Abstract
In this paper, we use molecular phylogenetics, micro-CT scanning, and morphological analyses to describe a new species of goby, Pascua marecoralliensis, and demonstrate that the genus Pascua is distinct from Hetereleotris, as supported by five diagnostic characters, including modified basicaudal scales and reduced sensory papillae patterns. Phylogenetic analysis places Pascua as sister to the Gobiodon group, while Hetereleotris forms a separate clade. The new species, P. marecoralliensis, differs from congeners in fin ray counts, cephalic pore patterns, and head morphology and exhibits unique live colouration. Additionally, we reclassify Hetereleotris readerae and H. sticta as Pascua readerae and P. sticta based on shared genus-specific traits. The distribution of Pascua spans the southern Pacific, suggesting a relict lineage or undiscovered diversity in the genus. This work underscores the importance of integrative taxonomic approaches for resolving cryptic diversity in gobioid fishes and highlights the need for further sampling in understudied regions.
Keywords:
coral reef; cryptic; cryptobenthic fishes; Gobioidei; Hetereleotris; morphology; osteology; phylogeny; tropical; Australia Key Contribution:
This study describes a new species of Pascua goby from Australia and demonstrates the validity of the genus Pascua (Gobiidae), which is distributed across the southern Pacific.
1. Introduction
The family Gobiidae is among the most speciose of fish families [1,2], and the rate of new species descriptions remains exceptionally high. Over the last decade (2016–2025), a new goby species has been described, on average, every sixteen days, and more species have been described from the Gobiidae than any other actinopterygian family except, perhaps, the Cyprinidae [2].
The phylogenetic relationships and taxonomy of genera within the Gobiidae are becoming increasingly well-resolved, and several problematic groups have recently undergone taxonomic reviews or revisions (e.g., Callogobius [3] and Glossogobius [4]). However, many more groups, such as Pascua, remain poorly understood. The genus was erected by Randall, 2005 [5], with the description of Pascua caudilinea [5,6], but the validity of the genus was immediately questioned by Hoese and Larson, 2005 [7].
Hoese and Larson [7] described two new species with similar traits to P. caudilinea but placed them in the genus Hetereleotris (H. sticta [8] and H. readerae [9]) and proposed that Pascua should be synonymised with Hetereleotris due to an apparent lack of diagnostic characters and several ambiguities in the description of the type species. Randall [10] then defended the validity of Pascua, highlighting five characters first identified by Hoese and Larson [7] that support the monophyly of P. caudilinea, P. sticta and P. readerae (Table 1). While these authors disagreed on the validity of Pascua, no formal nomenclatural acts were undertaken beyond the original species descriptions [6,8,9].
Regardless of their formal status, various authors and databases have considered the aforementioned species in different ways. Some recognise all three species as Pascua [11], while other authors comment on the problematic status of Pascua but provisionally recognise all species [12,13]. Some consider all species to be “Pascua-type Hetereleotris” [14], whereas others accept P. caudilinea and P. sticta but recognise H. readerae [15]. Others refrain from commenting on Pascua but note that Hetereleotris is probably not monophyletic [16,17]. This lack of clarity has led to a call for phylogenetic analysis of the genera Pascua and Hetereleotris [12].

Table 1.
The five characters that, in combination, distinguish Pascua from Hetereleotris [7,10,12]. Data from specimens, Kovacic, M. pers. comm., and [12,18,19].
Table 1.
The five characters that, in combination, distinguish Pascua from Hetereleotris [7,10,12]. Data from specimens, Kovacic, M. pers. comm., and [12,18,19].
| Character | Condition in Pascua | Condition in Hetereleotris (incl. Cerogobius) |
|---|---|---|
| Basicaudal scales | Two modified basicaudal scales on each side of the fish. On the dorsal and ventral margins of the basicaudal, these scales have enlarged posterior fields and a single row of extremely long ctenii extending over the caudal fin base. | Scales on basicaudal, where present, lack elongate ctenii. H. aurantiaca has three scales with slightly elongate ctenii along the base of the caudal fin. |
| Male urogenital papillae | Flattened and elongate, similar to those found in some species of Eviota. | Details unclear. Some may be short and rounded, similar in form to female; others longer and triangular. |
| Sensory papillae on cheek | Reduced pattern of sensory papillae on the cheek. Transverse suborbital rows 2 and 3 consist of a single papilla; rows 1 and 4 with five or fewer papillae. | Transverse suborbital papilla rows 2 and 3 consist of two or more papillae; row 1 usually with five or more papillae, row 4 often with nine or more papillae. |
| Posterior nares | Simple pores or with slightly elevated anterior margins. | Usually with an elevated rim or tube. |
| Mandibular papillae | Two papillae arranged mediolaterally immediately behind the mental frenum/ridge. Two parallel rows (e and i) of papillae following ventral margin of mandible. | Usually lacking papillae behind mental frenum. Two parallel rows (e and i) of papillae following ventral margin of mandible start on lateral margins of mental frenum. |
During the 2019 and 2020 “Coral Reef Health in the Coral Sea Marine Park” project [20], enclosed clove oil samples were undertaken to sample small cryptobenthic fish communities [21] on sixteen reefs spanning approximately 1400 km of the Coral Sea Marine Park. Although geographically extensive, the number of samples that could be collected on these trips (n = 47) amounted to a total reef area of just 188 m2. While the sampling covered an area smaller than a tennis court, the specimens collected have already yielded a new gobioid genus, Tempestichthys Goatley and Tornabene, 2022, the first tropical thalasseleotridid [22], as well as several other new species of gobies awaiting description. Included in this sampling was another unusual new species of goby that resembles other Pascua spp.
In addition, specimens of P. caudilinea were recently collected from Rapa Nui (Isla de Pascua), the type locality, providing the first genetic sample for the genus. With this new material, we aim to clarify the taxonomy of the genus Pascua and describe the new species from the Coral Sea using an integrative taxonomic approach that combines morphological characters, multivariate analyses, and molecular phylogenetics. Specifically, we (i) test whether the new species and P. caudilinea are sister species; (ii) assess whether they are closely related to (or nested within) Hetereleotris or instead are a distinct clade; and (iii) assess whether the molecular phylogenetic relationships are also supported by phenotypic characters. Our results ultimately provide evidence to support the validity of the previously disputed genus, Pascua Randall, 2005.
2. Materials and Methods
2.1. Specimen Collection
All specimens of the new species were collected by the first author, Dr. Penny Berents, and Dr. Renato Morais Araujo from the northern side of Lorna Cay on Lihou Reef in the Australian Coral Sea (17.12527° S, 151.82535° E) on 5 March 2020. The three specimens were collected from a single 4 m2 enclosed clove oil station, following [22].
2.2. Molecular Phylogenetics
We extracted DNA from a combination of fin and muscle tissue of a single specimen (smaller specimen in I.49536-038) of the new species using a Qiagen® DNEasy Blood and Tissue kit. Given that only three specimens of the new species exist, we chose to keep the remaining two specimens intact and not tissue sample them. Standard spin column protocols were followed, except the elution stage was modified, using triplicate rinses of 50 μL of AE buffer with a 1 min incubation before each centrifuging.
DNA extraction from two ethanol-preserved specimens of Pascua caudilinea from Rapa Nui (Isla de Pascua) stored at the Sala de Colecciones Biológicas, Facultad de Ciencias del Mar, Universidad Católica del Norte, Coquimbo, Chile (SCBUCN-8348 and SCBUCN-8455) was conducted using the Qiagen® DNEasy Blood and Tissue kit following the standard protocol.
We attempted to sequence segments of six genes (Table 2; [23,24]). PCR reactions and cycling conditions followed the protocol described in Goatley and Tornabene [22]. PCR products underwent Sanger sequencing at Molecular Cloning Laboratories (MCLAB), San Francisco, California and Macrogen, Santiago, Chile.
Sequence data were manually trimmed by removing low-quality bases at ends of raw reads and were aligned using Geneious Prime 2023 to create consensus sequences. Consensus sequences for RAG1, sreb2, and zic1 were concatenated and aligned with the five-gene (these three genes plus cytb and tRNAval) data set covering all families in the suborder Gobioidei [17,22,23,25]. In total, the data included 238 specimens from 229 species. New sequences generated in this study were deposited on GenBank (accession numbers PX240974–PX240980). Summary statistics for the alignment can be found in Supplemental Table S1. In order to investigate the phylogenetic placement of the new species and P. caudlinea within the Gobiidae, all major gobiid lineages (sensu [23]) were included, except Kraemeria, which was excluded as it led to instabilities in the phylogenetic reconstruction. Outgroups for the study follow [23] and include the gobioid families Rhyacichthyidae, Eleotridae, Odontobutidae, Butidae, Thalasseleotrididae, and Oxudercidae.
Data were analysed using Bayesian phylogenetic inference in the program MrBayes 3.2 [26] using the BEAGLE library [27]. The partitioning scheme and substitution model choice followed [23], which was determined using Bayesian Information Criterion. The analysis consisted of two parallel Markov Chain Monte Carlo (MCMC) runs, each with four chains, run for 40 × 106 generations, sampled every 1000 generations. Analyses were conducted through the NIH and NSF-funded CIPRES Science Gateway [28]. To determine model convergence, mixing, and appropriate burn-in values, we assessed MCMC logs using Tracer 1.7 [29].

Table 2.
Targeted gene regions and primers used for sequencing Pascua spp. * indicates sequences used to create phylogeny, † indicates PCR amplification failed with these primers and/or settings.
Table 2.
Targeted gene regions and primers used for sequencing Pascua spp. * indicates sequences used to create phylogeny, † indicates PCR amplification failed with these primers and/or settings.
| Gene Region | Primer Name | Primer Sequence (5′ to 3′) | Primer Design |
|---|---|---|---|
| RAG1 * | RAG1F1 | CTGAGCTGCAGTCAGTACCATAAGATGT | [30] |
| RAG1Ra | CGGGCRTAGTTCCCRTTCATCCTCAT | [31] | |
| sreb2 * | sreb2_F10 | ATGGCGAACTAYAGCCATGC | [32] |
| sreb2_R1094 | CTGGATTTTCTGCAGTASAGGAG | [32] | |
| zic1 * | zic1_F9 | GGACGCAGGACCGCARTAYC | [32] |
| zic1_R967 | CTGTGTGTGTCCTTTTGTGRATYTT | [32] | |
| Ptr | PtrF2 | TCGTTCATGGGATGTTTACAAAT | [33] |
| PtrR2 | GGATGAGCCAGAAGTTCCCCAGAG | [33] | |
| COI † | Fish F1 | TCAACCAACCACAAAGACATTGGCAC | [34] |
| Fish R1 | ACTTCAGGGTGACCGAAGAATCAGA | [34] | |
| cytb † | FishcytB-F | ACCACCGTTGTTATTCAACTACAAGAAC | [35] |
| TruccytB-R | CCGACTTCCGGATTACAAGACCG | [35] |
2.3. Micro-CT Scanning and Segmentation
We scanned the holotype and one paratype of the new species using the Friday Harbor Laboratories, Karel F. Liem Imaging Facility, Bruker SkyScan 1173 micro-CT scanner (Billerica, MA, USA), following the techniques described in [22]. The stacked scan (3579 slices) was made with voxel (i.e., 3D pixel) dimensions of 6 μm and a rotation step of 0.3° between images. The X-ray source voltage was 60 kV, and the current was 133 μA, with a 1 mm aluminium filter. CT scans were reconstructed using Bruker NRecon.
