Use of Essential Oil from Aloysia citrodora Paláu in Anesthesia and Simulated Transport of Tambaqui Colossoma macropomum (Cuvier 1826) at Two Different Cargo Densities
Abstract
1. Introduction
2. Materials and Methods
2.1. Acquisition and Maintenance Conditions of the Animals
2.2. Acquisition of the Essential Oil from Aloysia citrodora
2.3. Anesthetic Induction and Recovery Experiment
2.4. Simulated Transport Experiment
2.5. Sample Collection
2.6. Hematological, Plasmatic, and Liver Analysis
2.7. Ventilatory Rate (VR) Experiment
2.8. Statistical Analysis
3. Results
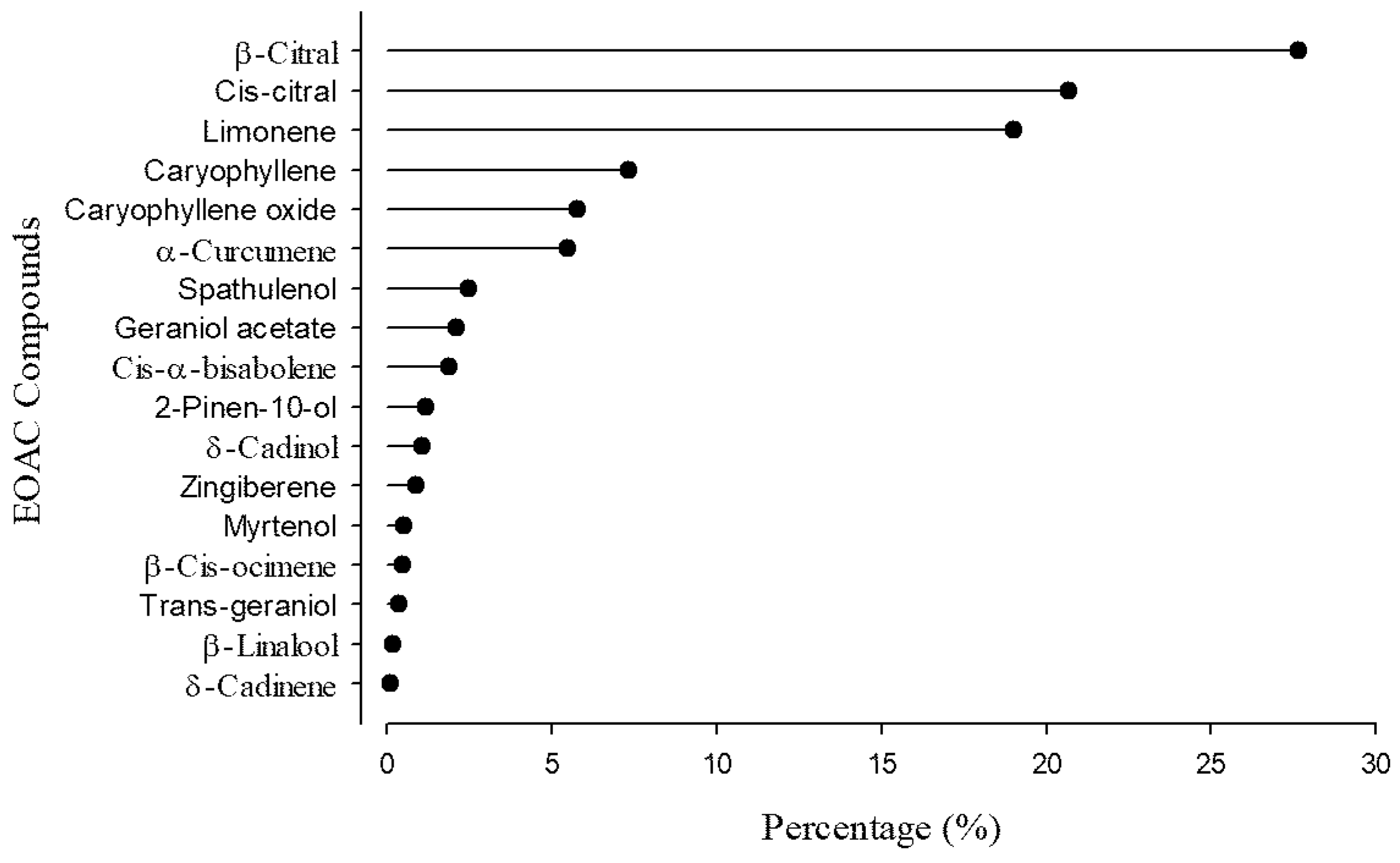
3.1. Composition of the Essential Oil from Aloysia citrodora
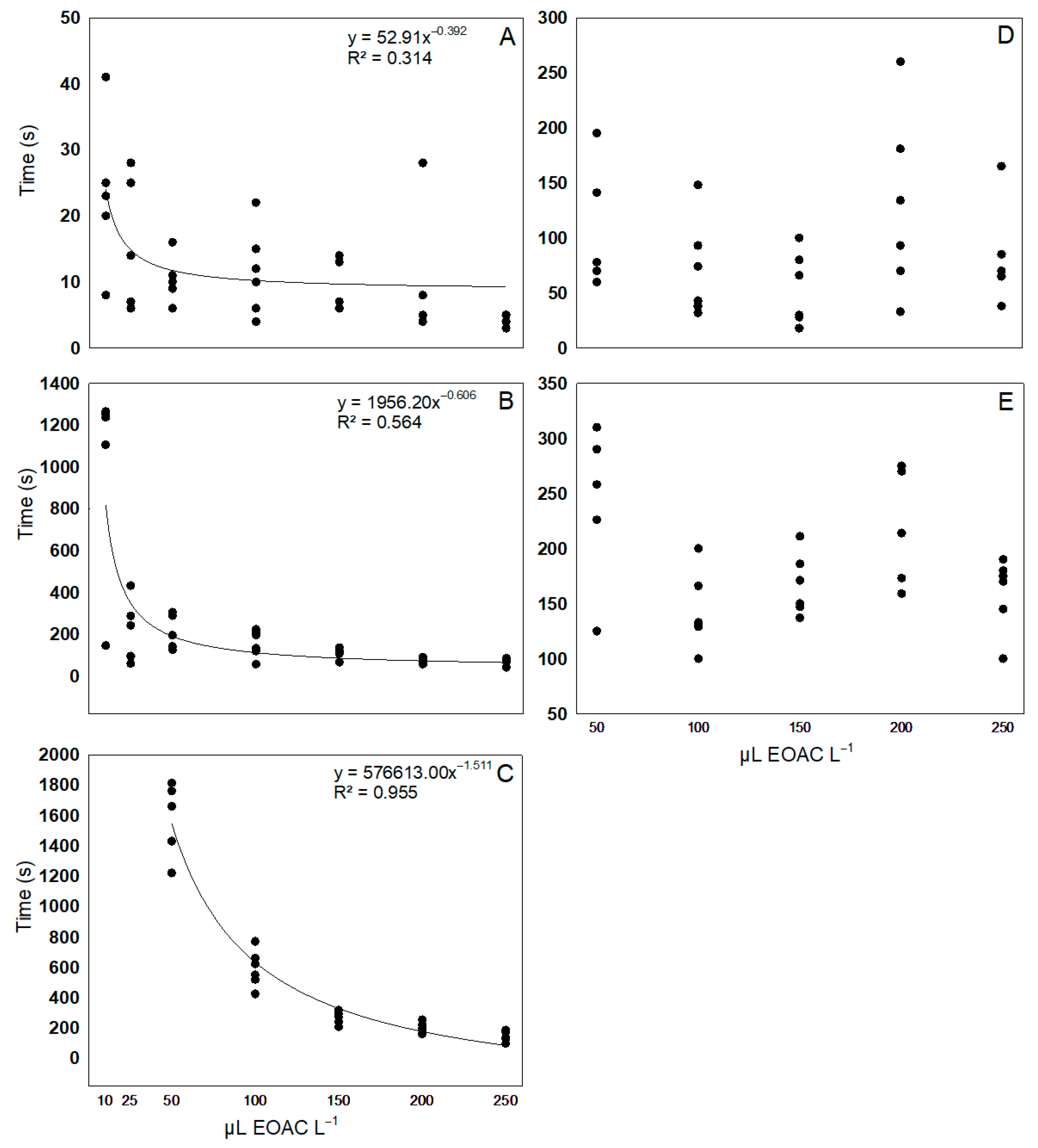
3.2. Experiment 1: Anesthetic Induction and Recovery
3.3. Experiment 2: Simulated Transport
3.3.1. Water Quality
3.3.2. Hematological Analysis
3.3.3. Biochemical Analysis
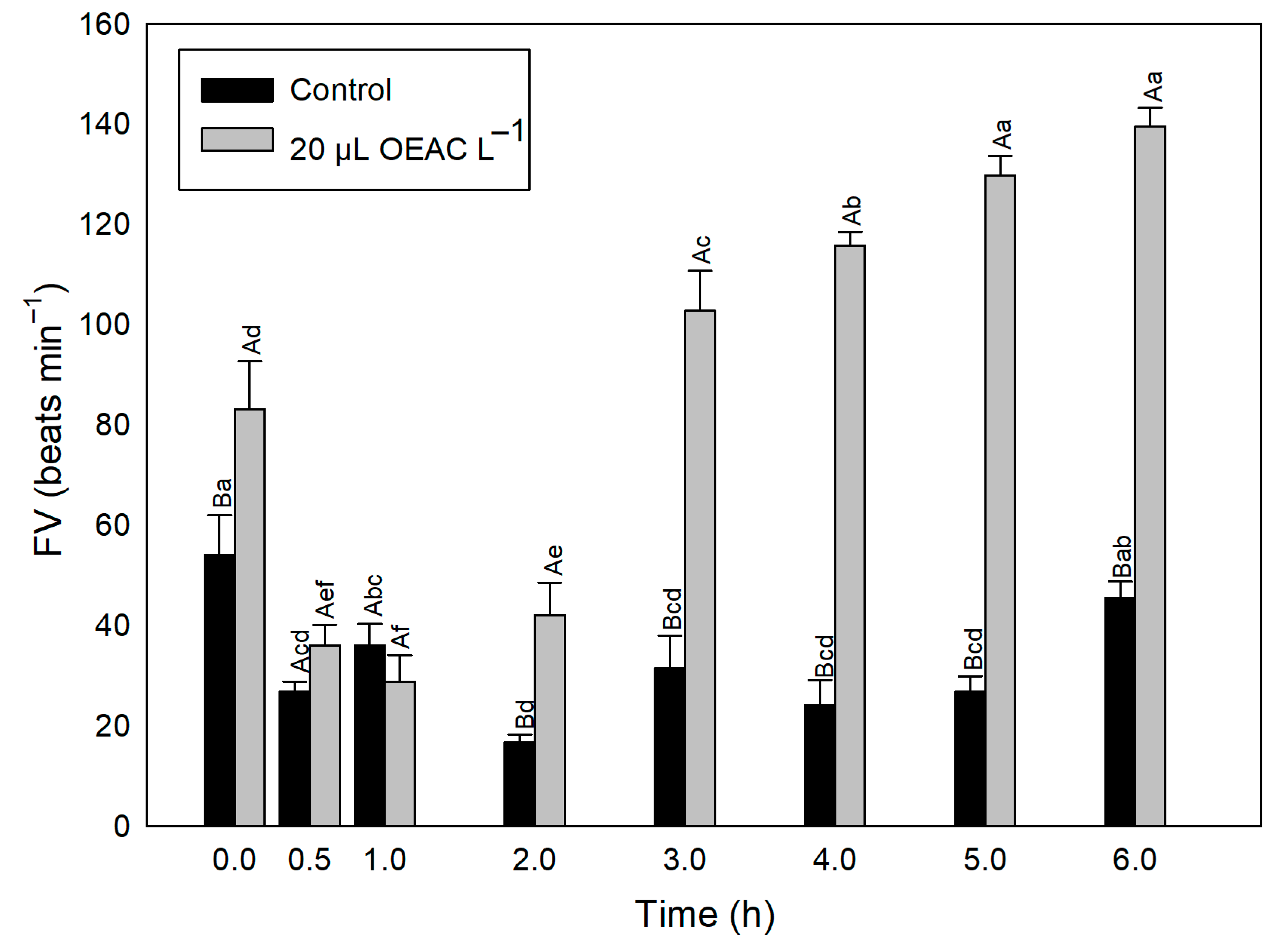
3.4. Experiment 3: Ventilatory Rate (VR)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Embrapa CIAqui—Centro de Inteligência e Mercado em Aquicultura: Comércio exterior—Exportação 2025. Available online: https://www.embrapa.br/cim-centro-de-inteligencia-e-mercado-em-aquicultura (accessed on 27 August 2025).
- Neves, L.; Favero, G.C.; Beier, S.L.; Ferreira, N.S.; Palheta, G.D.A.; Melo, N.F.A.C.; Luz, R.K. Physiological and metabolic responses in juvenile Colossoma macropomum exposed to hypoxia. Fish. Physiol. Biochem. 2020, 46, 2157–2167. [Google Scholar] [CrossRef]
- Chung, S.; Ribeiro, K.; Teixeira, D.V.; Copatti, C.E. Inclusion of essential oil from ginger in the diet improves physiological parameters of tambaqui juveniles (Colossoma macropomum). Aquaculture 2021, 543, 736934. [Google Scholar] [CrossRef]
- Sena, A.C.; Teixeira, R.R.; Ferreira, E.L.; Heinzmann, B.M.; Baldisserotto, B.; Caron, B.O.; Schmidt, D.; Couto, R.D.; Copatti, C.E. Essential oil from Lippia alba has anaesthetic activity and is effective in reducing handling and transport stress in tambacu (Piaractus mesopotamicus × Colossoma macropomum). Aquaculture 2016, 465, 374–379. [Google Scholar] [CrossRef]
- Teixeira, R.R.; Souza, R.C.; Sena, A.C.; Baldisserotto, B.; Heinzmann, B.M.; Couto, R.D.; Copatti, C.E. Essential oil of Aloysia triphylla in Nile tilapia: Anaesthesia, stress parameters and sensory evaluation of fillets. Aquac. Res. 2017, 48, 3383–3392. [Google Scholar] [CrossRef]
- Teixeira, R.R.; de Souza, R.C.; Sena, A.C.; Baldisserotto, B.; Heinzmann, B.M.; Copatti, C.E. Essential oil of Aloysia triphylla is effective in Nile tilapia transport. Bol. Inst. Pesca 2018, 44, 17–24. [Google Scholar] [CrossRef]
- Simões-Bueno, L.N.; Copatti, C.E.; Gomes, L.C.; Val, A.L.; Amanajás, R.D.; Caron, B.O.; Heinzmann, B.M.; Baldisserotto, B. Linalool chemotype essential oil from Lippia alba in the anesthesia of fat snook (Centropomus parallelus): Ventilatory rate, biochemical, antioxidant, and oxidative status parameters. Neotrop. Ichthyol. 2024, 22, e230114. [Google Scholar] [CrossRef]
