Transcriptomic and Metabolomic Analyses Provide Insights into Cryptocaryon irritans Resistance in Golden Pompano (Trachinotus ovatus)
Abstract
1. Introduction
2. Materials and Methods
2.1. Cryptocaryon irritans and Experimental Fish
2.2. Sample Collection
2.3. Transcriptome Analysis
2.4. Metabolome Analysis
2.5. Verification Results via qRT-PCR
2.6. Statistical Analysis
3. Results
3.1. Transcriptome Analysis
3.1.1. Sequencing, De Novo Assembly, and Gene Annotation
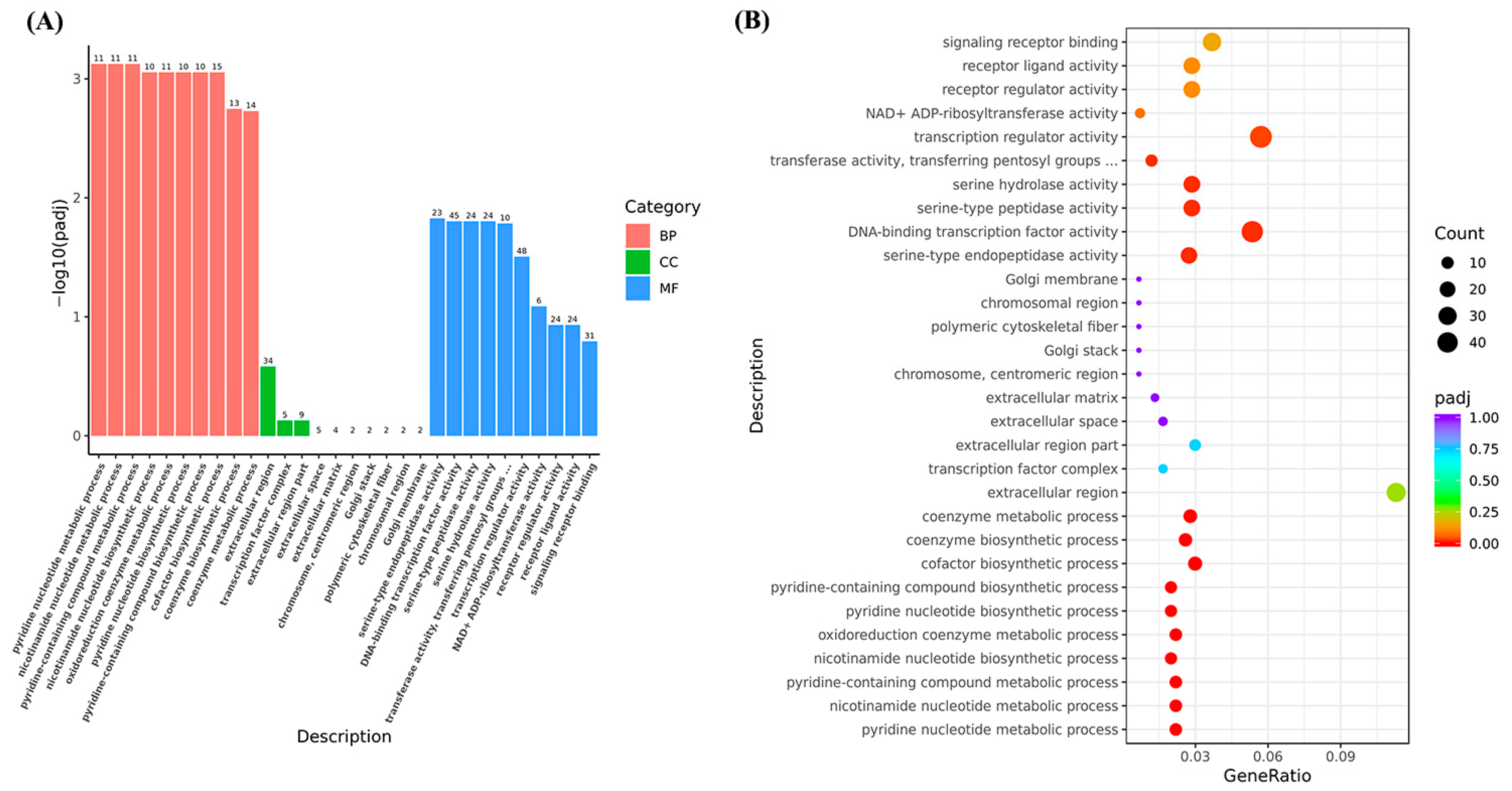
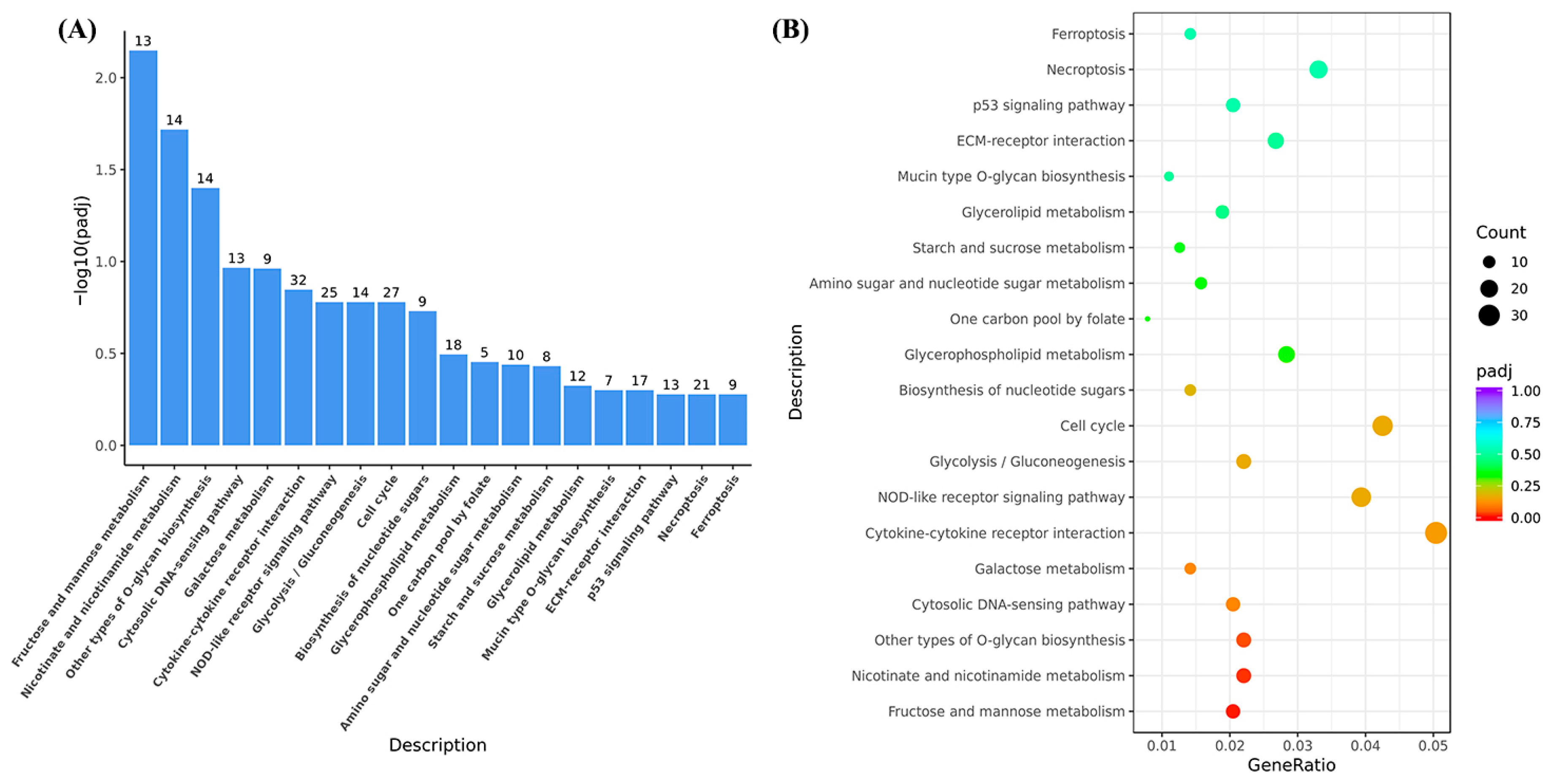
3.1.2. Functional Analysis of Liver Unigenes
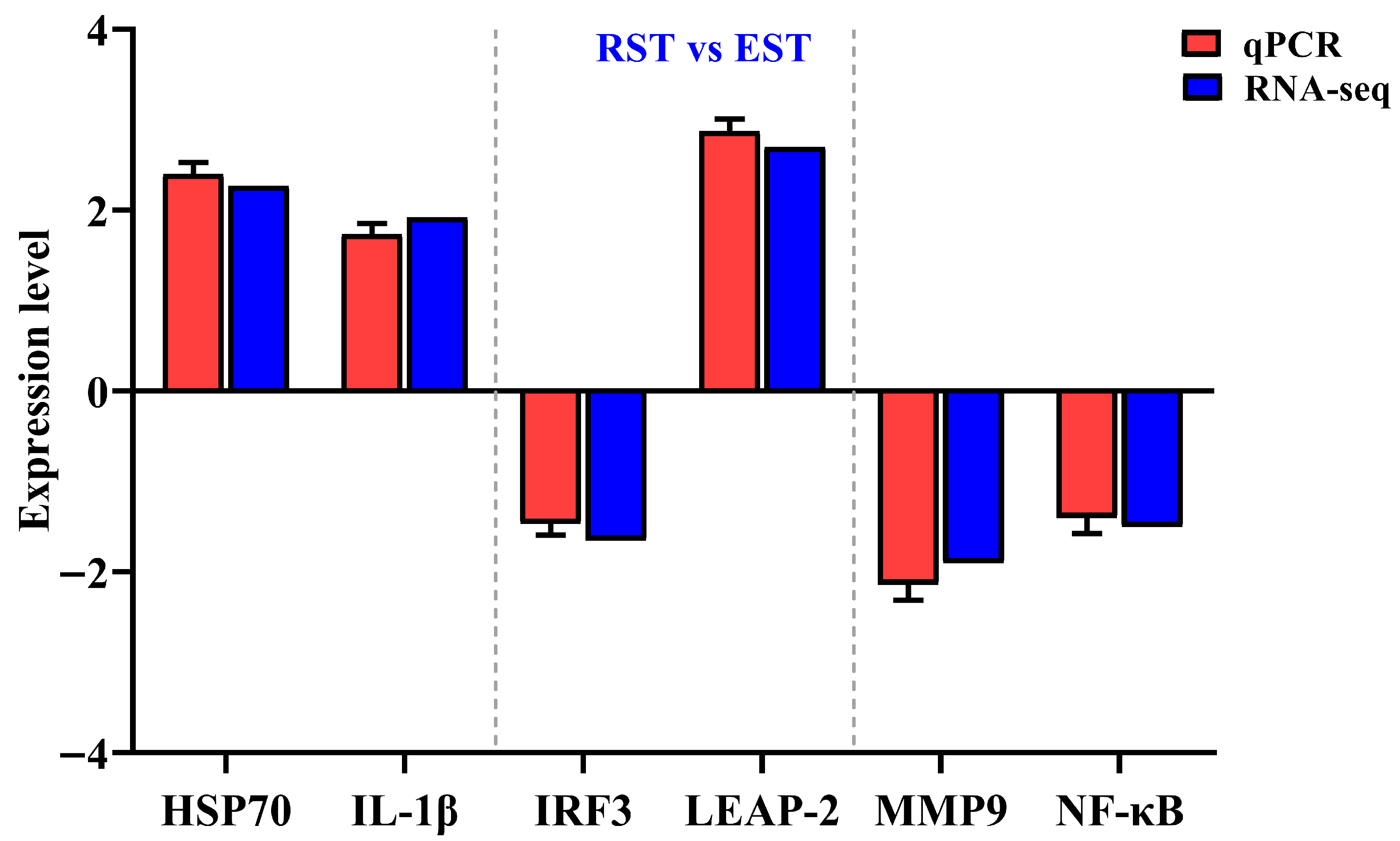
3.1.3. RNA-seq Data Validation
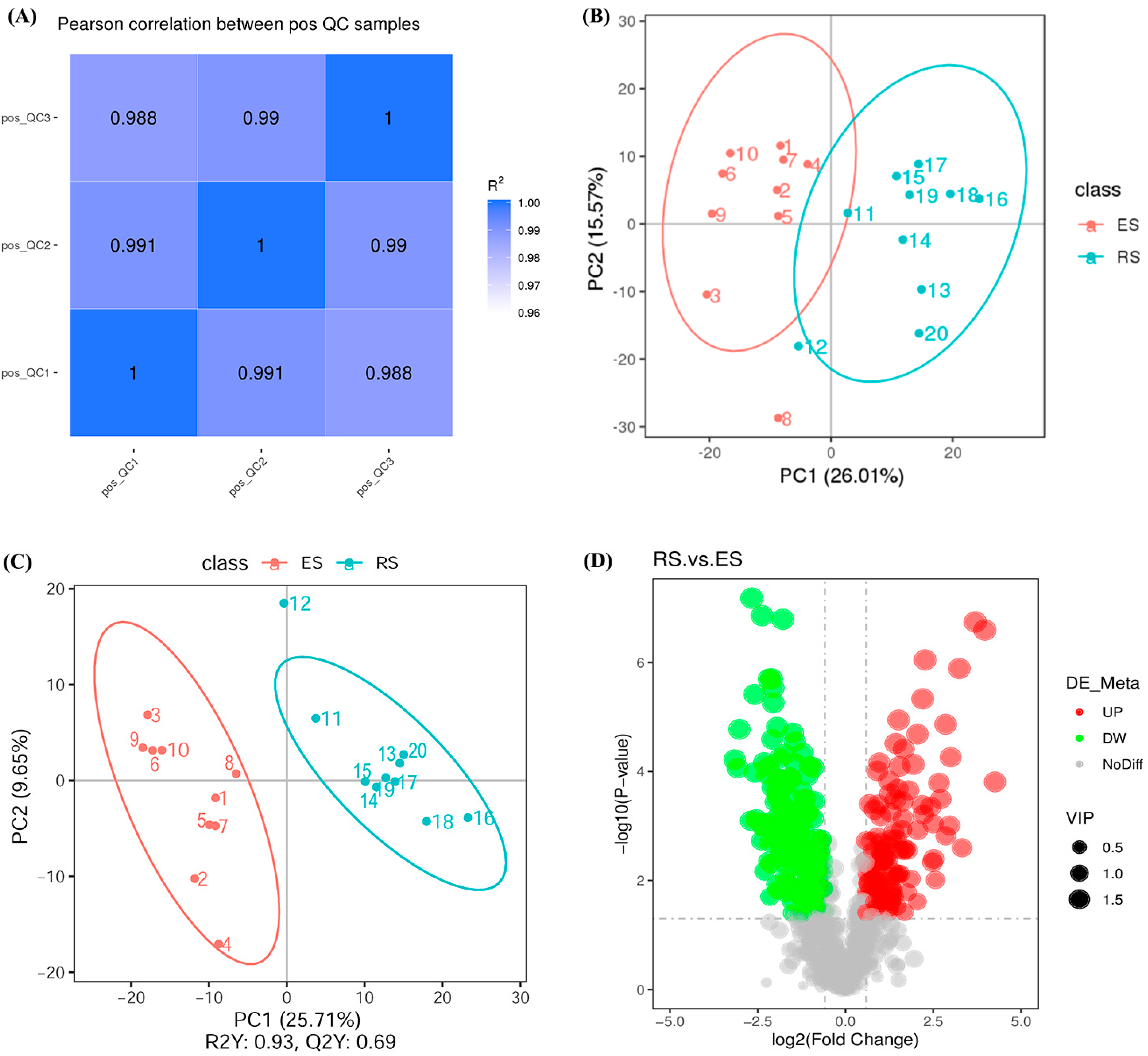
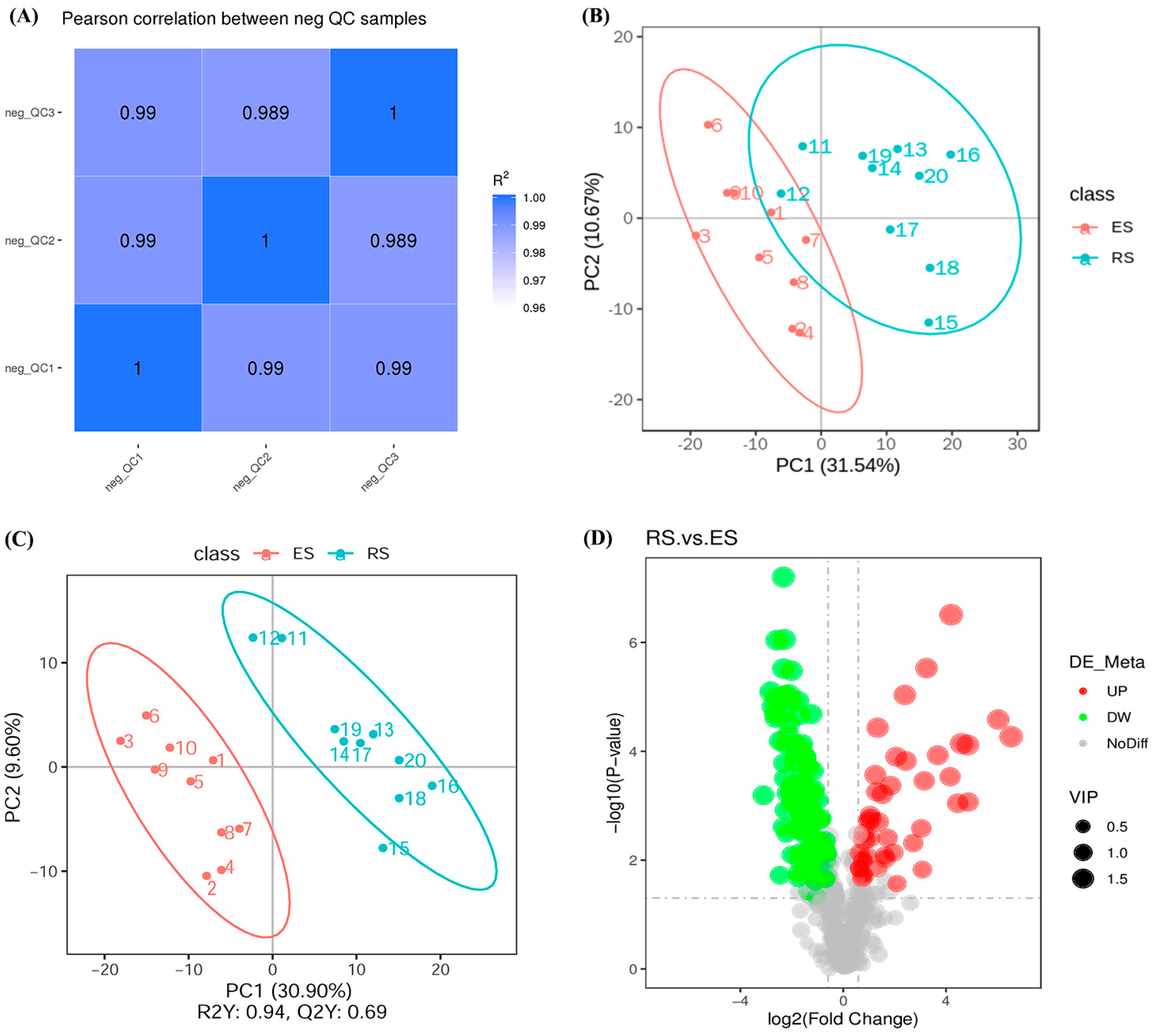
3.2. Metabolomics Analysis
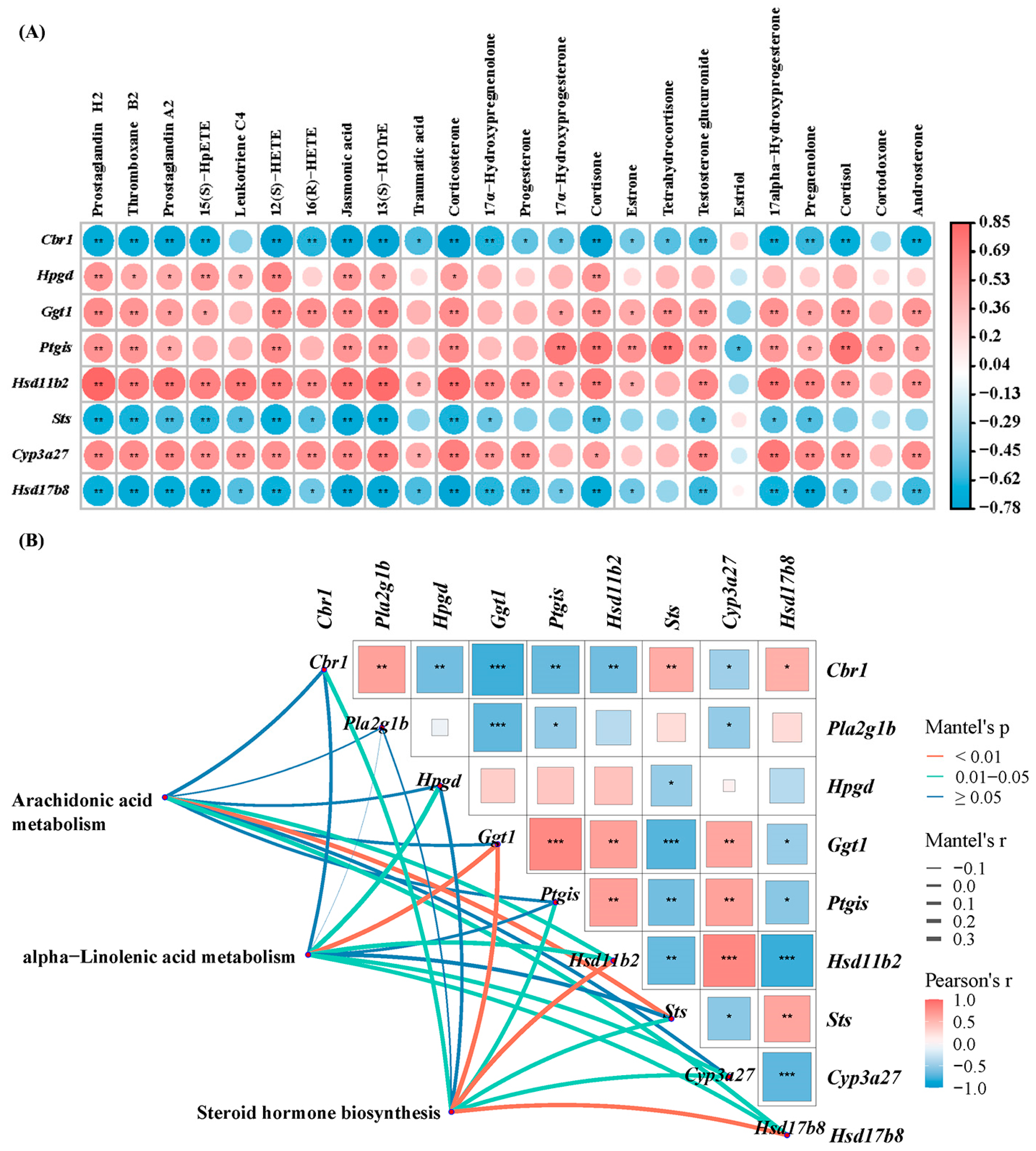
3.3. Combined Analysis of Transcriptomic and Metabolomic Data
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, B.; Guo, H.Y.; Liu, B.S.; Liu, B.S.; Zhang, N.; Yang, J.W.; Guo, L.; Jiang, S.G.; Zhang, D.C. Starvation and refeeding influence the growth, biochemical index, intestinal microbiota, and transcriptomic profiles of golden pompano Trachinotus ovatus (Linnaeus 1758). Front. Mar. Sci. 2022, 9, 998190. [Google Scholar] [CrossRef]
- Liu, B.S.; San, L.Z.; Guo, H.Y.; Zhu, K.C.; Zhang, N.; Yang, J.W.; Liu, B.; Hou, J.L.; Zhang, D.C. Transcriptomic Analysis Reveals Functional Interaction of mRNA-lncRNA-miRNA in Trachinotus ovatus Infected by Cryptocaryon irritans. Int. J. Mol. Sci. 2023, 24, 15886. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiang, B.; Zhong, Z.; Cao, J.; Li, H.; Wang, C.; Li, A. Skin transcriptomic analysis and immune-related gene expression of golden pompano (Trachinotus ovatus) after Amyloodinium ocellatum infection. Fish Shellfish. Immunol. 2022, 128, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Bushra; Maha, I.F.; Xie, X.; Yin, F. Integration of transcriptomic and metabolomic profiling of encystation in Cryptocaryon irritans regulated by rapamycin. Vet. Parasitol. 2023, 314, 109868. [Google Scholar] [CrossRef]
