Abstract
Histamine can damage the antioxidant and immune systems in fish and crustaceans. Rutin, a natural substance with a diverse phenolic structure, has demonstrated antioxidant and anti-inflammatory properties. However, whether rutin can mitigate histamine-induced negative effects remains uninvestigated in fish models. This study investigated the effect of 0.1–100 μM rutin preincubation on histamine (29.5 mM)-induced cytotoxicity in zebrafish liver cells (ZFL) and its potential mechanisms. Results showed that 0.1–100 μM rutin significantly improved ZFL cell survival following histamine stimulation and protected cellular morphology. Rutin inhibited the adverse effects of histamine on ZFL by scavenging or suppressing the accumulation of reactive oxygen species (ROS), H2O2, and malondialdehyde (MDA), while increasing the activities of superoxide dismutase (SOD), catalase (CAT), and total antioxidant capacity (T-AOC). At the protein level, 10 μM rutin significantly promoted Nrf2 protein expression. HO-1 protein was significantly up-regulated after preincubation with 0.1–10 μM rutin, whereas IL-1β protein levels were significantly down-regulated. The mechanism may involve activation of the Nrf2 antioxidant signaling pathway and inhibition of the NF-κB inflammatory signaling pathway. In summary, within the experimental concentration range, 10 μM rutin showed the strongest inhibitory effects on histamine-induced ZFL cell death and oxidative stress. This study provides a theoretical basis and data support for evaluating rutin’s feasibility as a green aquatic feed additive.
Key Contribution:
Rutin (0.1–100 μM) can protect zebrafish liver cells from histamine-induced damage by reducing oxidative stress (ROS, H2O2, MDA) and boosting antioxidant defenses (SOD, CAT, T-AOC), likely through activating the Nrf2 pathway and suppressing NF-κB/IL1β inflammation.
1. Introduction
Fish meals, as an important component in aquatic feed, will deteriorate and produce histamine if stored improperly. Histamine is produced by the decarboxylase action of histidine, and dietary histamine levels are negatively correlated with the freshness of fish meal [1]. In addition, some fish muscles are rich in free histidine, which can be used as a substrate and converted into histamine by histidine decarboxylase in certain gram-negative bacteria (such as Morganella morganii, Hafnia alvei, etc.) [2]. Histamine exerts its physiological effects mainly through four receptors: H1, H2, H3, and H4 [3]. In humans, histamine has a strong vasodilatory function, which can cause smooth muscle cell contraction, vascular dilation, vascular permeability, blood pressure changes, and also stimulate gastric acid secretion and pain nerve fibers [4]. Histamine in fish is mainly metabolized through a complementary pathway of imidazolyacetic acid produced by intestinal diamine oxidase and 1-methylhistamine produced by liver N-methyltransferase [5]. The toxicity of histamine in fish and crustaceans has been evaluated, such as inducing intestinal and liver damage [6,7], reducing antioxidant and immune function [6,8], inhibiting gonadal development, prolonging sexual maturation time [9], inhibiting melanin deposition [10], reducing survival rate and growth performance [1,6].
At present, certain antihistamines have been reported and utilized in aquaculture, such as probiotics [6], Lactobacilli [11], and stevia extract [12]. In addition, several polyphenolic compounds have been found to inhibit histamine release and have potential anti-allergic effects, such as grape seed anthocyanins [13], epigallocatechin gallate [14], and brown algae polyphenol derivatives [15]. Rutin (C27H30O16) belongs to a class of natural substances with variable phenolic structures and is present in many plants, especially in species of the Fagopyrum genus in the Polygonaceae family [16]. Rutin scavenges reactive oxygen species (ROS) by providing hydrogen atoms to peroxide radicals, superoxide anions (O2−), and hydroxyl radicals (OH·); It can also act as a terminating and chelating agent for metal ions, inhibiting lipid peroxidation [17,18]. Beyond its well-documented antioxidant properties, rutin exhibits a diverse array of biological activities encompassing anti-inflammatory [19] and neuroprotective [20] effects.