- Fang, D.; Mei, J.; Xie, J.; Qiu, W. The effects of transport stress (temperature and vibration) on blood biochemical parameters, oxidative stress, and gill histomorphology of pearl gentian groupers. Fishes 2023, 8, 218. [Google Scholar] [CrossRef]
- Hong, J.; Chen, X.; Liu, S.; Fu, Z.; Han, M.; Wang, Y.; Gu, Z.; Ma, Z. Impact of fish density on water quality and physiological response of golden pompano (Trachinotus ovatus) fingerlings during transportation. Aquaculture 2019, 507, 260–265. [Google Scholar] [CrossRef]
- Lima, A.F.; Oliveira, H.J.B.; Pereira, A.S.; Sakamoto, S.S. Effect of density of fingerling and juvenile pirarucu during transportation on water quality and physiological parameters. Acta Amaz. 2020, 50, 223–231. [Google Scholar] [CrossRef]
- Hohlenwerger, J.C.; Copatti, C.E.; Sena, A.C.; Couto, R.D.; Baldisserotto, B.; Heinzmann, B.M.; Caron, B.O.; Schmidt, D. Could the essential oil of Lippia alba provide a readily available and cost-effective anaesthetic for Nile tilapia (Oreochromis niloticus)? Mar. Freshw. Behav. Physiol. 2016, 49, 119–126. [Google Scholar] [CrossRef]
- Taheri Mirghaed, A.; Yasari, M.; Mirzargar, S.S.; Hoseini, S.M. Rainbow trout (Oncorhynchus mykiss) anesthesia with myrcene: Efficacy and physiological responses in comparison with eugenol. Fish. Physiol. Biochem. 2018, 44, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Aydın, B.; Barbas, L.A.L. Sedative and anesthetic properties of essential oils and their active compounds in fish: A review. Aquaculture 2020, 520, 734999. [Google Scholar] [CrossRef]
- Becker, A.G.; Parodi, T.V.; Zeppenfeld, C.C.; Salbego, J.; Cunha, M.A.; Heldwein, C.G.; Loro, V.L.; Heinzmann, B.M.; Baldisserotto, B. Pre-sedation and transport of Rhamdia quelen in water containing essential oil of Lippia alba: Metabolic and physiological responses. Fish. Physiol. Biochem. 2016, 42, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.P.B.; Lemos, C.H.P.; Felix e Silva, A.; Souza, S.A.; Albinati, A.C.L.; Lima, A.O.; Copatti, C.E. Use of eugenol for the anaesthesia and transportation of freshwater angelfish (Pterophyllum scalare). Aquaculture 2019, 513, 734409. [Google Scholar] [CrossRef]
- Hohlenwerger, J.C.; Baldisserotto, B.; Couto, R.D.; Heinzmann, B.M.; Silva, D.T.; Caron, B.O.; Schmidt, D.; Copatti, C.E. Essential oil of Lippia alba in the transport of Nile tilapia. Ciência Rural 2017, 47, e20160040. [Google Scholar] [CrossRef]
- Oliveira, I.C.; Oliveira, R.S.M.; Lemos, C.H.P.; Oliveira, C.P.B.; Felix e Silva, A.; Lorenzo, V.P.; Lima, A.O.; Cruz, A.L.; Copatti, C.E. Essential oils from Cymbopogon citratus and Lippia sidoides in the anesthetic induction and transport of ornamental fish Pterophyllum scalare. Fish. Physiol. Biochem. 2022, 48, 501–519. [Google Scholar] [CrossRef]