Specimens of the three putative Pascua species (2× P. caudilinea, 2× P. sticta and 1× P. readerae; hereafter referred to as belonging in Pascua) from the Australian Museum were scanned at the University of New England, Armidale, using a GE Phoenix V|tome|x S industrial micro-CT scanner (Boston, MA, USA). These scans were used for morphometric and meristic comparisons.
The models were segmented and visualised in the open-source software package 3D Slicer 5.8. To facilitate loading and modifying the high-resolution CT-scan data, we used the ImageStacks function of the SlicerMorph extension [36]. Scan data for the new species were accessioned on Morphosource.org (Table S2).
2.4. Morphology and Morphometrics
Meristic analyses and measurements of specimens were made from calibrated micrographs and micro-CT scans following [22]. The dorsal pterygiophore formula follows that of [37]. Definitions of all other morphological and meristic characters follow [38,39], using the standard uppercase Roman numerals to denote spines and Arabic numerals to indicate the number of segmented rays of the dorsal, anal, and pelvic fins.
Two multivariate analyses were conducted to compare the general body plans of Hetereleotris and Pascua spp. To support the validity of Pascua, the first analysis compared the body shape of Hetereleotris spp. with P. caudilinea, P. sticta and P. readerae. To support the placement of the new species within Pascua, the second analysis included specimens of the new species. Twenty-four images were compiled from online databases, published taxonomic works and museum collections (17 Hetereleotris images from 14 spp., 7 Pascua images including P. caudilinea, P. sticta and P. readerae). Images of all specimens of the new species were included in the second analysis. Images were selected if they showed a clear, direct lateral view of an intact specimen. While the level of replication in the multivariate analysis is limited, it represents all available images of Pascua and Hetereleotris and provides an opportunity to compare broad patterns in body plans among the genera.
From each image, 22 measurements were taken using the line properties tool of Adobe Illustrator 2025. Measurements were selected to capture broad differences in the shapes of the head, body and fins of the fishes (Figure S1; Table S3). All measurements were standardised against the standard length of the specimens and tested for allometric relationships following [40,41]; no relationships between body and trait sizes were revealed. Data were square root transformed and standardised across variables, then visualised using a threshold metric multidimensional scaling ordination (tmMDS) based on a Euclidean distance matrix. Threshold mMDS plots are useful where the relationship between distances in the two-dimensional mMDS and the Euclidean distance matrix does not pass through the origin in the Shepard plot. By removing the requirement for a zero-intercept, the tmMDS results in lower stress and a more accurate representation of relationships in the two-dimensional ordination [42]. The relative importance of each variable in shaping the morphospace was calculated using Pearson correlations, and those with correlations > 0.6 were plotted as vectors alongside the ordinations. Morphological differences among the groups were assessed using permutational multivariate analyses of variation (PERMANOVA) and subsequent pairwise tests where applicable.
3. Results
3.1. Molecular Phylogeny
The phylogenetic analysis (Figure 1 and Figure S2) strongly supports the placement of Pascua marecoralliensis sp. nov. alongside P. caudilinea as a well-supported sister group to the coral gobies in the Gobiodon group of [23]: Bryaninops, Eviota, and Gobiodon. The morphologically similar genus Hetereleotris (including Cerogobius) forms a separate, well-resolved monophyletic group sister to the Gobius group.
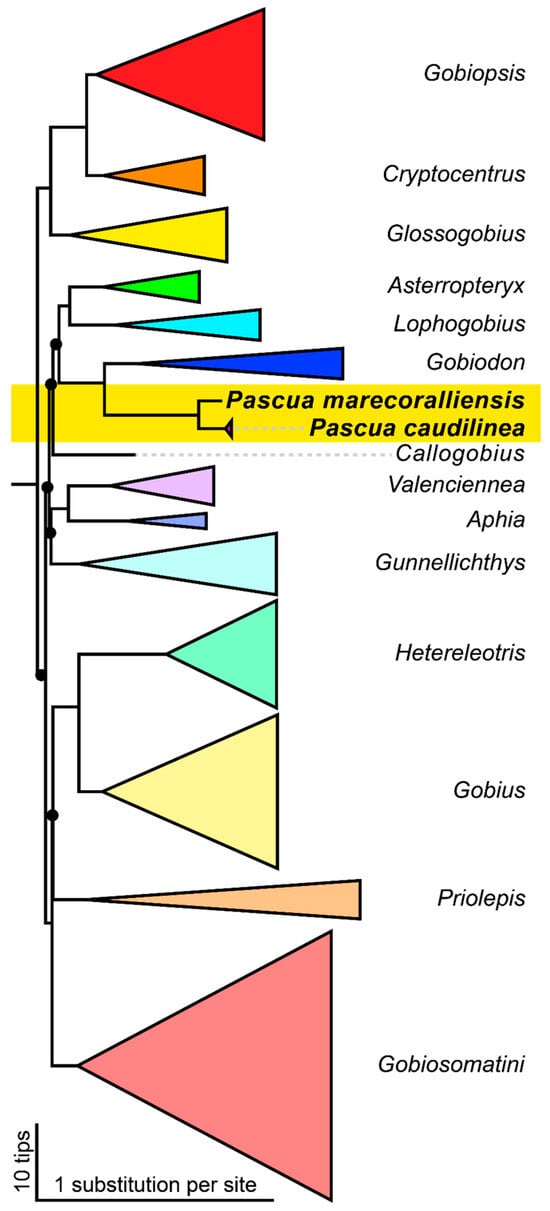
Figure 1.
Bayesian inference molecular phylogeny of Gobiidae inferred from up to 5704 bp of combined nuclear genes and mtDNA [17,23,25]. Branches of groups collapsed [23] to illustrate the relationships of Pascua marecoralliensis sp. nov. Heights of triangles represent number of collapsed tips. Bayesian posterior probability for all unannotated nodes = 1 ± 0 standard deviation, filled circles indicate nodes with probabilities < 1; see expanded phylogeny (Figure S2) for further information.
3.2. Taxonomy
3.2.1. Validation of the Genus Pascua Randall, 2005
Type species: Pascua caudilinea Randall, 2005
- Material Examined: Pascua caudilinea all from Rapa Nui (Isla de Pascua): AMS I. 17452-001, 25 mm SL; AMS I. 43246-001 24 mm SL; Pascua sticta: AMS I. 43612-001 (Paratypes), 2 (21–26.5 mm SL), Rapa Iti; Hetereleotris readerae: AMS I. 27149-048 (Holotype), 21 mm SL male, Elizabeth Reef; AMS I.27149–036 (Paratypes), 2 (20–21 mm SL), Elizabeth Reef; AMS I.27138–053 (Paratypes), 2 (19–24 mm SL), Middleton Reef; AMS I. 27139-030 (Paratype) 21 mm SL, Middleton Reef.
- Diagnosis: A gobiid with the first gill slit fully closed by a membrane. Dorsal rays VII, 7–9, dorsal pterygiophore formula 3-22110; two anal fin pterygiophores inserted anterior to first haemal spine; anal rays I, 7–9; pectoral rays 15–22, no rays free from membrane; pelvic fins I,5, fifth ray shorter and unbranched, widely separated at base and lacking an anterior frenum, distance between inner rays about equal to base of either fin; caudal fin rounded, with 17 segmented rays. Body with 22~29 scales in longitudinal series, ctenoid except a few scales above base of pectoral fin and on abdomen where cycloid; upper and lower basicaudal scales with enlarged posterior field and extremely long ctenii projecting over caudal fin; head, nape, prepectoral area, and chest naked. Vertebrae l0 + 17 [37]. Sensory papillae of cheek greatly reduced; second and third transverse rows reduced to single papillae, row d reduced to 2 papillae anterior of the ventral end of row 4 and row b consisting of 2 or 3 papillae extending forwards from the preopercular margin; two papillae behind mental frenum/ridge. Cephalic sensory pore system B’, C, D, E, F, G, H’ [43]; pores N’, O’ present in some species. Posterior nostril not tubular. Urogenital papillae elongated and flattened in males.
Remarks: Our phylogenetic analysis includes the first molecular data from Pascua caudilinea, the type species of the genus, and shows an obvious separation between Pascua and Hetereleotris. While the molecular data clearly separate the genera, there are a remarkable number of morphological similarities, which have contributed to the questionable validity of Pascua.
In his revision of Hetereleotris, Hoese [18] identified 11 characters, none of which are unique to Hetereleotris, but when considered in combination, define the genus:
- First gill slit closed by a membrane from the gill cover (present in some but not all Hetereleotris; partially closed in some Eviota, Tomiyamichthys, Callogobius species).
- Vertebrae 10 + 17, including urostyle (widely distributed among gobiids).
- First dorsal fin with 6 spines (widely distributed among gobiids).
- First dorsal pterygiophore insertion formula 3-22110 (widely distributed and common among gobiids).
- First dorsal fin usually connected to base of spine of second dorsal fin (present in some but not all Hetereleotris; extremely rare in gobiids).
- 17 segmented caudal fin rays, usually 15 branched (widely distributed among gobiids).
- Characteristic transverse papilla pattern (widely distributed among gobiids).
- Single-lobed mental frenum followed by two parallel rows of papillae (widely distributed among gobiids).
- Metapterygoid slender lacking ventral process extending across quadrate (not broadly surveyed across gobiids, extent of distribution unknown).
- Prominent median process on preoperculum reaching to upper part of symplectic (not broadly surveyed across gobiids, extent of distribution unknown)
- Lower hypural plate not fused with terminal vertebral element or upper hypural plate (widely distributed among gobiids).
With the possible exception of trait 11, which is poorly represented on the micro-CT scans used in this study (Table S2), all of these traits are present in Pascua [7] and many other gobiid genera. Recent descriptions of Hetereleotris spp. [16,17] have highlighted that the only unambiguous synapomorphy of Hetereleotris is the closure of the first gill slit. With Pascua possessing this character, and at least partially closed first gill slits in some Eviota [44], Tomiyamichthys [45,46] and Callogobius [18], Hetereleotris (like many of the >200 genera of Gobiidae) likely lacks a single morphological synapomorphy that hasn’t evolved independently in other lineages, further highlighting the need for a revision of this genus [7,10].
