Effects of Different Organic Carbon Sources on Water Quality and Growth of Mugil cephalus Cultured in Biofloc Technology Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Location
2.3. Pre-Acclimation to Culture Conditions
2.4. Experimental Conditions
2.5. Ex Situ Maturation of the Bioflocs
2.6. Water Quality Parameters
2.7. Feed
2.8. Growth Performance
2.9. Statistical Analysis
3. Results
3.1. Water Quality Parameters
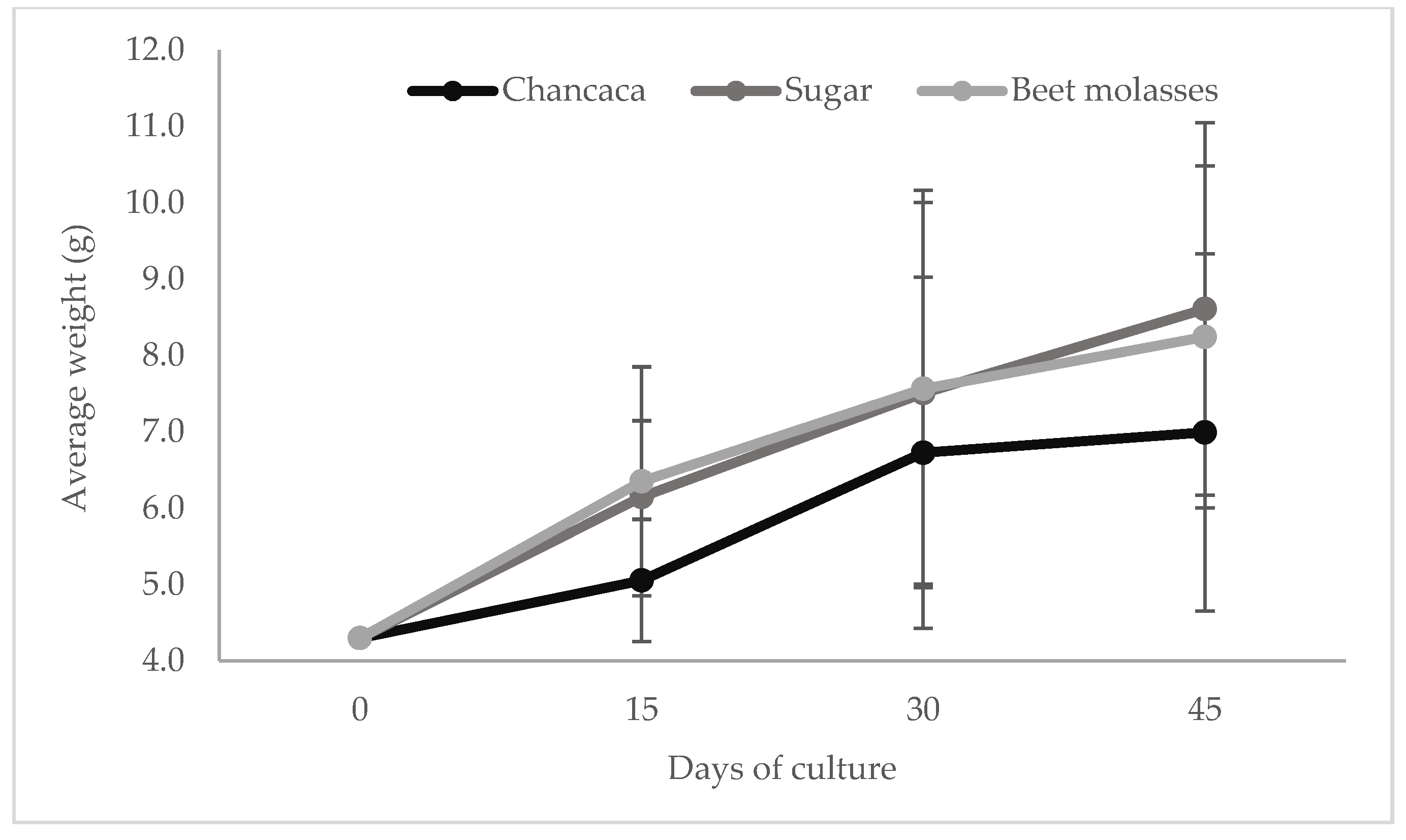
3.2. Growth Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koven, W.; Gisbert, E.; Meiri-Ashkenazi, I.; Nixon, O.; Israeli, D.; Tandler, A.; Rosenfeld, H. The effect of weaning diet type on grey mullet (Mugil cephalus) juvenile performance during the trophic shift from carnivory to omnivory. Aquaculture 2020, 518, 734848. [Google Scholar] [CrossRef]
- Sunarni, S.M.; Wairara, S.; Sisca, E. Distribution patterns and abundance of mullet fish populations (Mugil sp.) estuary areas. J. Phys. Conf. Ser. 2021, 1899, 012019. [Google Scholar] [CrossRef]
- Jamabo, N.A.; Maduako, N.C. Food and feeding habits of Mugil cephalus (Linnaeus, 1758) in Elechi Creek, Niger Delta, Nigeria. Int. J. Fish. Aquac. 2015, 7, 25–29. [Google Scholar] [CrossRef]
- Lawson, E.O.; Jimoh, A.A. Aspects of the biology of grey mullet (Mugil cephalus), in Lagos lagoon, Nigeria. Aquac. Aquar. Conserv. Legis. 2010, 3, 181–193. Available online: http://www.bioflux.com.ro/docs/2010.3.181-193.pdf?AdobeSystemsPDFv17=2e5f561ee1c0e839b1bc776aeffd9df47cfb381c (accessed on 26 November 2024).
