Reproductive Ecology of the Java Rabbitfish, Siganus javus, in the Southern South China Sea
Abstract
1. Introduction
2. Materials and Methods
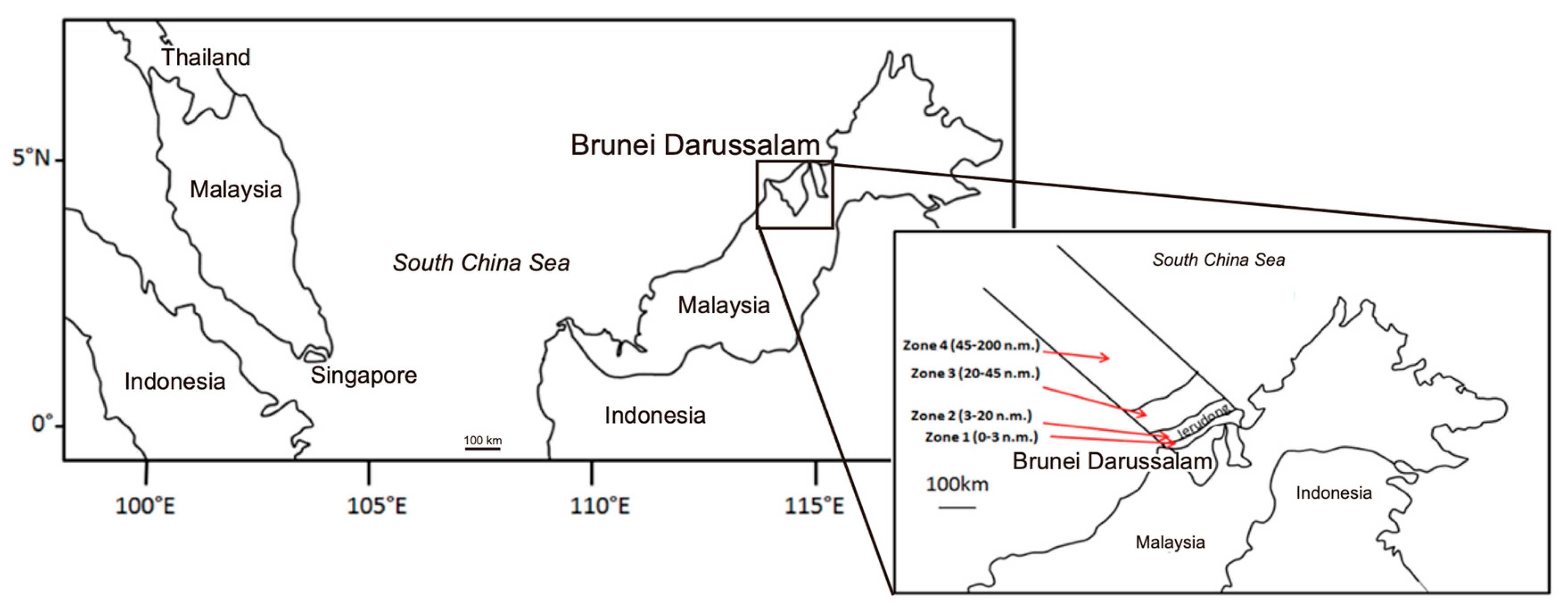
2.1. Study Area
2.2. Fish
2.3. Histology
2.4. Fecundity
2.5. Length at First Maturity
2.6. Statistics
3. Results
3.1. Sex Ratio
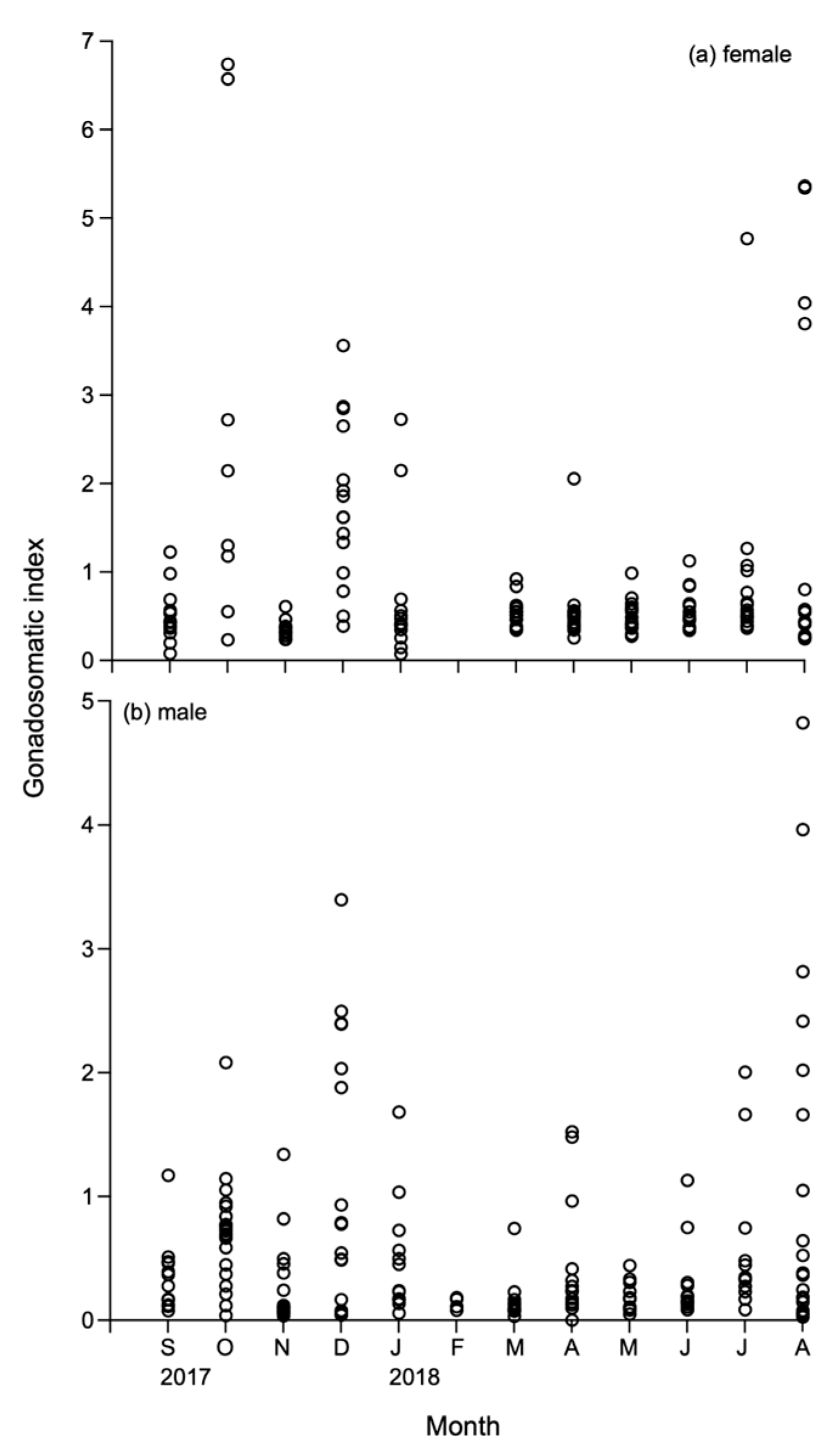
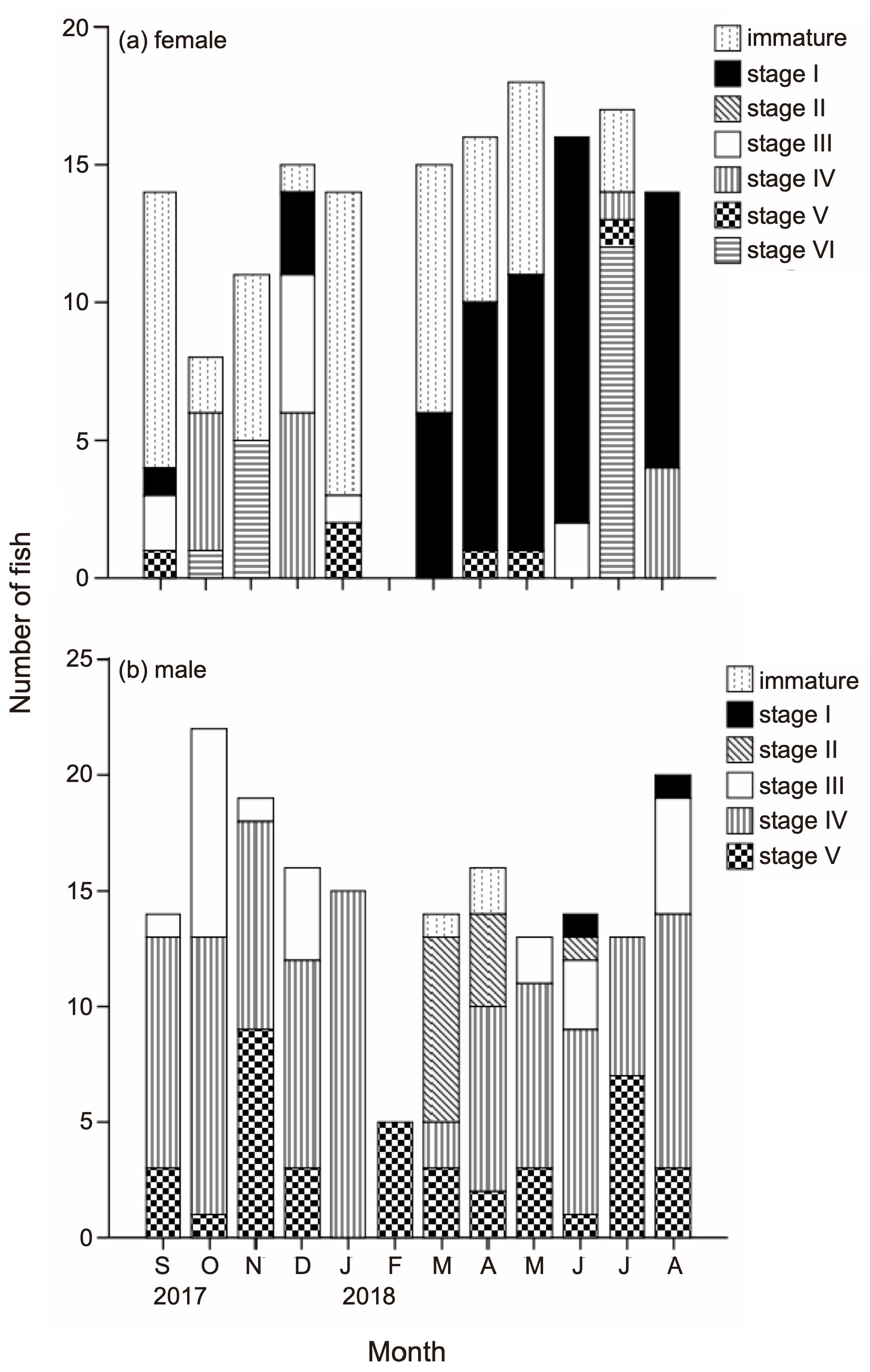
3.2. GSI and Histology in Female
3.3. Oocyte Development and Fecundity
3.4. GSI and Histology in Male
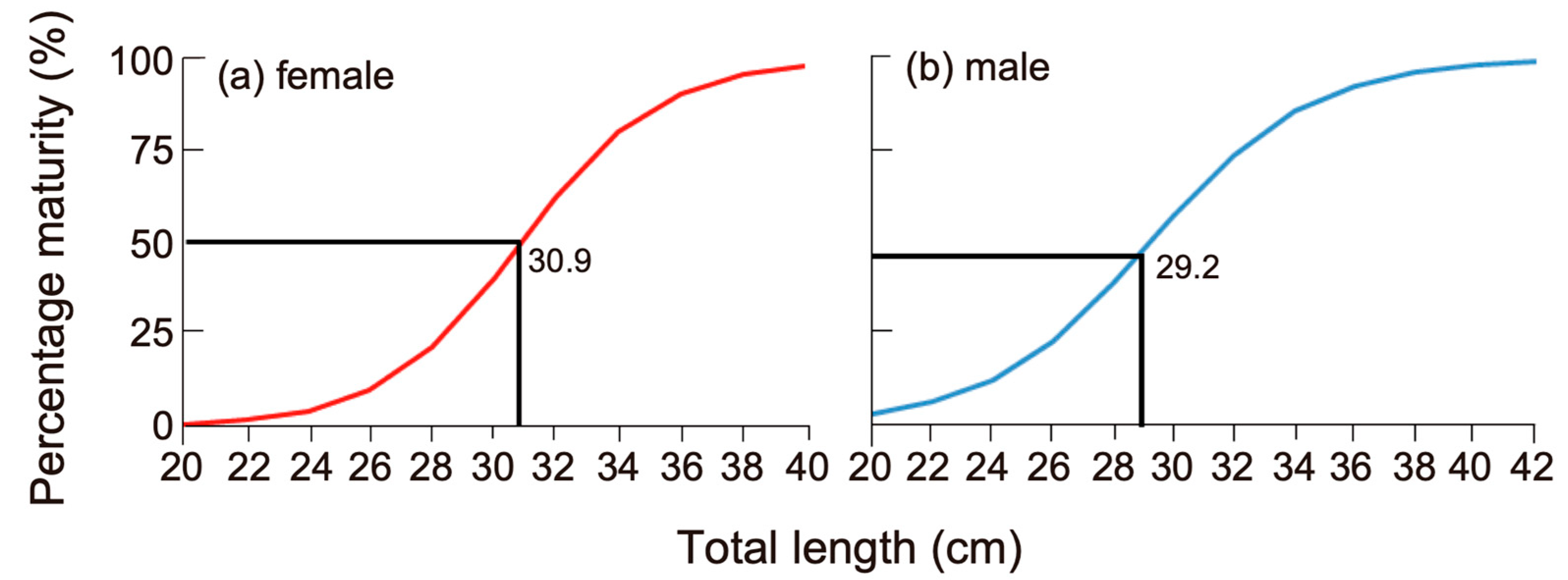
3.5. Length at First Maturity
4. Discussion
| Species | Country/Region | Spawning Period | Reference |
|---|---|---|---|
| S. canaliculatus | Singapore | January–April | [48] |
| Palau | March–May | [49] | |
| Asian estuaries | January–April | [50] | |
| Hong Kong | March–June | [51] | |
| Japan | April–June | [52] | |