- Chi, Y.; Mukiibi, R.; Zhang, H.; Zhang, H.; Li, W.; Robledo, D.; Chen, S.; Li, Y. Transcriptome Analysis Reveals the Immunosuppression in Tiger Pufferfish (Takifugu rubripes) under Cryptocaryon irritans Infection. Animals 2024, 14, 2058. [Google Scholar] [CrossRef]
- Wang, P.; Wang, J.; Su, Y.Q.; Mao, Y.; Zhang, J.S.; Wu, C.W.; Ke, Q.Z.; Han, K.H.; Zheng, W.Q.; Xu, N.D. Transcriptome analysis of the Larimichthys crocea liver in response to Cryptocaryon irritans. Fish Shellfish. Immunol. 2016, 48, 1–11. [Google Scholar] [CrossRef]
- Hu, Y.; Li, A.; Xu, Y.; Jiang, B.; Lu, G.; Luo, X. Transcriptomic variation of locally-infected skin of Epinephelus coioides reveals the mucosal immune mechanism against Cryptocaryon irritans. Fish Shellfish. Immunol. 2017, 66, 398–410. [Google Scholar] [CrossRef]
- Jiang, B.; Du, J.J.; Li, Y.W.; Ma, P.; Hu, Y.Z.; Li, A.X. Transcriptome analysis provides insights into molecular immune mechanisms of rabbitfish, Siganus oramin against Cryptocaryon irritans infection. Fish Shellfish. Immunol. 2019, 88, 111–116. [Google Scholar] [CrossRef]
- Chen, X.H.; Liu, S.R.; Peng, B.; Li, D.; Cheng, Z.X.; Zhu, J.X.; Zhang, S.; Peng, Y.M.; Li, H.; Zhang, T.T.; et al. Exogenous l-Valine Promotes Phagocytosis to Kill Multidrug-Resistant Bacterial Pathogens. Front. Immunol. 2017, 8, 207. [Google Scholar] [CrossRef]
- Cheng, J.; Lan, W.; Zheng, G.; Gao, X. Metabolomics: A High-Throughput Platform for Metabolite Profile Exploration. Methods Mol. Biol. 2018, 1754, 265–292. [Google Scholar] [CrossRef]