- Bhakta, D.; Das, B.K.; Kamble, S.P.; Solanki, S.; Solanki, J.K.; Sahoo, A.K.; Samanta, S. Reproductive biology of estuarine migrants (Mugil cephalus) from Narmada estuary, west coast of India. Reg. Stud. Mar. Sci. 2024, 70, 103371. [Google Scholar] [CrossRef]
- Trape, S.; Durand, J.; Guilhaumon, F.; Vigliola, L.; Panfili, J. Recruitment patterns of young-of-the-year mugilid fishes in a West African estuary impacted by climate change. Estuar. Coast. Shelf Sci. 2009, 85, 357–367. [Google Scholar] [CrossRef]
- Whitfield, A.K.; Panfili, J.; Durand, J.D. A global review of the cosmopolitan flathead mullet Mugil cephalus Linnaeus 1758 (Teleostei: Mugilidae), with emphasis on the biology, genetics, ecology and fisheries aspects of this apparent species complex. Rev. Fish Biol. Fish. 2012, 22, 641–681. [Google Scholar] [CrossRef]
- Ahammed, M.B.; Momin, A.; Sultana, S.; Sarkar, A. pH and Temperature Monitoring with a GSM-based Auto Feeding System of a Biofloc Technology. Int. J. Sci. Eng. Res. 2022, 13, 270–274. Available online: https://www.researchgate.net/profile/Anirban-Sarkar-25/publication/360053559 (accessed on 20 November 2024).
- Crab, R.; Chielens, B.; Wille, M.; Bossier, P.; Verstraete, W. The effect of different carbon sources on the nutritional value of bioflocs, a feed for Macrobrachium rosenbergii postlarvae. Aquac. Res. 2010, 41, 559–567. [Google Scholar] [CrossRef]
- Ahmed, N.; Thompson, S.; Glaser, M. Global aquaculture productivity, environmental sustainability, and climate change adaptability. Environ. Manag. 2019, 63, 159–172. [Google Scholar] [CrossRef]
- Servicio Nacional de Pesca y Acuicultura. Anuario Estadístico de Pesca y Acuicultura; Servicio Nacional de Pesca y Acuicultura SERNAPESCA: Victoria, Chile, 2024; Available online: http://www.sernapesca.cl/informacion-utilidad/anuarios-estadisticos-de-pesca-y-acuicultura (accessed on 13 January 2025).
- Souza, D.; Suita, S.; Romano, L.; Wasielesky, W., Jr.; Ballester, E. Use of molasses as a carbon source during the nursery rearing of Farfantepenaeus brasiliensis (Latreille, 1817) in a Biofloc technology system. Aquaculture 2014, 45, 270–277. [Google Scholar] [CrossRef]
- Mansour, A.; Esteban, M. Effects of carbon sources and plant protein levels in a biofloc system on growth performance, and the immune and antioxidant status of Nile tilapia (Oreochromis niloticus). Fish Shellfish. Immunol. 2017, 64, 202–209. [Google Scholar] [CrossRef] [PubMed]
- El-Hawarry, W.; Shourbela, R.; Haraz, Y.; Khatab, S.; Dawood, M. The influence of carbon source on growth, feed efficiency, and growth-related genes in Nile tilapia (Oreochromis niloticus) reared under biofloc conditions and high stocking density. Aquaculture 2021, 542, 736919. [Google Scholar] [CrossRef]
- Khanjani, M.; Alizadeh, M.; Mohammadi, M.; Sarsangi Aliabad, H. The effect of adding molasses in different times on performance of Nile tilapia (Oreochromis niloticus) raised in a low-salinity biofloc system. Ann. Anim. Sci. 2021, 21, 1435–1454. [Google Scholar] [CrossRef]
- Khanjani, M.; Sharifinia, M. Biofloc technology with addition molasses as carbon sources applied to Litopenaeus vannamei juvenile production under the effects of different C/N ratios. Aquac. Int. 2022, 30, 383–397. [Google Scholar] [CrossRef]
