Nunes, A.J.P.; Yamamoto, H.; Simões, J.P.; Pisa, J.L.; Miyamoto, N.; Leite, J.S. The Black Soldier Fly (Hermetia illucens) Larvae Meal Can Cost-Effectively Replace Fish Meal in Practical Nursery Diets for Post-Larval Penaeus Vannamei under High-Density Culture. Fishes 2023, 8, 605. [Google Scholar] [CrossRef]
- Alvanou, M.; Kyriakoudi, A.; Makri, V.; Lattos, A.; Feidantsis, K.; Papadopoulos, D.; Georgoulis, I.; Apostolidis, A.; Michaelidis, B.; Mourtzinos, I.; et al. Effects of Dietary Substitution of Fishmeal by Black Soldier Fly (Hermetia illucens) Meal on Growth Performance, Whole-Body Chemical Composition, and Fatty Acid Profile of Pontastacus Leptodactylus Juveniles. Front. Physiol. 2023, 14, 1156394. [Google Scholar] [CrossRef]
- Subchan, W.; Nawangsari, F.; Prihatin, J. The Effect of Flour-Based Feed Black Soldier Fly Larvae on Crayfish (Cherax Quadricarinatus von Martens) Growth. In Proceedings of the 5th International Conference on Life Sciences and Biotechnology (ICOLIB 2023) BIO Web of Conferences, Jember, India, 21 May 2024; Volume 101, p. 01005. [Google Scholar] [CrossRef]
- Yao, W.; Zhang, C.; Mao, H.; Hua, G.; Liu, Q.; Zhao, S.; Shuang, H.; Poolsawat, L.; Yuan, S.; Wang, J.; et al. Effects of Dietary Defatted Black Soldier Fly (Hermetia illucens) Larvae Meal Substituting Fish Meal on Growth, Antioxidative Capacity, Immunity, Intestinal Histology and Microbiota of Juvenile Chinese Mitten Crab (Eriocheir sinensis). Aquac. Rep. 2024, 38, 102302. [Google Scholar] [CrossRef]
- Li, M.; Li, M.; Wang, G.; Liu, C.; Shang, R.; Chen, Y.; Li, L. Defatted Black Soldier Fly (Hermetia illucens) Larvae Meal Can Partially Replace Fish Meal in Diets for Adult Chinese Soft-Shelled Turtles. Aquaculture 2021, 541, 736758. [Google Scholar] [CrossRef]
- Pornsuwan, R.; Pootthachaya, P.; Bunchalee, P.; Hanboonsong, Y.; Cherdthong, A.; Tengjaroenkul, B.; Boonkum, W.; Wongtangtintharn, S. Evaluation of the Physical Characteristics and Chemical Properties of Black Soldier Fly (Hermetia illucens) Larvae as a Potential Protein Source for Poultry Feed. Animals 2023, 13, 2244. [Google Scholar] [CrossRef]
- Dörper, A.; Veldkamp, T.; Dicke, M. Use of Black Soldier Fly and House Fly in Feed to Promote Sustainable Poultry Production. J. Insects Food Feed. 2021, 7, 761–780. [Google Scholar] [CrossRef]
- de Souza Vilela, J.; Andronicos, N.; Kolakshyapati, M.; Hilliar, M.; Sibanda, T.Z.; Andrew, N.; Swick, R.; Wilkinson, S.; Ruhnke, I. Black Soldier Fly Larvae in Broiler Diets Improve Broiler Performance and Modulate the Immune System. Anim. Nutr. 2021, 7, 695–706. [Google Scholar] [CrossRef]
- Stadtlander, T.; Stamer, A.; Buser, A.; Wohlfahrt, J.; Leiber, F.; Sandrock, C. Hermetia illucens Meal as Fish Meal Replacement for Rainbow Trout on Farm. J. Insects Food Feed. 2017, 3, 165–175. [Google Scholar] [CrossRef]
- Sealey, W.; Gaylord, G.; Barrows, F.; Tomberlin, J.; Mcguire, M.; Ross, C.; St-Hilaire, S. Sensory Analysis of Rainbow Trout, Oncorhynchus Mykiss, Fed Enriched Black Soldier Fly Prepupae, Hermetia illucens. J. World Aquac. Soc. 2011, 42, 34–45. [Google Scholar] [CrossRef]
- Turchini, G.M.; Mentasti, T.; Caprino, F.; Panseri, S.; Moretti, V.M.; Valfrè, F. Effects of Dietary Lipid Sources on Flavour Volatile Compounds of Brown Trout (Salmo trutta L.) Fillet. J. Appl. Ichthyol. 2004, 20, 71–75. [Google Scholar] [CrossRef]