- Reverter, M.; Sasal, P.; Banaigs, B.; Lecchini, D.; Lecellier, G.; Tapissier-Bontemps, N. Fish mucus metabolome reveals fish life-history traits. Coral Reefs 2017, 36, 463–475. [Google Scholar] [CrossRef]
- Peng, B.; Li, H.; Peng, X.X. Functional metabolomics: From biomarker discovery to metabolome reprogramming. Protein Cell 2015, 6, 628–637. [Google Scholar] [CrossRef]
- Zhang, J.; Shen, H.; Xu, W.; Xia, Y.; Barr, D.B.; Mu, X.; Wang, X.; Liu, L.; Huang, Q.; Tian, M. Urinary metabolomics revealed arsenic internal dose related metabolic alterations: A proof-of-concept study in a Chinese male cohort. Environ. Sci. Technol. 2014, 48, 12265–12274. [Google Scholar] [CrossRef]
- Buchmann, K.; Lindenstrom, T. Interactions between monogenean parasites and their fish hosts. Int. J. Parasitol. 2002, 32, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Maha, I.F.; Xie, X.; Zhou, S.; Yu, Y.; Liu, X.; Zahid, A.; Lei, Y.; Ma, R.; Yin, F.; Qian, D. Skin metabolome reveals immune responses in yellow drum Nibea albiflora to Cryptocaryon irritans infection. Fish Shellfish. Immunol. 2019, 94, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Kong, J.D.; Huang, J.S.; Zhou, Y.; Jiang, Y.; Miao, R.; Yin, F. Integration of metabolomic and transcriptomic analyses to characterize the influence of the gill metabolism of Nibea albiflora on the response to Cryptocaryon irritans infection. Vet. Parasitol. 2021, 298, 109533. [Google Scholar] [CrossRef]
- Dan, X.; Li, A.; Lin, X.; Teng, N.; Zhu, X.Q. A standardized method to propagate Cryptocaryon irritans on a susceptible host pompano Trachinotus ovatus. Aquaculture 2006, 258, 127–133. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Yang, Q.; Guo, L.; Liu, B.S.; Guo, H.Y.; Zhu, K.C.; Zhang, N.; Jiang, S.G.; Zhang, D.C. Effects of stocking density on the growth performance, serum biochemistry, muscle composition and HSP70 gene expression of juvenile golden pompano Trachinotus ovatus (Linnaeus, 1758). Aquaculture 2020, 518, 734841. [Google Scholar] [CrossRef]
