Abstract
Freshwater ecosystems are increasingly stressed by drought and anthropogenic inputs that can increase specific conductivity (SPC) and pH; however, little is known about how harsher conditions affect fish. We evaluated how fish growth and diet composition changed along a natural gradient in SPC and pH in Wyoming, USA using Northern plains killifish (Fundulus kansae) and Fathead minnows (Pimephales promelas). We surveyed 201 sites where we measured water chemistry, sampled fish, and assessed invertebrate prey availability from May to September 2024. Northern plains killifish and/or Fathead minnows inhabited 12 sites, which were the focus of our study. We measured otoliths to assess growth and stomach contents to estimate dietary selectivity. Growth decreased at higher SPC (486–23,500 µS/cm) for Fathead minnows and pH (7.2–9.0) for both species, suggesting an energy trade-off with osmoregulation. Dietary analyses revealed variable selection for Chironomidae larvae, while other taxa such as Gammaridae and Coleoptera were avoided at higher SPC and pH. Despite the extreme conditions, these fish maintained some dietary preference, highlighting behavioral plasticity. Our findings suggest that while these species can tolerate harsh environments, sublethal effects on growth and diet may limit long-term fitness. This research offers a framework for assessing the viability of fish populations inhabiting ecosystems with increasing salinity and pH that can inform conservation and management strategies under future environmental change.
Keywords:
osmoregulation; ion regulation; acidification tolerance; fish physiology; environmental stressors; fish distribution; aquatic ecosystems Key Contribution:
This study reveals that salinity and pH influence growth and dietary patterns in Northern plains killifish and Fathead minnows across Wyoming streams; with implications for understanding fish adaptability in changing aquatic environments.
1. Introduction
Freshwater ecosystems are particularly sensitive to changes in abiotic conditions such as salinity and pH, which play critical roles in shaping community structure, species physiology, and ecosystem processes [1]; however, relatively little is known about how these conditions alter the success of fish. Freshwater habitats are exposed to extreme environmental variation due to natural conditions and anthropogenic disturbances [2], and such effects can be pronounced in semi-arid and arid regions. Climate change, water extraction, drought and land use intensification contribute to altered hydrological regimes, exacerbating salinization and pH across river networks [3]. For instance, the application of road salt during winter months and fracking can lead to elevated and chronic chloride concentrations in surface waters, often exceeding the U.S. Environmental Protection Agency’s water-quality criteria [4]. As these stressors intensify, understanding how native fish species respond to such gradients is vital for the conservation and management of freshwater biodiversity [5].
Salinity in freshwater ecosystems is highly variable and can reach extreme concentrations in arid and isolated environments, posing significant challenges to aquatic life. Salinity is the concentration of salts in water and is often measured as specific conductivity (SPC). Salinity is typically low in freshwater ecosystems (<0.5 parts per thousand; <500 µS/cm); however, concentrations can be much higher in landlocked basins and disconnected stream pools in arid landscapes [6]. In such ecosystems, the concentration of salts can exceed those of brackish water and even approach or exceed marine concentrations due to evapoconcentration and limited freshwater inputs [7,8]. The average ocean salinity is ~50,000 µS/cm (~35 ppt), while hypersaline seas such as the Red Sea reach 59,727 µS/cm due to high evaporation and restricted circulation [9]. In contrast, the specific conductivity of groundwater can have higher concentrations of salt (≤100,000 µS/cm), which likely exceed the physiological limits of many freshwater fish species [8].
Salinization of freshwater ecosystems occurs through a combination of geological, climatic, and anthropogenic drivers. Naturally saline soils, groundwater inputs, and mineral weathering contribute baseline ions, but land use practices like agriculture and energy development often magnify salinity loads [7,10]. Irrigation return flows, road salting, and oil and gas development commonly introduce ions such as sodium, chloride, and sulfate into aquatic ecosystems, which can accumulate in isolated stream segments and floodplains. An excess of these ions disrupts the osmotic balance of aquatic organisms, forcing freshwater fish to expend more energy on osmoregulation—a cost that can reduce growth, reproductive output, and overall fitness [11,12,13]. For example, in highly saline environments, macroinvertebrate diversity is often diminished, forcing fish to rely on fewer or suboptimal prey types. This can lead to nutritional stress, stunted growth, and reduced reproductive performance [14].
In addition to salinity, pH is a critical chemical parameter in aquatic ecosystems, influencing the physiological performance, distribution, and survival of freshwater organisms [15]. Natural waters can exhibit pH values ranging from ~4.1 to 12.0 [16,17], though most freshwater fish thrive within a narrower range of 6.5 to 8.5. Deviations outside this optimal range can impair osmoregulation, enzymatic activity, and gill function, leading to reduced metabolic efficiency and increased energetic costs of homeostasis [18]. Acidic conditions (low pH) can increase the solubility of toxic metals such as aluminum, while alkaline conditions (high pH) can elevate un-ionized ammonia concentrations—both of which can be harmful to fish health [19,20]. Moreover, pH fluctuations can interfere with ion transport across epithelial membranes, disrupt acid-base balance, and negatively impact reproductive success and larval development.
High pH values can interact with salinity to compound stress responses in fish. For instance, extreme pH values can increase the toxicity of some ions and reduce ion exchange efficiency, intensifying the energetic burden of maintaining homeostasis [21,22,23]. These abiotic stressors not only influence fish physiology but also have rippling effects on behavior and ecological interactions, such as foraging and diet [24]. The availability and diversity of prey organisms tend to decrease as salinity and pH become more extreme which alters predator-prey dynamics and potentially leads to a less diverse diet or lower foraging success [25]. Additionally, sublethal effects—such as impaired osmoregulation, elevated metabolic costs, and altered behavior—can reduce overall fitness and resilience to additional stressors [26]. Evaluating diet in conjunction with growth provides a more comprehensive picture of how environmental stressors alter fish at both the individual and population levels [27,28].