| Japan | March–August | [38] | |
| Arabian Gulf UAE | April–July, November | [16] | |
| Oman | June–July, November–February | [39] | |
| S. sutor | Kenya | January–February, May–June | [35] |
| Kenya | June–July, November–January | [35] | |
| S. rivulatus | Northeastern Mediterranean | July–August | [40] |
| Lebanon | June–July | [37] | |
| S. spinus | Japan | June–August | [26] |
| S. luridus | Lebanon | June–July, October–January | [37] |
| S. argenteus | Guam | March–May, August–September | [29] |
| S. javus | Brunei Darussalam | July–August, October–December | This study |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bryant, D.; Burke, L.; McManus, J.; Spalding, M.D. Reefs at Risk: A Map-Based Indicator of Threats to the Worlds Coral Reefs; World Resources Institute: Washington, DC, USA, 1998; p. 56. [Google Scholar]
- Spalding, M.D.; Ravilious, C.; Green, E.P. World Atlas of Coral Reefs; UNEP World Conservation Monitoring Centre, University of California Press: Berkeley, CA, USA, 2001; p. 436. [Google Scholar]
- Whittingham, E.; Campbell, J.; Townsley, P. Poverty and Reefs. IMM Limited; DFID–IMM–IOC/UNESCO: Paris, France, 2003; p. 260. [Google Scholar]
- Arai, T. Diversity and conservation of coral reef fishes in the Malaysia South China Sea. Rev. Fish Biol. Fish. 2014, 25, 85–101. [Google Scholar] [CrossRef]
- Burke, L.; Selig, E.; Spalding, M. Reefs at Risk in Southeast Asia; World Resource Institute: Washington, DC, USA, 2002; p. 72. [Google Scholar]
- Longenecker, K.; Langston, R.; Bolick, H.; Crane, M.; Donaldson, T.J.; Franklin, E.C.; Kelokelo, M.; Kondio, U.; Potuku, T. Rapid reproduction analysis and length-weight relations for five species of coral reef fishes (actinopterygii) from Papua New Guinea: Nemipterus isacanthus, Parupeneus barberinus, Kyphosus cinerascens, Ctenochaetus striatus (Perciformes), and Balistapus undulatus. Acta Ichthyol. Piscat. 2017, 47, 107–124. [Google Scholar]
- Newton, K.; Isabelle, M.C.; Graham, M.P.; Simon, J.; Nicholas, K.D. Current and future sustainability of island coral reef fisheries. Curr. Biol. 2007, 17, 655–658. [Google Scholar] [CrossRef]
- Pauly, D. Major trends in small-scale fisheries, with emphasis on developing countries, and some implications for the social sciences. Marit. Stud. 2006, 4, 7–22. [Google Scholar]
- Béné, C. Small-Scale Fisheries: Assessing Their Rural Livelihoods in Developing Countries. FAO Fisheries Circular no. 1008, 2006; p. 42. Available online: http://www.fao.org/3/a-j7551e.pdf (accessed on 21 June 2021).
