Mechanical Characterization and Dual-Layer Discrete Element Modeling of Mactra veneriformis
Abstract
1. Introduction
- (1)
- The mechanical properties of the fruit shell and flesh were characterized experimentally, and the microstructure of the fruit shell (SEM) was recorded to guide the anisotropy of the model;
- (2)
- A two-layer flexible adhesive discrete element model (DEM) with anisotropic fruit shell–fruit shell and fruit shell–flesh contacts was developed; key contact parameters were calibrated using drop test results via Plackett–Burman screening, steepest ascent search, and Box–Behnken response surface design;
- (3)
- The model was experimentally validated, focusing on peak force and observed fracture behavior; the adaptability and limitations of the developed model were analyzed.
2. Materials and Methods
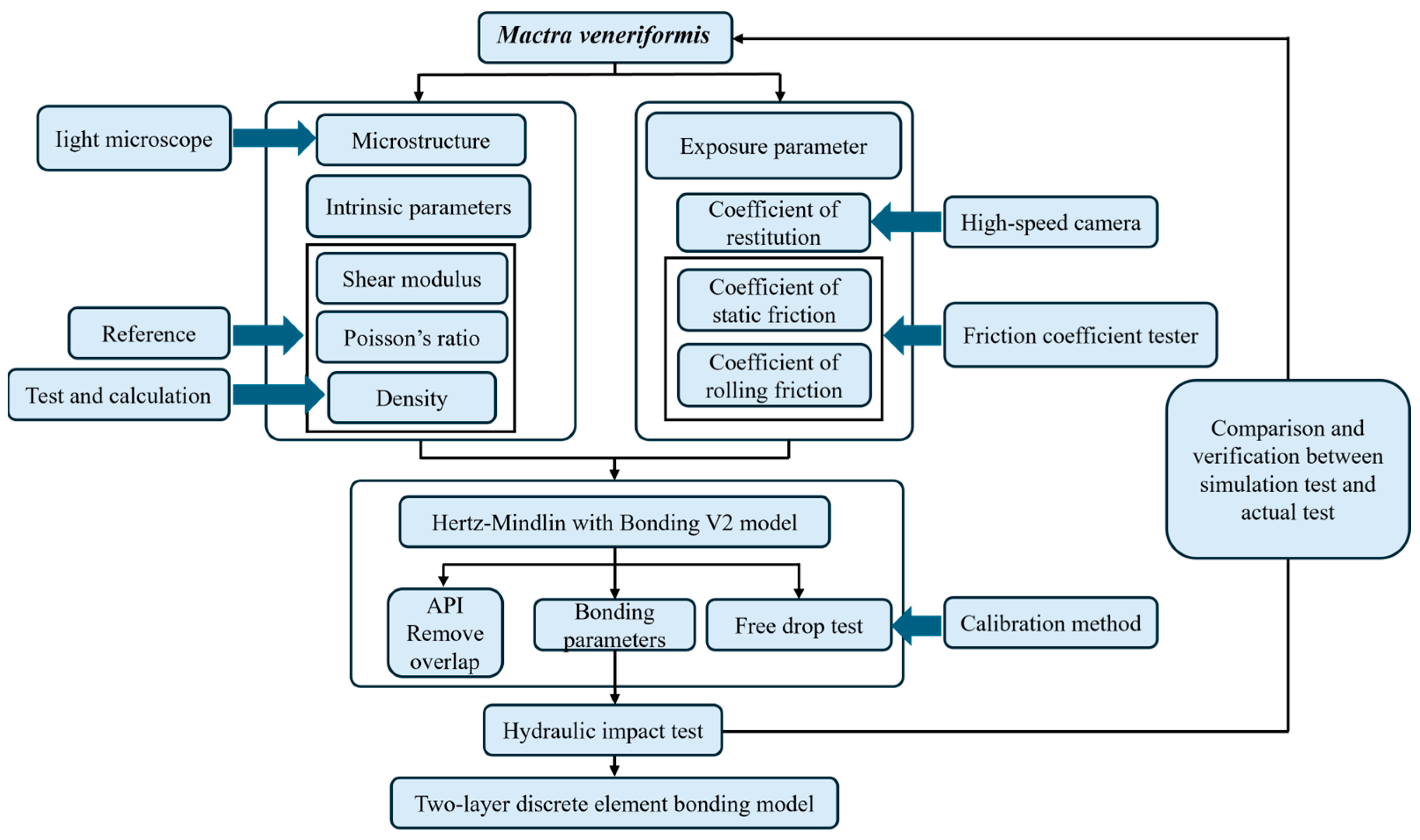
2.1. Overview of Research Methods
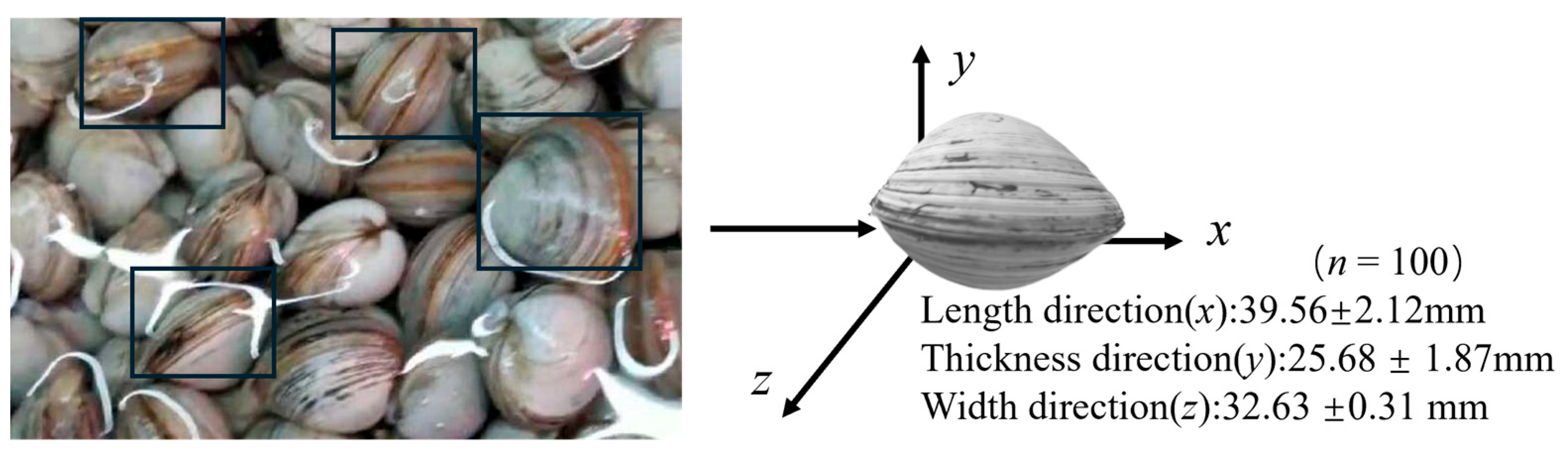
2.2. Determination of Micro-Morphology and Mechanical Properties
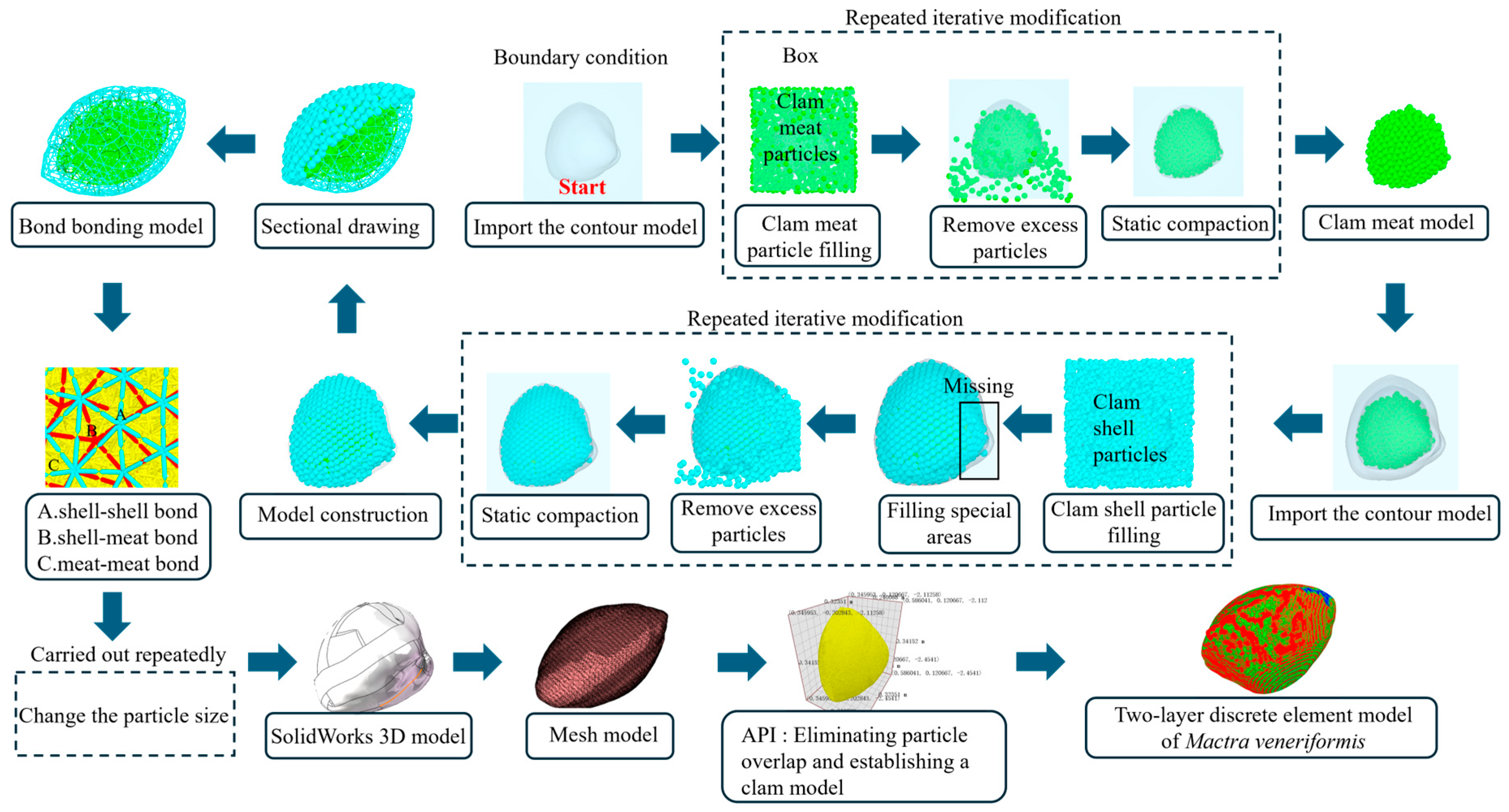
2.3. Construction of the Discrete Element Model of Mactra veneriformis
2.3.1. Contact Model Selection
2.3.2. Model-Building Process
2.4. Calibration and Verification Test
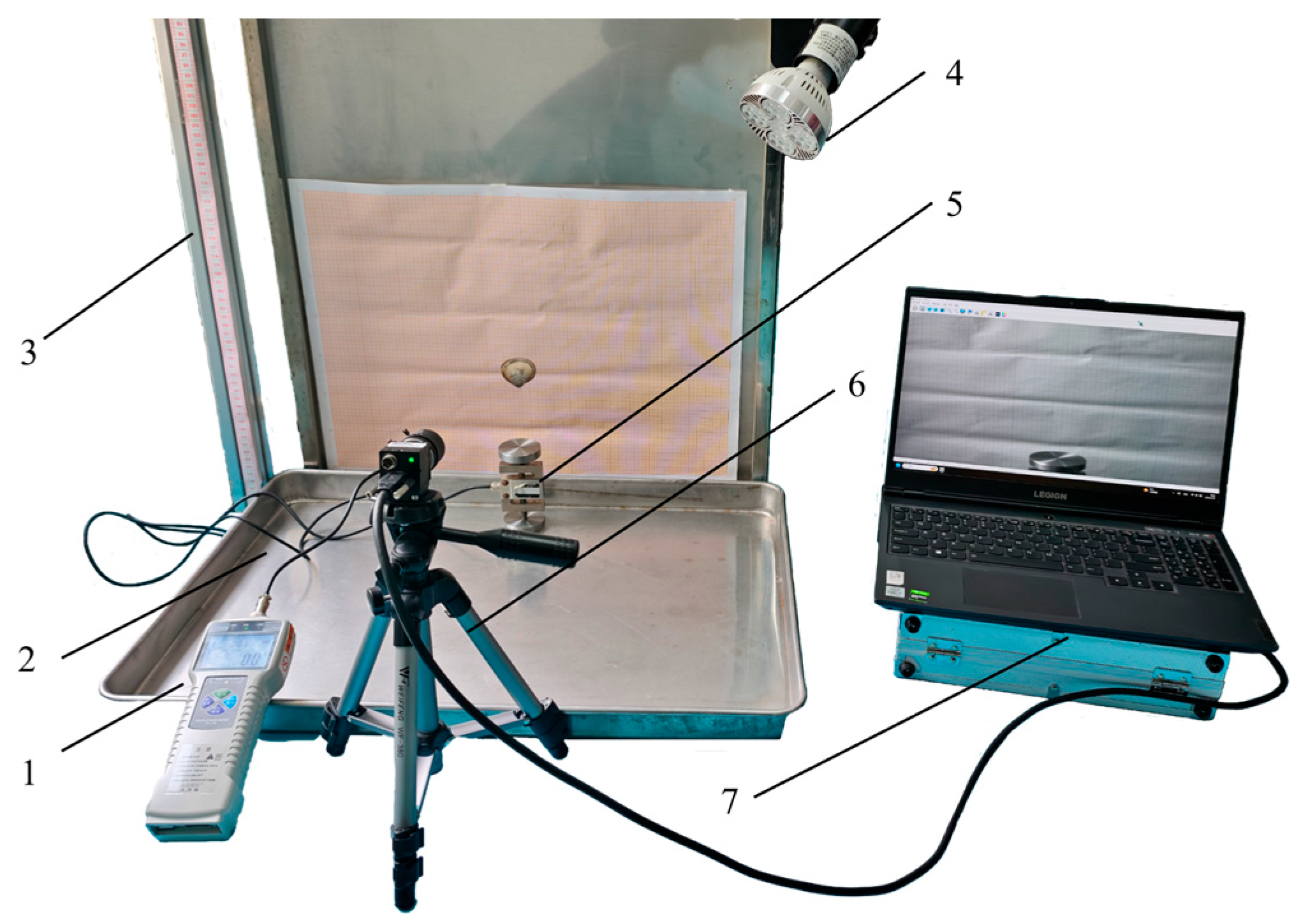
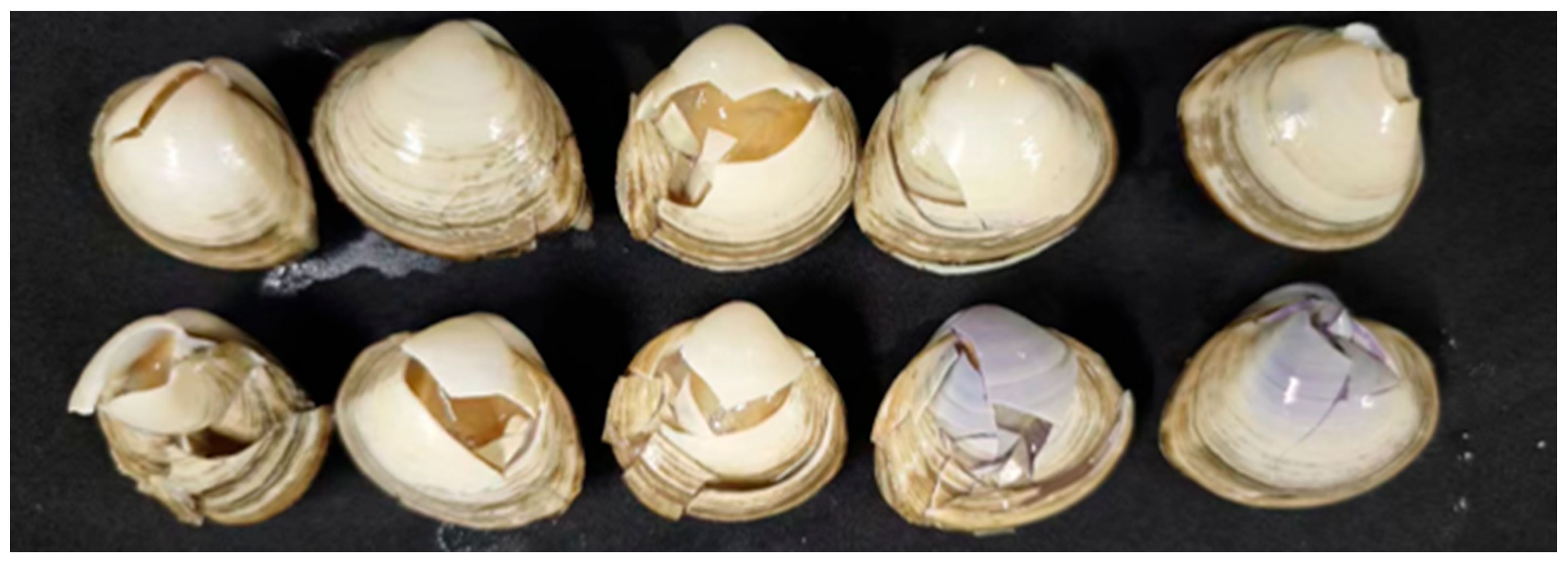
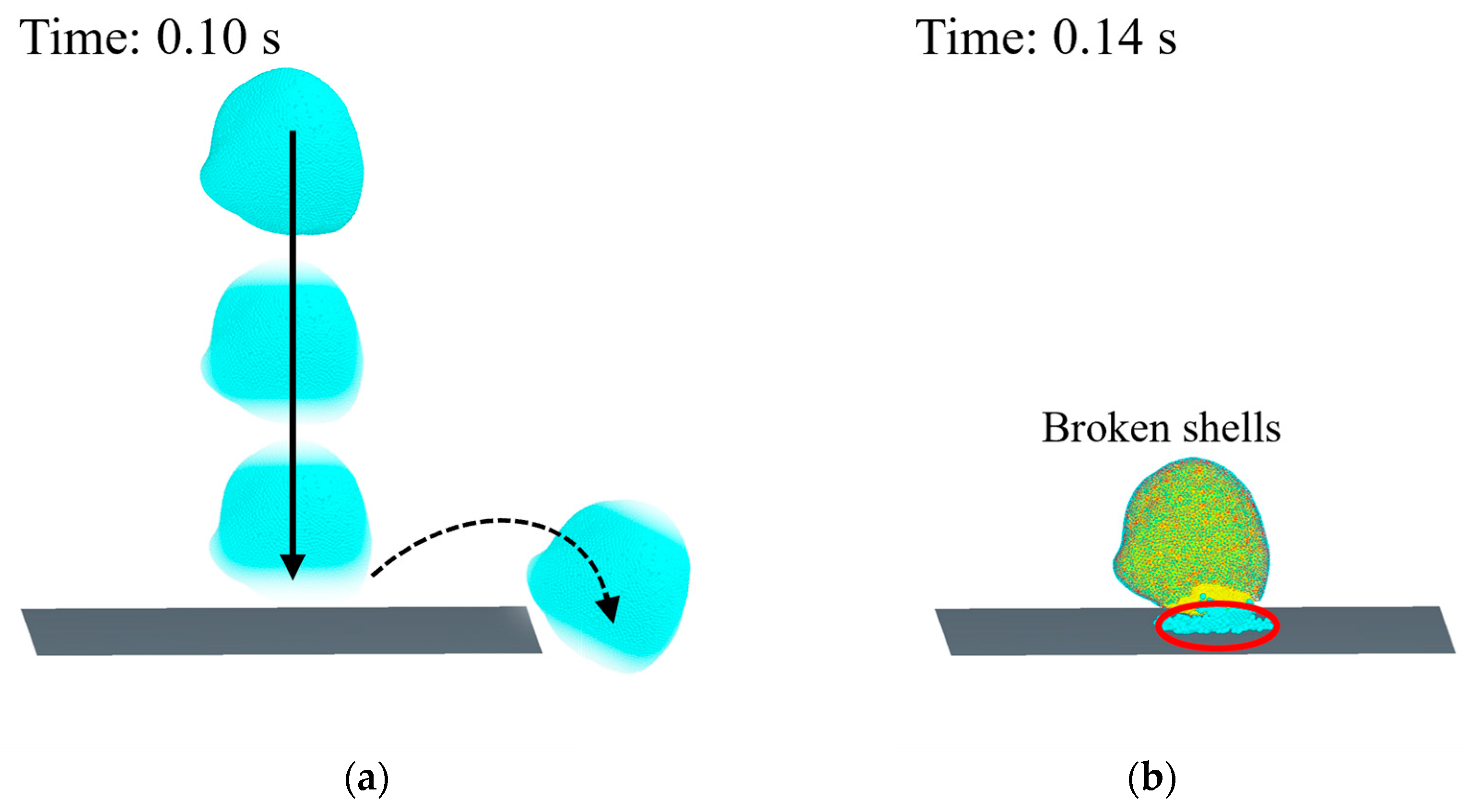
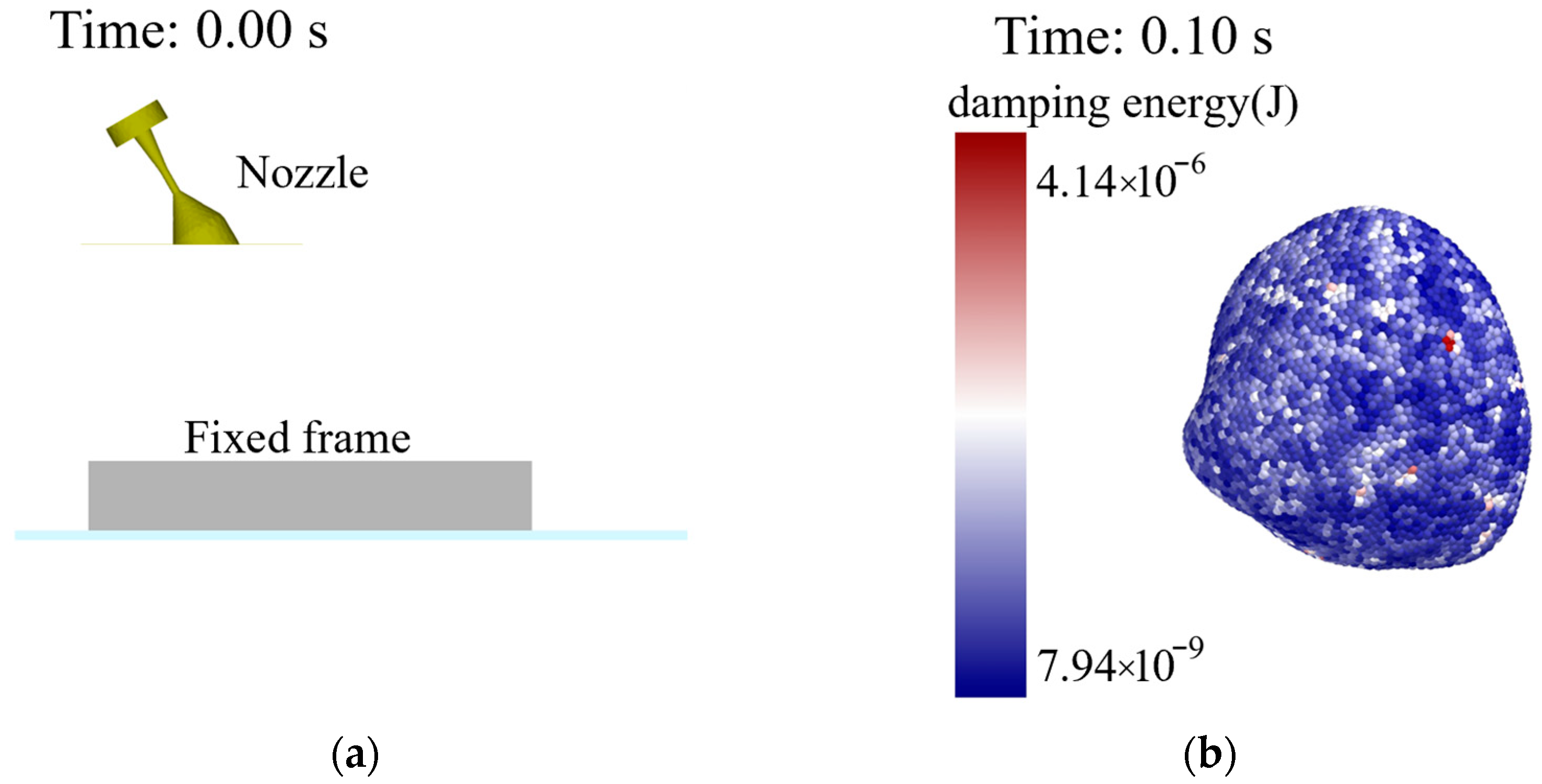
2.4.1. Free Drop Calibration Test of Clams
2.4.2. Verification Test of Hydraulic Impact Clams
3. Results and Discussion
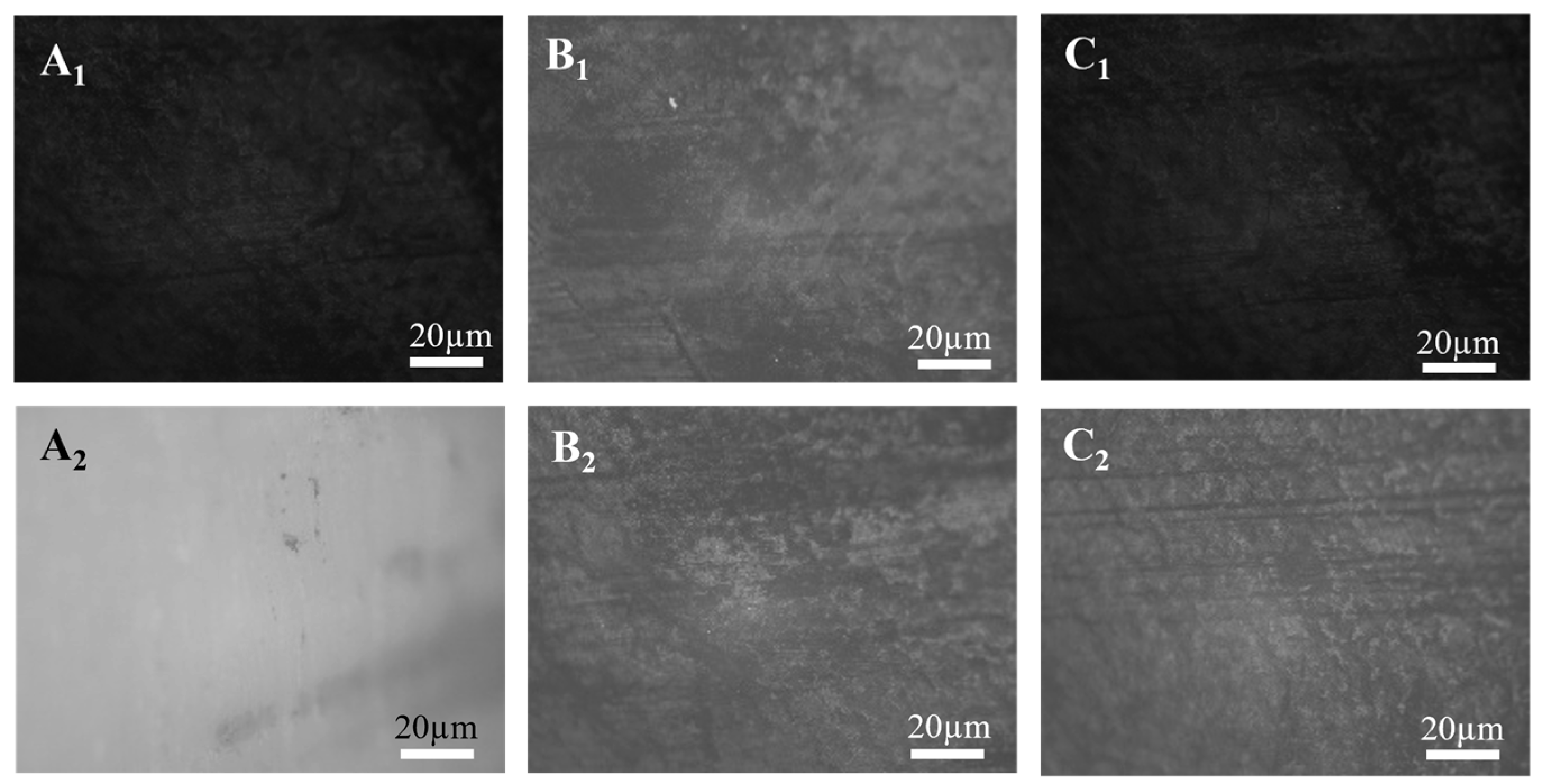
3.1. Microscopic Morphology Test Analysis and Simulation for Basic Parameter Determination