We studied the effects of salinity and pH on the growth and dietary composition in native prairie freshwater fish in streams. We assessed how environmental variability influenced physiological development and foraging patterns by assessing growth using otoliths and diet by analyzing stomach contents. We evaluated the degree to which fish selectively fed under high-stress conditions or shifted toward opportunistic feeding strategies as conditions became harsher by comparing dietary intake to local prey availability. Our findings contribute to a deeper understanding of how freshwater fish populations respond to salinization and pH fluctuations and offer valuable insights for conservation and management of inland ecosystems.
2. Materials and Methods
2.1. Study Area and Species
We conducted our study in the semi-arid basins of Wyoming, USA, a region characterized by wide elevational gradients, sparse vegetation, and variable hydrology [29,30]. Sampling took place on accessible public lands across a range of stream types, including perennial flowing streams, intermittent reaches, and isolated pools. Elevations at study sites ranged from ~1400 to 2500 m above sea level [29]. The region experiences a continental climate, with cold winters, hot summers, and low annual precipitation averaging 250–400 mm, much of which falls as snow [31]. Mean annual temperatures range from 2 °C to 9 °C, depending on elevation [31]. Riparian vegetation is typically dominated by willows (Salix spp.), sedges (Carex spp.), and occasional cottonwoods (Populus spp.), transitioning to sagebrush steppe and mixed-grass prairie in surrounding uplands [29]. These environmental gradients create a mosaic of aquatic habitats that vary seasonally in water availability, temperature, and salinity.
Northern plains killifish (Fundulus kansae) are a member of the family Fundulidae. The native range encompasses the Great Plains, specifically the Mississippi River drainage and its tributaries. This includes areas from north-central Missouri to central Wyoming and south to Texas [29,32]. They are recognized as a distinct species from the Plains killifish (Fundulus zebriunes) after phylogenetic analysis [33]. Northern plains killifish grow ≤ 100 mm in total length [33]. This species inhabits shallow streams, being most abundant in high salinity areas to avoid potential competitors [33,34]. Their diet primarily consists of insect larvae, especially Chironomidae larvae (Diptera) [35]. The conservation status of the Northern plains killifish became a concern after the species was split from the plains killifish. Their populations in Wyoming were deemed stable in 2017 [36].
The Fathead minnow (Pimephales promelas) is a member of the family Cyprinidae. Their native distribution is widespread, spreading across most of the United States. They are often used as baitfish and forage fish, which has contributed to their current distribution [37]. They have been introduced to drainages outside of their native range in 33 states [38]. Fathead minnows grow to ≤100 mm in total length [38]. The species is commonly associated with small muddy streams and pools [39,40]. They can tolerate low oxygen, highly turbid environments, and can tolerate salinities ≤25,200 µS/cm in experiments [41,42]. Fathead minnows are considered an acid-sensitive species. Small declines in pH from neutral can cause reductions in reproductive fitness and loss of populations [43]. These fish are omnivores, feeding on algae, detritus, and macroinvertebrates [40]. The Fathead minnow is considered to have a secure conservation status globally [37].
2.2. Field Sampling
Water chemistry was measured in each stream, and we accounted for microhabitat variability in intermittent streams by measuring water chemistry in each pool. We measured specific conductivity (µS/cm), pH, and water temperature (°C) in each stream using a calibrated Professional Plus sonde made by Yellow Spring Instrument, Ohio, USA. Specific conductivity and pH were not correlated (Figure S1).
We conducted fish surveys from May to September 2024 across Wyoming, USA. Fish were collected using seine nets in flowing streams and dip nets in stagnant pools of intermittent streams. A maximum of 10 individuals per species per day was collected to ensure ethical sampling limits under approved protocols (IACUC #2024-0118) at each stream. Fish collected were preserved in 95% ethanol in Whirl-Paks for subsequent analysis.
2.3. Fish Growth
Otolith microstructure analysis is a well-established method for assessing fish growth in relation to environmental conditions. Otoliths, or ear bones, grow incrementally throughout a fish’s life, incorporating chemical and physical signatures of surrounding conditions. Measuring rings in otoliths provides a time-resolved record of growth that can be linked to physiological stress [44,45]. When paired with stomach content analysis and assessments of prey availability, this approach allows researchers to infer how environmental gradients shape energetic input (diet) and growth output.
We extracted sagittal otoliths from the cranial region of preserved fish under a dissecting microscope to assess individual growth (Figure S2). Otoliths were cleaned, and annulus measurements were obtained using Evident CellSens software (Version 4.4) connected to an Evident Olympus CX43 compound microscope at 100× magnification. We measured otolith length, width, and annual growth rings to the nearest 0.01 µm. For comparative analysis, we focused on the first full annulus, representing growth from age 0 to age 1. These measurements were used to compare growth rates among individuals.
2.4. Fish Diet
We dissected fish stomachs from the esophagus to the anus to evaluate diet under a range of conditions. Stomach contents were examined under an Evident Olympus SZ61 dissecting microscope, and invertebrate prey items were identified and individuals were counted [46,47]. Partially digested or unidentifiable fragments, detritus, and plant matter were excluded from analysis to focus on active prey selection, and empty stomachs were recorded and included in the analysis along the gradients of SPC and pH.
We conducted aquatic invertebrate surveys at each stream using dip-net sampling by pool length in the same microhabitats that fish were captured to compare to stomach contents. We performed 10 dipnet jabs (500 µm mesh) per meter of pool. Prey items were collected before fish to minimize disturbance. Collected invertebrates were counted, identified to order, and returned to the stream. These counts were used to calculate the proportion of each prey type in each stream.
We calculated taxon-specific prey selection using a two-step approach to assess the degree to which the selectivity of diets changed over a gradient of SPC and pH. We calculated the taxa ratio (TR) for each prey type as follows:
TR = Ta/Pa
Taxon availability (Ta) refers to the number of individuals of each invertebrate group captured at each stream, while total prey availability (Pa) is the total number of invertebrates observed at each stream. Taxon ratio describes how common (near 1) or rare (near 0) a given invertebrate taxon was relative to the invertebrate’s community, and their availability as fish prey. We calculated the selection ratio (SR), which accounts for dietary intake relative to prey availability as follows:
SR = St/(TR * TS)
The abundance of a taxon in fish stomach (St) refers to the number of individuals of a specific taxon in the stomach, while total stomach abundance (TS) is the total number of invertebrate prey present in the stomach. An SR value >1 indicates selection of that taxon, while values < 1 suggest avoidance or underrepresentation in the diet. We calculated SR along the gradient of SPC and pH which allowed us to assess how environmental gradients changed foraging behavior and prey selectivity.