- Soliman, A.; Abdel-Tawwab, M. Effects of different carbon sources on water quality, biofloc quality, and the productivity of Nile tilapia reared in biofloc-based ponds. Ann. Anim. Sci. 2022, 22, 1281–1289. [Google Scholar] [CrossRef]
- Mansour, A.; Ashry, O.; Ashour, M.; Alsaqufi, A.; Ramadan, K.; Sharawy, Z. The optimization of dietary protein level and carbon sources on biofloc nutritive values, bacterial abundance, and growth performances of whiteleg shrimp (Litopenaeus vannamei) juveniles. Life 2022, 12, 888. [Google Scholar] [CrossRef]
- Huang, H.; Li, C.; Lei, Y.; Kuang, W.; Zou, W.; Yang, P. Bacterial composition and inferring function profiles in the biofloc system rearing Litopenaeus vannamei postlarvae at a low salinity. Isr. J. Aquac. Bamidgeh 2022, 74, 1–19. [Google Scholar] [CrossRef]
- Khanjani, M.H.; Sharifinia, M.; Hajirezaee, S. Recent progress towards the application of biofloc technology for tilapia farming. Aquaculture 2022, 552, 738021. [Google Scholar] [CrossRef]
- Mugwanya, M.; Dawood, M.; Kimera, F.; Sewilam, H. Biofloc Systems for Sustainable Production of Economically Important Aquatic Species: A Review. Sustainability 2021, 13, 7255. [Google Scholar] [CrossRef]
- Xu, W.; Xu, Y.; Su, H.; Hu, X.; Yang, K.; Wen, G.; Cao, Y. Characteristics of ammonia removal and nitrifying microbial communities in a hybrid biofloc-RAS for intensive Litopenaeus vannamei culture: A pilot-scale study. Water 2020, 12, 3000. [Google Scholar] [CrossRef]
- Ponce-Palafox, J.T.; Pavia, Á.A.; López, D.G.M.; Arredondo-Figueroa, J.L.; Lango-Reynoso, F.; Castañeda-Chávez, M.d.R.; Esparza-Leal, H.; Ruiz-Luna, A.; Páez-Ozuna, F.; Castillo-Vargasmachuca, S.G. Response surface analysis of temperature-salinity interaction effects on water quality, growth and survival of shrimp Penaeus vannamei postlarvae raised in biofloc intensive nursery production. Aquaculture 2019, 503, 312–321. [Google Scholar] [CrossRef]
- Esparza-Leal, H.; Amaral-Xavier, J.; Wasielesky, W. Performance of Litopenaeus vannamei postlarvae reared in indoor nursery tanks under biofloc conditions at different salinities and zero-water exchange. Aquac. Int. 2016, 24, 1435–1447. [Google Scholar] [CrossRef]
- Ray, A.; Lotz, J. Comparing salinities of 10, 20, and 30% in intensive, commercial-scale biofloc shrimp (Litopenaeus vannamei) production systems. Aquaculture 2017, 476, 29–36. [Google Scholar] [CrossRef]
- Cardona, L. Habitat selection by grey mullets (Osteichthytes: Mugilidae) in Mediterranean estuaries: The role of salinity. Sci. Mar. 2006, 70, 443–455. Available online: https://scientiamarina.revistas.csic.es/index.php/scientiamarina/article/view/96/93 (accessed on 26 November 2024). [CrossRef]
- Souza, J.; Cardozo, A.; Wasielesky, W., Jr.; Abreu, P. Does the biofloc size matter to the nitrification process in Biofloc Technology (BFT) Systems? Aquaculture 2019, 500, 443–450. [Google Scholar] [CrossRef]
- Ferreira, G.; Silva, V.; Martins, M.; Da Silva, A.C.; Machado, C.; Seiffert, W.; Do Nascimento-Vieira, F. Strategies for ammonium and nitrite control in Litopenaeus vannamei nursery systems with bioflocs. Aquac. Eng. 2020, 88, 102040. [Google Scholar] [CrossRef]
- Avnimelech, Y. Carbon/nitrogen ratio as a control element in aquaculture systems. Aquaculture 1999, 176, 227–235. [Google Scholar] [CrossRef]