- Thacker, R.W.; Ginsburg, D.W.; Paul, V.J. Effects of herbivore exclusion and nutrient enrichment on coral reef macroalgae and cyanobacteria. Coral Reefs 2001, 19, 318–329. [Google Scholar] [CrossRef]
- Folke, C.; Carpenter, S.; Walker, B.; Scheffer, M.; Elmqvist, T.; Gunderson, L.; Holling, C.S. Regime shifts, resilience and biodiversity in ecosystem management. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 557–581. [Google Scholar] [CrossRef]
- Fox, R.J.; Sunderland, T.L.; Hoey, A.S.; Bellwood, D.R. Estimating ecosystem function: Contrasting roles of closely related herbivorous rabbitfishes (Siganidae) on coral reefs. Mar. Ecol. Prog. Ser. 2009, 385, 261–269. [Google Scholar] [CrossRef]
- Bennett, S.; Bellwood, D.R. Latitudinal variation inmacroalgal consumption by fishes on the Great Barrier Reef. Mar. Ecol. Prog. Ser. 2011, 426, 241–252. [Google Scholar] [CrossRef]
- Hughes, T.P.; Rodrigues, M.J.; Bellwood, D.R.; Ceccarelli, D.; Hoegh-Guldberg, O.; McCook, L.; Moltschaniwskyj, N.; Pratchett, M.S.; Steneck, R.S.; Willis, B. Phase shifts, herbivory and the resilience of coral reefs to climate change. Curr. Biol. 2007, 17, 360–365. [Google Scholar] [CrossRef]
- Mehanna, S.F.; Abdallah, M. Population dynamics of the rabbitfish Siganus rivulatus from the Egyptian sector of the Red Sea. J. King Abdullah Univ. Mar. Sci. 2002, 13, 161–170. [Google Scholar] [CrossRef]
- Grandcourt, E.; Al-Abdessalaam, T.; Francis, F.; Al Shamsi, A. Population biology and assessment of the white-spotted spinefoot, Siganus canaliculatus (Park 1797), in the southern Arabian Gulf. J. App. Ichthyol. 2007, 23, 53–59. [Google Scholar] [CrossRef]
- Soliman, V.S.; Mendoza, A.B., Jr.; Yamaoka, K. Seaweed-associated fishes of Lagonoy Gulf in Bicol, the Philippines-with emphasis on siganids (Teleoptei: Siganidae). Kuroshio Sci. 2008, 2, 67–72. [Google Scholar]
- Woodland, D. Siganidae. Rabbitfishes (spinefoots). In FAO Identification Guide for Fishery Purposes; Carpenter, K.E., Niem, V., Eds.; The Western Central Pacific: Rome, Italy, 1997; pp. 837, 3627–3650. [Google Scholar]
- Alonso-Fernández, A.; Alós, J.; Grau, A.; Domínguez-Petit, R.; Saborido-Rey, F. The Use of Histological Techniques to Study the Reproductive Biology of the Hermaphroditic Mediterranean Fishes Coris julis, Serranus scriba, and Diplodus annularis. Mar. Coast. Fish. 2011, 3, 145–159. [Google Scholar] [CrossRef]
- Priyadharsin, S.; Manoharan, J.; Varadharajan, D.; Subramaniyan, A. Reproductive biology and histology study of red lionfish Pterois volitan from Cuddalore, South East Coast of India. Aquacult. Res. Develop. 2013, 4, 1–9. [Google Scholar]
- Sadovy de Mitcheson, Y.; Cornish, A.; Domeier, M.; Colin, P.; Russell, M.; Lindeman, K. A global baseline for spawning aggregations of reef fishes. Conserv. Biol. 2008, 22, 1233–1244. [Google Scholar] [CrossRef]
- Haji, P.K.R.B.P.; Rahim, A. Fishing in Brunei: Development of the fishing industry in the post-independence period, 1984–2000s. In Brunei History, Islam, Society and Contemporary Issues; Keat Gin, O., Ed., Routledge: London, United Kingdom, 2015; pp. 200–230. [Google Scholar]
- Allen, G.R. Reef Fishes of Brunei Darussalam; Department of Fisheries, Ministry of Industry and Primary Resources: Bandar Seri Begawan, Brunei Darussalam, 2009. [Google Scholar]