- Radhakrishnan, G.; Philip, A.J.P.; Caimi, C.; Lock, E.-J.; Araujo, P.; Liland, N.S.; Rocha, C.; Cunha, L.M.; Gasco, L.; Belghit, I. Evaluating the Fillet Quality and Sensory Characteristics of Atlantic Salmon (Salmo Salar) Fed Black Soldier Fly Larvae Meal for Whole Production Cycle in Sea Cages. Aquac. Rep. 2024, 35, 101966. [Google Scholar] [CrossRef]
- Bruni, L.; Randazzo, B.; Cardinaletti, G.; Zarantoniello, M.; Fabio, M.; Secci, G.; Francesca, T.; Olivotto, I.; Parisi, G. Dietary Inclusion of Full-Fat Hermetia illucens Prepupae Meal in Practical Diets for Rainbow Trout (Oncorhynchus Mykiss): Lipid Metabolism and Fillet Quality Investigations. Aquaculture 2020, 529, 735678. [Google Scholar] [CrossRef]
- Bruni, L.; Belghit, I.; Lock, E.-J.; Secci, G.; Taiti, C.; Parisi, G. Total Replacement of Dietary Fish Meal with Black Soldier Fly (Hermetia illucens) Larvae Does Not Impair Physical, Chemical or Volatile Composition of Farmed Atlantic Salmon (Salmo Salar L.). J. Sci. Food Agric. 2019, 100, 1038–1047. [Google Scholar] [CrossRef]
- Moutinho, S.; Pedrosa, R.; Magalhães, R.; Oliva-Teles, A.; Parisi, G.; Peres, H. Black Soldier Fly (Hermetia illucens) Pre-Pupae Larvae Meal in Diets for European Seabass (Dicentrarchus Labrax) Juveniles: Effects on Liver Oxidative Status and Fillet Quality Traits during Shelf-Life. Aquaculture 2020, 533, 736080. [Google Scholar] [CrossRef]
- Hervé; MK; Calice, M.D.; Dzepe, D.; Djouaka, R.F.; Chia, S.Y.; Efole, T.; Ndindeng, S.A. Black Soldier Fy (Hermetia illucens) Larvae Improve Growth Performance and Fesh Quality of African Catfsh (Clarias Gariepinus). Discov. Anim. 2025, 2, 9. [Google Scholar] [CrossRef]
- Salmon Bussines. 2025. Available online: https://www.salmonbusiness.com/new-study-finds-67-prefer-the-taste-of-salmon-fed-with-insect-based-feed/ (accessed on 6 June 2025).
- Roccatello, R.; Endrizzi, I.; Aprea, E.; Dabbou, S. Insect-Based Feed in Aquaculture: A Consumer Attitudes Study. Aquaculture 2024, 582, 740512. [Google Scholar] [CrossRef]
- Baldi, L.; Mancuso, T.; Peri, M.; Gasco, L.; Trentinaglia, M.T. Consumer Attitude and Acceptance toward Fish Fed with Insects: A Focus on the New Generations. J. Insects Food Feed. 2022, 8, 1249–1264. [Google Scholar] [CrossRef]
- Bazoche, P.; Poret, S. Acceptability of Insects in Animal Feed: A Survey of French Consumers. J. Consum. Behav. 2021, 20, 251–270. [Google Scholar] [CrossRef]
- Popoff, M.; MacLeod, M.; Leschen, W. Attitudes towards the Use of Insect-Derived Materials in Scottish Salmon Feeds. J. Insects Food Feed. 2017, 3, 131–138. [Google Scholar] [CrossRef]
- Rawski, M.; Mazurkiewicz, J.; Kierończyk, B.; Józefiak, D. Black Soldier Fly Full-Fat Larvae Meal Is More Profitable Than Fish Meal and Fish Oil in Siberian Sturgeon Farming: The Effects on Aquaculture Sustainability, Economy and Fish GIT Development. Animals 2021, 11, 604. [Google Scholar] [CrossRef] [PubMed]
- Limbu, S.M.; Shoko, A.P.; Ulotu, E.E.; Luvanga, S.A.; Munyi, F.M.; John, J.O.; Opiyo, M.A. Black Soldier Fly (Hermetia illucens L.) Larvae Meal Improves Growth Performance, Feed Efficiency and Economic Returns of Nile Tilapia (Oreochromis Niloticus L.) Fry. Aquac. Fish Fish. 2022, 2, 167–178. [Google Scholar] [CrossRef]
- Ouko, K.O. Socio-Economic Efficiency of Black Soldier Fly (Hermetia illucens L.) Larvae Meal for Aquaculture Production. Ph.D. Thesis, Jaramogi Oginga Odinga University of Science and Technology, Bondo Town, Kenya, 2023. Available online: https://agris.fao.org/search/en/providers/124791/records/676540f550e69ae818389e53 (accessed on 1 July 2025).