- Liu, B.; Liu, G.D.; Guo, H.Y.; Zhu, K.C.; Guo, L.; Zhang, N.; Liu, B.S.; Jiang, S.G.; Zhang, D.C. Characterization and functional analysis of liver-expressed antimicrobial peptide-2 (LEAP-2) from golden pompano Trachinotus ovatus (Linnaeus 1758). Fish Shellfish. Immunol. 2021, 104, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.J.; Guo, H.Y.; Liu, B.; Zhu, K.C.; Guo, L.; Liu, B.S.; Zhang, N.; Yang, J.W.; Jiang, S.G.; Zhang, D.C. Gill oxidative damage caused by acute ammonia stress was reduced through the HIF-1α/NF-κb signaling pathway in golden pompano (Trachinotus ovatus). Ecotoxicol. Environ. Saf. 2022, 222, 112504. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cao, Z.; Zhang, P.; Wei, C.; Li, J.; Wu, Y.; Zhou, Y. IFN regulatory factor 3 of golden pompano and its NLS domain are involved in antibacterial innate immunity and regulate the expression of type I interferon (IFNa3). Front. Immunol. 2023, 14, 1128196. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, K.C.; Pan, J.M.; Guo, H.Y.; Liu, B.S.; Zhang, N.; Yang, J.W.; Zhang, D.C. Characterization of the MMP9 Gene and Its Association with Cryptocaryon irritans Resistance Traits in Trachinotus ovatus (Linnaeus, 1758). Genes 2023, 14, 475. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, M.; Zhao, J.; Bai, H.; Zhang, X.; Wang, J.; Ke, Q.; Qu, A.; Pu, F.; Zheng, W.; et al. Comparative transcriptome analysis reveals immunoregulation mechanism of lncRNA-mRNA in gill and skin of large yellow croaker (Larimichthys crocea) in response to Cryptocaryon irritans infection. BMC Genom. 2022, 23, 206. [Google Scholar] [CrossRef]