- Slaoui, M.; Fiette, L. Histopathology procedures: From tissue sampling to hispathological evaluation. Meth. Mol. Biol. 2011, 691, 69–82. [Google Scholar]
- Arai, T.; Abdul Kadir, S.R. Opportunistic spawning of tropical anguillid eels Anguilla bicolor bicolor and A. bengalensis bengalensis. Sci. Rep. 2017, 7, 41649. [Google Scholar] [CrossRef] [PubMed]
- Harahap, A.P.; Takemura, A.; Nakamura, S.; Syadur Rahman, M.D.; Takano, K. Histological evidence of lunar-synchronized ovarian development and spawning in the spiny rabbitfish Siganus spinus (Linnaeus) around the Ryukyus. Fish. Sci. 2001, 67, 888–893. [Google Scholar] [CrossRef]
- Wassef, E.A.; Hady, H.A. Some biological studies and gonadal development of rabbitfish Siganus canaliculatus (Park) and Siganus spinus L. (F: Siganidae) from the Gulf Waters off Saudi Arabia. J. Kau. Mar. Sci. 2001, 12, 189–208. [Google Scholar] [CrossRef]
- Agembe, S. Estimation of importance reproductive parameter for management of the shoemaker spinefoot rabbitfish (Siganus sutor) in Southern Kenya. Int. J. Mar. Sci. 2012, 2, 24–30. [Google Scholar] [CrossRef]
- Taylor, B.M.; Gourley, J.; Trianni, M.S. Age, growth, reproductive biology and spawning periodicity of the forktail rabbitfish (Siganus argenteus) from the Mariana Island. Mar. Freshw. Res. 2016, 68, 1088–1097. [Google Scholar] [CrossRef]
- Rahman, M.D.S.; Takemura, A.; Takano, K. Annual changes in testicular activity and plasma steroid hormones in the golden rabbitfish Siganus guttatus. Fish. Sci. 2000, 66, 894–900. [Google Scholar] [CrossRef]
- Azzurro, E.; Carnevalli, O.; Bariche, M.; Andaloro, F. Reproductive features of the non-native Siganus luridus (Teleostei, Siganidae) during early colonization at Linosa Island (Sicily Strait, Mediterranean Sea). J. Appl. Ichthyol. 2007, 23, 640–645. [Google Scholar] [CrossRef]
- Lowerre-Barbieri, S.K.; Barbieri, L.R. A new method of oocyte separation and preservation for fish reproduction studies. Fish. Bull. 1993, 91, 165–170. [Google Scholar]
- Abdul Kadir, S.R.; Yamin, L.; Arai, T. Fecundity of the tropical catadromous eels Anguilla bicolor bicolor, A. bengalensis bengalensis and A. marmorata. Environ. Biol. Fish. 2017, 100, 1643–1648. [Google Scholar] [CrossRef]
- Karna, S.K.; Panda, S. Growth estimation and length at maturity of a commercially im- portant fish species i. e., Dayscieaena albida (Boroga) in Chilika Lagoon, India. Europ. J. Exp. Biol. 2011, 1, 84–91. [Google Scholar]
- De Souza, T. Reproduction, length-weight relationship and condition factor in Siganus sutor (Valenciennes, 1835) (Pisces: Siganidae) from the Kenyan waters of the western Indian Ocean. Kenya J. Sci. Ser. B 1988, 9, 89–101. [Google Scholar]
- Kamukuru, A.T. Trap fishery and reproductive biology of the white spotted rabbitfish Siganus sutor (Siganidae), within the Dar es Salaam Marine Reserves, Tanzania. West. Ind. Ocean J. Mar. Sci. 2009, 8, 75–86. [Google Scholar]
- Bariche, M.; Harmelin-vivien, M.; Quignard, J.P. Reproductive cycles and spawning periods of two Lessepsian siganids fishes on the Lebanese coast. J. Fish Biol. 2003, 62, 129–142. [Google Scholar] [CrossRef]
- Kanashiro, K.; Motonaga, F.; Kimura, F. Settlement of white-spotted spine-foot, Siganus canaliculatus (Pisces: Siganidae) in the coastal waters off Okinawa Island, Japan. Jpn. Soc. Fish. Sci. 1999, 65, 19–25. [Google Scholar] [CrossRef]