Nrf2 (Nuclear factor erythroid 2-related factor 2) signaling pathway is a classic antioxidant signaling pathway. Its core factor, Nrf2, effectively regulates the cellular defense capacity against toxic substances and oxidative damage by expressing genes involved in the oxidative stress response and drug detoxification (such as GPx4 and HO-1) [21]. It has been confirmed that rutin can promote the growth and improve the muscle quality of grass carp by regulating the Nrf2 and PPAR signaling pathways [19]. The NF-κB (nuclear factor kappa-B) pathway is an extremely important pathway in cellular signal transduction, playing a key role in regulating various physiological processes such as immune responses, inflammatory responses, and cell growth and death. This pathway can respond to a variety of stimuli, including promoting the expression of pro-inflammatory cytokines such as IL-1β and TNF-α [22]. Studies have shown that rutin hydrate can alleviate neuritis in zebrafish by inhibiting the NF-κB pathway [23]. However, whether rutin can inhibit the negative effects induced by histamine by regulating the Nrf2 and NF-κB signaling pathways remains unclear.
Previous research in our laboratory demonstrated that dietary rutin (100 mg/kg) alleviates the negative effects of feed histamine on the growth performance of Pelteobagrus fulvidraco, enhancing antioxidant capacity, improving gut microbiota composition, and improving liver health [24]. To further investigate the mechanisms underlying rutin’s protective effects against histamine-induced liver damage in fish, this study utilized zebrafish (Danio rerio) liver cells (ZFL) (a well-established fish model organism) to explore the effects and mechanisms of rutin on histamine-induced cytotoxicity.
2. Materials and Methods
2.1. Cell Culture
ZFLs were obtained from the China Zebrafish Resource Center (CZRC). Cells were initially seeded in T25 (25 cm2 growth area) or T75 (75 cm2 growth area) culture flasks (Corning, Corning, NY, USA) and maintained in a controlled environment incubator at 28 °C with 5% CO2 under constant humidity conditions. Medium replacement was performed at 48 h intervals throughout the culture period. The basal culture medium formulation consisted of a 1:1 volumetric mixture of Dulbecco’s Modified Eagle Medium (DMEM; Gibco, Grand Island, NY, USA) and Ham’s F12 nutrient mixture (Gibco, Grand Island, NY, USA), supplemented with 4% heat-inactivated fetal bovine serum (Zeta Life, San Francisco, CA, USA) and 1% penicillin–streptomycin solution (100 U/mL penicillin, 100 μg/mL streptomycin; Gibco, Grand Island, NY, USA). The method of cell culture refers to Cheng et al. [25].
Prior to experimental initiation, ZFL monolayers (>90% cellular density) underwent enzymatic digestion using 0.25% trypsin. The resulting cell suspension was subsequently seeded into 96-well plates at an optimized density of 4 × 105 cells/mL (100 μL/well). Following 72 h incubation with varying concentrations of rutin (0–100 μM; ≥94% purity by HPLC, Sigma-Aldrich, St. Louis, MO, USA), ZFL were exposed to 29.5 mM histamine (99.99% purity, MedChemExpress, Shanghai, China) for 24 h. The stock solutions of rutin and histamine were prepared with sterile PBS. In the formal experiment, the specified concentrations of rutin and histamine were diluted with basal culture medium. Concentration selection for both rutin and histamine was determined according to the pre-experiment (Supplementary Figure S1). The experimental groups were as follows:
- 0: 0 μM rutin;
- 0 + H: 0 μM rutin + 29.5 mM histamine;
- 0.1 + H: 0.1 μM rutin + 29.5 mM histamine;
- 1 + H: 1 μM rutin + 29.5 mM histamine;
- 10 + H: 10 μM rutin + 29.5 mM histamine;
- 100 + H: 100 μM rutin + 29.5 mM histamine.
The cell culture conditions were consistent with the above.