- Bahramsoltani, R.; Rostamiasrabadi, P.; Shahpiri, Z.; Marques, A.M.; Rahimi, R.; Farzaei, M.H. Aloysia citrodora Paláu (Lemon verbena): A review of phytochemistry and pharmacology. J. Ethnopharmacol. 2018, 222, 34–51. [Google Scholar] [CrossRef]
- Brandão, F.R.; Farias, C.F.S.; Souza, D.C.M.; Oliveira, M.I.B.; Matos, L.V.; Majolo, C.; Oliveira, M.R.; Chaves, F.C.M.; O’Sullivan, F.L.A.; Chagas, E.C. Anesthetic potential of the essential oils of Aloysia triphylla, Lippia sidoides and Mentha piperita for Colossoma macropomum. Aquaculture 2021, 534, 736275. [Google Scholar] [CrossRef]
- Santos, A.C.; Bianchini, A.E.; Bandeira Junior, G.; Garlet, Q.I.; Brasil, M.T.B.; Heinzmann, B.M.; Baldisserotto, B.; Caron, B.O.; Cunha, M.A. Essential oil of Aloysia citriodora Paláu and citral: Sedative and anesthetic efficacy and safety in Rhamdia quelen and Ctenopharyngodon idella. Vet. Anaesth. Analg. 2022, 49, 104–112. [Google Scholar] [CrossRef]
- Parodi, T.V.; Cunha, M.A.; Becker, A.G.; Zeppenfeld, C.C.; Martins, D.I.; Koakoski, G.; Barcellos, L.G.; Heinzmann, B.M.; Baldisserotto, B. Anesthetic activity of the essential oil of Aloysia triphylla and effectiveness in reducing stress during transport of albino and gray strains of silver catfish, Rhamdia quelen. Fish. Physiol. Biochem. 2014, 40, 323–334. [Google Scholar] [CrossRef]
- Ali, H.B.; Masoumeh, M.; Mohammad, S.; Mohammad, M. The effect of ethanol extract of Aloysia triphylla on anesthesia and improve the physiological parameters of rainbow trout (Oncorhynchus mykiss) after transfer. J. Aquat. Anim. Nutr. 2023, 9, 1–14. [Google Scholar]
- Burgess, D.R.; Linstrom, P.J.; Mallard, W.G. NIST Chemistry WebBook; Linstrom, PJ: Gaithersburg, MA, USA, 2016. [Google Scholar]
- Small, B.C. Anesthetic efficacy of metomidate and comparison of plasma cortisol responses to tricaine methanesulfonate, quinaldine and clove oil anesthetized channel catfish Ictalurus punctatus. Aquaculture 2003, 218, 177–185. [Google Scholar] [CrossRef]
- Gomes, L.C.; Araujo-Lima, C.A.R.M.; Roubach, R.; Chippari-Gomes, A.R.; Lopes, N.; Urbinati, E.C. Effect of fish density during transportation on stress and mortality of juvenile tambaqui Colossoma macropomum. J. World Aquac. Soc. 2003, 34, 76–84. [Google Scholar] [CrossRef]
- Becker, A.G.; Parodi, T.V.; Heldwein, C.G.; Zeppenfeld, C.C.; Heinzmann, B.M.; Baldisserotto, B. Transportation of silver catfish, Rhamdia quelen, in water with eugenol and the essential oil of Lippia alba. Fish. Physiol. Biochem. 2012, 38, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, F.D.F.; Freire, C.A. An overview of stress physiology of fish transport: Changes in water quality as a function of transport duration. Fish Fish. 2016, 17, 1055–1072. [Google Scholar] [CrossRef]