While Hetereleotris may be defined by a combination of widely distributed characters, the combination of five characters identified by Hoese [18], Randall [10] and Shibukawa [12] to distinguish Pascua from Hetereleotris remains largely valid (Table 1).
The presence of modified basicaudal scales in Pascua is particularly interesting and an important trait separating this genus from Hetereleotris. This feature is common in Atlantic and East Pacific gobiids but extremely rare in Indo-Pacific taxa (Table 3). The only Indo-Pacific gobiid species with modified basicaudal scales are Pascua, some Cabillus spp., members of the Callogobius sclateri group, and Hetereleotris aurantiaca (Figure 2)—all of which are distantly related genera based on the molecular phylogeny (Figure S2).
Pascua differs from Cabillus in form and in having a closed first gill slit and separated pelvic fins [47]. Callogobius occasionally displays partial closures of the first gill slit [18]. However, Pascua lacks the raised ridges of papillae on the head, which characterise Callogobius [3,48,49]. Pascua also has widely separated pelvic fins with no membrane linking them. All Callogobius species have a membrane linking their pelvic fins, although it may be small in C. sclateri [3].

Table 3.
Gobiid genera containing species with modified basicaudal scales with enlarged ctenii, as seen in Pascua. Lineage terminology follows [12], and location acronyms are as follows: WAC: West Atlantic and Caribbean, EP: East Pacific, EAM: East Atlantic and Mediterranean, RS: Red Sea, WIO: West Indian Ocean, TIP: Tropical Indo-Pacific. Locations determined from [15,16].
Table 3.
Gobiid genera containing species with modified basicaudal scales with enlarged ctenii, as seen in Pascua. Lineage terminology follows [12], and location acronyms are as follows: WAC: West Atlantic and Caribbean, EP: East Pacific, EAM: East Atlantic and Mediterranean, RS: Red Sea, WIO: West Indian Ocean, TIP: Tropical Indo-Pacific. Locations determined from [15,16].
| Genus/Species | Lineage | Location | Reference |
|---|---|---|---|
| Chriolepis | Gobiosoma | WAC, EP | [25] |
| Paedovaricus | Gobiosoma | WAC | |
| Pinnichthys | |||
| Varicus | |||
| Psilotris | Gobiosoma | WAC | [50] |
| Aboma | Gobiosoma | EP | [51] |
| Pariah | WAC | ||
| Risor | |||
| Birdsongichthys | Gobiosoma | WAC | [52] |
| Robinsichthys | |||
| Gobiosoma | Gobiosoma | WAC, EP | [53] |
| Odondebuenia | Gobius | EAM | [54] |
| Vanneaugobius | Gobius | EAM | [55] |
| Cabillus | Gobiopsis | Australia, TIP, WIO | [56,57,58] |
| Callogobius | Callogobius | Australia, RS, TIP | [48,59] |
| Hetereleotris aurantiaca * | Hetereleotris † | RS | [16] |
| Pascua caudilinea P. sticta comb. nov. | Gobiodon | EP | [7] |
| P. readerae comb. nov. | Gobiodon | Australia | |
| P. marecoralliensis sp. nov. | Herein |
* H. aurantiaca has scales with slightly extended ctenii along the caudal peduncle. A third central scale also shows these elongated ctenii [57]. † Hetereleotris is not included in the phylogeny of [23], but forms a sister group to the Gobius group in our phylogeny.
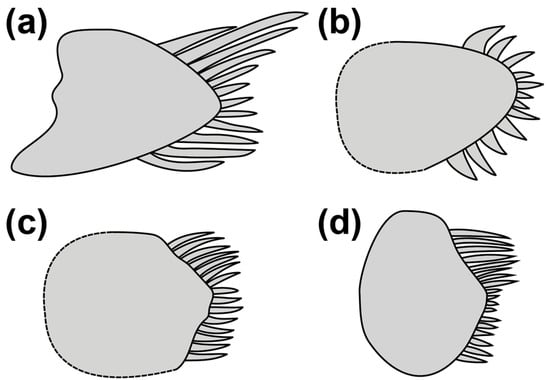
Figure 2.
Examples of basicaudal scales with elongated ctenii from Indo-Pacific gobiids: (a) Pascua readerae drawn from holotype; (b) Hetereleotris aurantiaca drawn from [16]; (c) Cabillus nigromarginatus modified from [57]; (d) Callogobius flavobrunneus adapted with permission from Ref. [3], Copyright © 2018 by Naomi R Delventhal [3].
The basicaudal scales in Hetereleotris aurantiaca possess only slightly elongated ctenii present on three scales arranged in a triangle covering the mid-lateral two-thirds of the basicaudal, rather than the well-separated dorsal and ventral scales in Pascua. Furthermore, the basicaudal scales in H. aurantiaca only protrude slightly over the caudal fin base, whereas the enlarged posterior field and elongated ctenii in Pascua protrude well beyond the caudal fin base.
Of the other characters, all remain robust. However, differentiating the papillae on the lower jaw and cheek of Pascua and Hetereleotris can be challenging, particularly in small Hetereleotris specimens, which may have few papillae.
In addition to possessing the five diagnostic characters of the genus, Pascua caudilinea, P. sticta, and P. readerae also differ from Hetereleotris species in their general body plans. Our morphological multivariate analysis clearly distinguishes Pascua and Hetereleotris (Figure 3; PERMANOVA; Pseudo-F1,22 = 8.65, p(perm) ≤ 0.001). Pascua spp. are characterised by possessing relatively larger eyes, longer fins and deeper bodies than Hetereleotris, which are characterised by having longer insertion lengths of the second dorsal and anal fins.
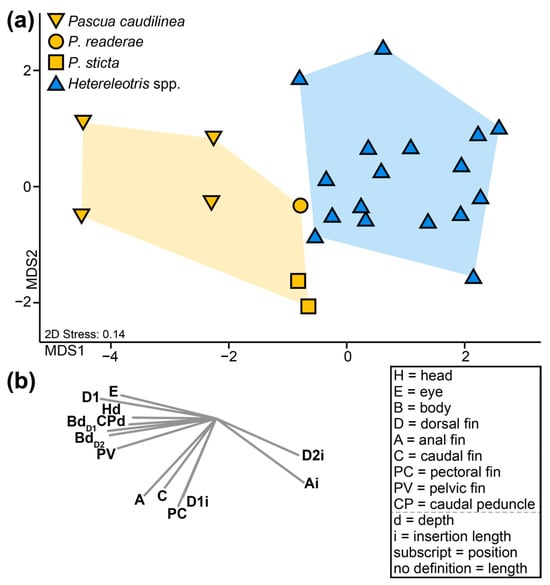
Figure 3.
(a) Threshold metric multidimensional scaling ordination comparing the body plans of Pascua caudilinea, P. sticta and P. readerae with 17 specimens from 14 species of Hetereleotris. Coloured polygons represent groupings supported by PERMANOVA (p(perm) ≤ 0.001). (b) Vectors associated with the tmMDS calculated using Pearson correlation, displaying morphological variables with correlation scores > 0.6 and explanations of morphological trait acronyms used.
Given the above information, as Hetereleotris readerae Hoese and Larson, 2005 and H. sticta Hoese and Larson, 2005 both possess the unique combination of characters ascribed to the genus Pascua (Table 1), it is parsimonious to follow the taxonomy of Fricke et al. [11] and redescribe these species as Pascua readerae (Hoese and Larson, 2005) comb. nov. and Pascua sticta (Hoese and Larson, 2005) comb. nov. A key to the identification of species in the genus Pascua is provided in Appendix A.
3.2.2. Pascua readerae (Hoese and Larson, 2005) comb. nov.
Sally’s Pascua goby
(Figure 4)
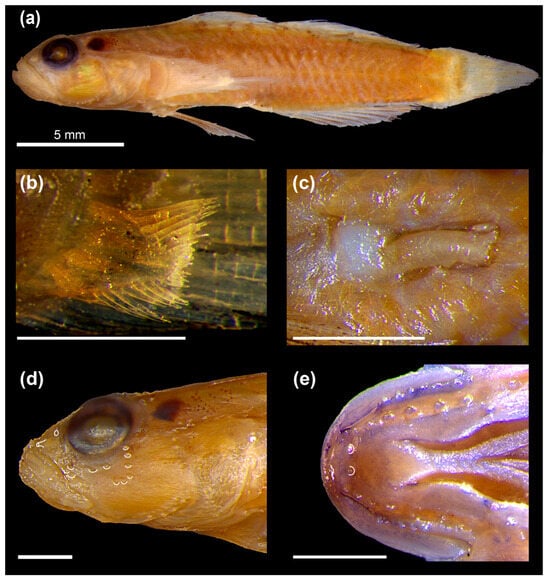
Figure 4.
(a) Pascua readerae (Hoese and Larson 2005) comb. nov. AMS I.27149-048 (holotype). (b) Lower basicaudal scale showing elongate posterior field and extremely elongated ctenii in AMS I.27138-053 (paratype). (c) Elongate and flattened urogenital papilla of male specimen AMS I.27138-053 (paratype). (d) Magnified view of head of AMS I.27149-036 (paratype), displaying the reduced pattern of sensory papillae on cheek (highlighted with white crescents) compared to that of Hetereleotris and posterior naris reduced to a simple pore (outlined in white). (e) Chin of holotype displaying the two papillae immediately behind the mental frenum, highlighted with white crescents. The parallel bands of papillae following the mandibular margin are also visible. Scale bars = 1 mm unless otherwise noted.
Hetereleotris readerae Hoese and Larson, 2005: 11, Figure 5 (Elizabeth Reef, Australia).
Hetereleotris sp. Gill and Reader, 1992: 92 (Middleton and Elizabeth Reefs).
- Material Examined: AMS I.27149-048 (Holotype; Figure 4) 21 mm SL male, Elizabeth Reef; AMS I.27149–036 (Paratypes), 2 (20–21 mm SL), Elizabeth Reef; AMS I.27138–053 (Paratypes), 2 (19–24 mm SL), Middleton Reef; AMS I. 27139-030 (Paratype) 21 mm SL, Middleton Reef.
- Diagnosis: Complete description provided in Hoese and Larson [7]: (1) extremely large ctenii on its basicaudal scales (Figure 4b), (2) an elongate, flattened urogenital papilla (Figure 4c), (3) a reduced transverse papilla pattern on the cheek (second and third transverse rows reduced to single papillae (Figure 4d), (4) the posterior naris a simple pore with no elevated margins (Figure 4d), and (5) two papillae behind the mental ridge (Figure 4e).