2.2. Morphological Observation and Survival Rate Detection of ZFL
After treatment, the morphology and adhesion of ZFL were observed under an optical microscope. Subsequently, cellular specimens were harvested through enzymatic dissociation with 0.25% trypsin (Gibco, Grand Island, NY, USA), followed by fixation in 2.5% glutaraldehyde solution for ultrastructural preservation. Transmission electron microscopy (TEM) was then employed to observe intracellular architecture, including but not limited to nuclear membrane integrity, nucleolar morphology, mitochondrial cristae structure, and lysosomal distribution.
To assess cellular viability post-treatment, ZFL cells underwent triple PBS washing (Gibco, Grand Island, NY, USA) followed by quantitative analysis using CCK-8 assay (Cat No. CK04, Dojindo, Kumamoto, Japan). Specifically, washed cells were incubated with 100 μL DMEM-F12 medium (Gibco, Grand Island, NY, USA) supplemented with 10 μL CCK-8 reagent at 37 °C for 3 h under controlled conditions. After that, the absorbance at 450 nm of each well was determined by a microplate reader (Tecan, Männedorf, Switzerland). The survival rate of cells in each group was calculated as follows:
Cell survival rate (%) = [(ODtreatment − ODblank)/(ODcontrol − ODblank)] × 100%
In the formula, treatment represents 0.1–100 μM rutin groups; control represents 0 μM rutin group; blank represents pure culture medium groups without cells.
2.3. Detection of Mitochondrial Membrane Potential
JC-1 staining kit (Cat No. M8650, Solarbio, Beijing, China) was used to monitor changes in mitochondrial transmembrane potential in ZFL. The experimental process followed the manufacturer’s guidelines to prepare the working solution and washing buffer. After pre-treatment, the old culture medium was discarded, and 100 μL JC-1 staining working solution was added to each well. The cells were stained at 28 °C in the dark for 20 min. After staining, the cells were washed three times, and then 100 μL of fresh culture medium was added to each well. The stained cells were analyzed using dual channel imaging under a fluorescence microscope, and the ratio of red and green fluorescence intensity was calculated to quantitatively analyze the depolarization level of mitochondrial membrane potential.
2.4. Detection of Peroxide Levels
The DCFH-DA probe (Cat No. S0033M, Beyotime, Shanghai, China) was diluted 1000 times with PBS and used to detect the level of total ROS in ZFL. After incubation of ZFL in 96-well plates, cells were washed three times with PBS, then 100 μL of diluted DCHF-DA probe was added to each well and incubated at 37 °C against light for 30 min. After incubation, the cells were washed three times with PBS. Finally, the fluorescence intensity of each group was observed and photographed on a fluorescence microscope, and the fluorescence intensity of each group was analyzed in Image J (version: 1.53).
Intracellular H2O2 levels and total protein levels in ZFL were determined using the H2O2 assay kit and total protein (TP) determination kit (CAT No.A064-1-1, CAT No.A045-2-2, Nanjing Jiancheng, China), respectively. Cells post-treatment in Section 2.1 were collected and placed in 2 mL centrifuge tubes and were broken in an ultrasonic cell crusher at 4 °C for 20 min. Subsequently, it was centrifuged at 12,000 rpm for 10 min at 4 °C, and the supernatant was collected for later use. TP and H2O2 levels were detected and calculated according to the instructions provided in the kit.
2.5. Detection of Antioxidant Indicators
The levels of malondialdehyde (MDA) (CAT No.A003-1-2), superoxide dismutase (SOD) (CAT No.A003-1-2), catalase (CAT) (CAT No.A007-1-1), and total antioxidant capacity (T-AOC) (CAT No.A015-2-1) in ZFL were detected using kits from Nanjing Jiancheng Bioengineering Institute (Nianjing, China). The collected cells were crushed according to the above method (in Section 2.4), and the supernatant was collected for later use. The cell supernatant was tested according to the steps described in the instructions of the kits, and the levels of various antioxidant indicators were calculated based on formulas or standard curves.