- Al-Marzouqi, A.; Jayabalan, N.; Al-Nahdi, A.; Al-Anbory, I. Reproductive biology of the white-spotted rabbitfish, Siganus canaliculatus (Park, 1797) in the Arabian Sea coast of Oman. West. Ind. Ocean J. Mar. Sci. 2011, 10, 73–82. [Google Scholar]
- Yeldan, H.; Avsar, D. A preliminary study on the reproduction of the rabbitfish (Siganus rivulatus (Forsskal, 1775)) in the Northeastern Mediterranean. Tur. J. Zool. 2000, 24, 173–182. [Google Scholar]
- Takemura, A.; Rahman, M.S.; Nakamura, S.; Park, Y.J.; Takano, K. Lunar cycles and reproductive activity in reef fishes with particular attention to rabbitfishes. Fish Fish. 2004, 5, 317–328. [Google Scholar] [CrossRef]
- Loubens, G. Biologie de queleus especes de poisons du lagon Neo-Caledonia II. Sexualite et reproduction. Cah. De L’indo-Pac. 1980, 2, 41–72. [Google Scholar]
- Sadovy, Y.J. Reproduction of reef fishery species. In Reef Fisheries; Polunin, N.V.C., Roberts, C.M., Eds.; Fish and Fisheries Series; Chapman & Hall: London, UK, 1996; Volume 20, pp. 15–59. [Google Scholar]
- Freitas, M.O.; De Moura, R.L.; Francini-Filho, R.B.; Minte-Vera, C.V. Spawning patterns of commercially important reef fish (Lutjanidae and Serranidae) in the tropical western South Atlantic. Sci. Mar. 2011, 75, 135–146. [Google Scholar] [CrossRef]
- Kahar, R.; Ahmad, N.; Arai, T. Year-round spawning of three tropical Cypriniformes fishes in Southeast Asia. Sci. Rep. 2023, 13, 8971. [Google Scholar] [CrossRef]
- Rick, M.R.; Tomkiewicz, J. Skipped spawning in fishes: More common than you think. Fish. Repr. Biol. 2011, 3, 176–189. [Google Scholar]
- Wootton, R.J. Ecology of Teleost Fishes; Kluwer Academic Publishers: London, UK, 1998. [Google Scholar]
- Soh, C.L. Some aspects of the Biology of Siganus canaliculatus (Park) 1797. Ph.D. Dissertation, National University of Singapore, Singapore, 1976; p. 293. [Google Scholar]
- Hasse, J.J.; Madraisau, B.B.; McVey, J.P. Some aspects of the life history of Siganus canaliculatus (Park) (Pisces: Siganidae) in Palau. Micronesica 1977, 13, 297–312. [Google Scholar]
- Jayaseelan, M.J.P. Manual of Fish Eggs and Larvae from Asian Mangrove Waters; UNESCO: Paris, France, 1998; p. 193. [Google Scholar]
- Sadovy, Y.J. Patterns of reproduction in marine fishes of Hong Kong and adjacent waters. In The Marine Biology of the South China Sea, Proceedings of Third International Conference of the Marine Biology of the South China Sea, Hong Kong, China, 28 October–1 November 1996; Morton, B., Ed.; Hong Kong University Press: Hong Kong, China, 1998; pp. 261–274. [Google Scholar]
- Hoque, M.M.; Takemura, A.; Matsuyama, M.; Matsura, S.; Takno, K. Lunar spawning Siganus canaliculatus. J. Fish Biol. 1999, 55, 1213–1222. [Google Scholar] [CrossRef]
- Lima, A.F. The influence of sex ratio on the reproduction of pirarucu, Arapaima gigas, in captivity. Acta Amaz. 2018, 48, 38–41. [Google Scholar] [CrossRef]
- Barson, N.J.; Aykanat, T.; Hindar, K.; Baranski, M.; Bolstad, G.H.; Fiske, P.; Jacq, C.; Jensen, A.J.; Johnston, S.E.; Karlsson, S.; et al. Sex-dependent dominance at a single locus maintains variation in age at maturity in salmon. Nature 2015, 528, 405–408. [Google Scholar] [CrossRef]
- Roff, D.A. The Evolution of Life Histories: Theory and Analysis; Chapman and Hall: New York, NY, USA, 1992. [Google Scholar]