- Dongare, M.L.; Buchade, P.B.; Awatade, M.N.; Shaligram, A.D. Mathematical modeling and simulation of refractive index based Brix measurement system. Optik 2014, 125, 946–949. [Google Scholar] [CrossRef]
- Ayazo-Genes, J.; Pertúz-Buelvas, V.; Espinosa-Araujo, J.; Jiménez-Velásquez, C.; Atencio-García, V.; Prieto-Guevara, M. Desempeño de bocachico (Prochilodus magdalenae) en sistemas intensivos de producción con tecnología biofloc. Biotecnol. Sect. Agropecu. Agroindustrial 2018, 16, 91–101. [Google Scholar] [CrossRef]
- Lara, G.; Poersch, L.; Wasielesky, W., Jr. The quantity of artificial substrates influences the nitrogen cycle in the biofloc culture system of Litopenaeus vannamei. Aquac. Eng. 2021, 94, 102171. [Google Scholar] [CrossRef]
- Avnimelech, Y. Feeding with microbial flocs by tilapia in minimal discharge bio-flocs technology ponds. Aquaculture 2007, 264, 140–147. [Google Scholar] [CrossRef]
- APHA (American Public Health Association). Standard Methods for the Examination of Water and Wastewater, 20th ed; APHA (American Public Health Association): Washington, DC, USA, 2016; p. 1546. [Google Scholar]
- Kibenge, F.S.B. Descriptions of major farmed aquatic animal species. In Aquaculture Pathophysiology; Frederick, S.B., Kibenge, B.B., Roger, S.M.C., Eds.; Academic Press: New York, NY, USA, 2022; Volume 1, pp. 1–44. [Google Scholar] [CrossRef]
- Prakoso, V.A.; Kim, K.T.; Ryu, J.H.; Min, B.H.; Chang, Y.J. Oxygen consumption of Mugil cephalus on several temperatures under brackish water conditions. IOP Conf. Ser. Earth Environ. Sci. 2019, 278, 012060. [Google Scholar] [CrossRef]
- Whitfield, A.K.; Taylor, R.H.; Fox, C.; Cyrus, D.P. Fishes and salinities in the St Lucia system—A review. Rev. Fish Biol. Fish. 2006, 6, 1–20. [Google Scholar] [CrossRef]
- Borges, B.A.A.; Rocha, J.L.; Pinto, P.H.O.; Zacheu, T.; Chede, A.C.; Magnotti, C.C.F.; Arana, L.A.V. Integrated culture of white shrimp (Litopenaeus vannamei) and mullet (Mugil liza) on biofloc technology: Zootechnical performance, sludge generation, and Vibrio spp. reduction. Aquaculture 2020, 524, 735234. [Google Scholar] [CrossRef]
- Garcés, S.; Lara, G. Applying Biofloc Technology in the Culture of Mugil cephalus in Subtropical Conditions: Effects on Water Quality and Growth Parameters. Fishes 2023, 8, 420. [Google Scholar] [CrossRef]
- Ebeling, J.; Timmons, M.; Bisogni, J. Engineering analysis of the stoichiometry of photoautotrophic, autotrophic, and heterotrophic removal of ammonia–nitrogen in aquaculture systems. Aquaculture 2006, 257, 346–358. [Google Scholar] [CrossRef]
- Emerenciano, M.; Martínez-Córdova, L.; Martínez-Porchas, M.; Miranda-Baeza, A. Biofloc Technology (BFT): A Tool for Water Quality Management in Aquaculture. Water Qual. 2017, 5, 92–109. [Google Scholar] [CrossRef]
- Biswas, G.; De, D.; Thirunavukkarasu, A.; Natarajan, M.; Sundaray, J.; Kailasam, M.; Sarkar, A. Effects of stocking density, feeding, fertilization and combined fertilization-feeding on the performances of striped grey mullet (Mugil cephalus L.) fingerlings in brackishwater pond rearing systems. Aquaculture 2012, 338, 284–292. [Google Scholar] [CrossRef]