2.6. Detection of Protein Expression in Antioxidant Pathways and Inflammatory Pathways
The expression of nuclear factor erythroid 2-related factor 2 (Nrf2), glutathione peroxidase 4 (GPx4), heme oxygenase-1 (HO-1) proteins in the antioxidant pathway, and nuclear factor kappa-B p65 (NF-κB p65), tumor necrosis factor-α (TNF-α), and interleukin-1β (IL-1β) proteins in the inflammatory pathway were detected by enzyme-linked immunosorbent assay (ELISA). The cells in Section 2.1 were collected and counted, and an equal number of cells were taken from each group to be placed in a 2 mL centrifuge tube. ZFL was lysed in an ultrasonic cell disruptor at 4 °C for 20 min, then centrifuged at 12,000 rpm for 10 min at 4 °C, and the supernatant (sample) was collected for later use. In the ELISA experiment, 50 μL of sample was added to the corresponding wells of the ELISA plate, and then 50 μL of antibody mixture was added to each well. The ELISA plate was incubated at 37 °C for 30 min. After incubation, 1×ELISA washing buffer was used to wash the ELISA plate five times, followed by adding 100 μL of TMB substrate to each well. The enzyme-linked immunosorbent assay (ELISA) plate was incubated in the dark for 30 min, and then 100 μL of STOP solution was added to each well. The absorbance at 450 nm was detected using a microplate reader (Tecan, Mannedorf, Switzerland) within 15 min.
2.7. Statistical Analysis
Statistical analyses were conducted using SPSS 20.0 software, with one-way ANOVA followed by Tukey’s post hoc test to evaluate intergroup variations. Quantitative data are presented as mean ± standard deviation (SD), and statistical significance was defined at p < 0.05.
3. Results
3.1. Effect of Rutin on the Survival Rate of ZFL After Histamine Stimulation
As shown in Figure 1, compared with the 0 group, the survival rate of ZFL in the 0 + H group significantly decreased to 44.54% (p < 0.05). Compared with the 0 + H group, incubation with varying concentrations of rutin (0.1 μM, 1 μM, 10 μM, and 100 μM) significantly increased the survival rate of ZFL cells following histamine stimulation (p < 0.05), reaching 55.86%, 63.07%, 68.59%, and 74.16%, respectively, demonstrating a certain dose-dependent effect. Among them, there was no significant difference in the survival rate of ZFL in the 1 + H, 10 + H, and 100 + H groups (p > 0.05).
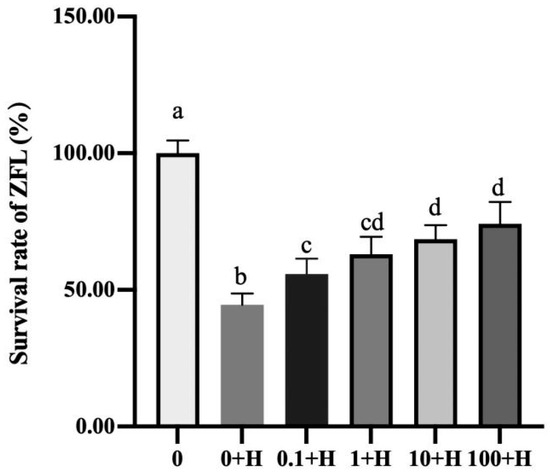
Figure 1.
Effects of different concentrations of rutin (0.1–100 µM) on the survival rate of ZFL after histamine stimulation. Different letters (a–d) represent p < 0.05.
3.2. Effect of Rutin on the Morphological Changes of ZFL After Histamine Stimulation
The morphology and internal structure of ZFL in each treatment group were observed under optical microscopy and transmission electron microscopy, respectively. As shown in Figure 2A, the single-layer cells in the 0 group were evenly distributed with strong adhesion, no plaques, clear cell edges, and consistent size. ZFL in the 0 + H group has poor adhesion and uneven distribution, with large areas of plaque at the bottom of the flask and numerous vacuoles inside the cells. Compared with the 0 + H group, preincubation with 0.1 μM to 100 μM rutin for 72 h improved the adhesion of ZFL after histamine stimulation, reduced plaque area, and was positively correlated with rutin concentration.