- Pramod, P.K.; Ramachandran, A.; Sajeevan, T.P.; Thampy, S.; Pai, S.S. Comparative efficacy of MS-222 and benzocaine as anaesthetics under simulated transport conditions of a tropical ornamental fish Puntius filamentosus (Valenciennes). Aquac. Res. 2010, 41, 309–314. [Google Scholar] [CrossRef]
- CONCEA. Diretrizes da Prática de Eutanásia do Conselho Nacional de Controle de Experimentação Animal; Ministério da Ciência, Tecnologia e Inovação: Brasília, Brazil, 2018. [Google Scholar]
- Blaxhall, P.C.; Daisley, K.W. Routine haematological methods for use with fish blood. J. Fish. Biol. 1973, 5, 771–781. [Google Scholar] [CrossRef]
- Bidinotto, P.M.; Souza, R.H.S.; Moraes, G. Hepatic glycogen in eight tropical freshwater teleost fish: A procedure for field determinants of microsamples. Bol. Téc. CEPTA 1997, 10, 53–60. [Google Scholar]
- Silva, H.N.P.; Carvalho, B.C.F.; Maia, J.L.D.S.; Becker, A.G.; Baldisserotto, B.; Heinzmann, B.M.; Mourão, R.H.V.; Silva, L.V.F. Anesthetic potential of the essential oils of Lippia alba and Lippia origanoides in tambaqui juveniles. Ciência Rural 2019, 49, e20181059. [Google Scholar] [CrossRef]
- Santos, A.C.; Bandeira Junior, G.; Zago, D.C.; Zeppenfeld, C.C.; Silva, D.T.; Heinzmann, B.M.; Baldisserotto, B.; Cunha, M.A. Anesthesia and anesthetic action mechanism of essential oils of Aloysia triphylla and Cymbopogon flexuosus in silver catfish (Rhamdia quelen). Vet. Anaesth. Analg. 2017, 44, 106–113. [Google Scholar] [CrossRef]
- Limma-Netto, J.D.; Sena, A.C.; Copatti, C.E. Essential oils of Ocimum basilicum and Cymbopogon flexuosus in the sedation, anesthesia and recovery of tambacu (Piaractus mesopotamicus male x Colossoma macropomum Female). Bol. Inst. Pesca 2016, 42, 727–733. [Google Scholar] [CrossRef]
- Lopes, J.M.; Souza, C.F.; Schindler, B.; Pinheiro, C.G.; Salbego, J.; Siqueira, J.C.; Heinzmann, B.M.; Baldisserotto, B. Essential oils from Citrus x aurantium and Citrus x latifolia (Rutaceae) have anesthetic activity and are effective in reducing ion loss in silver catfish (Rhamdia quelen). Neotrop. Ichthyol. 2018, 16, e170152. [Google Scholar] [CrossRef]
- Silva, R.C.; Silva, L.R.; França, I.F.; Lopes, J.M.; Pantoja, B.T.S.; Pereira, M.M.; Ramos, L.R.V. Anesthetic effect and acute toxicity of Citrus sinensis essential oil in betta. Bol. Inst. Pesca 2023, 49, e816. [Google Scholar] [CrossRef]
- Tarkhani, R.; Imani, A.; Jamali, H.; Farsani, H.G. Anaesthetic efficacy of eugenol on various size classes of angelfish (Pterophyllum scalare Schultze, 1823). Aquac. Res. 2017, 48, 5263–5270. [Google Scholar] [CrossRef]
- Wang, Z.J.; Heinbockel, T.T. Essential oils and their constituents targeting the GABAergic system and sodium channels as treatment of neurological diseases. Molecules 2018, 23, 1061. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.A.R.D.A.; Kohn, D.O.; Lima, V.M.; Gargano, A.C.; Flório, J.C.; Costa, M. The GABAergic system contributes to the anxiolytic-like effect of essential oil from Cymbopogon citratus (lemongrass). J. Ethnopharmacol. 2011, 137, 828–836. [Google Scholar] [CrossRef]