2.5. Data Analysis
We used statistical modeling, ecological indices, and spatial analysis to evaluate how environmental variables influenced fish growth and foraging behavior. Regression analyses were used to analyze otolith annulus length as the dependent variable, with specific conductivity (SPC) and pH as predictors; an interaction term between SPC and pH was included to assess compounding effects. We fitted linear mixed-effects models using the lme4 package [48], treating pH and SPC as fixed effects and sampling location as a random intercept to account for site-level variation. To assess foraging patterns, we calculated selection indices and compared stomach contents to prey availability; selection ratios were plotted across environmental gradients to visualize shifts in foraging strategy. All data were analyzed using R Statistical Software (R Version 4.4.2) [49] within the RStudio IDE (Version 2024.12.1+402) [50]. We used the tidyverse package for data manipulation [51]; ggplot2, patchwork, and ggpubr for data visualization and figure composition [52,53,54]; and sf, ggmap, and ggspatial for spatial analysis and map creation [55,56,57].
3. Results
We sampled 201 streams where SPC ranged from 177.5 to 109,625 µS/cm with an average of 9304.96 µS/cm, and pH varied between 7.2 and 10.03 with an average of 8.28. Twelve streams were inhabited by both Northern plains killifish and Fathead minnows which we focused our study on (Figure 1). At these sites, SPC ranged from 486 to 23,500 µS/cm with an average of 6819.52 µS/cm and pH ranged from 7.2 to 9.0 with an average of 8.14. Out of all the streams with fish, 20.5% of the streams contained only Northern plains killifish and/or Fathead minnows. A representative pool where fish were observed under high salinity and alkaline conditions is presented in Figure 2.
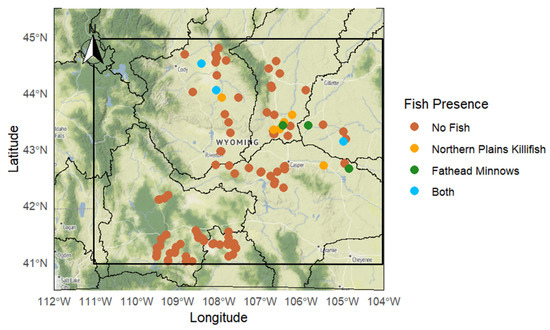
Figure 1.
Geographic distribution of fish presence across sampling sites in Wyoming (n = 201). Points represent sites categorized by fish presence: Northern plains killifish (yellow, n = 3), Fathead minnows (green, n = 3), both species (blue n = 6), or neither species detected (orange, n = 189).

Figure 2.
A representative pool at one of the study sites where fish were observed (SPC = 9330 µS/cm, pH = 8.17). The pool measured approximately 1.5 m in length and 10 cm in depth. The white deposits surrounding the pool are dried salt.
3.1. Growth Patterns
A total of 40 Northern plains killifish were collected, with total lengths ranging from 43 to 87 mm and 1 to 3 years old. Growth of the first otolith annuli did not change as SPC increased (Figure 3A; p = 0.22, β = −0.001, R2 = 0.046). Conversely, growth decreased at more basic pH values (Figure 4A; p = 0.0382, β = −15.823, R2 = 0.131). An interaction between SPC and pH indicated that more extreme values of both decreased growth further (β = 0.0671, SE = 0.0326, z = 2.055, p = 0.0399).
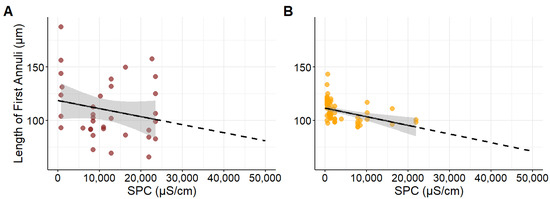
Figure 3.
Relationship between specific conductivity (SPC) and the length of the first annulus on otoliths for (A) Northern plains killifish (Fundulus kansae; n = 33, p = 0.22) and (B) Fathead minnows (Pimephales promelas; n = 65, p < 0.05). Each point represents an individual fish. The black line shows the mean trend estimated by a linear regression model and the shaded gray area indicates the 95% confidence interval. The solid portion of the line corresponds to the observed data range, while the dotted line indicates projected trends.
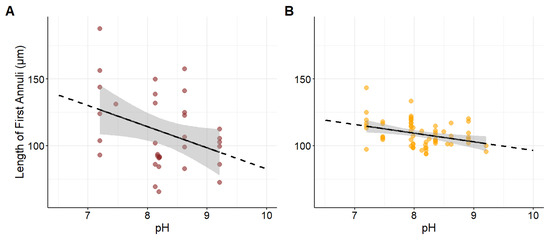
Figure 4.
(A) The relationship between the growth of Northern plains killifish (n = 33, p < 0.05) and (B) Fathead minnow (n = 65, p < 0.05) using the first annulus of otolith along a gradient of natural pH values. The black line represents the mean trend estimated by a linear regression model, and the shaded gray area indicates the 95% confidence interval. Each point represents an individual otolith measurement. The solid portion of the line corresponds to the observed data range, while the dotted line indicates projected trends.
A total of 65 Fathead minnows were collected, with total lengths ranging from 37 to 78 mm and 1 to 3 years old. Growth of the first annulus decreased as SPC increased (Figure 3B; p = 0.0006, β = −0.001, R2 = 0.171). Additionally, growth decreased at more basic pH values (Figure 4B; p = 0.0054, β = −6.487, R2 = 0.116). An interaction between SPC and pH indicated that higher values of both further decreased growth for Fathead minnows (β = 0.0671, SE = 0.0326, z = 2.055, p = 0.0399).