- Kong, S.; Ke, Q.; Chen, L.; Zhou, Z.; Pu, F.; Zhao, J.; Bai, H.; Peng, W.; Xu, P. Constructing a High-Density Genetic Linkage Map for Large Yellow Croaker (Larimichthys crocea) and Mapping Resistance Trait Against Ciliate Parasite Cryptocaryon irritans. Mar. Biotechnol. 2019, 21, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Z.; Fraslin, C.; Chi, Y.; Mukiibi, R.; Hu, Y.; Wang, J.; Li, W.; Li, W.; Houston, R.S.; Robledo, D.; et al. A newly developed 20 K SNP array reveals QTLs for disease resistance to Cryptocaryon irritans in tiger pufferfish (Takifugu rubripes). Aquaculture 2025, 595, 741652. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, J.; Tong, B.; Ke, Q.; Bai, Y.; Gong, J.; Zeng, J.; Deng, Y.; Lan, B.; Zhou, T.; et al. Effects of artificial mating on genomic selection of resistance against Cryptocaryon irritans in large yellow croaker. Aquaculture 2022, 561, 738617. [Google Scholar] [CrossRef]
- Zhao, J.; Bai, H.; Ke, Q.; Li, B.; Zhou, Z.; Wang, H.; Chen, B.; Pu, F.; Zhou, T.; Xu, P. Genomic selection for parasitic ciliate Cryptocaryon irritans resistance in large yellow croaker. Aquaculture 2021, 531, 735786. [Google Scholar] [CrossRef]
- Wu, H.; Lai, X.; Guo, W.; Li, X.; Hu, Y.; Dan, X.; Li, Y.; Mo, Z. Manganese improved Trachinotus ovatus immune against Cryptocaryon irritans infection. Aquaculture 2023, 576, 739835. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhao, J.; Li, R.; Ke, Q.; Wang, J.; Li, Y.; Hu, S.; Zeng, J.; Pu, F.; Li, N.; et al. Immune regulation differences in Large yellow croaker with varied resistance to Cryptocaryon irritans infection. Fish Shellfish. Immunol. 2025, 158, 110159. [Google Scholar] [CrossRef]
- Han, Q.; Mo, Z.; Lai, X.; Guo, W.; Hu, Y.; Chen, H.; He, Z.; Dan, X.; Li, Y. Mucosal immunoglobulin response in Epinephelus coioides after Cryptocaryon irritans infection. Fish Shellfish. Immunol. 2022, 128, 436–446. [Google Scholar] [CrossRef]