- Nesterkina, M.; Kravchenko, I. Synthesis and pharmacological properties of novel esters based on monocyclic terpenes and GABA. Pharmaceuticals 2016, 9, 32. [Google Scholar] [CrossRef]
- Hacke, A.C.M.; Miyoshi, E.; Marques, J.A.; Pereira, R.P. Anxiolytic properties of Cymbopogon citratus (DC.) Stapf extract, essential oil and its constituents in zebrafish (Danio rerio). J. Ethnopharmacol. 2020, 260, 113036. [Google Scholar] [CrossRef] [PubMed]
- Heldwein, C.G.; Silva, L.L.; Reckziegel, P.; Barros, F.M.C.; Bürger, M.E.; Baldisserotto, B.; Mallmann, C.A.; Schmidt, D.; Caron, B.O.; Heinzmann, B.M. Participation of the GABAergic system in the anesthetic effect of Lippia alba (Mill.) N.E. Brown essential oil. Braz. J. Med. Biol. Res. 2012, 45, 436–443. [Google Scholar] [CrossRef]
- Vale, T.G.; Matos, F.J.A.; Lima, T.C.M.; Viana, G.S.B. Behavioral effects of essential oils from Lippia alba (Mill.) N.E. Brown chemotypes. J. Ethnopharmacol. 1999, 167, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Yoshioka, M.; Yokogoshi, H. Sub-chronic effects of s-limonene on brain neurotransmitter levels and behavior of rats. J. Nutr. Sci. Vitaminol. 2009, 55, 367–373. [Google Scholar] [CrossRef]
- Song, Y.; Seo, S.; Lamichhane, S.; Seo, J.; Hong, J.T.; Cha, H.J.; Yun, J. Limonene has anti-anxiety activity via adenosine A2A receptor-mediated regulation of dopaminergic and GABAergic neuronal function in the striatum. Phytomedicine 2021, 83, 153474. [Google Scholar] [CrossRef]
- He, R.; Lei, B.; Su, Y.; Wang, A.; Cui, K.; Shi, X.; Chen, X. Effectiveness of eugenol as an anesthetic for adult spotted sea bass (Lateolabrax maculatus). Aquaculture 2020, 523, 735180. [Google Scholar] [CrossRef]
- Lam, P.H.; Vo, H.D.N.; Truong, L.M.T.; Dang, D.M.T.; Dang, C.M.; Doan, T.C.D.; Mollaamin, F.; Monajjemi, M. Anesthetic effects of clove basil essential oil (Ocimum gratissimum) microemulsion on asian redtail catfish (Hemibagrus wyckioides) and Its Biochemical Stress Indicators. Fishes 2025, 10, 104. [Google Scholar] [CrossRef]
- Roohi, Z.; Imanpoor, M.R. The efficacy of the oils of spearmint and methyl salicylate as new anesthetics and their effect on glucose levels in common carp (Cyprinus carpio L., 1758) juveniles. Aquaculture 2015, 437, 327–332. [Google Scholar] [CrossRef]
- Ventura, A.S.; Corrêa Filho, R.A.C.; Teodoro, G.C.; Laice, L.M.; Barbosa, P.T.L.; Stringhetta, G.R.; Jerônimo, G.T.; Povh, J.A. Essential oil of Ocimum basilicum and eugenol as sedatives for Nile tilapia. J. Agric. Stud. 2020, 8, 657–665. [Google Scholar] [CrossRef]
- Boaventura, T.P.; Souza, C.F.; Ferreira, A.L.; Favero, G.C.; Baldissera, M.D.; Heinzmann, B.M.; Baldisserotto, B.; Luz, R.K. Essential oil of Ocimum gratissimum (Linnaeus, 1753) as anesthetic for Lophiosilurus alexandri: Induction, recovery, hematology, biochemistry and oxidative stress. Aquaculture 2020, 529, 735676. [Google Scholar] [CrossRef]