- Auzins, A.; Leimane, I.; Reissaar, R.; Brobakk, J.; Sakelaite, I.; Grivins, M.; Zihare, L. Assessing the Socio-Economic Benefits and Costs of Insect Meal as a Fishmeal Substitute in Livestock and Aquaculture. Animals 2024, 14, 1461. [Google Scholar] [CrossRef]
- Maroušek, J.; Strunecký, O.; Maroušková, A. Insect Rearing on Biowaste Represents a Competitive Advantage for Fish Farming. Rev. Aquac. 2023, 15, 965–975. [Google Scholar] [CrossRef]
- Ramzy, R.R.; Goenka, V.; El-Dakar, M.A.; Lee, J.S.H. Assessing the Environmental Impacts of the Black Soldier Fly-Based Circular Economy and Decentralized System in Singapore: A Case Study. Sustainability 2025, 17, 6115. [Google Scholar] [CrossRef]
- Vala, R.B.; Borichangar, R.V.; Solanki, H.G.; Patel, M.R.; Parmar, A.M.; Varma, A. Insect Meal: An Emerging Eco-Friendly Approach for Sustainable Aquaculture Feed Industry. J. Exp. Zool. India 2024, 27, 1549–1559. [Google Scholar] [CrossRef]
- Eide, L.H.; Rocha, S.D.C.; Morales-Lange, B.; Kuiper, R.V.; Dale, O.B.; Djordjevic, B.; Hooft, J.M.; Øverland, M. Black Soldier Fly Larvae (Hermetia illucens) Meal Is a Viable Protein Source for Atlantic Salmon (Salmo Salar) during a Large-Scale Controlled Field Trial under Commercial-like Conditions. Aquaculture 2024, 579, 740194. [Google Scholar] [CrossRef]
- Cho, J.-H.; Bae, J.; Hwang, I.J. Effects of Black Soldier Fly (Hermetia illucens) Pre-Pupae Meal on the Growth, Stress, and Immune Responses of Juvenile Rainbow Trout (Oncorhynchus Mykiss) Reared at Different Stocking Densities. Aquac. Rep. 2022, 25, 101202. [Google Scholar] [CrossRef]
- Nekrasov, R.; Ivanov, G.; Chabaev, M.G.; Zelenchenkova, A.A.; Bogolyubova, N.; Nikanova, D.; Sermyagin, A.; Bibikov, S.; Shapovalov, S. Effect of Black Soldier Fly (Hermetia illucens L.) Fat on Health and Productivity Performance of Dairy Cows. Animals 2022, 12, 2118. [Google Scholar] [CrossRef]
- Huseynli, L.; Parviainen, T.; Kyllönen, T.; Aisala, H.; Vene, K. Exploring the Protein Content and Odor-Active Compounds of Black Soldier Fly Larvae for Future Food Applications. Future Foods 2023, 7, 100224. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Harahap, I.A.; Osei-Owusu, J.; Saikia, T.; Wu, Y.S.; Fernando, I.; Perestrelo, R.; Câmara, J.S. Bioconversion of Organic Waste by Insects—A Comprehensive Review. Process Saf. Environ. Prot. 2024, 187, 1–25. [Google Scholar] [CrossRef]
- Rushikesh, J.; Milesh, L.; Twinkle, M.; Madhu, R.; Vendra, R.; Ruti, M.; Haldar, S.; Kumar, K.; Anjlina, D. Black Soldier Fly/Larvae: A Weapon for Solid Waste Management and Alternative Feed for Poultry and Aquatic Industries. Int. J. Pharma Bio Sci. 2020, 10, 113–120. [Google Scholar] [CrossRef]
- Vauterin, A.; Steiner, B.; Sillman, J.; Kahiluoto, H. The Potential of Insect Protein to Reduce Food-Based Carbon Footprints in Europe: The Case of Broiler Meat Production. J. Clean. Prod. 2021, 320, 128799. [Google Scholar] [CrossRef]
- Invertebrate Welfare—April 2020. Available online: https://www.invertebratewelfare.org/newsletter/2020-04 (accessed on 5 June 2025).