- Stamps, J.; Krishnan, V.V. Sexual bimaturation and sexual size dimorphism in animals with asymptotic growth after maturity. Evol. Ecol. 1997, 11, 21–39. [Google Scholar] [CrossRef]
- Diego, R.B.; Ross Roberson, D.R.; Craig, R.W.; Dustin, J.M. Fish reproductive-energy output increases disproportionately with body size. Science 2018, 360, 642–645. [Google Scholar] [CrossRef] [PubMed]
- Ntiba, M.J.; Jaccarini, V. Age and growth parameters of Siganus sutor in Kenyan marine inshore water, derived from number of otolith microbands and fish length. J. Fish Biol. 1988, 33, 465–470. [Google Scholar] [CrossRef]
- Chitravadivelu, K. Observation on the biology of three siganids Siganus lineatus, Siganus javus and Siganus canaliculatus from waters around Jaffna. J. Sci. 1987, 2, 1–13. [Google Scholar]
| Sex | Maturation Stage | Number of Specimens | Total Length (cm) | Body Weight (g) | Gonadosomatic Index | Oocyte Diameter (µm) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | |||
| female | 0 | 55 | 30.53 ± 4.79 | 19.00–42.00 | 380.36 ± 163.40 | 99.85–1001.93 | 0.46 ± 0.26 | 0.15–2.15 | 58.25 ± 10.49 | 40.96–98.57 |
| I | 53 | 29.53 ± 4.70 | 21.00–40.00 | 363.27 ± 182.10 | 128.15–935.75 | 0.53 ± 0.21 | 0.25–1.62 | 72.08 ± 24.37 | 47.70–206.52 | |
| II | 0 | |||||||||
| III | 10 | 28.68 ± 4.96 | 22.50–37.00 | 350.90 ± 161.50 | 147.26–632.63 | 1.13 ± 0.58 | 0.08–1.92 | 193.03 ± 47.88 | 106.35–261.57 | |
| IV | 16 | 31.18 ± 3.22 | 26.00–37.50 | 421.14 ± 126.88 | 276.70–667.29 | 3.65 ± 1.69 | 1.30–6.74 | 268.48 ± 28.97 | 235.49–332.71 | |
| V | 6 | 32.05 ± 5.00 | 27.50–39.00 | 492.82 ± 210.39 | 259.57–696.73 | 1.22 ± 0.10 | 0.08–2.73 | 112.06 ± 66.76 | 41.20–189.35 | |
| VI | 18 | 30.76 ± 3.53 | 23.00–36.20 | 373.07 ± 108.47 | 172.48–579.31 | 0.64 ± 0.30 | 0.25–1.27 | 61.05 ± 27.59 | 41.34–168.89 | |
| male | 0 | 3 | 25.83 ± 8.22 | 16.50–32.00 | 315.94 ± 221.97 | 71.16–504.16 | 0.06 ± 0.06 | 0.01–0.13 | ||
| I | 2 | 20.85 ± 0.21 | 20.70–21.00 | 134.13 ± 12.08 | 125.59–142.67 | 0.08 ± 0.04 | 0.05–0.11 | |||
| II | 13 | 31.54 ± 4.21 | 24.00–40.00 | 465.29 ± 185.22 | 192.26–898.48 | 0.22 ± 0.17 | 0.08–0.74 | |||
| III | 25 | 28.16 ± 3.6 | 22.00–38.50 | 300.50 ± 123.34 | 165.59–740.59 | 0.80 ± 0.10 | 0.06–4.83 | |||
| IV | 98 | 29.17 ± 4.02 | 21.50–41.50 | 336.90 ± 132.80 | 143.54–925.90 | 0.71 ± 0.77 | 0.06–3.96 | |||
| V | 40 | 30.35 ± 4.97 | 19.80–38.50 | 366.55 ± 168.25 | 115.24–790.73 | 0.13 ± 0.09 | 0.03–0.39 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arai, T.; Tan, I.V.; Ching, F.F.; Ahmad, N. Reproductive Ecology of the Java Rabbitfish, Siganus javus, in the Southern South China Sea. Fishes 2025, 10, 441. https://doi.org/10.3390/fishes10090441
Arai T, Tan IV, Ching FF, Ahmad N. Reproductive Ecology of the Java Rabbitfish, Siganus javus, in the Southern South China Sea. Fishes. 2025; 10(9):441. https://doi.org/10.3390/fishes10090441
Chicago/Turabian StyleArai, Takaomi, Iy Vonne Tan, Fui Fui Ching, and Norhayati Ahmad. 2025. "Reproductive Ecology of the Java Rabbitfish, Siganus javus, in the Southern South China Sea" Fishes 10, no. 9: 441. https://doi.org/10.3390/fishes10090441
APA StyleArai, T., Tan, I. V., Ching, F. F., & Ahmad, N. (2025). Reproductive Ecology of the Java Rabbitfish, Siganus javus, in the Southern South China Sea. Fishes, 10(9), 441. https://doi.org/10.3390/fishes10090441