3.2. Diet and Prey Selection
A total of 44 Northern plains killifish stomachs and 67 Fathead minnow stomachs were analyzed in total. Of those stomachs, seven killifish stomachs were empty and 34 Fathead minnow stomachs were empty. Eight Northern plains killifish stomachs and seven Fathead minnow stomachs had detritus/plant matter in their stomach, which were not included in the analysis. Of all streams that Northern plains killifish and/or Fathead minnows were present, Diptera (true flies) represented 7.0%, Gammaridae (scuds) represented 50.2%, Notonectidae represented 2.7%, Corixidae represented 31.2%, Gastropoda (snails) represented 3.7%, and Coleoptera represented 5.2% of selected prey items available.
Northern plain killifish diet varied as SPC increased and pH became harsher. Chironomidae larvae were selected across nearly all SPC and pH values (Figure 5A); however, Gammaridae were selected under the lowest SPC and most neutral pH but quickly were consumed at a proportional level (Figure 5B). Notonectidae were avoided at all values (Figure 5C). Corixidae and Gastropoda had inconsistent patterns of selection, being consumed at a proportional level (Figure 5D,E). Coleoptera were selected at lower salinities and neutral pH values and consumed at a proportional level at higher SPC and pH (Figure 5F).
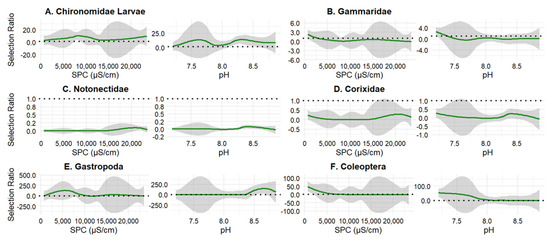
Figure 5.
Northern plains killifish selection ratios for six invertebrate taxa across gradients of specific conductivity (SPC) and pH: (A) Chironomidae larvae, (B) Gammaridae, (C) Notonectidae, (D) Corixidae, (E) Gastropoda, and (F) Coleoptera (adults and larvae). Green lines represent smoothed estimates of selection ratio, with shaded areas indicating 95% confidence intervals. The horizontal dashed line at 1 denotes neutral selection; values >1 suggest positive selection, while values < 1 indicate avoidance or negative selection (n = 37). Left panels show patterns across a gradient of SPC, and the right panels for each group show patterns across pH.
Fathead minnow diet varied as SPC increased and pH became harsher. Chironomidae larvae were selected across nearly all pH values but proportionally consumed at all SPC (Figure 6A); however, Gammaridae were proportionally consumed at all levels (Figure 6B). Notonectidae were avoided at all values (Figure 6C) Corixidae was consumed at a proportional level (Figure 6D). Gastropoda was avoided at all SPC and pH levels (Figure 6E). Coleoptera were selected at neutral pH values and consumed at a proportional level at all SPC values (Figure 6F). A higher proportion of Northern plains killifish and Fathead minnows had empty stomachs at higher SPC and pH values (Figure 7).
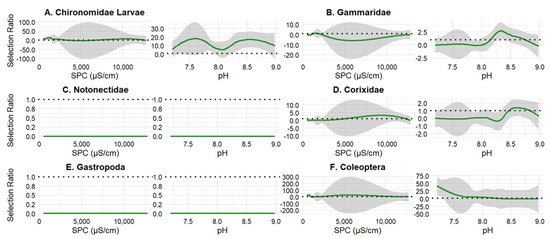
Figure 6.
Fathead minnow selection ratios for six invertebrate taxa across gradients of specific conductivity (SPC) and pH: (A) Chironomidae larvae, (B) Gammaridae, (C) Notonectidae, (D) Corixidae, (E) Gastropoda, and (F) Coleoptera (adults and larvae). Green lines represent smoothed estimates of selection ratio, with shaded areas indicating 95% confidence intervals. The horizontal dashed line at 1 denotes neutral selection; values >1 suggest positive selection, while values < 1 indicate avoidance or negative selection (n = 33). Left panels show patterns across a gradient of SPC, and the right panels for each group show patterns across pH.
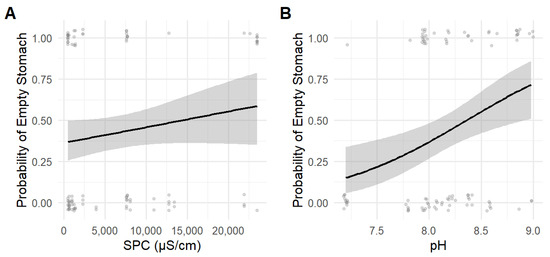
Figure 7.
Probability of empty stomachs in fish along gradients of (A) specific conductivity and (B) pH. Points represent individual fish (n = 111), with curves showing logistic regression fits and 95% confidence intervals (shaded). A total of 44 Northern plains killifish stomachs and 67 Fathead minnow stomachs were analyzed. Of these, 7 Northern plains killifish and 34 Fathead minnow stomachs were empty.
4. Discussion
This study provides evidence that fish can exhibit sublethal responses to harsher values of salinity and pH, and they can persist in these high-stress environments; however, their tolerance has limits. Although both species are known for their physiological tolerance to salinity extremes, the observed reductions in growth suggest a reallocation of energetic resources from somatic development to osmoregulatory processes [11]. These fish faced reduced food availability in more extreme environments in addition to higher physiological demands. The interaction between salinity and pH further influenced growth, indicating that compounding environmental stressors may limit fish development even in species considered tolerant.
The use of the first full otolith annulus allowed us to infer cumulative growth responses to long-term environmental exposure despite our study being limited to a single season. This growth marker integrates responses over approximately one year of habitat conditions, accounting for short-term variability and confirming that fish were exposed to these gradients.