- Janssen, G.J.A.; Jerrett, A.R.; Black, S.E.; Forster, M.E. The effects of progressive hypoxia and re-oxygenation on cardiac function, white muscle perfusion and haemoglobin saturation in anaesthetised snapper (Pagrus auratus). J. Comp. Physiol. B 2010, 180, 503–510. [Google Scholar] [CrossRef]
- Luz, R.K.; Favero, G.C. Use of salt, anesthetics, and cargo density in transport of live fish: A review. Fishes 2024, 9, 286. [Google Scholar] [CrossRef]
- Aride, P.H.R.; Roubach, R.; Val, A.L. Tolerance response of tambaqui Colossoma macropomum (Cuvier) to water pH. Aquac. Res. 2007, 38, 588–594. [Google Scholar] [CrossRef]
- Zimmer, A.M. Ammonia excretion by the fish gill: Discoveries and ideas that shaped our current understanding. J. Comp. Physiol. B 2024, 194, 697–715. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Kou, N.; Liu, X.; Yang, D. Oxygen Consumption and Ammonia Excretion of Marphysa sanguinea (Polychaeta: Eunicidae) in relation to body mass and temperature. Fishes 2021, 6, 52. [Google Scholar] [CrossRef]
- Fazio, F. Fish hematology analysis as an important tool of aquaculture: A review. Aquaculture 2019, 500, 237–242. [Google Scholar] [CrossRef]
- Witeska, M.; Kondera, E.; Ługowska, K.; Bojarski, B. Hematological methods in fish—Not only for beginners. Aquaculture 2022, 547, 737498. [Google Scholar] [CrossRef]
- Davis, A.K.; Maney, D.L.; Maerz, J.C. The use of leukocyte profiles to measure stress in vertebrates: A review for ecologists. Funct. Ecol. 2008, 22, 760–772. [Google Scholar] [CrossRef]
- Dinesh, R.; Daniel, N.; Stephen, J.; Kumar, S. Haematological parameters as reliable stress indicators in fish. J. Agric. Environ. 2021, 2, 48–52. [Google Scholar]
- Wendelaar-Bonga, S.E. The stress response in fish. Physiol. Rev. 1997, 77, 591–625. [Google Scholar] [CrossRef]
- Dai, Y.; Shen, Y.; Gu, J.; Yang, H.; Chen, F.; Zhang, W.; Wu, W.; Xu, X.; Li, J. Glycolysis and gluconeogenesis are involved of glucose metabolism adaptation during fasting and re-feeding in black carp (Mylopharyngodon piceus). Aquac. Fish. 2024, 9, 226–233. [Google Scholar] [CrossRef]
- Milligan, C.L. A regulatory role for cortisol in muscle glycogen metabolism in rainbow trout Oncorhynchus mykiss Walbaum. J. Exp. Biol. A 2003, 206, 3167–3173. [Google Scholar] [CrossRef]
- Polakof, S.; Míguez, J.M.; Moon, T.W.; Soengas, J.L. Evidence for the presence of a glucosensor in hypothalamus, hindbrain, and Brockmann bodies of rainbow trout. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R1657–R1666. [Google Scholar] [CrossRef][Green Version]