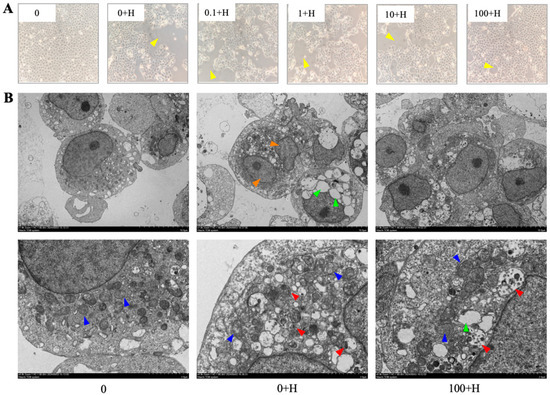
Figure 2.
Observation of ZFL cell morphology and internal structure. (A) The effect of different concentrations of rutin on the morphology of ZFL stimulated by histamine. Optical microscope, magnification of 200×. (B) The effect of rutin on the internal structure of ZFL after histamine stimulation. Transmission electron microscope with magnifications of 1000× and 4000×. Arrow annotation: Yellow: plaques; Blue: mitochondria; Orange: nucleus; Red: autophagic lysosomes; Green: cytoplasmic vacuolization.
The internal structures of ZFL in 0, 0 + H, and 100 + H obtained by transmission electron microscopy are shown in Figure 2B. The cells in group 0 had a complete membrane structure, clear nuclei and nucleoli, normal oval-shaped mitochondria, uniform distribution, and clear mitochondrial cristae. In the 0 + H group, the cell membrane remained intact, with numerous vacuoles visible inside the cell. Mitochondria were obvious but reduced in number, and some nuclei were lysed, indicating the possibility of apoptotic necrosis of the cell; the appearance of a large number of autophagic lysosomes inside the cell indicated that autophagy may occur. In the 100 + H group, the cell membrane was intact, the intracellular vacuoles were reduced compared to the 0 + H group, the single nucleus was clear, the nucleolus was obvious, the mitochondria were normal, oval-shaped, and autophagic lysosomes appeared inside the cells, indicating that autophagy may occur in the cells.
3.3. Effect of Rutin on the Mitochondrial Membrane Potential of ZFL After Histamine Stimulation
Figure 3 shows the changes in mitochondrial membrane potential of ZFL in each treatment group, where red fluorescence represents JC-1 aggregates, i.e., high membrane potential; green fluorescence represents JC-1 monomer, i.e., low membrane potential, and the depolarization of mitochondrial membrane potential is generally indicated by the ratio of red to green fluorescence. The results showed that there was no significant change in mitochondrial membrane potential of ZFL among the groups (p > 0.05).
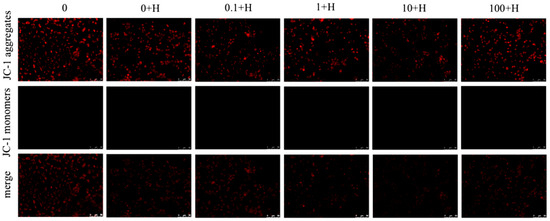
Figure 3.
Effects of different concentrations of rutin on mitochondrial membrane potential of ZFL cells after histamine stimulation. Red fluorescence: JC-1 aggregate, high membrane potential; Green fluorescence: JC-1 monomer, low membrane potential.
3.4. Effect of Rutin on Peroxide Levels of ZFL After Histamine Stimulation
For the ROS levels in ZFL, as shown in Figure 4A, compared with the 0 group, the ROS levels in the 0 + H group were significantly increased (p < 0.05). Compared with the 0 + H group, the ROS levels in each group were dose-dependent. There was no significant change in ROS levels in the 0.1 + H and 1 + H groups (p > 0.05), while ROS levels significantly decreased in the 10 + H and 100 + H groups (p < 0.05).