3.2.3. Pascua sticta (Hoese and Larson, 2005) comb. nov.
Spotted Pascua goby
(Figure 5)
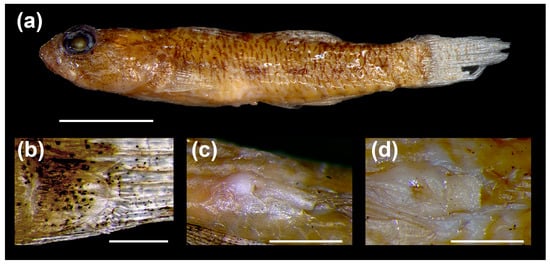
Figure 5.
(a) Pascua sticta (Hoese and Larson 2005) comb. nov. Larger of two preserved specimens in lot AMS I.43612-001 (paratype). Scale bar = 5 mm. (b) Basicaudal scale with enlarged posterior field and elongate ctenii from the same specimen. Scale bar = 0.5 mm. (c) Male urogenital papilla from smaller specimen in lot. Scale bar = 1 mm. (d) Female urogenital papilla from larger specimen in lot. Scale bar = 1 mm.
Hetereleotris sticta Hoese and Larson, 2005: 8, Figure 4 (Rapa Iti, French Polynesia).
- Material Examined: AMS I.43612-001 (Paratypes, taken with holotype; Figure 5), 2 (21 mm SL male and 26.5 mm SL female), “just south of Isle Tauna at mouth of Haurei Bay Rapa Iti” [7], approximately 27.608° S, 144.304° W.
- Diagnosis: Complete description provided in Hoese and Larson [7]: (1) extremely large ctenii on its basicaudal scales (Figure 5b), (2) an elongate, flattened urogenital papilla in males (Figure 5c), the female papilla is broad and rectangular (Figure 5d), (3) a reduced transverse papilla pattern on the cheek (second and third transverse rows reduced to single papillae), (4) the posterior naris a simple pore with no elevated margins, and (5) two papillae behind the mental ridge. The last three characters were observed in the preserved specimens, but photomicrographs were not collected.
3.2.4. Pascua marecoralliensis sp. nov.
Coral Sea Pascua goby

Figure 6.
The three specimens of Pascua marecoralliensis sp. nov. collected from Lorna Cay, Lihou Reef in the Central Coral Sea, Australia. (a) AMS I.49536-002 (holotype), (b) AMS I.49536-038 specimen 1 (paratype; specimen damaged during description and molecular sampling), (c) AMS I.49536-038 specimen 2 (paratype).
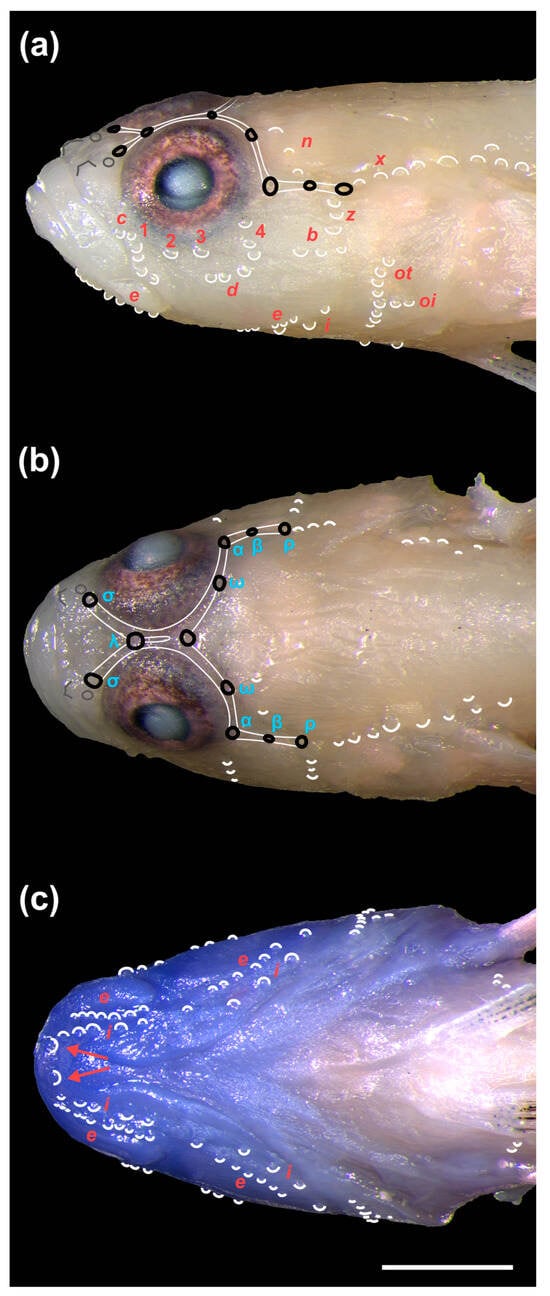
Figure 7.
(a) Lateral view of the head of Pascua marecoralliensis sp. nov. (AMS I.49536-002, holotype) with cephalic sensory papillae highlighted with white crescents. Red labels follow the nomenclature of [12,54,60]. Cephalic pores are labelled with black ovoids linked by the canals in white. Anterior and posterior nares are outlined in grey. (b) Dorsal view with cephalic pores with labels in cyan following [43]. (c) Ventral view of specimen. The diagnostic pair of sensory papillae behind the mental ridge are highlighted with red arrows. Scale bar = 1 mm.
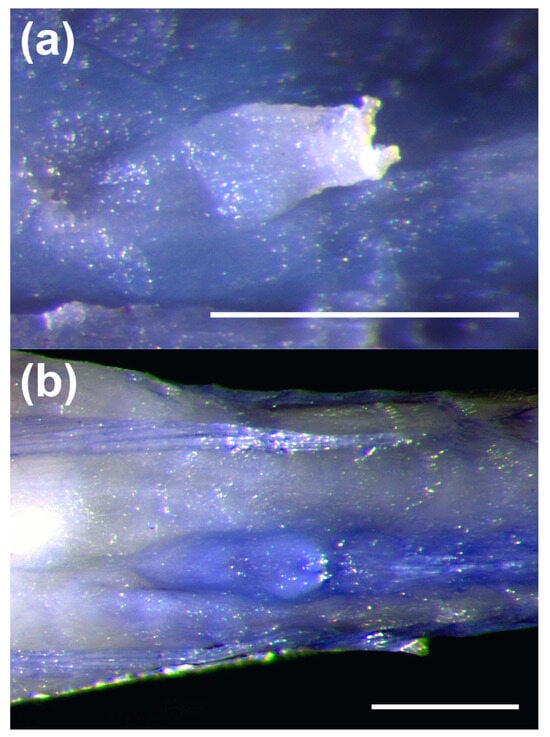
Figure 8.
Urogenital papillae of Pascua marecoralliensis (a) male specimen (AMS I.49536-002, holotype) displaying characteristic elongate, flattened papilla (b) female (AMS I.49536-038, paratype) papilla short with paired finger-like projections. Scale bars = 0.5 mm.
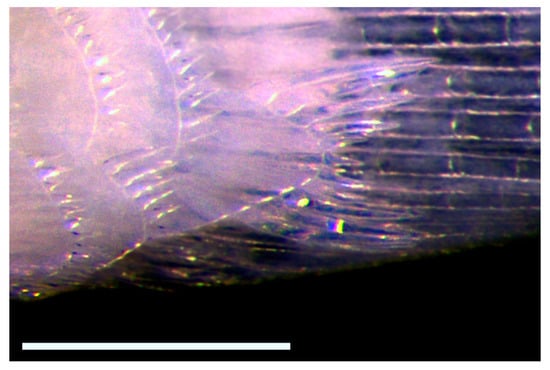
Figure 9.
Lower left basicaudal scale of Pascua marecoralliensis (AMS I.49536-038, paratype) displaying the enlarged posterior field and extremely long ctenii, characteristic of this genus. Scale bar = 0.5 mm.
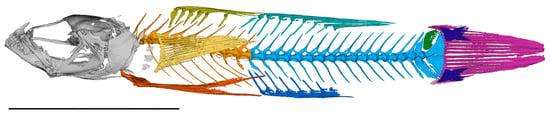
Figure 10.
Segmented micro-CT scan of Pascua marecoralliensis AMS I.49536-002 (holotype). Precaudal vertebrae, pale orange; caudal vertebrae, light blue; epural, green; basicaudal scales, dark blue. Scale bar = 5 mm.
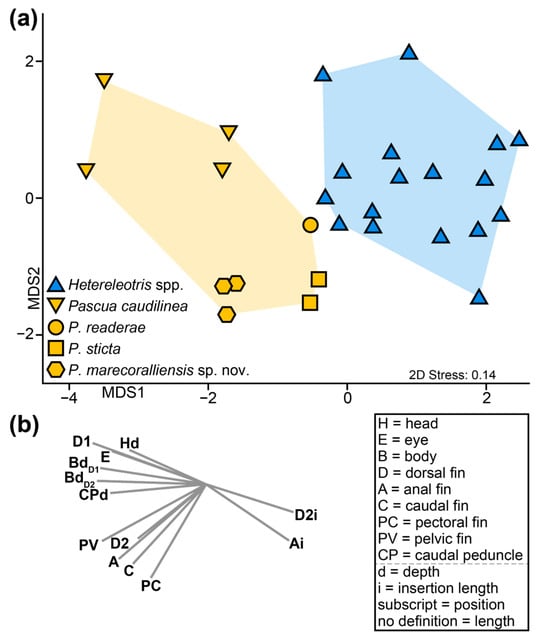
Figure 11.
(a) Morphospace obtained from the threshold metric multidimensional scaling (tmMDS) ordination of Pascua (blue) and Hetereleotris (yellow) specimens. Morphospace occupied by each taxon is enclosed within a convex polygon, denoting the statistical grouping of Pascua s.l. and Pascua marecoralliensis derived from the PERMANOVA tests (p(perm) ≤ 0.05 for all groupings). (b) Vectors associated with the tmMDS calculated using Pearson correlation, displaying morphological variables with correlation scores > 0.6, and explanations of morphological trait acronyms used.

Figure 12.
Preserved specimen of Pascua marecoralliensis sp. nov. (AMS I.49536-002, holotype). The dark spot on the dorsal and pigments on the pelvic fins remain in preservation in ethanol.
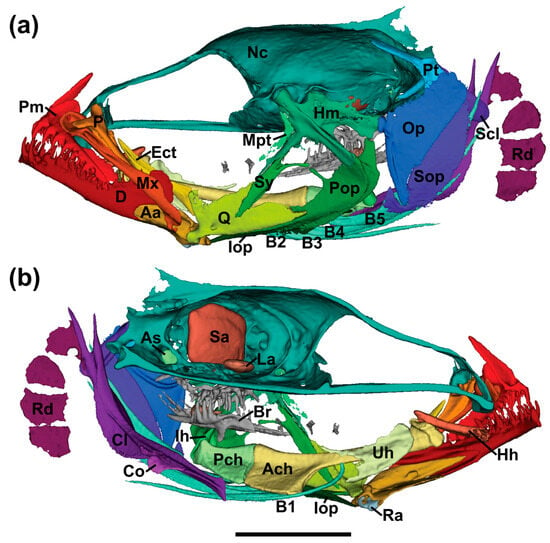
Figure 13.