3.1.1. Micro-Morphology Test Results and Analysis
3.1.2. Determination of Simulation Basic Parameters
3.2. Calibration Test Results and Bonding Parameter Analysis
3.2.1. Results of Drop Test
3.2.2. Screening Analysis of Significant Factors
3.3. Parameter Optimization and Verification Analysis
3.3.1. Optimal Parameters and Verification
3.3.2. Hydraulic Impact Verification Results
3.4. Applicability, Limitations, and Future Work
4. Conclusions
- (1)
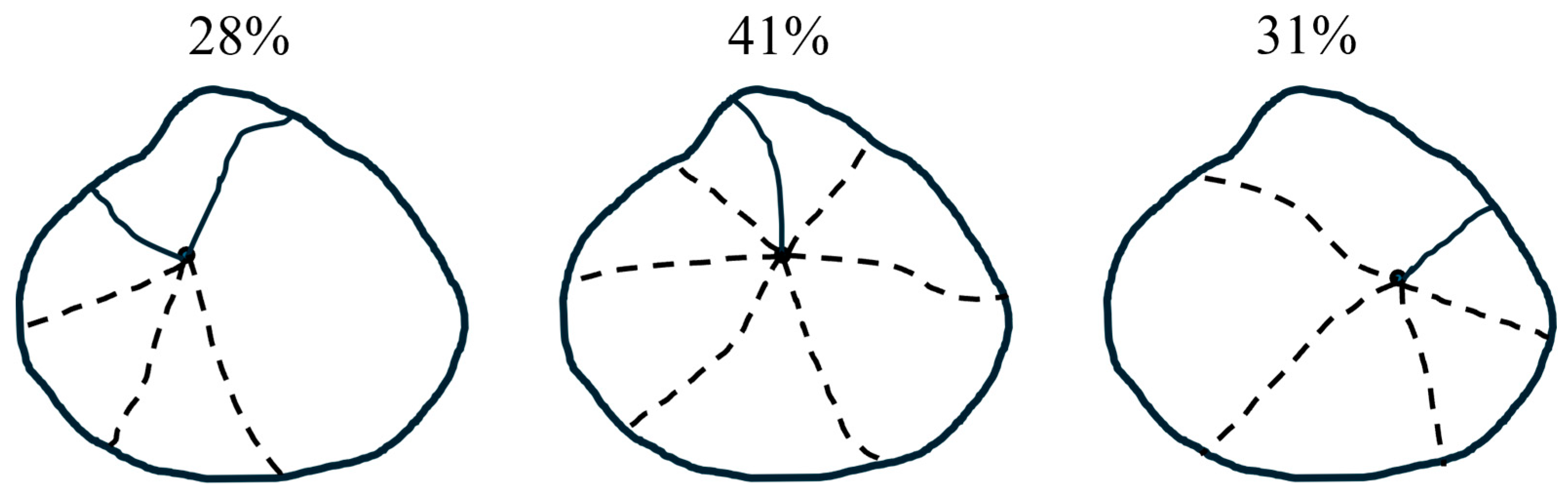
- Microscopic observation revealed that M. veneriformis shells exhibit distinct anisotropic layered structures, with significant differences in morphology, texture, and mechanical properties across different regions. These findings provide a structural basis for determining bonding parameters in DEM modeling while deepening the understanding of shell fracture resistance mechanisms, offering theoretical support for solving practical shell damage problems.