- Hoang, M.N.; Nguyen, P.N.; Bossier, P. Water quality, animal performance, nutrient budgets and microbial community in the biofloc-based polyculture system of white shrimp (Litopenaeus vannamei) and gray mullet (Mugil cephalus). Aquaculture 2020, 515, 734610. [Google Scholar] [CrossRef]
- Legarda, E.C.; Aranha Martins, M.; Moreira Pereira, P.K.; Siqueira Carneiro, R.F.; Pinheiro, I.C.; Seiffert, W.Q.; Vieira, F.D.N. Shrimp rearing in biofloc integrated with different mullet stocking densities. Aquac. Res. 2020, 51, 3571–3581. [Google Scholar] [CrossRef]
- Legarda, E.C.; da Silva, D.; Miranda, C.S.; Pereira, P.K.M.; Martins, M.A.; Machado, C.; do Nascimento Vieira, F. Sea lettuce integrated with Pacific white shrimp and mullet cultivation in biofloc impact system performance and the sea lettuce nutritional composition. Aquaculture 2021, 534, 736265. [Google Scholar] [CrossRef]
- El-Sayed, A. Use of biofloc technology in shrimp aquaculture: A comprehensive review, with emphasis on the last decade. Rev. Aquac. 2021, 13, 676–705. [Google Scholar] [CrossRef]
- Soaudy, M.R.; Ghonimy, A.; Greco, L.S.L.; Chen, Z.; Dyzenchauz, A.; Li, J. Total suspended solids and their impact in a biofloc system: Current and potentially new management strategies. Aquaculture 2023, 572, 739524. [Google Scholar] [CrossRef]
- Hargreaves, J.A. Biofloc Production Systems for Aquaculture; Southern Regional Aquaculture Center: Stoneville, MS, USA, 2013; Volume 4503, pp. 1–11. [Google Scholar]
- Vinatea, L.; Malpartida, J.; Carbó, R.; Andree, K.B.; Gisbert, E.; Estevez, A. A comparison of recirculation aquaculture systems versus biofloc technology culture system for on-growing of fry of Tinca (Cyprinidae) and fry of grey Mugil cephalus (Mugilidae). Aquaculture 2018, 482, 155–161. [Google Scholar] [CrossRef]
- Haridas, H.; Chadha, N.K.; Sawant, P.B.; Deo, A.D.; Ande, M.P.; Syamala, K.; Lingam, S.S. Different carbon sources influences the growth and digestive enzyme activity of grey mullet (Mugil cephalus) in biofloc based nursery rearing system. J. Environ. Biol. 2021, 42, 1249–1256. [Google Scholar] [CrossRef]
- Dauda, A.B.; Romano, N.; Ebrahimi, M.; Karim, M.; Natrah, I.; Kamarudin, M.S.; Ekasari, J. Different carbon sources affects biofloc volume, water quality and the survival and physiology of African catfish (Clarias gariepinus) fingerlings reared in an intensive biofloc technology system. Fish. Sci. 2017, 83, 1037–1048. [Google Scholar] [CrossRef]
- Panigrahi, A.; Saranya, C.; Ambiganandam, K.; Sundaram, M.; Sivakumar, M.R. Evaluation of biofloc generation protocols to adopt high density nursery rearing of Penaeus vannamei for better growth performances, protective responses and immuno modulation in biofloc based technology. Aquaculture 2020, 522, 735095. [Google Scholar] [CrossRef]
- Xu, W.; Xu, Y.; Su, H.; Hu, X.; Xu, Y.; Li, Z.; Cao, Y. Production performance, inorganic nitrogen control and bacterial community characteristics in a controlled biofloc-based system for indoor and outdoor super-intensive culture of Litopenaeus vannamei. Aquaculture 2021, 531, 735749. [Google Scholar] [CrossRef]
- Mohammadi, G.; Rafiee, G.; Tavabe, K.R.; Abdel-Latif, H.M.; Dawood, M.A. The enrichment of diet with beneficial bacteria (single-or multi-strain) in biofloc system enhanced the water quality, growth performance, immune responses, and disease resistance of Nile tilapia (Oreochromis niloticus). Aquaculture 2021, 539, 736640. [Google Scholar] [CrossRef]