- Jiang, S.; Huang, X.; Li, T.; Zhang, Y.; Zhang, J. Immune response of large yellow croaker Larimichthys crocea towards a recombinant vaccine candidate targeting the parasitic ciliate Cryptocaryon irritans. Aquac. Int. 2023, 31, 3383–3402. [Google Scholar] [CrossRef]
- Huang, K.; Jiang, L.; Huang, W.; Li, X.; Yuan, L.; Jiang, J.; Zhou, S.; Wang, Y.; Xie, J. Investigating the interplay between Cryptocaryon irritans ectoparasite infection and the immune responses of the head kidney in silver pomfret (Pampus argenteus). Aquaculture 2023, 575, 739780. [Google Scholar] [CrossRef]
- Mo, Z.Q.; Wu, H.C.; Hu, Y.T.; Lu, Z.J.; Lai, X.L.; Chen, H.P.; He, Z.C.; Luo, X.C.; Li, Y.W.; Dan, X.M. Transcriptomic analysis reveals innate immune mechanisms of an underlying parasite-resistant grouper hybrid (Epinephelus fuscogutatus × Epinephelus lanceolatus). Fish Shellfish. Immunol. 2021, 119, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, J.J.; Li, S.; Liu, H.H.; Xu, D.D.; Chi, C.F.; Zheng, L.B. Transcriptomic analysis of large yellow croaker (Larimichthys crocea) reveals the suppression of the inflammatory response from Cryptocaryon irritans infection. Fish Shellfish. Immunol. 2024, 144, 109258. [Google Scholar] [CrossRef]
- Guo, X.; Huang, W.; Xu, Y.; Zhan, Q.; Sun, P.; Hu, H. Metabolomic changes in Cryptocaryon irritans from Larimichthys crocea after exposure to copper plate. Front. Cell. Infect. Microbiol. 2024, 14, 1424669. [Google Scholar] [CrossRef]
- Sun, J.L.; Jiang, T.; Gu, Y.; Song, F.B.; Wen, X.; Luo, J. Differential immune and metabolic responses underlie differences in the resistance of Siganus oramin and Trachinotus blochii to Cryptocaryon irritans infection. Fish Shellfish. Immunol. 2022, 120, 166–179. [Google Scholar] [CrossRef]



| Primer Name | Primer Sequences (5′–3′) | Reference |
|---|---|---|
| HSP70-F | TTGAGGAGGCTGCGCACAGCTTGTG | Yang et al. [19] |
| HSP70-R | ACGTCCAGCAGCAGCAGGTCCT | |