Segmented micro-CT scan of the head of Pascua marecoralliensis AMS I.49536-002 (holotype). (a) lateral view, (b) medial view, digitally dissected along midline. Scan data available at Morphosource.org (ark:/87602/m4/768084). Abbreviations: Aa anguloarticular, Ach anterior ceratohyal, As asteriscus, Br branchial arch, B1-6 branchiostegals 1-6, Cl cleithrum, Co coracoid, D dentary, Ect ectopterygoid, Hh hypohyal, Hm hyomandibula, Ih interhyal, Iop interoperculum, La lapillus, Mpt metapterygoid, Mx maxilla, Nc neurocranium, Op operculum, P palatine, Pch posterior ceratohyal, Pm premaxilla, Pop preoperculum, Pt posttemporal, Q quadrate, Ra retroarticular, Rd radials, Sa sagitta, Scl supercleithrum, Sop suboperculum, Sy symplectic, Uh urohyal.
LSID: urn:lsid:zoobank.org:act:BCC2C231-3EB1-40AD-8985-ED1F1CF8B248
- Holotype: AMS I.49536-002, 13.8 mm SL, collected using an enclosed clove oil station at 11 m depth from reef to the north of Lorna Cay, Lihou Reef in the Australian Coral Sea 17.12527° S, 151.82535° E, from the vessel Iron Joy (RB Holdings). Collectors C. Goatley, P. Berents and R. Morais-Araujo.
- Paratypes: Two specimens, AMS I.49536-038, collected with holotype. Smaller with posterior half damaged following initial photography.
- Etymology: The specific epithet is an adjective combining the Latin mare (sea; n., nom.), corallium (coral; n., nom.) and the suffix -ensis (from; f., nom.). The epithet is feminine in correspondence with the generic name and, together, refers to the type locality of the specimen, the Coral Sea. The common name refers to the type localities of the species and the genus, Rapa Nui, colonially named Isla de Pascua or Easter Island.
- Generic placement: Alongside the results of the phylogenetic analysis, the generic placement of P. marecoralliensis is supported by a closed first gill slit and the possession of the five morphological characters which separate Pascua from Hetereleotris [7,10]:
- The posterior nares are simple pores lacking any elevated margins (Figure 7).
- The male urogenital papilla is flattened and elongate (Figure 8).
- The sensory papillae on the cheek are reduced. The second and third transverse rows reduced to single papillae, row d reduced to 2 papillae anterior of the ventral end of row 4 and row b consisting of 2 or 3 papillae extending forwards from the preopercular margin (Figure 7a).
- Two papillae are found immediately behind the mental ridge (although the mental frenum/flap is absent or reduced to a small ridge). These are followed by two rows of papillae (e and i) along the preopercular-mandibular margin (Figure 7c).
The morphometrics in the tmMDS (Figure 11) and PERMANOVA (Pseudo-F2,24 = 6.57, p(perm) ≤ 0.001) revealed clear differences in body shape among Hetereleotris and the three current Pascua species (t = 2.86, p(perm) ≤ 0.001), and Hetereleotris vs. P. marecoralliensis sp. nov. (t = 2.63, p(perm) = 0.002). However, the new species could not be resolved from Pascua s.l. (t = 1.34, p(perm) = 0.104). The primary morphological features characterising the new species are elongate rays in all fins except the first dorsal fin.
- Diagnosis: P. marecoralliensis sp. nov. differs from all other species in the genus Pascua in the following five characters:
- Fewer unpaired fin rays D. VI + I,7; A. I,7. All Pascua and Hetereleotris have eight or more dorsal fin rays, except for one specimen of P. readerae that has been reported to have seven anal fin rays (Table 4).
 Table 4. The cephalic pore pattern and second dorsal (DII) and anal (A) fin element counts in Cerogobius, Hetereleotris and Pascua spp. Pore nomenclatures from [43,60,61] are presented. Data from [13,16,17,18,62,63]. + indicates the possession of the pore, − the lack of the pore, and ? suggests this character is ambiguous or variable.
Table 4. The cephalic pore pattern and second dorsal (DII) and anal (A) fin element counts in Cerogobius, Hetereleotris and Pascua spp. Pore nomenclatures from [43,60,61] are presented. Data from [13,16,17,18,62,63]. + indicates the possession of the pore, − the lack of the pore, and ? suggests this character is ambiguous or variable. - Fewer pectoral fin rays (15[2], 16[1]) than other Pascua spp. (17–22, usually 18–20).
- Head dorsoventrally compressed (height 70–80% of width) compared to rounded or laterally compressed in other Pascua spp.
- The first and second dorsal fins are separate in P. marecoralliensis sp. nov.; they are connected by a rudimentary membrane in other Pascua spp. [7].
In addition to the aforementioned characters, the colouration of P. marecoralliensis differs significantly from the eastern Pacific members of the genus. The body of P. marecoralliensis is primarily pale tan with a series of pink/orange marks, and the fins are mostly yellow (Figure 6). By contrast, P. caudilinea and P. sticta are both primarily mottled brown with few distinctive markings. The live colouration of the other Australian Pascua species, P. readerae, is unknown in life, but it displays a large spot of melanophores behind the eye (Figure 4), which remains visible following preservation. All specimens of P. marecoralliensis lack any markings on the body in preservation, with only markings on the dorsal and pelvic fins remaining visible (Figure 12).
- Description:
General shape (see Table 5 for morphometrics): Body elongate, with dorsoventrally compressed head. All fins appear elongated, especially the pelvics (Figure 6).

Table 5.
Morphometrics of all specimens of Pascua marecoralliensis sp. nov. All values except standard length (SL) are in % SL.
Fins: dorsal fin elements VI + I,7; first dorsal fin triangular, tips of spines protruding slightly from fin membrane, second or third element longest, reaching first soft ray of second dorsal fin when adpressed, second dorsal fin soft rays 4–7 branched, last ray branched to base; anal-fin I,7, final four soft rays branched, last ray branched to base; pectoral fin rays 15 (16 in 1 specimen), rays 2–4 branched (possibly more rays branched, but damaged in holotype), rounded, reaching past insertion of second dorsal fin; pelvic fins I,5, 5th ray unbranched, 30% length of 4th ray, extending beyond anal fin insertion, fins separated by a distance similar to the width of one pelvic fin base, lacking any connecting membrane or frenum; caudal-fin with 17 rays, rounded 28.5% SL.
Squamation: 23–24 lateral line scales; 6 transverse rows; body scales ctenoid, cycloid on belly, extending forward to ventral margin of pectoral fin base, no scales on head.
Genitalia: male papillae elongate and flattened, female flattened with several finger-like protrusions (Figure 8), both similar to those of Eviota [64].
Head: length 28% standard length; eye large, 9% SL; front of head angular, pointed at an angle of 33° from horizontal axis; mouth slanted upwards at an angle of 44° from horizontal axis; lower jaw projecting slightly; maxilla extending posteriorly to slightly in front of pupil midline; anterior nares tubular and translucent extending to, or in front of, anterior margin of upper lip; cephalic sensory pore system described in diagnosis; cutaneous sensory papilla system described in generic placement (Figure 7), as described for the three species in Hoese and Larson, 2005 [7].
Dentition: dentary with single row of small caniniform teeth along posterior half of toothed portion, anterior half with interior (lingual) row of larger caniniform teeth, two to four large fang-like caniniform teeth at the anterior tip of dentary along outer (labial) surface; premaxilla with two rows of caniniform teeth along whole length, inner row small and tightly packed, outer row larger and widely spaced, third interior row of ~5 mid-sized teeth along anterior of bone (Figure 13); fifth ceratobranchial with three rows of widely spaced conical-caniniform teeth; pharyngobranchial teeth in similar arrangement to those on ceratobranchial.
Select osteological characters: Pterygiophore insertion formula for first dorsal fin, 3-22110 [37]; two anal fin pterygiophores before first haemal spine; ten precaudal vertebrae, seventeen caudal vertebrae (including urostylar complex; Figure 10); Metapterygoid slender lacking ventral process extending across quadrate; prominent median process on preoperculum reaching to upper part of symplectic; sagittal otolith dorsoventrally compressed hexagon with slight posterodorsal and anteroventral projections (Figure 13).
Colour in life: Body pale tan with series of pink/orange marks along dorsal midline linking to marks along ventral midline with faint barring or x-shaped marks; four marks along second dorsal fin base, three to four marks along anal fin base; cheek and operculum speckled with red, coalescing into three bars below the eye, the anterior of which is more yellowish. Iris red. Dorsal fins primarily yellow, the first spine of both with alternating red and white bands; first dorsal fin has a large black spot at the posterior base of the fin. Pelvic fin dark orange-red, pale on smallest specimen; base of pectoral fin opaque white extending diagonally onto pelvic fin rays towards the ventral margin of fin. Anal and caudal fins pale yellow, paler and translucent on smallest specimen.
4. Discussion
The validity of the genus Pascua is shown using molecular and morphological evidence, and supports the assignment of Hetereleotris readerae [9] and H. sticta [8] in Pascua. This results in Pascua containing four species with a disparate distribution across the southern Pacific. P. caudilinea is endemic to Rapa Nui (Isla de Pascua) in the eastern South Pacific, and P. sticta is found around Rapa Iti, French Polynesia, and possibly Pitcairn. P. readerae and P. marecoralliensis are described, respectively, from Elizabeth and Lihou Reefs, Australia (Figure 14).
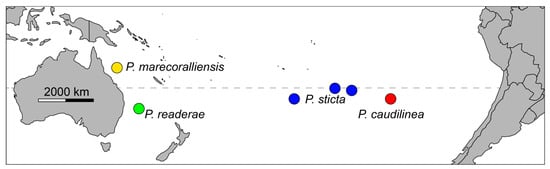
Figure 14.
Map of known collection sites for all Pascua spp. dashed line represents the Tropic of Capricorn (23.4° S).
The separation of the eastern and western species is at least 5400 km, almost half the width of the Pacific Ocean at this latitude, yet may be explained by several factors. First, Pascua may be a relict genus, with a few surviving species in isolated peripheral reef locations [65,66,67]. Second, there may be more species of Pascua yet to be discovered and/or described from the central and western Pacific. The number of new cryptobenthic species being described is high, and relatively few studies using the anaesthetic collection techniques most suited for cryptobenthic fishes are being conducted in the central and southern Pacific [21]. Third, Pascua spp. may have been misidentified or described as other genera. While this may be true, the most likely misidentification of Pascua spp. is as Hetereleotris due to the closed first gill slit and the numerous shared characters mentioned above. There are currently no Hetereleotris spp. described from the central Pacific, with distributions of the only Pacific members of this genus, H. exilis Shibukawa, 2010 and H. poecila (Fowler, 1946) found no further east than the Ryukyu Islands, Japan.