Although our study species are recognized for their tolerance of harsh environmental conditions, they did not inhabit the most extreme lotic environments observed in this study. These high-stress stream conditions were present in close proximity to occupied sites, suggesting that the species likely encountered them but either actively avoided them or experienced mortality or outmigration upon exposure. Of the 201 sites surveyed, only 12 supported populations of our target fish species. Despite an extensive sampling effort, the low occupancy rate indicates that the presence of these species in such habitats is uncommon, reinforcing the idea that extreme environmental conditions may exceed even their notable physiological and ecological tolerances.
The sites used in our analysis were characterized by low macrophyte density and a tendency to dry rapidly. These habitats were largely dependent on episodic flooding events, which temporarily filled isolated pools and potentially trapped fish within them. Usually, the only water source would come from flooding events that would fill up pools and trap these fish.
Environmental stressors in naturally freshwater ecosystems rarely occur in isolation. Streams with elevated salinity often experience additional challenges such as extreme pH values, low dissolved oxygen, and thermal variability, particularly in isolated pools with limited inflow [58]. For example, fish in the North Platte River, USA must cope with osmotic stress due to isolation from low snowpack, regulated flows, and water extraction. Isolation also increases stress from reduced habitat complexity and limited access to refugia [59]. Isolated pools may act as physiological traps, where tolerant species survive but exhibit reduced performance [60], and our results support this conclusion. This underscores the importance of evaluating the cumulative impacts of multiple stressors on freshwater fish in arid and semi-arid landscapes.
Our findings suggest that fish engage in selective feeding at more extreme values of SPC and pH rather than switching entirely to opportunistic foraging; however, the composition of aquatic invertebrates they selected changed. Chironomidae larvae were consistently selected across most gradients, while other taxa, which were selected in milder conditions but avoided under harsher conditions, were generally not very abundant. This pattern indicates that fish exhibit prey preference even as prey richness declines, demonstrating that fish are not consuming all available taxa equally. Limited availability of some taxa may reduce their ability to maintain optimal diets. In instances where prey items were consumed in proportion to their availability, this likely reflects a scarcity of food resources rather than active dietary choice, suggesting that fish may have had limited foraging options in these ephemeral and resource-constrained environments. Laboratory studies with controlled food items would be valuable to clarify specific prey selection patterns under different stress levels. Selection ratios for Gastropoda remained relatively stable across gradients. Northern plains killifish were either selecting the snails due to the low variety of prey items, or Gastropoda (snails) were a favored prey item when available. The increase in empty stomachs at higher salinity and pH may reflect reduced prey capture success or decreased prey availability, but the consistency of selection patterns supports that behavioral selectivity persists despite environmental stress.
This study was conducted in conjunction with a long-term project initiated in 2010 that investigates Hygrotus diversepes, a rare aquatic beetle native to Wyoming. For our analysis, we assumed that a single measurement of SPC and pH adequately represents the water chemistry experienced by fish in April through October. Of the 12 sites included in this study, five overlap with sites from the ongoing beetle research. Previous data from that study indicate that water chemistry at these locations remains highly consistent across seasons and times of day. Based on this consistency, we justified the use of one-time water chemistry measurements to represent long-term conditions at each site [61]. These core sites were sampled monthly, but all other sites were haphazardly selected.
While the broad geographic scope of our sampling effort helped capture natural variability across harsh environments, it also resulted in a relatively small number of occupied sites, limiting the statistical power of some analyses. Nevertheless, our invertebrate sampling provides a representative snapshot of prey availability, allowing us to make robust inferences about dietary preferences and trophic responses under varying environmental stress.
The methodological approach of our study provides a novel framework for evaluating sublethal stress responses in freshwater fish. Our design allowed us to capture natural variability, but our widespread sampling to find these sites limited our ability to track seasonal patterns or within-individual changes over time. Future studies could improve resolution by incorporating repeated sampling over multiple years or including laboratory-controlled experiments to isolate the combined effects of salinity and pH. Including more sensitive species in future comparisons would also clarify whether these patterns are generalizable or specific to the tolerant species we used in our study.
Previous research has examined how various fish species respond to environmental stressors such as elevated salinity and pH. Shifts in fish assemblages often accompany abrupt increases in salinity, with some species demonstrating greater vulnerability than others [62]. For example, Nile tilapia (Oreochromis niloticus), a primarily freshwater species, exhibit enhanced osmoregulatory performance at optimal salinities, which supports improved growth outcomes [63]. In laboratory settings, Fundulus heteroclitus (a euryhaline killifish) altered salinity preference depending on diet composition [64], while reductions in salinity negatively affected growth in the marine European bass (Dicentrarchus labrax) [65]. These findings suggest that both dietary patterns and growth trajectories are influenced by salinity and other environmental factors; however, we found no studies examining the combined salinity and pH levels observed in the habitats occupied by our focal species. Our study contributes to this knowledge gap by documenting sublethal responses in fish inhabiting naturally extreme freshwater systems, providing insight into how these stressors influence species considered tolerant to such conditions.
The habitats sampled in this study represent extreme freshwater environments where salinity exceeded concentrations experienced in marine ecosystems. The resilience of Northern Plains killifish and Fathead minnows comes with tradeoffs in growth and diet. These results provide a strong foundation for similar studies in regions with brackish or fluctuating water quality, including coastal zones or other arid inland basins. Replicating this approach in more sensitive species, such as salmonids or stenohaline minnows, could help us to measure whether observed responses reflect general physiological limits or species-specific adaptations.
5. Conclusions
Establishing methods for assessing population-level stress in freshwater ecosystems impacted by shifting salinization and pH is critical to understanding changes to fish. Using a combination of dietary analysis and growth metrics to monitor fish species can help managers assess how changes in water quality can affect populations. Such studies can ascertain if streams are approaching thresholds that could compromise fish populations. Monitoring water quality is essential for long-term conservation planning in critical habitats given the unique geology and variable hydrology of many streams in semi-arid and arid ecosystems. Similar mechanisms of energy reallocation and dietary simplification may occur in fish inhabiting coastal and estuarine ecosystems, especially as sea levels rise. Understanding how fish species respond to stress can inform conservation ranking and predictions about vulnerability in changing aquatic environments worldwide.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fishes10090423/s1, Figure S1. Relationship between pH and the logarithm of specific conductivity (log(SPC)) across sampling sites. Each blue dot represents an individual observation at a given site. A smoothed regression line (black) with 95% confidence interval (gray shaded area) (n = 201); Figure S2. Sagittal otolith from a Northern Plains Killifish (Fundulus kansae). The black arrow indicates the region and direction corresponding to the first year of growth used for measurement.