| LEAP-2-F | AGTGGCGCTGTGCATTGT | Liu et al. [20] |
| LEAP-2-R | GTGTGTGGACGCTGTGTTC | |
| IL-1β-F | CGGACTCGAACGTGGTCACATTC | Liu et al. [21] |
| IL-1β-R | AATATGGAAGGCAACCGTGCTCAG | |
| IRF3-F | ACAAGAACGAAACCGCTAACCC | Sun et al. [22] |
| IRF3-R | TCATCAAAGCACGAGACCACC | |
| MMP9-F | CACCAGTGAGGGACGAG | Liu et al. [23] |
| MMP9-R | GGCTGCCACCAGAAACA | |
| NF-κB-F | CGTGAGGTCAGCGAGCCAATG | Liu et al. [21] |
| NF-κB-R | ATGTGCCGTCTATCTTGTGGAATGG | |
| EF-1α-F | CCCCTTGGTCGTTTTGCC | Liu et al. [1] |
| EF-1α-R | GCCTTGGTTGTCTTTCCGCTA |
| Group | Library | Raw Reads | Clean Reads | Clean Bases | Q20 (%) | Q30 (%) | G + C (%) |
|---|---|---|---|---|---|---|---|
| EST_1 | FRAS230314133-1r | 50,202,762 | 7.53G | 45,489,766 | 6.82G | 98.07 | 96.36 |
| EST_2 | FRAS230314134-1r | 48,618,656 | 7.29G | 44,361,892 | 6.65G | 98.1 | 96.5 |
| EST_3 | FRAS230314135-1r | 48,861,698 | 7.33G | 48,730,290 | 7.31G | 98.11 | 96.54 |
| EST_4 | FRAS230314136-1r | 46,984,622 | 7.05G | 44,201,420 | 6.63G | 98.04 | 96.42 |
| EST_5 | FRAS230314137-1r | 47,799,178 | 7.17G | 44,371,490 | 6.66G | 98.1 | 96.5 |
| EST_6 | FRAS230314138-1r | 46,545,882 | 6.98G | 43,242,526 | 6.49G | 97.93 | 96.12 |
| EST_7 | FRAS230314139-1r | 49,982,384 | 7.5G | 45,714,808 | 6.86G | 97.97 | 96.22 |
| EST_8 | FRAS230314140-1r | 53,002,766 | 7.95G | 48,986,010 | 7.35G | 97.96 | 96.23 |
| EST_9 | FRAS230314141-1r | 46,084,420 | 6.91G | 43,220,576 | 6.48G | 97.98 | 96.26 |
| EST_10 | FRAS230314142-1r | 49,690,014 | 7.45G | 46,634,750 | 7.0G | 98.02 | 96.32 |
| RST_1 | FRAS230314143-1r | 48,088,756 | 7.21G | 45,985,858 | 6.9G | 97.98 | 96.23 |
| RST_2 | FRAS230314144-1r | 49,700,112 | 7.46G | 46,766,754 | 7.02G | 98.12 | 96.53 |
| RST_3 | FRAS230314145-1r | 47,244,240 | 7.09G | 44,333,416 | 6.65G | 98.14 | 96.54 |
| RST_4 | FRAS230314146-1r | 47,768,858 | 7.17G | 43,853,000 | 6.58G | 98.13 | 96.54 |