The results of our phylogenetic analysis place Pascua as sister to the Gobiodon group of [23]. While our analysis is based only on a handful of loci, a recent phylogenomic study on the interrelationships of gobiids using hundreds of loci showed highly concordant intergeneric topologies with our study and previous studies using similar loci to ours [23,68]. The inclusion of Pascua in future genome-wide phylogenetic studies would be useful to confirm the position recovered here.
Nevertheless, the observed phylogenetic relationship is supported morphologically by the presence of flattened urogenital papillae similar to Eviota and some members of Bryaninops [12,69], and separate pelvic fins, characteristic of Eviota [12]. The presence of 27 vertebral elements (10 + 17) in Pascua is somewhat surprising in this group, with most other species possessing 25 (10 + 15) or 26 (10 + 16) vertebrae [37]. Some species of Eviota display 27 (10 + 17) vertebrae [37], including E. shibukawai [70], and occasional reports from E. distigma, E. epiphanes, E. herrei, E. melasma, E. nigripinna, E. queenslandica, E. smaragdus [71] and E. albolineata [72]. Morphological examinations of Sueviota atrinasa reveal that it also possesses 27 (10 + 17) vertebrae in all specimens inspected [73].
The generic placement of Pascua marecoralliensis sp. nov. is well supported by morphological characters and our molecular phylogeny, yet it bears some striking morphological and biogeographical differences from other members of the genus. First, the colouration of P. marecoralliensis differs significantly from the eastern Pacific members of the genus, which are both noticeably drab and mottled brown (the live colouration of P. readerae remains unknown).
Second, this species is the smallest known member of the genus. The largest specimen of P. marecoralliensis collected was 13.8 mm SL. It displayed bright colouration along with well-developed urogenital papillae and cephalic pores characteristic of adult stages in other coral gobies. Specimens of other Pascua spp. are usually much larger. In P. caudilinea, the average length of specimens examined is 26.0 ± 1.0 mm (mean SL ± standard error; range 18–32 mm); in P. sticta, 19.5 ± 1.4 mm (11-30.5 mm); in P. readerae, 21 ± 0.7 mm (19–24 mm) [7]. Specimens of P. marecoralliensis may grow larger than those described herein, but the adult traits displayed in the specimens suggest that it is likely that they would still be smaller than the maximum sizes reported for the other Pascua species.
Finally, this species is the most northerly distributed Pascua. All other species are described from subtropical reef systems, with the second most northerly species, P. sticta, being reported from Oeno Atoll, Pitcairn, just south of the Tropic of Capricorn at 23.9° S. The type locality of P. marecoralliensis is at 17.1° S, around 750 km north (Figure 14). The tropical distribution of this species may also be linked to this species’ small body size. Bergmann’s rule describes the trend in increasing body size at higher latitudes in homeotherms [74] but has repeatedly been shown to hold true for ectothermic marine fishes [75,76].
These unusual morphological and biogeographic characters bear a striking similarity to those of the recently described thalasseleotridid, Tempestichthys bettyae Goatley and Tornabene, 2022. This species was the fourth member of the family Thalasseleotrididae and is distinguished by being the smallest member of the family, the only species lacking mottled brown colouration, and the first known to inhabit tropical waters [22]. Here, the parallels between P. marecoralliensis and T. bettyae highlight the potential broad distributions and morphological diversity that may exist within gobiiform taxa and the value of combining molecular and morphological techniques to study the systematics of the order. Future taxonomic work on cryptobenthic fishes would benefit from the preservation of tissue samples or whole specimens using ethanol, cryopreservation or other DNA-friendly protocols [77,78] alongside routine use of X-ray or micro-CT analyses to facilitate osteological analyses of specimens [36,79].
In addition to providing opportunities to assess the osteology of P. marecoralliensis, our micro-CT scans provided us with insights into the ecology of this species. In the largest specimen, we found several fish scales in the gut (Figure S3). These scales may have been ingested in collection bags as anaesthesia subsided or incidentally ingested with gill ventilation during anaesthesia [80]. The first of these scenarios is unlikely, as feeding rates usually decrease under stress [81]. The second scenario is feasible, but as several of the ingested scales were still linked by integument and in the digestive tract rather than the mouth, they were likely bitten off a whole fish by deliberate predation prior to collection, rather than ingested incidentally.
The diet of most cryptobenthic fishes is poorly known, but, where assessed, a large proportion, across multiple species, consists of microcrustaceans [82,83,84]. This is likely true for P. marecoralliensis, but the demand for growth to increase fecundity [85] and reduce predation risk [86] may give rise to opportunistic feeding on high-energy prey, such as mucus and scales [87]. Gaining further information on the diets of cryptobenthic fishes using visual, isotopic or molecular techniques is important to improve our understanding of the role that these short-lived species play in coral reef trophodynamics.
This paper describes the second new species identified during the 2019–2020 Coral Reef Health in the Coral Sea Marine Park surveys [20,22], with additional specimens awaiting formal description. Despite the Coral Sea’s relative remoteness, extensive ichthyological collections have been made in the region (e.g., [88,89]). The discovery of multiple new species within our limited sampling area (188 m2) highlights that cryptobenthic fishes represent a remarkable reservoir of undescribed biodiversity on coral reefs around the world. Like other lineages of Indo-Pacific cryptobenthic fishes, it is possible that these previously unrecognised species found in the Coral Sea display high levels of endemism and microhabitat specialisation, making them particularly vulnerable to local disturbances and thus important for conservation and management.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/fishes10090449/s1, Figure S1: Measurements taken from each fish for morphological comparisons; Figure S2: Complete phylogeny; Figure S3: Micro-CT scan of fish scales in the gut of Pascua marecoralliensis; Table S1: Summary statistics for alignment of molecular data; Table S2: MorphoSource.org ark ID numbers for Pascua spp. scanned for this project; Table S3: Descriptions of measurements used for morphological analysis.
Author Contributions
Conceptualisation, C.H.R.G. and L.T.; methodology, C.H.R.G. and L.T.; data collection, all authors; formal analysis, C.H.R.G. and L.T.; resources, all authors; data curation, all authors; writing—original draft preparation, C.H.R.G.; writing—review and editing, all authors; visualisation, C.H.R.G.; project administration, C.H.R.G. All authors have read and agreed to the published version of the manuscript.
Funding
The fieldwork for this project was jointly funded by the Director of National Parks, Australia and ARC Centre of Excellence for Coral Reef Studies, JCU (CE140100020). Micro-CT scanning at the Karel F. Liem Bioimaging Center was supported by the NSF (oVert, Award Number 1701665, L.T.). Collaborative research was funded by a Fulbright Postdoctoral Future Fellowship funded by the Kinghorn Foundation and a University of New England Postdoctoral Research Fellowship (C.G.). Funding to J.S. was provided by grants ANID- Anillo BiodUCCT ATE220044 and FONDECYT 1241386.
Institutional Review Board Statement
This research was conducted in the Coral Sea Marine Park under Permit No. AU-COM2018-403 and animal ethics approval AEC19-105 (valid 12 November 2019–12 November 2022) from UNE, Australia. Rapa Nui specimens were collected under the permission Res. Ext No. 3685/2016 from SUBPESCA (Chile) to Universidad Católica del Norte.
Data Availability Statement
Micro CT scan data are available on MorphoSource.org (Table S2); DNA sequence data are available through NCBI GenBank (accession numbers PX240974–PX240980). Morphological data are available on Figshare doi: 10.6084/m9.figshare.29950199.
Acknowledgments
The authors wish to thank the relevant staff at Parks Australia and Andrew Hoey, Morgan Pratchett, Andrew Baird, Hugo Harrison, Penny Berents and Renato Morais Araujo, Rob and Anita Benn and the crew of the Iron Joy for field assistance; Yi-Kai Tea, Tony Gill, Kerryn Parkinson, Adam Summers, Stephen Wroe, Richard Flavel for taxonomic and imaging assistance; Amanda Hay, Joseph DiBattista, Kerryn Parkinson, Sally Reader, and Katherine Maslenikov for assistance with museum specimens; Naomi Delventhal for permitting use of redrawn figure.
Conflicts of Interest
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. The findings and views expressed are those of the authors and do not necessarily represent the views of Parks Australia, the Director of National Parks or the Australian Government.
Appendix A. Key to Species in the Genus Pascua Randall, 2005
|
|
|
|
|
References
- Nelson, J.S.; Grande, T.C.; Wilson, M.V.H. Fishes of the World: Fifth Edition; John Wiley & Sons: Hoboken, NJ, USA, 2016; p. 707. [Google Scholar]
- Fricke, R.; Eschmeyer, W.N.; Fong, J.D. Eschmeyer’s Catalog of Fishes, Species by Family/Subfamily. Available online: http://researcharchive.calacademy.org/research/ichthyology/catalog/SpeciesByFamily.asp (accessed on 17 April 2023).
- Delventhal, N.R. Systematics of Callogobius (Teleostei: Gobiidae). Ph.D. Thesis, University of Manitoba, Winnipeg, MB, Canada, 2018. [Google Scholar]
- Hammer, M.P.; Taillebois, L.; King, A.J.; Crook, D.A.; Wedd, D.; Adams, M.; Unmack, P.J.; Hoese, D.F.; Bertozzi, T. Unravelling the taxonomy and identification of a problematic group of benthic fishes from tropical rivers (Gobiidae: Glossogobius). J. Fish Biol. 2021, 99, 87–100. [Google Scholar] [CrossRef]
- Randall, J.E. Pascua caudilinea, a new genus and species of gobiid fish (Perciformes: Gobiidae) from Easter Island. Zool. Stud. 2005, 44, 19–25. [Google Scholar]
- Zoobank. Pascua caudilinea. LSID: Urn:lsid:zoobank.org:act:D0C8AA1B-2822-4311-8DCB-E6A7F70D300A. Available online: https://zoobank.org/NomenclaturalActs/D0C8AA1B-2822-4311-8DCB-E6A7F70D300A (accessed on 1 April 2025).
- Hoese, D.F.; Larson, H.K. Description of two new species of Hetereleotris (Gobiidae) from the south Pacific, with a revised key to species and synonymization of the genus Pascua with Hetereleotris. Zootaxa 2005, 1096, 1–16. [Google Scholar] [CrossRef]
- Zoobank. Hetereleotris sticta. LSID: Urn:lsid:zoobank.org:act:F77184F4-DA4F-46C3-9C74-D2B6A9B5B3F2. Available online: https://zoobank.org/NomenclaturalActs/F77184F4-DA4F-46C3-9C74-D2B6A9B5B3F2 (accessed on 1 April 2025).