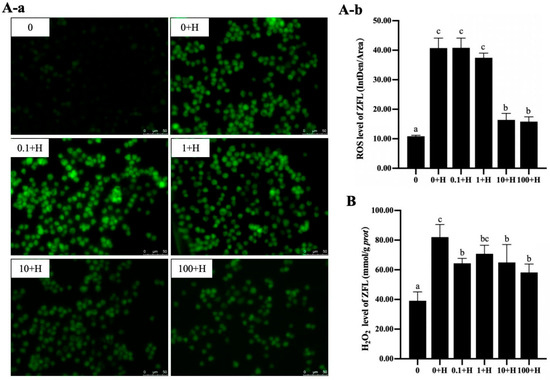
Figure 4.
Effects of different concentrations of rutin on the levels of ROS (A) and H2O2 (B) in ZFL stimulated by histamine. (A-a): the fluorescence visualization of ROS, green fluorescence represents the location of ROS in ZFL. (A-b): the statistical of the mean fluorescence intensity of ROS. Different letters (a–c) indicate p < 0.05.
The changes in H2O2 levels are shown in Figure 4B. Compared with the 0 group, the H2O2 level in the 0 + H group was significantly increased (p < 0.05). Compared with the 0 + H group, the H2O2 levels in the 0.1 + H, 1 + H, 10 + H, and 100 + H were significantly down-regulated (p < 0.05).
3.5. Effect of Rutin on Antioxidant Enzyme Levels of ZFL After Histamine Stimulation
The levels of MDA, SOD, CAT, and T-AOC in each group of ZFL were shown in Figure 5. Compared with the 0 group, the level of MDA in the 0 + H group significantly increased (p < 0.05), while the levels of SOD and CAT significantly decreased (p < 0.05). Compared with the 0 + H group, 0.1 μM rutin significantly reduced MDA levels in ZFL after histamine stimulation, 1 μM rutin increased SOD levels in ZFL after histamine stimulation, and 10 μM and 100 μM rutin increased CAT levels in ZFL after histamine stimulation. 1 μM to 100 μM rutin significantly increases the T-AOC level of ZFL in a dose-dependent manner.
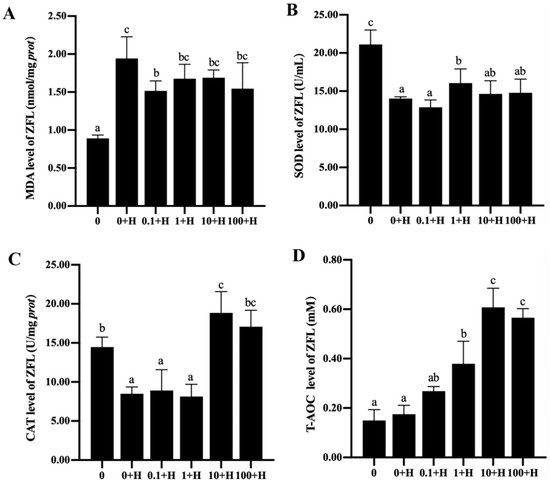
Figure 5.
Effects of different concentrations of rutin on the levels of MDA (A), SOD (B), CAT (C), and T-AOC (D) in ZFL stimulated by histamine. Different letters (a–c) indicate p < 0.05.
3.6. Expression of Key Proteins in Antioxidant and Inflammatory Pathways
As shown in Figure 6A, for the Nrf2 signaling pathway, compared with group 0, the protein levels of Nrf2 and GPx4 in ZFL in group 0 + H had no significant changes (p > 0.05), while the protein levels of HO-1 were significantly down-regulated (p < 0.05). After incubation with different concentrations of rutin, 10 μM rutin significantly promoted the expression of Nrf2 protein (p < 0.05). HO-1 protein was significantly up-regulated in 0.1 μM, 1 μM, and 10 μM rutin treatment groups (p < 0.05), and there was no significant difference in HO-1 protein level among the three groups (p > 0.05). The level of HO-1 protein in the 100 + H group was not significantly different from that in the 0 + H group (p > 0.05). There were no significant differences in GPx4 protein levels among all groups (p > 0.05).