Author Contributions
Conceptualization, M.M., A.L. and L.T.; Data curation, M.M. and A.L.; Formal analysis, M.M., A.L. and L.T.; Funding acquisition, M.M. and A.L.; Investigation, M.M., A.L. and L.T.; Methodology, M.M., A.L. and L.T.; Project administration, M.M., A.L. and L.T.; Resources, M.M. and L.T.; Software, M.M. and A.L.; Supervision, A.L. and L.T.; Validation, M.M., A.L. and L.T.; Visualization, M.M., A.L. and L.T.; Writing—original draft, M.M.; Writing—review and editing, M.M., A.L. and L.T. All authors have read and agreed to the published version of the manuscript.
Funding
This undergraduate research was funded by the Wyoming Research Scholars Program (WRSP) at the University of Wyoming.
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee of Institutional Animal Care and Use Committee (IACUC) (#2024-0118, approved on 14 May 2024).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
Miles Milbrath conducted this project as an undergraduate research project at the University of Wyoming. Thank you to Aspen Gair for the lab work with stomach and otolith dissection. Thank you to Nina Crawford, Katrina Cook and Michelle Weschler who provided helpful edits on a previous version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Velasco, J.; Gutiérrez-Cánovas, C.; Botella-Cruz, M.; Sánchez-Fernández, D.; Arribas, P.; Carbonell, J.A.; Millán, A.; Pallarés, S. Effects of salinity changes on aquatic organisms in a multiple stressor context. Philos. Trans. R. Soc. B 2019, 374, 20180011. [Google Scholar] [CrossRef] [PubMed]
- Brahney, J.; Ballantyne, A.P.; Kociolek, P.; Leavitt, P.R.; Farmer, G.L.; Neff, J.C. Ecological changes in two contrasting lakes associated with human activity and dust transport in western Wyoming. Limnol. Oceanogr. 2015, 60, 678–695. [Google Scholar] [CrossRef]
- Olson, J.R. Predicting combined effects of land use and climate change on river and stream salinity. Philos. Trans. R. Soc. B 2019, 374, 20180005. [Google Scholar] [CrossRef]
- Corsi, S.R.; Graczyk, D.J.; Geis, S.W.; Booth, N.L.; Richards, K.D. A fresh look at road salt: Aquatic toxicity and water-quality impacts on local, regional, and national scales. Environ. Sci. Technol. 2010, 44, 7376–7382. [Google Scholar] [CrossRef]
- Kaushal, S.S.; Likens, G.E.; Pace, M.L.; Utz, R.M.; Haq, S.; Gorman, J.; Grese, M. Freshwater salinization syndrome on a continental scale. Proc. Natl. Acad. Sci. USA 2018, 115, E574–E583. [Google Scholar] [CrossRef]
- USGS. New Study Demonstrates How Climate and Irrigation Influence Salinity of Waters in the Upper Colorado Basin; U.S. Geological Survey: Reston, VA, USA, 2024. [Google Scholar]
- Bern, C.R.; Clark, M.L.; Schmidt, T.S.; Holloway, J.M.; McDougal, R.R. Soil disturbance as a driver of increased stream salinity in a semiarid watershed undergoing energy development. J. Hydrol. 2015, 524, 123–136. [Google Scholar] [CrossRef]
- Taboga, K.G.; Stafford, J.E. Groundwater salinity in Wyoming: Wyoming State Geological Survey Open File Report. 2020; 2020, 29p. Available online: https://www.wsgs.wyo.gov/products/wsgs-2020-ofr-06.pdf (accessed on 15 January 2025).
- NOAA. Sea Water Salinity. National Oceanic and Atmospheric Administration. 2025. Available online: https://www.noaa.gov/jetstream/ocean/sea-water (accessed on 15 January 2025).
- Hastie, L.C.; Young, M.R.; Boon, P.J.; Cosgrove, P.J.; Henninger, B. Sizes, densities and age structures of Scottish Margaritifera margaritifera (L.) populations. Aquat. Conserv. Mar. Freshw. Ecosyst. 2000, 10, 229–247. [Google Scholar] [CrossRef]
- Bœuf, G.; Payan, P. How should salinity influence fish growth? Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2001, 130, 411–423. [Google Scholar] [CrossRef]
- Lydeard, C.; Cowie, R.H.; Ponder, W.F.; Bogan, A.E.; Bouchet, P.; Clark, S.A.; Cummings, K.S.; Frest, T.J.; Gargominy, O.; Herbert, D.G.; et al. The global decline of nonmarine mollusks. BioScience 2004, 54, 321–330. [Google Scholar] [CrossRef]
- Walker, R.H.; Smith, G.D.; Hudson, S.B.; French, S.S.; Walters, A.W. Warmer temperatures interact with salinity to weaken physiological facilitation to stress in freshwater fishes. Conserv. Physiol. 2020, 8, coaa107. [Google Scholar] [CrossRef] [PubMed]
- Williams, W.D. Anthropogenic salinisation of inland waters. In the Saline Lakes, Proceedings of the 7th International Conference on Salt Lakes; California, CA, USA, 20–23 September 1999, Melack, J.M., Jellison, R., Herbst, D.B., Eds.; Springer: New York, NY, USA, 2001; pp. 329–337. [Google Scholar]
- LIFE. pH Requirements of Freshwater Aquatic Life. Freshwater Aquatic LIFE Program; 2004. Available online: https://semspub.epa.gov/work/03/2244701.pdf (accessed on 9 May 2025).