| RST_5 | FRAS230314147-1r | 46,331,646 | 6.95G | 46,199,834 | 6.93G | 98.05 | 96.39 |
| RST_6 | FRAS230314148-1r | 47,009,362 | 7.05G | 43,610,810 | 6.54G | 98.01 | 96.34 |
| RST_7 | FRAS230314149-1r | 46,743,660 | 7.01G | 43,711,294 | 6.56G | 97.96 | 96.19 |
| RST_8 | FRAS230314150-1r | 47,852,908 | 7.18G | 44,328,694 | 6.65G | 98.01 | 96.33 |
| RST_9 | FRAS230314151-1r | 47,766,414 | 7.16G | 44,969,728 | 6.75G | 98.05 | 96.4 |
| RST_10 | FRAS230314152-1r | 48,105,080 | 7.22G | 45,513,244 | 6.83G | 97.99 | 96.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Liu, B.-S.; Yang, J.-W.; Guo, H.-Y.; Zhang, N.; Zhu, T.-F.; Xian, L.; Zhu, K.-C.; Zhang, D.-C. Transcriptomic and Metabolomic Analyses Provide Insights into Cryptocaryon irritans Resistance in Golden Pompano (Trachinotus ovatus). Fishes 2025, 10, 426. https://doi.org/10.3390/fishes10090426
Liu B, Liu B-S, Yang J-W, Guo H-Y, Zhang N, Zhu T-F, Xian L, Zhu K-C, Zhang D-C. Transcriptomic and Metabolomic Analyses Provide Insights into Cryptocaryon irritans Resistance in Golden Pompano (Trachinotus ovatus). Fishes. 2025; 10(9):426. https://doi.org/10.3390/fishes10090426
Chicago/Turabian StyleLiu, Bo, Bao-Suo Liu, Jing-Wen Yang, Hua-Yang Guo, Nan Zhang, Teng-Fei Zhu, Lin Xian, Ke-Cheng Zhu, and Dian-Chang Zhang. 2025. "Transcriptomic and Metabolomic Analyses Provide Insights into Cryptocaryon irritans Resistance in Golden Pompano (Trachinotus ovatus)" Fishes 10, no. 9: 426. https://doi.org/10.3390/fishes10090426
APA StyleLiu, B., Liu, B.-S., Yang, J.-W., Guo, H.-Y., Zhang, N., Zhu, T.-F., Xian, L., Zhu, K.-C., & Zhang, D.-C. (2025). Transcriptomic and Metabolomic Analyses Provide Insights into Cryptocaryon irritans Resistance in Golden Pompano (Trachinotus ovatus). Fishes, 10(9), 426. https://doi.org/10.3390/fishes10090426