- (2)
- A dual-layer DEM model of shell and flesh was developed based on the Hertz–Mindlin with Bonding V2 contact model. Through free-fall tests and experimental designs (Plackett–Burman screening, steepest ascent method, and Box–Behnken optimization), four key bonding parameters were identified and optimized: flesh–flesh normal/tangential stiffness (X1) = 3.64 × 1011 N/m3, shell–flesh normal/tangential stiffness (X3) = 1.48 × 1013 N/m3, shell–shell tangential stiffness (X6) = 3.23 × 1012 N/m3, and shell–shell normal strength (X7) = 8.35 × 106 Pa.
- (3)
- Under optimal parameters, the relative error of the maximum impact force between simulated and actual drop tests was 4.89%. In hydraulic impact tests, the CFD–DEM simulations agreed well with experiments and showed no shell fractures, confirming model accuracy under complex loading and demonstrating the feasibility of hydraulic harvesting for preserving shellfish integrity. The established model provides a reliable tool for optimizing M. veneriformis harvesting equipment, is transferable to fragile bivalves such as R. philippinarum, and supports simulation of representative mechanized modes to relate shell damage to equipment parameters and guide low-damage design; future integration of substrate–bivalve aggregate models will further strengthen engineering applicability.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bureau of Fisheries, Ministry of Agriculture and Rural Affairs. China Fishery Statistical Yearbook 2024; China Agriculture Press: Beijing, China, 2024.