- Baker, J.P.; Gherini, S.A.; Munson, R.K.; Christensen, S.W.; Driscoll, C.T.; Gallagher, J.; Newton, R.M.; Reckhow, K.H.; Schofield, C.L. Adirondack Lakes Survey: An Interpretive Analysis of Fish Communities and Water Chemistry, 1984–1987; Oak Ridge National Lab. (ORNL) A: Oak Ridge, TN, USA; Adirondack Lakes Survey Corp B: Ray Brook, NY, USA, 1990. [Google Scholar]
- Folger, P.F.; Tiemann, M.; Bearden, D.M. The EPA draft report of groundwater contamination near Pavillion, Wyoming: Main findings and stakeholder responses. Congr. Res. Serv. 2012, 42327. Available online: https://wyofile.com/wp-content/uploads/2012/01/R42327-2.pdf (accessed on 9 May 2025).
- Evans, D.H.; Piermarini, P.M.; Choe, K.P. The multifunctional fish gill: Dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol. Rev. 2005, 85, 97–177. [Google Scholar] [CrossRef] [PubMed]
- Mount, D.I. Chronic effect of low pH on fathead minnow survival, growth and reproduction. Water Res. 1973, 7, 987–993. [Google Scholar] [CrossRef]
- Clearwater, S.J.; Farag, A.M.; Meyer, J.S. Bioavailability and toxicity of dietborne copper and zinc to fish. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2002, 132, 269–313. [Google Scholar] [CrossRef]
- Sibly, R.M.; Hone, J. Population growth rate and its determinants: An overview. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2002, 357, 1153–1170. [Google Scholar] [CrossRef]
- Fung, L.; Rae, J.; Baylis, A.; Juteau, C.; Mageroy, J. Status of the western pearlshell mussel in the Little Campbell River: Comparison between 2009 and 2015. Northwest Sci. 2016, 90, 317–328. [Google Scholar]
- Geist, J. Strategies for the conservation of endangered freshwater pearl mussels (Margaritifera margaritifera L.): A synthesis of conservation genetics and ecology. Hydrobiologia 2010, 644, 69–88. [Google Scholar] [CrossRef]
- Canosa, L.F.; Bertucci, J.I. The effect of environmental stressors on growth in fish and its endocrine control. Front. Endocrinol. 2023, 14, 1109461. [Google Scholar] [CrossRef] [PubMed]
- Anthony, J.L.; Kesler, D.H.; Downing, W.L.; Downing, J.A. Length-specific growth rates in freshwater mussels (Bivalvia: Unionidae): Extreme longevity or generalized growth cessation? Freshw. Biol. 2001, 46, 1349–1359. [Google Scholar] [CrossRef]
- Barton, B.A.; Iwama, G.K. Physiological changes in fish from stress in aquaculture with emphasis on the response and effects of corticosteroids. Annu. Rev. Fish Dis. 1991, 1, 3–26. [Google Scholar] [CrossRef]
- Wootton, R.J. Ecology of Teleost Fishes; Springer Science and Business Media: New York, NY, USA, 2012. [Google Scholar]
- Schull, Q.; Beauvieux, A.; Viblanc, V.A.; Metral, L.; Leclerc, L.; Romero, D.; Pernet, F.; Quéré, C.; Derolez, V.; Munaron, D.; et al. An integrative perspective on fish health: Environmental and anthropogenic pathways affecting fish stress. Mar. Pollut. Bull. 2023, 194, 115318. [Google Scholar] [CrossRef]
- Knight, D.H.; Jones, G.P.; Reiners, W.A.; Romme, W.H. Mountains and Plains: The Ecology of Wyoming Landscapes; Yale University Press: London, UK, 2014. [Google Scholar]
- NRCS. Wyoming Hydrologic Unit Map; U.S. Department of Agriculture Natural Resources Conservation Service: Washington, DC, USA, 2020. [Google Scholar]
- PRISM Climate Group. 30-Year Normals (1991–2020) for Wyoming; Oregon State University: Corvallis, OR, USA, 2024. [Google Scholar]
- Rahel, F.J.; Thel, L.A. Plains Killifish (Fundulus zebrinus): A Technical Conservation Assessmen; USDA Forest Service, Rocky Mountain Region: Ogden, UT, USA, 2004. [Google Scholar]
- Eberle, M.E. Type locality and conservation status of the northern plains killifish (Fundulus kansae: Fundulidae) in Kansas. Trans. Kans. Acad. Sci. 2009, 112, 87–97. [Google Scholar]
- Cross, F.B.; Moss, R.E.; Collins, J.T. Assessment of Dewatering Impacts on Stream Fisheries in the Arkansas and Cimarron Rivers; University of Kansas: Lawrence, KS, USA, 1985. [Google Scholar]
- Minckley, C.O.; Klaassen, H.E. Life history of the plains killifish, Fundulus kansae (Garman), in the Smoky Hill River, Kansas. Trans. Am. Fish. Soc. 1969, 98, 460–465. [Google Scholar] [CrossRef]
- WGFD. Wyoming Statewide Angling Regulations. Wyoming Game and Fish Department. 2017. Available online: https://wgfd.wyo.gov/media/1453/download?inline (accessed on 13 March 2025).
- Nico, L.G.; Fuller, P.L.; Neilson, M.; Pimephales promelas—Fathead Minnow. USGS Nonindigenous Aquatic Species Database. 2025. Available online: https://nas.er.usgs.gov/queries/factsheet.aspx?SpeciesID=621 (accessed on 13 March 2025).