- Zoobank. Hetereleotris readerae. LSID: Urn:lsid:zoobank.org:act:9F50CB57-0FF6-4D3A-9F5D-E16EAC784990. Available online: https://zoobank.org/NomenclaturalActs/9F50CB57-0FF6-4D3A-9F5D-E16EAC784990 (accessed on 1 April 2025).
- Randall, J.E. Validation of the gobiid fish genus Pascua. Aqua. Int. J. Ichthyol. 2006, 12, 35–38. [Google Scholar]
- Fricke, R.; Eschmeyer, W.N.; van der Laan, R. Eschmeyer’s Catalog of Fishes: Genera, Species, References. Available online: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp (accessed on 9 June 2023).
- Shibukawa, K. Hetereleotris exilis, a new goby (Teleostei, Perciformes, Gobiidae) from the Ryukyu Islands, Japan. Bull. Natl. Mus. Nat. Sci. Ser. A 2010, S4, 89–95. [Google Scholar]
- Kovačić, M.; Bogorodsky, S.V. A new species of Hetereleotris (Perciformes: Gobiidae) from the Red Sea. Zootaxa 2014, 3764, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Shibukawa, K.; Iwata, A. Grallenia, a new goby genus from the Western Pacific, with descriptions of two new species (Perciformes: Gobiidae: Gobiinae). Bull. Natl. Mus. Nat. Sci. Ser. A 2007, Supp. 1, 123–136. [Google Scholar]
- Froese, R.; Pauly, D. FishBase. Available online: www.fishbase.se (accessed on 1 April 2025).
- Kovačić, M.; Bogorodsky, S.V.; Mal, A.O. Two new species of Hetereleotris (Perciformes: Gobiidae) from the Red Sea. Zootaxa 2019, 4608, 501–516. [Google Scholar] [CrossRef]
- Kovačić, M.; Bogorodsky, S.V.; Zajonz, U.; Tornabene, L. A new species of Hetereleotris (Teleostei: Gobiidae) from the Socotra Archipelago (north-western Indian Ocean), a rare case of a hole-associated adaptation in gobiid fishes. Zootaxa 2021, 4996, 283–300. [Google Scholar] [CrossRef]
- Hoese, D.F. Descriptions of two new species of Hetereleotris (Pisces: Gobiidae) from the Western Indian Ocean, with discussion of related species. Ser. Publ. J.L.B. Smith Inst. Ichthyol. 1986, 41, 1–25. [Google Scholar]
- Gill, A.C. Hetereleotris georgegilli, a new species of gobiid fish, with notes on other Mauritian Hetereleotris species. Bull. Nat. Hist. Mus. Zool. 1998, 64, 91–95. [Google Scholar]
- Hoey, A.S.; Pratchett, M.S.; Harrison, H.B. Coral Reef Health in the Coral Sea Marine Park: Report on Reef Surveys April 2018 to March 2020; Parks Australia: Canberra, Australia, 2020; p. 170. [Google Scholar]
- Brandl, S.J.; Goatley, C.H.R.; Bellwood, D.R.; Tornabene, L. The hidden half: Ecology and evolution of cryptobenthic fishes on coral reefs. Biol. Rev. 2018, 93, 1846–1873. [Google Scholar] [CrossRef]
- Goatley, C.H.R.; Tornabene, L. Tempestichthys bettyae, a new genus and species of ocean sleeper (Gobiiformes, Thalasseleotrididae) from the central Coral Sea. Syst. Biodivers. 2022, 20, 1–15. [Google Scholar] [CrossRef]
- Agorreta, A.; San Mauro, D.; Schliewen, U.; Van Tassell, J.L.; Kovačić, M.; Zardoya, R.; Rüber, L. Molecular phylogenetics of Gobioidei and phylogenetic placement of European gobies. Mol. Phylogenet. Evol. 2013, 69, 619–633. [Google Scholar] [CrossRef]
- Tornabene, L.; Ahmadia, G.N.; Berumen, M.L.; Smith, D.J.; Jompa, J.; Pezold, F.L. Evolution of microhabitat association and morphology in a diverse group of cryptobenthic coral reef fishes (Teleostei: Gobiidae: Eviota). Mol. Phylogenet. Evol. 2013, 66, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Tornabene, L.; Van Tassell, J.L.; Gilmore, R.G.; Robertson, D.R.; Young, F.; Baldwin, C.C. Molecular phylogeny, analysis of character evolution, and submersible collections enable a new classification of a diverse group of gobies (Teleostei: Gobiidae: Nes subgroup), including nine new species and four new genera. Zool. J. Linn. Soc. 2016, 177, 764–812. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Ayres, D.L.; Darling, A.; Zwickl, D.J.; Beerli, P.; Holder, M.T.; Lewis, P.O.; Huelsenbeck, J.P.; Ronquist, F.; Swofford, D.L.; Cummings, M.P.; et al. BEAGLE: An application programming interface and high-performance computing library for statistical phylogenetics. Syst. Biol. 2012, 61, 170–173. [Google Scholar] [CrossRef]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. The CIPRES science gateway: A community resource for phylogenetic analyses. In Proceedings of the 2011 TeraGrid Conference: Extreme Digital Discovery, Salt Lake City, UT, USA, 18–21 July 2011; p. 8. [Google Scholar]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef]
- López, J.A.; Chen, W.-J.; Ortí, G. Esociform Phylogeny. Copeia 2004, 2004, 449–464. [Google Scholar] [CrossRef]
- Tornabene, L.; Pezold, F.L. Phylogenetic analysis of Western Atlantic Bathygobius (Teleostei: Gobiidae). Zootaxa 2011, 3042, 27–36. [Google Scholar] [CrossRef]
- Li, C.; Ortí, G.; Zhang, G.; Lu, G. A practical approach to phylogenomics: The phylogeny of ray-finned fish (Actinopterygii) as a case study. BMC Evol. Biol. 2007, 7, 44. [Google Scholar] [CrossRef]
- Yamada, T.; Sugiyama, T.; Tamaki, N.; Kawakita, A.; Kato, M. Adaptive radiation of gobies in the interstitial habitats of gravel beaches accompanied by body elongation and excessive vertebral segmentation. BMC Evol. Biol. 2009, 9, 145. [Google Scholar] [CrossRef]
- Ward, R.D.; Zemlak, T.S.; Innes, B.H.; Last, P.R.; Hebert, P.D.N. DNA barcoding Australia’s fish species. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 1847–1857. [Google Scholar] [CrossRef]
- Sevilla, R.G.; Diez, A.; Norén, M.; Mouchel, O.; Jérôme, M.; Verrez-Bagnis, V.; Van Pelt, H.; Favre-Krey, L.; Krey, G.; The Fishtrace Consortium; et al. Primers and polymerase chain reaction conditions for DNA barcoding teleost fish based on the mitochondrial cytochrome b and nuclear rhodopsin genes. Mol. Ecol. Notes 2007, 7, 730–734. [Google Scholar] [CrossRef]
- Rolfe, S.; Pieper, S.; Porto, A.; Diamond, K.; Winchester, J.; Shan, S.; Kirveslahti, H.; Boyer, D.; Summers, A.; Maga, A.M. SlicerMorph: An open and extensible platform to retrieve, visualize and analyse 3D morphology. Methods Ecol. Evol. 2021, 12, 1816–1825. [Google Scholar] [CrossRef]
- Birdsong, R.S.; Murdy, E.O.; Pezold, F.L. A study of the vertebral column and median fin osteology in gobioid fishes with comments on gobioid relationships. Bull. Mar. Sci. 1988, 42, 174–214. [Google Scholar]
- Böhlke, J.E.; Robins, C.R. The taxonomic position of the west Atlantic goby, Eviota personata, with descriptions of two new related species. Proc. Acad. Nat. Sci. Phila. 1962, 114, 175–189. [Google Scholar]
- Van Tassell, J.L.; Tornabene, L.; Colin, P.L. Review of the western Atlantic species of Bollmannia (Teleostei: Gobiidae: Gobiosomatini) with the description of a new allied genus and species. Aqua Int. J. Ichthyol. 2012, 18, 61–94. [Google Scholar]
- Heiple, Z.; Huie, J.M.; Medeiros, A.P.M.; Hart, P.B.; Goatley, C.H.R.; Arcila, D.; Miller, E.C. Many ways to build an angler: Diversity of feeding morphologies in a deep-sea evolutionary radiation. Biol. Lett. 2023, 19, 20230049. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, D.K.; Karan, E.A.; Collar, D.C. Evolutionary patterns of scale morphology in damselfishes (Pomacentridae). Biol. J. Linn. Soc. 2022, 135, 138–158. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N.; Somerfield, P.J.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation; PRIMER-E Ltd.: Plymouth, UK, 2014. [Google Scholar]
- Akihito, P.; Hayashi, M.; Yoshino, T.; Shimada, K.; Senou, H.; Yamamoto, T. Suborder Gobioidei. In The Fishes of the Japanese Archipelago, 2nd ed.; Masuda, H., Amaoka, K., Araga, C., Uyeno, T., Yoshino, T., Eds.; Tokai University Press: Tokyo, Japan, 1988; pp. 236–289. [Google Scholar]
- Gill, A.C.; Mooi, R.D. Thalasseleotrididae, new family of marine gobioid fishes from New Zealand and temperate Australia, with a revised definition of its sister taxon, the Gobiidae (Teleostei: Acanthomorpha). Zootaxa 2012, 52, 41–52. [Google Scholar] [CrossRef]
- Shibukawa, K.; Suzuki, T.; Senou, H.; Yano, K. Records of three shrimp-goby species (Teleostei, Perciformes, Gobiidae) from the Ryukyu Archipelago, Japan. Bull. Natl. Sci. Mueum Tokyo Ser. A 2005, 31, 191–204. [Google Scholar]
- Hoese, D.F.; Shibukawa, K.; Johnson, J.E. Description of a new species of Tomiyamichthys from Australia with a discussion of the generic name. Zootaxa 2016, 4079, 582–594. [Google Scholar] [CrossRef]
- Randall, J.E.; Sakamoto, K.; Shibukawa, K. Cabillus atripelvicus, a new species of gobiid fish from the Ogasawara Islands, with a key to species of the genus. Ichthyol. Res. 2007, 54, 38–43. [Google Scholar] [CrossRef]
- Delventhal, N.R.; Mooi, R.D.; Bogorodsky, S.V.; Mal, A.O. A review of the Callogobius (Teleostei: Gobiidae) from the Red Sea with the description of a new species. Zootaxa 2016, 4179, 225–243. [Google Scholar] [CrossRef]