- Cheng, J.; Wang, H.; Sun, D. An Overview of Tropomyosin as an Important Seafood Allergen: Structure, Cross-reactivity, Epitopes, Allergenicity, and Processing Modifications. Comp. Rev. Food Sci. Food Saf. 2022, 21, 127–147. [Google Scholar] [CrossRef]
- Zhang, T.; Hua, Y.; Zhou, C.; Xiong, Y.; Pan, D.; Liu, Z.; Dang, Y. Umami Peptides Screened Based on Peptidomics and Virtual Screening from Ruditapes Philippinarum and Mactra Veneriformis Clams. Food Chem. 2022, 394, 133504. [Google Scholar] [CrossRef]
- Li, F.-J.; He, X.-R.; Li, D.-X.; Yang, Y.; Kang, S.; Liu, Q.-M.; Luo, L.-Z.; Chen, G.-X.; Liu, G.-M. Effect of Maillard Reaction on the Allergenicity of Crude Extract of Mactra Quadrangularis. Food Res. Int. 2025, 200, 115473. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Bardhan, S.; Mallick, S.; Roy, A. Repair Scars on Mactra Violacea from the Eastern Coast of India: A New Classification and a Model for Describing Shell Breakage on Bivalves. Palaeontol. Electron. 2014, 17, 1–13. [Google Scholar] [CrossRef]
- Li, H.; Mu, G.; Li, X.; Wu, H.; Bin, X.; Liu, F.; Sun, Z.; Zhang, Q.; Zhang, H.; Xin, M.; et al. Design and Testing of the Clam Vibration Harvester’s V-Shaped Double-Spiral Harvesting Roller Brush Based on DEM-MBD. Aquac. Eng. 2025, 111, 102582. [Google Scholar] [CrossRef]
- Li, H.; Zhang, G.; Liu, F.; Li, X.; Wang, Y.; Mu, G. Design and Testing of Mudflat Shellfish Vibrating Harvester Based on DEM-MBD Simulation. Trans. Chin. Soc. Agric. Mach. 2024, 55, 361–370. [Google Scholar]
- Kaplan, D.L. Mollusc Shell Structures: Novel Design Strategies for Synthetic Materials. Curr. Opin. Solid State Mater. Sci. 1998, 3, 232–236. [Google Scholar] [CrossRef]
- Zhang, P.; Tang, J.; Tang, Q.; Zhang, M.; Shen, L.; Tian, W.; Zhang, Y.; Sun, Z. Shell Powder as a Novel Bio-Filler for Thermal Insulation Coatings. Chin. J. Chem. Eng. 2019, 27, 452–458. [Google Scholar] [CrossRef]
- Panigrahi, A.; Jena, H.; Surekha, B. Effect of Clams Shell in Impact Properties of Jute Epoxy Composite. Mater. Today Proc. 2018, 5, 19997–20001. [Google Scholar] [CrossRef]
- Li, T.; Zeng, K. Nano-Hierarchical Structure and Electromechanical Coupling Properties of Clamshell. J. Struct. Biol. 2012, 180, 73–83. [Google Scholar] [CrossRef]
- Wang, L.C.; Di, L.Q.; Liu, R.; Wu, H. Characterizations and Microsphere Formulation of Polysaccharide from the Marine Clam (Mactra Veneriformis). Carbohydr. Polym. 2013, 92, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.H.; Chai, X.W.; Yan, Z.F.; Chen, D.H.; Man, J. Clam Shell Compression Characteristics and Bond Strength. Adv. Mater. Res. 2012, 472–475, 2554–2557. [Google Scholar] [CrossRef]
- Fakayode, O.A. Size-Based Physical Properties of Hard-Shell Clam (Mercenaria mercenaria) Shell Relevant to the Design of Mechanical Processing Equipment. Aquac. Eng. 2020, 89, 102056. [Google Scholar] [CrossRef]
- Vasconcelos, P.; Morgado-André, A.; Morgado-André, C.; Gaspar, M.B. Shell Strength and Fishing Damage to the Smooth Clam (Callista Chione): Simulating Impacts Caused by Bivalve Dredging. ICES J. Mar. Sci. 2011, 68, 32–42. [Google Scholar] [CrossRef]
- Mu, G.; Duan, F.; Zhang, G.; Li, X.; Ding, X.; Zhang, L. Microstructure and Mechanical Property of Ruditapes Philippinarum Shell. J. Mech. Behav. Biomed. Mater. 2018, 85, 209–217. [Google Scholar] [CrossRef]
- Giordano, V.; Castagnoli, A.; Pecorini, I.; Chiarello, F. Identifying Technologies in Circular Economy Paradigm through Text Mining on Scientific Literature. PLoS ONE 2024, 19, e0312709. [Google Scholar] [CrossRef]
- Li, H.; Zhang, G.; Li, X.; Zhang, H.; Zhang, Q.; Liu, W.; Mu, G. Calibration of the Discrete Element Method Parameters in Living Juvenile Manila Clam (Ruditapes Philippinarum) and Seeding Verification. AgriEngineering 2021, 3, 894–906. [Google Scholar] [CrossRef]
- Zhang, Y.; Gai, G.; Zong, W.; Chen, P.; Wang, F.; Wang, X. Crushing Mechanism of Walnut under Extrusion-Shear Load Based on FDEM. Trans. Chin. Soc. Agric. Mach. 2025, 56, 291–300. [Google Scholar]
- Tang, H.; Zhu, G.; Wang, Z.; Xu, W.; Xu, C.; Wang, J. Prediction Method for Maize Kernel Impact Breakage Based on High-Speed Camera and FEM. Powder Technol. 2024, 444, 120002. [Google Scholar] [CrossRef]
- Tang, H.; Zhu, G.; Sun, Z.; Xu, C.; Wang, J. Impact Damage Evolution Rules of Maize Kernel Based on FEM. Biosyst. Eng. 2024, 247, 162–174. [Google Scholar] [CrossRef]
- Guan, X.; Li, T.; Zhou, F. Determination of Bruise Susceptibility of Fresh Corn to Impact Load by Means of Finite Element Method Simulation. Postharvest Biol. Technol. 2023, 198, 112227. [Google Scholar] [CrossRef]
- Hou, J.; Park, B.; Li, C.; Wang, X. A Multiscale Computation Study on Bruise Susceptibility of Blueberries from Mechanical Impact. Postharvest Biol. Technol. 2024, 208, 112660. [Google Scholar] [CrossRef]
- Zhang, G.; Li, Z.; Liu, W.; Zhang, Q.; Chen, L.; Pei, L.; Liu, H. Finite Element Simulation and Experiment of Impact Damage of Water Chestnuts. Trans. Chin. Soc. Agric. Eng. 2025, 41, 40–49. [Google Scholar]
- Lou, Y.; Zhang, G.; Li, X.; Zhang, Q.; Zhang, H.; Zhao, J.; Li, H.; Wu, H.; Ye, Z.; Zhang, Z.; et al. Experiment and Calibration of Contact Parameters of Mactra Veneriformis Based on DEM. Comput. Part. Mech. 2025, 12, 313–326. [Google Scholar] [CrossRef]