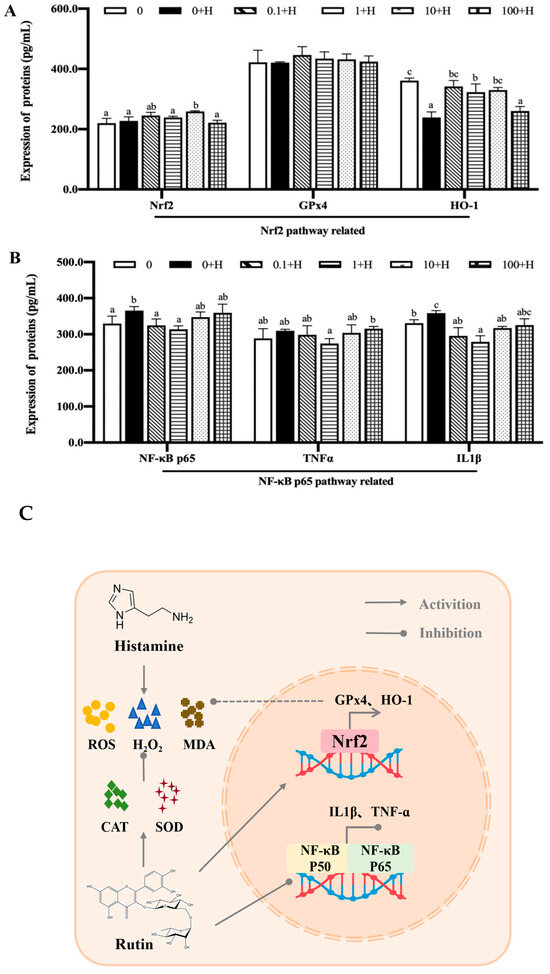
Figure 6.
Effects of different concentrations of rutin on the expression levels of key proteins in the Nrf2 signaling pathway (A) and NF-κB signaling pathway (B) in ZFL after histamine stimulation. Visualization of the possible mechanism of rutin in inhibiting histamine-induced cytotoxicity (C). Different letters (a–c) indicate p < 0.05.
For the NF-κB signaling pathway, the levels of NF-κB p65 and IL1β in group 0 + H were significantly increased compared with group 0 (p < 0.05). Compared with the 0 + H group, NF-κB p65 protein in 0.1 + H and 1 + H groups was significantly down-regulated (p < 0.05), and IL1β protein level in 0.1 + H, 1 + H, and 10 + H groups was significantly down-regulated (p < 0.05). There were no significant changes in TNFα protein in all treatment groups (p > 0.05).
4. Discussion
Rutin, also known as vitamin P, is a flavonoid compound widely present in various plants. Due to its antioxidant properties and potential health benefits, rutin is commonly used as a dietary supplement and therapeutic adjuvant [26]. Previous studies have shown that rutin has some protective effects on cells, and in Alzheimer’s disease, rutin has been shown to inhibit Aβ aggregation and cytotoxic effects [27]; Li et al. [28] reported that rutin can improve the survival rate of SH-SY5Y cells after lead treatment. In this study, 0.1 μM, 1 μM, 10 μM, and 100 μM rutin all improved the survival rate of ZFL after histamine incubation, and the survival rate of ZFL increased with the increase in rutin concentration, indicating that rutin had an inhibitory effect on histamine-induced cell death of ZFL. The above results have also been confirmed in human disease studies, where rutin concentrations of 10 μmol/L, 20 μmol/L, and 40 μmol/L can significantly improve the survival rate of stromal cells after H2O2 treatment, reaching 70.1%, 86.8% and 80.3%, respectively [29]. In terms of cell morphology, histamine stimulation caused a decrease in the adhesion of ZFL and a large number of vacuoles in the cells, while after incubation with rutin, the adhesion of ZFL and cell state were significantly improved. Singh et al. [30] also found that rutin protects red blood cell morphology by reducing ROS levels.