- Aboseif, A.M.; Flefil, N.S.; Taha, M.K.; Tahoun, U.M.; Mola, H.R.A.; El-Haroun, E.; Goda, A.M.A. Influence of dietary C: N: P ratios on Nile tilapia (Oreochromis niloticus) growth performance and formation of water biotic communities within a biofloc system containment. Aquac. Rep. 2022, 24, 101136. [Google Scholar] [CrossRef]
- Holanda, M.; Santana, G.; Furtado, P.; Rodrigues, R.V.; Cerqueira, V.R.; Sampaio, L.A.; Wasielesky, W., Jr.; Poersch, L.H. Evidence of total suspended solids control by Mugil liza reared in an integrated system with pacific white shrimp Litopenaeus vannamei using biofloc technology. Aquac. Rep. 2020, 18, 100479. [Google Scholar] [CrossRef]
- Lara, G.; Furtado, P.S.; Hostins, B.; Poersch, L.; Wasielesky, W., Jr. Addition of sodium nitrite and biofilm in a Litopenaeus vannamei biofloc culture system. Lat. Am. J. Aquat. Res. 2015, 44, 760–768. [Google Scholar] [CrossRef]
- Uawisetwathana, U.; Situmorang, M.L.; Arayamethakorn, S.; Haniswita Suantika, G.; Panya, A.; Rungrassamee, W. Supplementation of ex-situ biofloc to improve growth performance and enhance nutritional values of the Pacific white shrimp rearing at low salinity conditions. Appl. Sci. 2021, 11, 4598. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, J.; Dong, D.; Li, M.; Yang, X.; Song, X.; Li, X. Effects of carbon source addition strategies on water quality, growth performance, and microbial community in shrimp BFT aquaculture systems. Aquaculture 2024, 578, 740027. [Google Scholar] [CrossRef]
- Abakari, G.; Luo, G.; Kombat, E.O.; Alhassan, E.H. Supplemental carbon sources applied in biofloc technology aquaculture systems: Types, effects and future research. Rev. Aquac. 2021, 13, 1193–1222. [Google Scholar] [CrossRef]
- Wu, J.; Xu, W.; Xu, Y.; Su, H.; Hu, X.; Cao, Y.; Wen, G. Impact of organic carbons addition on the enrichment culture of nitrifying biofloc from aquaculture water: Process, efficiency, and microbial community. Microorganisms 2024, 12, 703. [Google Scholar] [CrossRef] [PubMed]
- Durand, J.D.; Shen, K.N.; Chen, W.J.; Jamandre, B.W.; Blel, H.; Diop, K.; Borsa, P. Systematics of the grey mullets (Teleostei: Mugiliformes: Mugilidae): Molecular phylogenetic evidence challenges two centuries of morphology-based taxonomy. Mol. Phylogenetics Evol. 2012, 64, 73–92. [Google Scholar] [CrossRef]
- Parrino, V.; Cappello, T.; Costa, G.; Cannavà, C.; Sanfilippo, M.; Fazio, F.; Fasulo, S. Comparative study of haematology of two teleost fish (Mugil cephalus and Carassius auratus) from different environments and feeding habits. Eur. Zool. J. 2018, 85, 193–199. [Google Scholar] [CrossRef]
- Talukdar, A.; Deo, A.D.; Sahu, N.P.; Sardar, P.; Aklakur, M.; Prakash, S.; Shamna, N.; Kumar, S. Effects of dietary protein on growth performance, nutrient utilization, digestive enzymes and physiological status of grey mullet, (Mugil cephalus L). fingerlings reared in inland saline water. Aquac. Nutr. 2020, 26, 921–935. [Google Scholar] [CrossRef]