- Conde-Sieira, M.; Aguilar, A.J.; López-Patiño, M.A.; Míguez, J.M.; Soengas, J.L. Stress alters food intake and glucosensing response in hypothalamus, hindbrain, liver, and Brockmann bodies of rainbow trout. Physiol. Behav. 2010, 101, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Javed, M.; Usmani, N. Stress response of biomolecules (carbohydrate, protein and lipid profiles) in fish Channa punctatus inhabiting river polluted by thermal power plant effluent. Saudi J. Biol. Sci. 2015, 22, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Lei, C.; Li, Z.; Lei, Y.; Luo, C.; Shao, L.; Huang, C.; Yang, P. Effects of a diet of Phragmites australis instead of Triticum aestivum L. on immune performance and liver tissue structure of Ctenopharyngodon idellus. Fishes 2022, 7, 378. [Google Scholar] [CrossRef]
| Variables | Non-Transported | Control–LCD | Control–SCD | EOAC-LCD | EOAC-SCD |
|---|---|---|---|---|---|
| DO | 4.50 ± 1.22 | 8.63 ± 0.20 Aa* | 8.54 ± 0.22 Aa* | 8.71 ± 0.18 Aa* | 8.75 ± 0.20 Aa* |
| Alkalinity | 42.50 ± 2.04 | 45.00 ± 4.08 Aa | 45.00 ± 4.08 Aa | 35.00 ± 4.08 Bb | 45.00 ± 4.08 Aa |
| Hardness | 115.00 ± 4.08 | 120.00 ± 8.16 Aa | 125.00 ± 4.08 Aa | 125.00 ± 4.08 Aa | 115.00 ± 4.08 Aa |
| Total ammonia | 0.13 ± 0.02 | 1.50 ± 0.14 Aa* | 1.75 ± 0.14 Aa* | 1.00 ± 0.25 Ab* | 1.75 ± 0.25 Aa* |
| UIA | 0.72 ± 0.06 | 1.68 ± 0.15 Ab* | 2.21 ± 0.17 Aa* | 1.15 ± 0.16 Bb | 1.67 ± 0.21 Ba* |
| Nitrite | 0.013 ± 0.01 | 0.013 ± 0.01 Aa | 0.025 ± 0.004 Aa | 0.013 ± 0.010 Aa | 0.025 ± 0.004 Aa |
| Temperature | 28.63 ± 0.10 | 28.83 ± 0.14 Aa | 28.88 ± 0.10 Aa | 28.90 ± 0.08 Aa | 28.85 ± 0.12 Aa |
| pH | 6.90 ± 0.08 | 6.20 ± 0.20 Aa* | 6.25 ± 0.15 Aa* | 6.21 ± 0.23 Aa* | 6.13 ± 0.10 Aa* |
| Variables | Non-Transported | Control–LCD | Control–SCD | EOAC-LCD | EOAC-SCD |
|---|---|---|---|---|---|
| Erythrocytes | 1.60 ± 0.04 | 1.77 ± 0.05 Ab | 1.96 ± 0.06 Aa* | 1.53 ± 0.08 Ba | 1.50 ± 0.03 Ba |
| Hemoglobin | 7.33 ± 0.22 | 8.40 ± 0.22 Aa* | 9.01 ± 0.39 Aa* | 7.15 ± 0.34 Ba | 6.90 ± 0.19 Ba |
| Hematocrit | 21.33 ± 0.80 | 23.33 ± 0.71 Aa | 25.50 ± 1.33 Aa* | 19.50 ± 1.18 Ba | 20.16 ± 0.60 Ba |
| MCV | 133.83 ± 2.46 | 131.16 ± 1.93 Aa | 129.50 ± 3.53 Aa | 126.83 ± 2.83 Aa | 134.50 ± 1.72 Aa |
| HCM | 46.16 ± 0.65 | 47.17 ± 0.94 Aa | 45.83 ± 0.65 Aa | 46.83 ± 0.70 Aa | 46.00 ± 0.68 Aa |
| MCHC | 34.66 ± 0.49 | 36.00 ± 0.51 Aa | 35.50 ± 0.61 Aa | 37.00 ± 0.81 Aa* | 34.00 ± 0.25 Ab |
| Total leukocytes | 2.66 ± 0.25 | 3.26 ± 0.28 Aa | 3.83 ± 0.22 Aa* | 2.45 ± 0.23 Ba | 2.31 ± 0.39 Ba |
| Heterophils | 54.66 ± 2.27 | 47.17 ± 0.94 Aa* | 44.33 ± 0.84 Aa* | 42.00 ± 1.18 Ba* | 42.17 ± 0.83 Aa* |
| Lymphocytes | 42.83 ± 2.10 | 50.33 ± 0.88 Bb* | 53.33 ± 0.99 Aa* | 55.16 ± 0.91 Aa* | 55.16 ± 0.87 Aa* |
| Eosinophils | 1.16 ± 0.16 | 1.33 ± 0.21 Aa | 1.16 ± 0.16 Aa | 1.50 ± 0.22 Aa | 1.33 ± 0.21 Aa |
| Monocytes | 1.33 ± 0.21 | 1.16 ± 0.16 Aa | 1.16 ± 0.16 Aa | 1.13 ± 0.21 Aa | 1.33 ± 0.21 Aa |
| Variables | Non-Transported | Control–LCD | Control–SCD | EOAC-LCD | EOAC-SCD |
|---|---|---|---|---|---|
| Plasma | |||||
| Glucose | 73.80 ± 2.57 | 77.80 ± 8.80 Ab | 127.70 ± 7.81 Aa* | 74.90 ± 9.82 Ab | 107.30 ± 5.85 Aa* |