- Kafashan, J.; Wiacek, J.; Ramon, H.; Mouazen, A.M. Modelling and Simulation of Fruit Drop Tests by Discrete Element Method. Biosyst. Eng. 2021, 212, 228–240. [Google Scholar] [CrossRef]
- Gai, X.; Xie, S.; Deng, W.; Lu, K.; Ji, X. Construction and Parameter Calibration of Potato Double-layer Flexible Bonding Model Based on Discrete-element Method. J. Food Process Eng. 2024, 47, e14602. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, G.; Yong, Z.; Liu, H.; Tang, N.; Kang, Q.; Zhao, Z. Establishment of Discrete Element Flexible Model of the Tiller Taro Plant and Clamping and Pulling Experiment. Front. Plant Sci. 2022, 13, 1019017. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; He, X.; Shang, S.; Zhao, Z.; Wang, H.; Tan, Y.; Li, C.; Wang, D. Evaluation of Soil-Cutting and Plant-Crushing Performance of Rotary Blades with Double-Eccentric Circular-Edge Curve for Harvesting Cyperus Esculentus. Agriculture 2022, 12, 862. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, D.; Shang, S.; Guo, P.; Gao, Z.; Xia, C.; Yan, H.; Hou, J. Design and Test of a Dual-Axis Layered Rotary Tillage Stubble Exterminator in Saline-Alkali Land Based on Discrete Elements. Int. J. Agric. Biol. Eng. 2024, 17, 163–175. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Du, D.; Long, S.; Wang, Y.; Han, C.; Xu, Y. Optimization and Validation of Root-Cutting Device for Chinese Cabbage Harvester Based on Discrete Element Method. Comput. Electron. Agric. 2023, 214, 108314. [Google Scholar] [CrossRef]
- Luan, Y. Discrete Element Simulation Parameter Calibration for Smart Shellfish Seeding Devices. J. Intell. Agric. Mech. 2025, 6, 69–78. [Google Scholar]
- Zeng, H.; Xu, W.; Zang, M.; Yang, P. Calibration of DEM-FEM Model Parameters for Traction Performance Analysis of an off-Road Tire on Gravel Terrain. Powder Technol. 2020, 362, 350–361. [Google Scholar] [CrossRef]
- Chen, X.; Wang, L.G.; Morrissey, J.P.; Ooi, J.Y. DEM Simulations of Agglomerates Impact Breakage Using Timoshenko Beam Bond Model. Granul. Matter 2022, 24, 74. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, R.; Cao, Q.; Zhang, Y.; Fu, J.; Wen, X.; Yuan, H. A Calibration Method for Contact Parameters of Agricultural Particle Mixtures Inspired by the Brazil Nut Effect (BNE): The Case of Tiger Nut Tuber-Stem-Soil Mixture. Comput. Electron. Agric. 2023, 212, 108112. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, L.; Liu, H.; Dong, Z.; Zhang, H.; Zhou, Y. Calibration and Experiments of the Discrete Element Simulation Parameters for Water Chestnut. Trans. Chin. Soc. Agric. Eng. 2022, 38, 41–50. [Google Scholar]
- Liu, F.; Tang, H.; Shahin, M.A.; Zhao, H.; Karrech, A.; Zhu, F.; Zhou, H. Multiscale Simulation Study for Mechanical Characteristics of Coral Sand Influenced by Particle Breakage. Powder Technol. 2025, 449, 120387. [Google Scholar] [CrossRef]
- Chen, T.; Yi, S.; Li, Y.; Tao, G.; Qu, S.; Li, R. Establishment of Discrete Element Model and Parameter Calibration of Alfalfa Stem in Budding Stage. Trans. Chin. Soc. Agric. Mach. 2023, 54, 91–100. [Google Scholar]
- Zhou, B.L. Bio-Inspired Study of Structural Materials. Mater. Sci. Eng. C 2000, 11, 13–18. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, J.; Wu, C. The Micro/Nanostructure Characteristics and the Mechanical Properties of Hemifusus Tuba Conch Shell. J. Bionic Eng. 2010, 7, 307–313. [Google Scholar] [CrossRef]
- Yang, W.; Kashani, N.; Li, X.-W.; Zhang, G.-P.; Meyers, M.A. Structural Characterization and Mechanical Behavior of a Bivalve Shell (Saxidomus Purpuratus). Mater. Sci. Eng. C 2011, 31, 724–729. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, Q.; Li, X.; Zhang, Z.; Ren, L. Study of the Microstructure and Mechanical Properties of White Clam Shell. Micron 2016, 87, 10–17. [Google Scholar] [CrossRef]
- Zuschin, M.; Stanton, R.J. Experimental Measurement of Shell Strength and Its Taphonomic Interpretation. Palaios 2001, 16, 161–170. [Google Scholar] [CrossRef]
- Menig, R.; Meyers, M.H.; Meyers, M.A.; Vecchio, K.S. Quasi-Static and Dynamic Mechanical Response of Haliotis Rufescens (Abalone) Shells. Acta Mater. 2000, 48, 2383–2398. [Google Scholar] [CrossRef]
- Zhou, J.; Yu, X.; Ding, C.; Wang, Z.; Zhou, Q.; Pao, H.; Cai, W. Optimization of Phenol Degradation by Candida Tropicalis Z-04 Using Plackett-Burman Design and Response Surface Methodology. J. Environ. Sci. 2011, 23, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Meng, Y.; Qi, X.; Wang, Y.; Li, Y.; Li, M. Discrete Element Modelling Method and Parameter Calibration of Garlic Species Based on Bonding V2 Model. Trans. Chin. Soc. Agric. Mach. 2025, 56, 150–157. [Google Scholar]



| Level | Parameter | Value Ranges |
|---|---|---|
| X1 | Flesh–flesh normal (tangential) stiffness per unit area (N·m3) | 1 × 1011~1 × 1012 |
| X2 | Flesh–flesh normal (tangential) strength (Pa) | 1 × 1015~5 × 1015 |
| X3 | Shell–flesh normal (tangential) stiffness per unit area (N·m3) | 4 × 1012~4 × 1013 |
| X4 | Shell–flesh normal (tangential) strength (Pa) | 4 × 107~4 × 108 |
| X5 | Shell–shell normal stiffness per unit area (N·m3) | 1 × 1011~9 × 1012 |