- Bensaâd-Bendjedid, L.; Tahri, M.; Mokrani, A.; Mounira, R. Growth, mortality and exploitation level of (Mugil cephalus) (Linnaeus, 1758) in El Mellah Lagoon (Algeria). Egypt. J. Aquat. Biol. 2022, 26, 1381–1393. Available online: https://www.researchgate.net/publication/363147860_Growth_mortality_and_exploitation_level_of_Mugil_cephalus_Linnaeus_1758_in_El_Mellah_Lagoon_Algeria (accessed on 13 January 2025). [CrossRef]
- Hoang, M.; Nguyen, P.; Le, D.; Nguyen, D.; Bossier, P. Effects of stocking density of gray mullet (Mugil cephalus) on water quality, growth performance, nutrient conversion rate, and microbial community structure in the white shrimp (Litopenaeus vannamei) integrated system. Aquaculture 2018, 496, 123–133. [Google Scholar] [CrossRef]
- Gisbert, E.; Mozanzadeh, M.T.; Kotzamanis, Y.; Estévez, A. Weaning wild flathead grey mullet (Mugil cephalus) fry with diets with different levels of fish meal substitution. Aquaculture 2016, 462, 92–100. [Google Scholar] [CrossRef]
- Mehrim, A.; Refaey, M.; Khalil, F.; Shaban, Z. Impact of Mono- and Polyculture Systems on Growth Performance, Feed Utilization, and Economic Efficiency of Oreochromis niloticus, Mugil cephalus, and Mugil capito. J. Anim. Poult. Prod. 2018, 9, 393–400. [Google Scholar] [CrossRef]
- Biswas, G.; Kumar, P.; Ghoshal, T.K.; Kailasam, M.; De, D.; Bera, A.; Mandal, B.; Sukumaran, K.; Vijayan, K.K. Integrated multi-trophic aquaculture (IMTA) outperforms conventional polyculture with respect to environmental remediation, productivity and economic return in brackish water ponds. Aquaculture 2020, 516, 734626. [Google Scholar] [CrossRef]
- Elhetawy, A.I.; El-Dahhar, A.A.; Elebiary, E.H.; Abo El-Wafa, M.A.; Lotfy, A.M.; Emelianova, N. Effect of biofloc system at different salinities and crude protein levels on water quality, growth performance, and survival rate of flathead grey mullet (Mugil Cephalus). Egypt. J. Aquac. 2021, 11, 41–67. [Google Scholar] [CrossRef]
- Magana-Gallegos, E.; Gonzalez-Zuniga, R.; Arevalo, M.; Cuzon, G.; Chan-Vivas, E.; Lopez-Aguiar, K.; Gaxiola, G. Biofloc and food contribution to grow out and broodstock of Farfantepenaeus brasiliensis (Latreille, 1817) determined by stable isotopes and fatty acids. Aquac. Res. 2018, 49, 1782–1794. [Google Scholar] [CrossRef]
- Dauda, A.B. Biofloc technology: A review on the microbial interactions, operational parameters and implications to disease and health management of cultured aquatic animals. Rev. Aquac. 2020, 12, 1193–1210. [Google Scholar] [CrossRef]
- Debnath, S.; Ahmed, M.U.; Parvez, M.S.; Karmokar, A.K.; Ahsan, M.N. Effect of stocking density on growth performance and body composition of climbing perch (Anabas testudineus) in biofloc system. Aquac. Int. 2022, 30, 1089–1100. [Google Scholar] [CrossRef]
- Ekasari, J.; Angela, D.; Waluyo, S.; Bachtiar, T.; Surawidjaja, E.; Bossier, P.; De Schryver, P. The size of biofloc determines the nutritional composition and the nitrogen recovery by aquaculture animals. Aquaculture 2014, 426, 105–111. [Google Scholar] [CrossRef]
- Becerril-Cortés, D.; Monroy-Dosta, M.; Coelho-Emerenciano, M.; Castro-Mejía, G.; Cienfuegos-Martínez, K.; Lara-Andrade, D. Nutritional importance for aquaculture and ecological function of microorganisms that make up Biofloc, a review. Int. J. Aquat. Sci. 2017, 8, 69–77. [Google Scholar]