- López-Gámez, G.; del Pino-García, R.; López-Bascón, M.A.; Verardo, V. From Feed to Functionality: Unravelling the Nutritional Composition and Techno-Functional Properties of Insect-Based Ingredients. Food Res. Int. 2024, 178, 113985. [Google Scholar] [CrossRef]
- Lee, C.G.; Silva, C.A.D.; Lee, J.-Y.; Hartl, D.; Elias, J.A. Chitin Regulation of Immune Responses: An Old Molecule with New Roles. Curr. Opin. Immunol. 2008, 20, 684–689. [Google Scholar] [CrossRef]
- Caligiani, A.; Marseglia, A.; Leni, G.; Baldassarre, S.; Maistrello, L.; Dossena, A.; Sforza, S. Composition of Black Soldier Fly Prepupae and Systematic Approaches for Extraction and Fractionation of Proteins, Lipids and Chitin. Food Res. Int. 2017, 105, 812–820. [Google Scholar] [CrossRef]
- Vargas, A.; Randazzo, B.; Riolo, P.; Truzzi, C.; Gioacchini, G.; Giorgini, E.; Loreto, N.; Ruschioni, S.; Zarantoniello, M.; Antonucci, M.; et al. Rearing Zebrafish on Black Soldier Fly (Hermetia illucens): Biometric, Histological, Spectroscopic, Biochemical, and Molecular Implications. Zebrafish 2018, 15, 404–419. [Google Scholar] [CrossRef]
- Zarantoniello, M.; Randazzo, B.; Truzzi, C.; Giorgini, E.; Marcellucci, C.; Vargas, A.; Zimbelli, A.; Annibaldi, A.; Parisi, G.; Francesca, T.; et al. A Six-Months Study on Black Soldier Fly (Hermetia illucens) Based Diets in Zebrafish. Sci. Rep. 2019, 9, 8598. [Google Scholar] [CrossRef]
- Tang, C.; Yang, D.; Liao, H.; Sun, H.; Liu, C.; Wei, L.; Li, F. Edible Insects as a Food Source: A Review. Food Prod. Process. Nutr. 2019, 1, 8. [Google Scholar] [CrossRef]
- Lalander, C.; Lopes, I. Advances in Substrate Source Composition for Rearing Black Soldier Fly Larvae as a Protein Source. In Burleigh Dodds Series in Agricultural Science; Burleigh Dodds Science Publishing: Cambridge, UK, 2024; ISBN 978-1-80146-584-7. [Google Scholar]
- Niassy, S.; Ekesi, S.; Hendriks, S.; Haller-Barker, A. Legislation for the Use of Insects as Food and Feed in the South African Context. In Edible Insects in Sustainable Food Systems; Springer Nature: Cham, Switzerland, 2018; pp. 457–470. ISBN 978-3-319-74010-2. [Google Scholar]
| Parameter | Fishmeal | BSFL | ||||
|---|---|---|---|---|---|---|
| Dry matter (%) | 92.10 | 91.70 | 94.10 | - | - | 93.19 |
| Protein content (%) | 65.20 | 37.70 | 48.60 | 42.10 | 39.38–48.20 | 52.46 |
| Fat content (%) | 9.20 | 32.60 | 32.00 | 26.00 | 25.69–38.36 | 9.29 |
| Ash content (%) | 16.80 | 10.70 | 58.50 | - | 7.26–8.27 | 7.80 |
| NDF (%) | 5.30 | 16 | 181.10 | - | - | - |
| ADF (%) | 0.50 | 8.40 | 92.50 | - | - | 22.10 |
| Gross energy (kcal/kg) | 4.510 | 5.660 | - | - | - | - |
| Gross energy (MJ/kg) | 18.90 | 23.70 | 26.60 | - | - | 22.76 |