- Akihito, P.; Meguro, K. Five species of the genus Callogobius in Japan and their relationships. Jpn. J. Ichthyol. 1977, 24, 113–127. [Google Scholar]
- Tornabene, L.; Baldwin, C.C. Psilotris vantasselli, a new species of goby from the tropical western Atlantic (Teleostei: Gobiidae: Gobiosomatini: Nes subgroup). Zootaxa 2019, 4624, 191–204. [Google Scholar] [CrossRef]
- Van Tassell, J.L. Gobiiformes of the Americas. In The Biology of Gobies; Patzner, R.A., Van Tassell, J.L., Kovačić, M., Kapoor, B.G., Eds.; CRC Press: Boca Raton, FL, USA, 2011; pp. 139–176. [Google Scholar]
- Tornabene, L.; Manning, R.; Robertson, D.R.; Van Tassell, J.L.; Baldwin, C.C. A new lineage of deep-reef gobies from the Caribbean, including two new species and one new genus (Teleostei: Gobiidae: Gobiosomatini). Zool. J. Linn. Soc. 2023, 197, 322–343. [Google Scholar] [CrossRef]
- Tornabene, L.; Van Tassell, J.L. Redescription of the goby genus Gobiosoma (Teleostei: Gobiidae: Gobiosomatini), with the synonymy of the genus Enypnias. J. Nat. Hist. 2014, 48, 1413–1437. [Google Scholar] [CrossRef]
- Miller, P.J. Gobiidae. In Fishes of the North-Eastern Atlantic and the Mediterranean; Whitehead, P.J.P., Bauchot, M.-L., Hureau, J.-C., Nielsen, J., Tortonese, E., Eds.; UNESCO: Paris, France, 1986; Volume 3, pp. 1019–1085. [Google Scholar]
- Van Tassell, J.L.; Miller, P.J.; Brito, A. A revision of Vanneaugobius (Teleostei: Gobiidae), with description of a new species. J. Nat. Hist. 1988, 22, 545–567. [Google Scholar] [CrossRef]
- Greenfield, D.W.; Randall, J.E. The marine gobies of the Hawaiian Islands. Proc. Calif. Acad. Sci. 2004, 55, 498–549. [Google Scholar]
- Kovačić, M.; Bogorodsky, S.V. Two new species of Cabillus (Perciformes: Gobiidae) and the first record of Cabillus macrophthalmus from the Western Indian Ocean. Zootaxa 2013, 3717, 179–194. [Google Scholar] [CrossRef]
- Shibukawa, K.; Aizawa, M. Cabillus pexus, a new marine goby (Teleostei, Gobiidae) from Amami-Oshima Island, Ryukyu Islands, Japan. Bull. Natl. Mus. Nat. Sci. Ser. A 2013, 39, 133–142. [Google Scholar]
- Delventhal, N.R.; Mooi, R.D. Callogobius winterbottomi, a new species of goby (Teleostei: Gobiidae) from the Western Indian Ocean. Zootaxa 2013, 3630, 155–164. [Google Scholar] [CrossRef]
- Sanzo, L. Distribuzione delle papille cutanee (organi ciatiformi) e suo valore sistematico nei Gobi. Mitteilungen Aus Der Zool. Stn. Zu Neapel 1911, 20, 249–328. [Google Scholar]
- Lachner, E.A.; McKinney, J.F. Barbuligobius boehlkei, a new Indo-Pacific genus and species of Gobiidae (Pisces), with notes on the genera Callogobius and Pipidonia. Copeia 1974, 1974, 869–879. [Google Scholar] [CrossRef]
- Kovačić, M.; Bogorodsky, S.V.; Mal, A.O. A new species of Hetereleotris (Perciformes: Gobiidae) from Farasan Island (Red Sea). Zootaxa 2014, 3846, 119–126. [Google Scholar] [CrossRef]
- Kovačić, M.; Bogorodsky, S.V.; Troyer, E.M.; Tornabene, L. Cerogobius petrophilus (Perciformes: Gobiidae), a new gobiid genus and species from the Red Sea. Zootaxa 2019, 4565, 171–189. [Google Scholar] [CrossRef]
- Greenfield, D.W. An overview of the dwarfgobies, the second most speciose coral-reef fish genus (Teleostei: Gobiidae: Eviota). J. Ocean. Sci. Found. 2017, 54, 32–54. [Google Scholar]
- Hodge, J.R.; Read, C.I.; van Herwerden, L.; Bellwood, D.R. The role of peripheral endemism in species diversification: Evidence from the coral reef fish genus Anampses (Family: Labridae). Mol. Phylogenet. Evol. 2012, 62, 653–663. [Google Scholar] [CrossRef]
- Bowen, B.W.; Rocha, L.A.; Toonen, R.J.; Karl, S.A.; Laboratory, T.T. The origins of tropical marine biodiversity. Trends Ecol. Evol. 2013, 28, 359–366. [Google Scholar] [CrossRef]
- van der Meer, M.H.; Horne, J.B.; Gardner, M.G.; Hobbs, J.P.; Pratchett, M.; van Herwerden, L. Limited contemporary gene flow and high self-replenishment drives peripheral isolation in an endemic coral reef fish. Ecol. Evol. 2013, 3, 1653–1666. [Google Scholar] [CrossRef]
- Johnson, K.; Tornabene, L.; Li, C.; Rüber, L.; Schliewen, U.; Hogan, D.; Pezold, F. Exon-capture data resolve relationships resulting from a rapid radiation within family Gobiidae. Mol. Phylogenet. Evol. 2025, 212, 108424. [Google Scholar] [CrossRef]
- Larson, H.K. A revision of the gobiid genus Bryaninops (Pisces), with a description of six new species. Beagle 1985, 2, 57–93. [Google Scholar] [CrossRef]
- Suzuki, T.; Greenfield, D.W. Two new dwarfgobies from the Ryukyu Islands, Japan: Eviota shibukawai and Eviota filamentosa (Teleostei: Gobiidae). J. Ocean. Sci. Found. 2014, 11, 32–39. [Google Scholar]
- Lachner, E.A.; Karnella, S.J. Fishes of the Indo-Pacific genus Eviota with descriptions of eight new species (Teleostei, Gobiidae). Smithson. Contrib. Zool. 1980, 315, 1–127. [Google Scholar] [CrossRef]
- Jewett, S.L.; Lachner, E.A. Seven new species of the Indo-Pacific genus Eviota (Pisces: Gobiidae). Proc. Biol. Soc. Wash. 1983, 96, 780–806. [Google Scholar]
- Vaz, D.F.B.; Goatley, C.H.R.; Tornabene, L. Osteology of dwarfgobies Eviota and Sueviota (Gobiidae: Gobiomorpharia), with phylogenetic inferences within coral gobies. J. Morphol. 2025, 286, e70039. [Google Scholar] [CrossRef] [PubMed]
- Meiri, S.; Dayan, T. On the validity of Bergmann’s rule. J. Biogeogr. 2003, 30, 331–351. [Google Scholar] [CrossRef]
- Fernández-Torres, F.; Martínez, P.A.; Olalla-Tárraga, M.Á. Shallow water ray-finned marine fishes follow Bergmann’s rule. Basic Appl. Ecol. 2018, 33, 99–110. [Google Scholar] [CrossRef]
- Troyer, E.M.; Betancur, R.R.; Hughes, L.C.; Westneat, M.; Carnevale, G.; White, W.T.; Pogonoski, J.J.; Tyler, J.C.; Baldwin, C.C.; Orti, G.; et al. The impact of paleoclimatic changes on body size evolution in marine fishes. Proc. Natl. Acad. Sci. USA 2022, 119, e2122486119. [Google Scholar] [CrossRef]
- Chakraborty, A.; Sakai, M.; Iwatsuki, Y. Museum fish specimens and molecular taxonomy: A comparative study on DNA extraction protocols and preservation techniques. J. Appl. Ichthyol. 2006, 22, 160–166. [Google Scholar] [CrossRef]
- Moreau, C.S.; Wray, B.D.; Czekanski-Moir, J.E.; Rubin, B.E.R. DNA preservation: A test of commonly used preservatives for insects. Invertebr. Syst. 2013, 27, 81–86. [Google Scholar] [CrossRef]
- Faulwetter, S.; Vasileiadou, A.; Kouratoras, M.; Dailianis, T.; Arvanitidis, C. Micro-computed tomography: Introducing new dimensions to taxonomy. ZooKeys 2013, 263, 1–45. [Google Scholar] [CrossRef]
- Robertson, D.R.; Baldwin, C.C.; Bellwood, D.; Pyle, R.; Smith-Vaniz, W.F.; Tornabene, L.; Van Tassell, J.L. Aspiration or expiration: Hypoxia and the interpretation of fish predation in the fossil record. Palaios 2019, 34, 245–247. [Google Scholar] [CrossRef]
- Leal, E.; Fernandez-Duran, B.; Guillot, R.; Rios, D.; Cerda-Reverter, J.M. Stress-induced effects on feeding behavior and growth performance of the sea bass (Dicentrarchus labrax): A self-feeding approach. J. Comp. Physiol. B 2011, 181, 1035–1044. [Google Scholar] [CrossRef]
- Brandl, S.J.; Casey, J.M.; Meyer, C.P. Dietary and habitat niche partitioning in congeneric cryptobenthic reef fish species. Coral Reefs 2020, 39, 305–317. [Google Scholar] [CrossRef]
- Depczynski, M.; Bellwood, D.R. The role of cryptobenthic reef fishes in coral reef trophodynamics. Mar. Ecol. Prog. Ser. 2003, 256, 183–191. [Google Scholar] [CrossRef]
- Kramer, M.J.; Bellwood, O.; Bellwood, D.R. The trophic importance of algal turfs for coral reef fishes: The crustacean link. Coral Reefs 2013, 32, 575–583. [Google Scholar] [CrossRef]
- Depczynski, M.; Bellwood, D.R. Extremes, plasticity, and invariance in vertebrate life history traits: Insights from reef fishes. Ecology 2006, 87, 3119–3127. [Google Scholar] [CrossRef] [PubMed]
- Mihalitsis, M.; Morais, R.A.; Bellwood, D.R. Small predators dominate fish predation in coral reef communities. PLoS Biol. 2022, 20, e3001898. [Google Scholar] [CrossRef] [PubMed]
- Grutter, A.S.; Bshary, R. Cleaner fish, Labroides dimidiatus, diet preferences for different types of mucus and parasitic gnathiid isopods. Anim. Behav. 2004, 68, 583–588. [Google Scholar] [CrossRef]
- GBIF. The Global Biodiversity Information Facility. Available online: https://www.gbif.org (accessed on 1 April 2025).
- Winterbottom, R.; Hoese, D.F. A revision of the Australian species of Trimma (Actinopterygii, Gobiidae), with descriptions of six new species and redescriptions of twenty-three valid species. Zootaxa 2015, 3934, 1–102. [Google Scholar] [CrossRef] [PubMed][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).