- Castro, L.; Pinto, R.; Nunes, A. Nutrient value and contribution of microbial floc to the growth performance of juvenile shrimp (Litopenaeus vannamei), fed fatty acid and amino acid restrained diets under a zero-water exchange intensive system. Aquaculture 2021, 531, 735789. [Google Scholar] [CrossRef]
- Abakari, G.; Wu, X.; He, X.; Fan, L.; Luo, G. Bacteria in biofloc technology aquaculture systems: Roles and mediating factors. Rev. Aquac. 2022, 14, 1260–1284. [Google Scholar] [CrossRef]

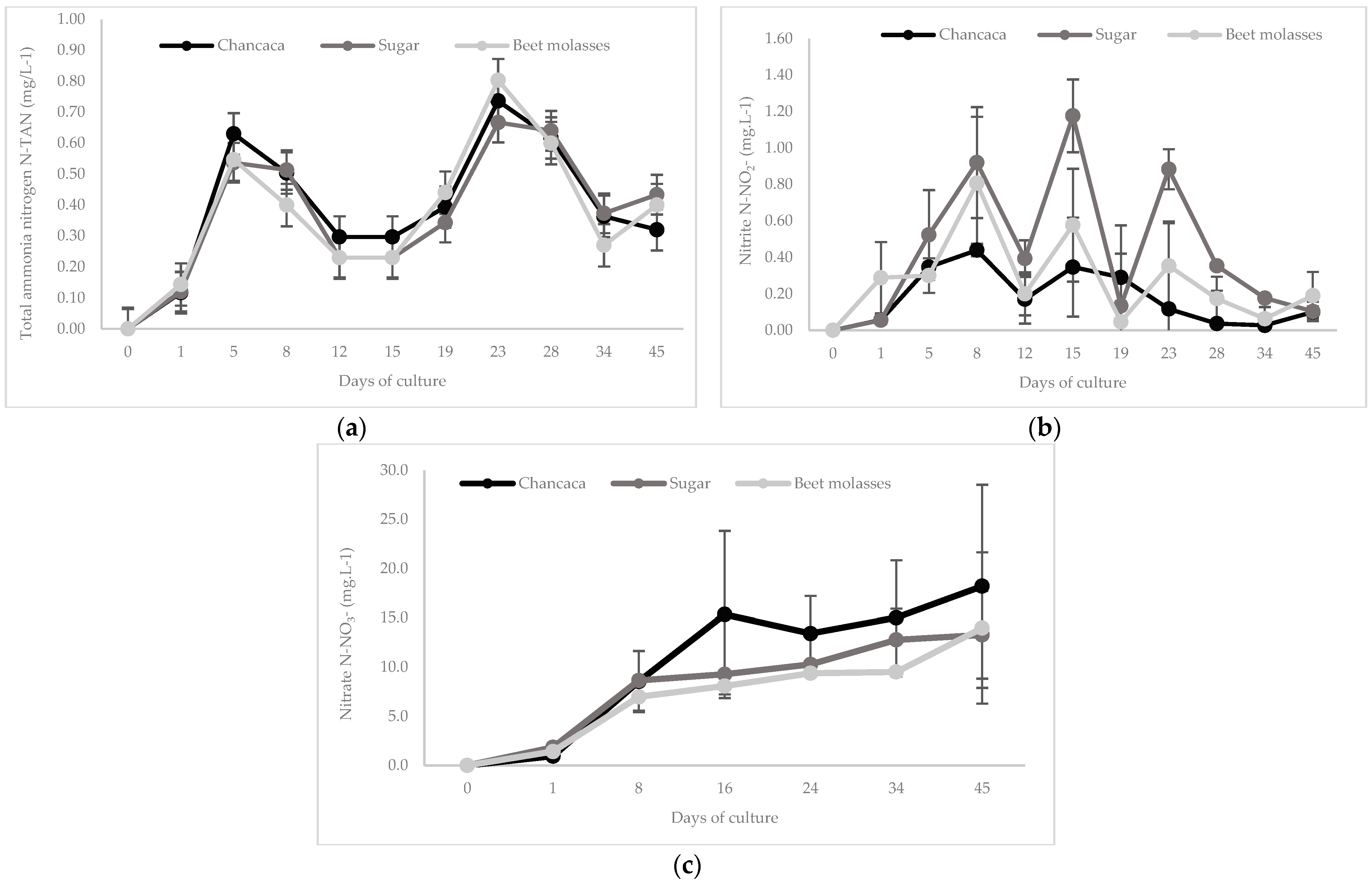
| Nitrogen Compounds | Average | Maximum | Minimum * |
|---|---|---|---|
| Total ammonia nitrogen (N-TAN) | |||
| Chancaca | 0.39 ± 0.25 | 0.95 | 0.00 *–0.03 ** |
| Sucrose | 0.37 ± 0.23 | 0.85 | 0.00 *–0.12 ** |
| Beet molasses | 0.37 ± 0.25 | 0.83 | 0.00 *–0.03 ** |
| Nitrite (mg·L−1 N-NO2−) | |||
| Chancaca | 0.17 ± 0.24 b | 0.80 | 0.00 *–0.020 ** |
| Sucrose | 0.43 ± 0.43 a | 1.65 | 0.00 *–0.037 ** |
| Beet molasses | 0.28 ± 0.28 ab | 1.17 | 0.00 *–0.030 ** |
| Nitrate (mg·L−1 N-NO3−) | |||
| Chancaca | 10.20 ± 8.43 | 30.10 | 0.00 *–0.60 ** |
| Sucrose | 8.00 ± 5.19 | 18.40 | 0.00 *–0.15 ** |
| Beet molasses | 7.04 ± 5.24 | 22.20 | 0.00 *–0.70 ** |
| Growth Parameters | Chancaca | Sucrose | Beet Molasses |
|---|---|---|---|
| Initial weight (g) | 4.33 ± 2.09 | 4.33 ± 2.09 | 4.33 ± 2.09 |
| Initial length (cm) | 6.40 ± 1.15 | 6.40 ± 1.15 | 6.40 ± 1.15 |
| Final average weight (g) | 6.99 ± 0.16 b | 8.59 ± 0.51 a | 8.15 ± 0.77 ab |
| Final average length (cm) | 7.76 ± 0.11 b | 8.21 ± 0.12 a | 8.20 ± 0.09 a |
| WG (g) | 2.66 ± 0.16 b | 4.26 ± 0.51 a | 3.82 ± 0.77 ab |
| TL (cm) | 1.36 ± 0.10 b | 1.81 ± 0.11 a | 1.80 ± 0.08 a |
| AWG (g) | 0.05 ± 0.00 b | 0.09 ± 0.01 a | 0.08 ± 0.01 ab |
| GF3 | 0.79 ± 0.04 b | 1.27 ± 0.15 a | 1.14 ± 0.23 ab |
| Initial biomass (kg/m3) | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.01 |
| Final biomass (kg/m3) | 0.05 ± 0.00 | 0.06 ± 0.01 | 0.05 ± 0.01 |
| FCR | 2.40 ± 0.00 | 2.33 ± 0.23 | 2.73 ± 0.92 |
| %S * | 100.0 ± 0.00 | 95.3 ± 8.08 | 90.3 ± 16.74 |
| Composition | Chancaca | Sucrose | Beet Molasses |
|---|---|---|---|
| %CP | 5.1 ± 0.01 b | 8.2 ± 0.01 a | 10.2 ± 0.02 a |
| %Ash | 25.6 ± 0.01 | 25.6 ± 0.01 | 27.6 ± 0.02 |
| %CF | 10.1 ± 0.01 | 10.8 ± 0.01 | 8.8 ± 0.02 |
| %Fat | 5.0 ± 0.01 | 5.0 ± 0.01 | 5.0 ± 0.01 |
| Kcal * | 0.3 ± 0.2 c | 3.44 ± 0.2 a | 2.46 ± 0.2 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayazo Genes, J.E.; Holanda, M.; Lara, G. Effects of Different Organic Carbon Sources on Water Quality and Growth of Mugil cephalus Cultured in Biofloc Technology Systems. Fishes 2025, 10, 427. https://doi.org/10.3390/fishes10090427
Ayazo Genes JE, Holanda M, Lara G. Effects of Different Organic Carbon Sources on Water Quality and Growth of Mugil cephalus Cultured in Biofloc Technology Systems. Fishes. 2025; 10(9):427. https://doi.org/10.3390/fishes10090427
Chicago/Turabian StyleAyazo Genes, Julia Eva, Mariana Holanda, and Gabriele Lara. 2025. "Effects of Different Organic Carbon Sources on Water Quality and Growth of Mugil cephalus Cultured in Biofloc Technology Systems" Fishes 10, no. 9: 427. https://doi.org/10.3390/fishes10090427
APA StyleAyazo Genes, J. E., Holanda, M., & Lara, G. (2025). Effects of Different Organic Carbon Sources on Water Quality and Growth of Mugil cephalus Cultured in Biofloc Technology Systems. Fishes, 10(9), 427. https://doi.org/10.3390/fishes10090427