| Calcium (g/kg) | 41.30 | 30.30 | 6.80 | 7.56 | 13.00–21.17 | 3.64 |
| Iron (mg/kg) | 352 | 375 | 1600 | - | - | - |
| Sodium (g/kg) | 10.57 | 0.91 | 1.20 | - | 3.38–5.02 | - |
| Zinc (mg/kg) | 99 | 93 | 154.0 | - | 300–1200 | - |
| Phosphorus (g/kg) | 26.40 | 6.60 | 9.30 | 9 | 6.00–9.10 | 10.1 |
| Magnesium (g/kg) | 2.20 | 2.90 | 3.30 | - | 2.50–3.31 | - |
| References | [53] | [54] | [55] | [56] | [57] | [58] |
| Types of Substrates | References |
|---|---|
| Degassed sludge and chicken feed | [60] |
| Pineapple waste, jackfruit waste, rumen content, fish offal, and mixed substrates | [61] |
| Market waste: fruits, vegetables, meat, and fish in decomposition; hotel waste: cooked foods and vegetable and non-vegetable wastes | [62] |
| Animal waste (slaughterhouse remains, fish, mussels, butcher waste, etc.), fodder waste (wheat bran, soy flour, cornmeal, dog food, old bread, alfalfa, etc.), fermentation products (by-products from winemaking, beer waste, and tofu yeast), food waste (waste from markets, canteens, hotels, municipal organic waste, brown algae, etc.), fruits, garden waste, vegetables, and mixed waste | [63] |
| Soybeans | [64] |
| Spent mushroom substrate | [65] |
| Rice bran, fruit waste (papaya and bananas), vegetable waste (mustard leaves and watercress), tofu by-products, liquid palm sugar, and sago (Putak flour) | [66] |
| Aquaculture solid waste | [67] |
| Fresh soybean curd residue and coconut endosperm | [68] |
| Sesbania grandiflora and Moringa oleifera leaves and agro-industrial by-products, including soybean waste, wheat pollard, rice bran, and milk-extracted coconut meat | [69] |
| Parameter | Fishmeal | BSFL | ||||
|---|---|---|---|---|---|---|
| Arginine (%) | 5.70 | 18.70 | 19.90 | 22.00 | 1.94 | 1.80–2.55 |
| Histidine (%) | 2.41 | 11.70 | 13.80 | 13.40 | 1.32 | 2.08–2.77 |
| Isoleucine (%) | 4.74 | 15.8 | 19.10 | 19.30 | 1.57 | 1.76–2.40 |
| Leucine (%) | 7.74 | 26.30 | 30.60 | 30.00 | 2.59 | 2.67–3.62 |
| Lysine (%) | 7.91 | 21.80 | 23.00 | 27.70 | 2.22 | 2.44–3.60 |
| Methionine (%) | 3.02 | 6.80 | 7.10 | 7.60 | 0.58 | 0.61–1.07 |
| Cysteine (%) | 0.94 | 2.40 | 2.20 | 3.30 | 0.28 | 0.12–0.16 |
| Phenylalanine (%) | 4.12 | 15.30 | 16.40 | 17.10 | 1.51 | 1.35–2.11 |
| Tyrosine (%) | 3.33 | 21.40 | - | 26.50 | 2.30 | 1.71–3.09 |
| Threonine (%) | 4.37 | 14.50 | 16.20 | 18.40 | 1.42 | 1.42–1.94 |
| Tryptophan (%) | 1.18 | 5.40 | 5.40 | - | 0.53 | - |
| Valine (%) | 5.43 | 22.6 | 28.20 | 29.40 | 2.25 | 2.29–3.09 |
| References | [53] | [54] | [42] | [55] | [78] | [57] |
| Parameter | Fishmeal | BSFL | ||||
|---|---|---|---|---|---|---|
| C8:0 caprylic acid (%) | - | - | 0.003 | - | - | - |
| C10:0 capric acid (%) | - | - | 0.201 | 0.70 | 0.86 | 0.44–0.85 |