| Triglycerides | 59.30 ± 2.67 | 53.00 ± 1.46 Aa | 46.50 ± 6.25 Aa | 45.60 ± 5.61 Aa | 48.10 ± 6.54 Aa |
| Total protein | 2.18 ± 0.05 | 2.10 ± 0.11 Ab | 2.33 ± 0.08 Aa | 2.10 ± 0.02 Aa | 2.24 ± 0.06 Aa |
| Albumin | 0.60 ± 0.03 | 0.32 ± 0.08 Bb* | 0.50 ± 0.09 Aa | 0.49 ± 0.07 Aa | 0.52 ± 0.04 Aa |
| Total cholesterol | 101.50 ± 2.60 | 99.70 ± 3.92 Aa | 111.20 ± 3.86 Aa | 97.40 ± 12.66 Aa | 94.30 ± 10.85 Aa |
| HDL | 4.00 ± 0.52 | 5.80 ± 0.48 Aa | 4.80 ± 0.79 Aa | 6.10 ± 0.46 Aa* | 5.60 ± 0.53 Aa |
| LDL | 85.60 ± 2.02 | 83.20 ± 3.68 Aa | 97.00 ± 4.05 Aa | 82.20 ± 11.51 Aa | 79.10 ± 9.69 Aa |
| VLDL | 11.90 ± 0.53 | 10.60 ± 0.29 Aa | 9.30 ± 1.25 Aa | 9.10 ± 1.12 Aa | 9.60 ± 1.3 Aa |
| ALT | 10.70 ± 0.61 | 6.40 ± 1.05 Ab* | 9.50 ± 0.89 Aa | 8.30 ± 0.42 Ab | 11.90 ± 1.47 Aa |
| AST | 73.70 ± 5.94 | 64.30 ± 7.83 Aa | 73.30 ± 9.66 Aa | 55.00 ± 4.42 Aa | 70.70 ± 11.16 Aa |
| Liver | |||||
| Glycogen | 16.90 ± 1.17 | 15.83 ± 1.79 Bb | 25.54 ± 2.00 Aa* | 23.28 ± 2.26 Aa | 30.44 ± 3.78 Aa* |
| Total protein | 0.48 ± 0.03 | 0.76 ± 0.07 Ba* | 0.62 ± 0.08 Ba* | 1.10 ± 0.06 Ab* | 1.31 ± 0.07 Aa* |
| AST | 54.75 ± 2.88 | 34.45 ± 3.66 Aa* | 40.92 ± 3.98 Aa | 22.08 ± 1.63 Ba* | 18.74 ± 1.51 Ba* |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro Neto, O.P.d.A.; Correia-Silva, P.J.; Silva, I.S.; Santos, A.d.A.; Rocha, A.d.S.; Couto, R.D.; Silva, E.d.S.; Schmidt, D.; Copatti, C.E. Use of Essential Oil from Aloysia citrodora Paláu in Anesthesia and Simulated Transport of Tambaqui Colossoma macropomum (Cuvier 1826) at Two Different Cargo Densities. Fishes 2025, 10, 448. https://doi.org/10.3390/fishes10090448
Castro Neto OPdA, Correia-Silva PJ, Silva IS, Santos AdA, Rocha AdS, Couto RD, Silva EdS, Schmidt D, Copatti CE. Use of Essential Oil from Aloysia citrodora Paláu in Anesthesia and Simulated Transport of Tambaqui Colossoma macropomum (Cuvier 1826) at Two Different Cargo Densities. Fishes. 2025; 10(9):448. https://doi.org/10.3390/fishes10090448
Chicago/Turabian StyleCastro Neto, Orlando Pinto de Almeida, Patrick Jordan Correia-Silva, Isabelle Santos Silva, Aline dos Anjos Santos, Aline da Silva Rocha, Ricardo David Couto, Erick dos Santos Silva, Denise Schmidt, and Carlos Eduardo Copatti. 2025. "Use of Essential Oil from Aloysia citrodora Paláu in Anesthesia and Simulated Transport of Tambaqui Colossoma macropomum (Cuvier 1826) at Two Different Cargo Densities" Fishes 10, no. 9: 448. https://doi.org/10.3390/fishes10090448
APA StyleCastro Neto, O. P. d. A., Correia-Silva, P. J., Silva, I. S., Santos, A. d. A., Rocha, A. d. S., Couto, R. D., Silva, E. d. S., Schmidt, D., & Copatti, C. E. (2025). Use of Essential Oil from Aloysia citrodora Paláu in Anesthesia and Simulated Transport of Tambaqui Colossoma macropomum (Cuvier 1826) at Two Different Cargo Densities. Fishes, 10(9), 448. https://doi.org/10.3390/fishes10090448