| X6 | Shell–shell tangential stiffness per unit area (N·m3) | 1 × 1011~9 × 1012 |
| X7 | Shell–shell normal strength (Pa) | 1 × 106~9 × 107 |
| X8 | Shell–shell tangential strength (Pa) | 1 × 106~9 × 107 |
| Parameter | Value | Parameter | Value |
|---|---|---|---|
| Clam shell Poisson‘s ratio | 0.28 | Shell–flesh collision recovery coefficient | 0.08 |
| Clam shell density (kg·m−3) | 2600 | Shell–flesh static friction coefficient | 0.65 |
| Shear modulus of clam shell (Pa) | 1.5 × 109 | Shell–flesh dynamic friction coefficient | 0.30 |
| Poisson‘s ratio of clam flesh | 0.48 | Flesh–flesh collision recovery coefficient | 0.15 |
| Clam flesh density (kg·m−3) | 1050 | Flesh–flesh static friction coefficient | 0.45 |
| Clam flesh shear modulus (Pa) | 2 × 104 | Flesh–flesh dynamic friction coefficient | 0.30 |
| Poisson‘s ratio of stainless steel | 0.30 | Restitution coefficient of shell–steel collision | 0.28 |
| Stainless steel density (kg·m−3) | 7800 | Shell–steel static friction coefficient | 0.62 |
| Stainless steel shear modulus (Pa) | 7.8 × 1012 | Shell–steel dynamic friction coefficient | 0.16 |
| Shell–shell collision recovery coefficient | 0.22 | Restitution coefficient of flesh–steel collision | 0.10 |
| Shell–shell static friction coefficient | 0.41 | Flesh–steel static friction coefficient | 0.35 |
| Shell–shell dynamic friction coefficient | 0.23 | Flesh–steel dynamic friction coefficient | 0.2 |
| No. | Drop Height | Fractured | Intact | Fracture Rate |
|---|---|---|---|---|
| 1 | 95 | 1 | 9 | 10% |
| 2 | 100 | 5 | 5 | 50% |
| 3 | 105 | 7 | 3 | 70% |
| 4 | 110 | 6 | 4 | 60% |
| 5 | 115 | 2 | 8 | 20% |
| 6 | 120 | 4 | 6 | 40% |
| 7 | 125 | 9 | 1 | 90% |
| 8 | 130 | 10 | 0 | 100% |
| 9 | 135 | 10 | 0 | 100% |
| No. | X1 | X2 | X3 | X4 | X5 | X6 | X7 | X8 | Fsim (N) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | −1 | 1 | 1 | −1 | 1 | 1 | 1 | 6.33 |
| 2 | −1 | −1 | −1 | 1 | −1 | 1 | 1 | −1 | 5.49 |
| 3 | 1 | 1 | −1 | 1 | 1 | 1 | −1 | −1 | 4.73 |
| 4 | 1 | −1 | −1 | −1 | 1 | −1 | 1 | 1 | 5.24 |
| 5 | −1 | 1 | 1 | −1 | 1 | 1 | 1 | −1 | 6.15 |
| 6 | 1 | 1 | 1 | −1 | −1 | −1 | 1 | −1 | 5.76 |
| 7 | −1 | 1 | −1 | 1 | 1 | −1 | 1 | 1 | 4.37 |
| 8 | 1 | 1 | −1 | −1 | −1 | 1 | −1 | 1 | 4.89 |
| 9 | −1 | −1 | 1 | −1 | 1 | 1 | −1 | 1 | 4.42 |
| 10 | 1 | −1 | 1 | 1 | 1 | −1 | −1 | −1 | 5.11 |
| 11 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | 4.21 |
| 12 | −1 | 1 | 1 | 1 | −1 | −1 | −1 | 1 | 4.41 |
| No. | X1 | X3 | X6 | X7 | Fsim (N) | E (%) |
|---|---|---|---|---|---|---|
| 1 | 1 × 1011 | 4 × 1012 | 1 × 1011 | 1 × 106 | 4.20 | 10.07 |
| 2 | 2.8 × 1011 | 1.13 × 1013 | 1.92 × 1012 | 1.92 × 107 | 4.70 | 0.71 |
| 3 | 4.6 × 1011 | 1.86 × 1013 | 3.74 × 1012 | 3.74 × 107 | 4.73 | 1.35 |
| 4 | 6.4 × 1011 | 2.50 × 1013 | 5.56 × 1012 | 5.56 × 107 | 4.75 | 1.79 |
| 5 | 8.2 × 1011 | 3.32 × 1013 | 7.38 × 1012 | 7.38 × 107 | 4.81 | 2.42 |
| 6 | 1 × 1012 | 4 × 1013 | 9 × 1012 | 9 × 107 | 5.24 | 11.20 |
| No. | X1 | X3 | X6 | X7 | Fsim (N) | E (%) |
|---|---|---|---|---|---|---|
| 1 | 0 | 0 | 0 | 0 | 4.630 | 0.85 |
| 2 | 0 | 0 | −1 | −1 | 4.075 | 12.74 |
| 3 | 0 | −1 | 0 | −1 | 4.654 | 0.35 |
| 4 | 0 | 1 | 0 | −1 | 4.382 | 6.17 |
| 5 | −1 | 0 | 0 | −1 | 4.371 | 6.41 |
| 6 | −1 | 0 | 1 | 0 | 4.764 | 2.01 |
| 7 | 1 | 0 | 0 | −1 | 4.656 | 0.31 |
| 8 | 1 | 0 | 1 | 0 | 4.563 | 2.29 |
| 9 | 0 | 1 | 1 | 0 | 4.587 | 1.77 |
| 10 | −1 | −1 | 0 | 0 | 4.353 | 6.79 |
| 11 | −1 | 0 | −1 | 0 | 3.925 | 15.96 |
| 12 | 0 | 0 | 1 | 1 | 4.631 | 0.83 |
| 13 | 0 | 1 | 0 | 1 | 4.731 | 1.31 |
| 14 | 0 | 0 | 0 | 0 | 4.631 | 0.84 |
| 15 | 0 | 1 | −1 | 0 | 3.961 | 15.19 |
| 16 | 0 | −1 | 0 | −1 | 4.309 | 7.74 |
| 17 | 0 | 0 | 1 | −1 | 4.670 | 0.01 |
| 18 | 1 | −1 | 0 | 0 | 4.748 | 1.68 |
| 19 | 0 | 0 | 1 | 1 | 4.798 | 2.74 |
| 20 | 0 | −1 | 1 | 0 | 4.789 | 2.55 |
| 21 | 0 | −1 | −1 | 0 | 3.955 | 15.31 |
| 22 | 1 | 1 | 0 | 0 | 4.730 | 1.28 |
| 23 | 0 | 0 | −1 | 1 | 4.263 | 8.70 |
| 24 | 1 | 0 | 0 | 1 | 4.800 | 2.78 |
| 25 | 1 | 0 | −1 | 0 | 4.065 | 12.95 |
| 26 | −1 | 0 | 0 | 1 | 4.506 | 3.52 |
| 27 | −1 | 1 | 0 | 0 | 4.468 | 4.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, B.; Yang, Y.; Li, H.; Chen, G.; Chang, Y.; Guo, F.; Wu, H.; Zhao, J.; Liu, Z.; Zhang, G.; et al. Mechanical Characterization and Dual-Layer Discrete Element Modeling of Mactra veneriformis. Fishes 2025, 10, 429. https://doi.org/10.3390/fishes10090429
Xu B, Yang Y, Li H, Chen G, Chang Y, Guo F, Wu H, Zhao J, Liu Z, Zhang G, et al. Mechanical Characterization and Dual-Layer Discrete Element Modeling of Mactra veneriformis. Fishes. 2025; 10(9):429. https://doi.org/10.3390/fishes10090429
Chicago/Turabian StyleXu, Bin, Yazhou Yang, Hangqi Li, Guangcong Chen, Yizhi Chang, Feihong Guo, Hao Wu, Jixuan Zhao, Zijing Liu, Guochen Zhang, and et al. 2025. "Mechanical Characterization and Dual-Layer Discrete Element Modeling of Mactra veneriformis" Fishes 10, no. 9: 429. https://doi.org/10.3390/fishes10090429
APA StyleXu, B., Yang, Y., Li, H., Chen, G., Chang, Y., Guo, F., Wu, H., Zhao, J., Liu, Z., Zhang, G., Li, X., Zhang, H., Zhang, Q., & Mu, G. (2025). Mechanical Characterization and Dual-Layer Discrete Element Modeling of Mactra veneriformis. Fishes, 10(9), 429. https://doi.org/10.3390/fishes10090429