| C11:0 undecanoic acid (%) | - | - | 0.004 | - | - | - |
| C12:0 lauric acid (%) | 0.09 | 122 | 8.567 | 14.10 | 45.97 | 17.89–37.18 |
| C13:0 tridecanoic acid (%) | - | 0.009 | - | - | - | |
| C14:0 myristic acid (%) | 4.10 | 18.10 | 2.488 | 1.90 | 8.70 | 5.21–11.77 |
| C14:1 myristoleic acid (%) | - | - | 0.042 | - | - | - |
| C15:0 pentadecanoic acid (%) | - | - | 0.048 | - | 0.15 | - |
| C15:1 pentadecenoic acid (%) | - | - | 0.009 | - | - | - |
| C16:0 palmitic acid (%) | 12 | 32.80 | 8.870 | 5.30 | 12.21 | 20.65–24.59 |
| C16:1 palmitoleic acid (%) | 4.80 | 11.80 | 0.924 | 1.10 | 1.91 | 1.75–2.67 |
| C17:0 heptadecanoic acid (%) | - | - | 0.053 | - | 0.20 | - |
| C17:1 heptadecanoic acid (%) | - | - | 0.032 | - | 0.20 | - |
| C18:0 stearic acid (%) | 2.40 | 6 | 1.194 | 0.90 | 2.53 | 2.95–4.42 |
| C18:1 oleic acid (%) | 8.20 | 32.30 | 8.869 | 7.30 | 11.24 | 9.28–15.35 |
| C18:2 linoleic acid (%) | 1.40 | 19.70 | 3.815 | 2.70 | 14.07 | 4.71–24.08 |
| C18:3 linolenic acid (%) | 1.20 | 2.80 | 0.400 | 0.20 | 1.65 | 0.32–1.99 |
| C18:4 stearidonic acid (%) | 1 | 0.80 | - | - | - | - |
| C20:0 arachidic acid (%) | 0.20 | 0 | 0.041 | 0.0 | 0.10 | - |
| C20:1 eicosenoic acid (%) | 4.50 | 0 | 0.034 | - | 0.06 | ND–0.46 |
| C20:3 eicosatrienoic acid (%) | - | - | 0.008 | - | - | ND–0.38 |
| C20:4 arachidonic acid (%) | 1.60 | 0 | 0.118 | - | 0.14 | - |
| C20:5 eicosapentaenoic acid (%) | 6 | 1.30 | 0.079 | 0.30 | - | - |
| C22:0 behenic acid (%) | 0.20 | 0 | - | - | - | ND–0.26 |
| C22:1 erucic acid (%) | 5.20 | 0 | - | - | - | - |
| C22:2 docosadienoic acid (%) | - | 0.20 | 0.081 | - | - | - |
| C22:5 docosapentaenoic acid (%) | 1.80 | - | - | - | - | - |
| C22:6 docosahexaenoic acid (%) | 4.40 | 0.60 | - | - | - | - |
| C24:0 lignoceric acid (%) | 0 | 0 | - | - | - | - |
| References | [53] | [54] | [47] | [78] | [100] | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dîrvariu, L.; Barbacariu, C.-A.; Burducea, M.; Simeanu, D. Feed Sources for Sustainable Aquaculture: Black Soldier Fly Larvae (BSFL). Fishes 2025, 10, 464. https://doi.org/10.3390/fishes10090464
Dîrvariu L, Barbacariu C-A, Burducea M, Simeanu D. Feed Sources for Sustainable Aquaculture: Black Soldier Fly Larvae (BSFL). Fishes. 2025; 10(9):464. https://doi.org/10.3390/fishes10090464
Chicago/Turabian StyleDîrvariu, Lenuța, Cristian-Alin Barbacariu, Marian Burducea, and Daniel Simeanu. 2025. "Feed Sources for Sustainable Aquaculture: Black Soldier Fly Larvae (BSFL)" Fishes 10, no. 9: 464. https://doi.org/10.3390/fishes10090464
APA StyleDîrvariu, L., Barbacariu, C.-A., Burducea, M., & Simeanu, D. (2025). Feed Sources for Sustainable Aquaculture: Black Soldier Fly Larvae (BSFL). Fishes, 10(9), 464. https://doi.org/10.3390/fishes10090464