- Nico, L.G.; Fuller, P.L. Spatial and temporal patterns of nonindigenous fish introductions in the United States. Fisheries 1999, 24, 16–27. [Google Scholar] [CrossRef]
- Page, L.M.; Burr, B.M. A Field Guide to Freshwater Fishes: North America North of Mexico; Houghton Mifflin Harcourt: Boston, MA, USA, 1991. [Google Scholar]
- Matern, S.A.; Moyle, P.B.; Pierce, L.C. Native and alien fishes in a California estuarine marsh: Twenty-one years of changing assemblages. Trans. Am. Fish. Soc. 2002, 131, 797–816. [Google Scholar] [CrossRef]
- Carlander, K.D. Handbook of Freshwater Fishery Biology; Iowas State University Press: Ames, IA, USA, 1951. [Google Scholar]
- Nelson, J.S. Salinity tolerance of brook sticklebacks, Culaea inconstans, freshwater ninespine sticklebacks, Pungitius pungitius, and freshwater fourspine sticklebacks, Apeltes quadracus. Can. J. Zool. 1968, 46, 663–667. [Google Scholar] [CrossRef]
- Palmer, R.E.; Klauda, R.J.; Jepson, M.A.; Perry, E.S. Acute sensitivity of early life stages of fathead minnow (Pimephales promelas) to acid and aluminum. Water Res. 1989, 23, 1039–1047. [Google Scholar] [CrossRef]
- Brunel, T.; Piet, G.J. Is age structure a relevant criterion for the health of fish stocks? ICES J. Mar. Sci. 2013, 70, 270–283. [Google Scholar] [CrossRef]
- Sturrock, A.M.; Hunter, E.; Milton, J.A.; Johnson, E.R.C.; Waring, C.P.; Trueman, C.N. Quantifying physiological influences on otolith microchemistry. Methods Ecol. Evol. 2015, 6, 806–816. [Google Scholar] [CrossRef]
- Merritt, R.W.; Cummins, K.W.; Berg, M.B. Trophic relationships of macroinvertebrates. In Methods in Stream Ecology; Hauer, F.R., Lamberti, G., Eds.; Academic Press: New York NY, USA, 2017; Volume 1, pp. 413–433. [Google Scholar]
- Cummins, K.W.; Merritt, R.W.; Berg, M.B. Ecology and distribution of aquatic insects. In An Introduction to the Aquatic Insects of North America; Kendall/Hull Publishing: Dubuque, IA, USA, 1996; Volume 3, pp. 74–86. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Team, R.D.C. R: A Language and Environment for Statistical Computing. 2010. Available online: https://www.r-project.org/ (accessed on 9 May 2025).
- Posit, P.B.C. RStudio: Integrated Development Environment for R, version 2024.09. 0 Build 375. Crunchbase: San Francisco, CA, USA, 2024.
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.A.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Wickham, H.; Chang, W.; Wickham, M.H. Package‘Ggplot2’: Create Elegant Data Visualisations Using the Grammar of Graphics. 2016. version 2. pp. 1–189. Available online: https://cran.r-project.org/web/packages/ggplot2/index.html (accessed on 9 May 2025).
- Pedersen, T.L. Patchwork: The Composer of Plots. CRAN: Contributed Packages. 2019. Available online: https://cran.r-project.org/web/packages/patchwork/index.html (accessed on 9 May 2025).
- Kassambara, A. Ggpubr:‘Ggplot2′ Based Publication Ready Plots, R package version 0.4.0. 2020. Available online: https://cran.r-project.org/web/packages/ggpubr/index.html (accessed on 9 May 2025).
- Pebesma, E. Simple features for R: Standardized support for spatial vector data. R J. 2018, 10, 439–446. [Google Scholar] [CrossRef]
- Kahle, D.; Wickham, H. Ggmap: Spatial Visualization with ggplot2. R J. 2013, 5, 144–146. [Google Scholar]
- Dunnington, D.; Thorne, B.; Hernangómez, D. Ggspatial: Spatial Data Annotation Layers for Ggplot2, version 1.1.7. 2023. Available online: https://cran.r-project.org/web/packages/ggspatial/ggspatial.pdf (accessed on 9 May 2025).
- Kaushal, S.S.; Mayer, P.M.; Likens, G.E.; Reimer, J.E.; Maas, C.M.; Rippy, M.A.; Grant, S.B.; Hart, I.; Utz, R.M.; Shatkay, R.R.; et al. Five state factors control progressive stages of freshwater salinization syndrome. Limnol. Oceanogr. Lett. 2023, 8, 190–211. [Google Scholar] [CrossRef] [PubMed]
- Poff, N.L.; Olden, J.D.; Merritt, D.M.; Pepin, D.M. Homogenization of regional river dynamics by dams and global biodiversity implications. Proc. Natl. Acad. Sci. USA 2007, 104, 5732–5737. [Google Scholar] [CrossRef] [PubMed]
- Vander Vorste, R.; Obedzinski, M.; Nossaman Pierce, S.; Carlson, S.M.; Grantham, T.E. Refuges and ecological traps: Extreme drought threatens persistence of an endangered fish in intermittent streams. Glob. Change Biol. 2020, 26, 3834–3845. [Google Scholar] [CrossRef]
- Tronstad, L.M.; Lindsteadt, A.; Hotaling, S. Integrating historical and contemporary data for narrow-foot hygrotus diving beetle (Hygrotus diversipes L., 1966): Perspectives studying invertebrates of management and conservation concern. West. N. Am. Nat. 2024, 84, 447–477. [Google Scholar] [CrossRef]
- Trombley, C.A.; Hardy, T.B.; Schwalb, A.N. Disturbance-driven changes in fish assemblages caused by a sudden increase in salinity in a perennial desert stream. Environ. Biol. Fishes 2018, 101, 791–798. [Google Scholar] [CrossRef]
- Iqbal, K.J.; Qureshi, N.A.; Ashraf, M.; Rehman, M.H.U.; Khan, N.; Javid, A.; Abbas, F.; Mushtaq, M.M.H.; Rasool, F.; Majeed, H. Effect of different salinity levels on growth and survival of Nile tilapia (Oreochromis niloticus). J. Anim. Plant Sci. 2012, 22, 919–922. [Google Scholar]
- Bucking, C.; Wood, C.M.; Grosell, M. Diet influences salinity preference of an estuarine fish, the killifish Fundulus heteroclitus. J. Exp. Biol. 2012, 215, 1965–1974. [Google Scholar] [CrossRef]
- Dendrinos, P.; Thorpe, J.P. Effects of reduced salinity on growth and body composition in the European bass Dicentrarchus labrax (L.). Aquaculture 1985, 49, 333–358. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).