Mitochondria are important physiological sources of ROS. Mitochondrial membrane permeability changes increase the formation of reactive oxygen species by inhibiting the respiratory chain [31], and mitochondria play a key role in cell cycle regulation [32,33]. Studies have shown that histamine can cause mitochondrial membrane potential disorders in primary rat lung fibroblasts [34]. However, in the current study, no significant changes were detected in the mitochondrial membrane potential of ZFL after histamine stimulation. Combined with the results observed under transmission electron microscopy, it was found that there was no significant change in the mitochondrial morphology of ZFL after histamine treatment, but the number decreased. The reason for this difference may be the difference in the concentration and time of histamine treatment, as well as differences in cell types. It could also be because cells precisely clear functionally impaired mitochondria (such as those with reduced membrane potential) through autophagic mechanisms while retaining healthy ones, resulting in a decrease in the total number. However, the remaining mitochondria function normally; hence, the membrane potential remains unchanged. There were no significant changes in mitochondrial membrane potential of ZFL cells in all groups after preincubation with rutin, suggesting that preincubation with 0.1–100 μM rutin had no negative effects on mitochondrial membrane potential of ZFL. At the same time, it cannot be ruled out that the subtle changes in ZFL mitochondria after incubation with histamine or rutin exceed the detection sensitivity of the JC-1 probe.
Reactive oxygen species (ROS) are potent intracellular oxidants and have been proposed as key regulators of apoptosis [35]. Rutin is an antioxidant with the ability to reduce ROS accumulation to prevent oxidative damage, thereby protecting cells from subsequent cellular damage [36,37]. This is because the structure of rutin contains a catalytic domain, which can directly clear the generated ROS [38,39,40]. In a rat model of acute inflammation, rutin showed anti-inflammatory effects by regulating ROS levels, apoptosis, and cell periodicity [41]. Similar results were obtained in the current study, where ROS levels in ZFL were significantly elevated after histamine stimulation, suggesting that histamine stimulation caused severe oxidative stress in ZFL, while high concentrations (10 μM and 100 μM) of rutin preincubated cleared or inhibited ROS in ZFL. H2O2 also belongs to the ROS and is a strong intracellular oxidant that can induce oxidative reactions in the body. Previous studies have extensively explored the inhibitory effect of rutin on H2O2-induced oxidative reactions. Sun et al. [28] reported that rutin can alleviate H2O2-induced oxidative damage and cell apoptosis in interstitial cells by activating the PI3K/Akt signaling pathway; Singh et al. [42] also found that rutin can alleviate the decrease in antioxidant enzyme activities such as SOD, CAT, GPx induced by H2O2. However, the effect of rutin on the production or accumulation of H2O2 has not been reported. The current study examined the level of H2O2 in ZFL after the preincubation of rutin and histamine stimulation, and the results showed that the preincubation of rutin from 0.1 μM to 100 μM could reverse the accumulation of H2O2 in ZFL caused by histamine. Therefore, we speculate that rutin may also inhibit the occurrence of oxidation in the body by clearing H2O2.
Antioxidant enzyme activity can directly reflect the antioxidant capacity of the body. In human medical studies, rutin has been shown to reduce the production of MDA, ROS, NO, and pro-inflammatory cytokines. In addition, rutin can increase the level of antioxidant enzymes such as CAT, SOD, GSH, etc. [26]. In fish, Pês et al. [43] reported that adding 0.15% rutin to the diet of silver catfish can increase the activity of SOD, CAT, and glutathione S-transferase (GST) in brain, kidney, liver, and muscle. In the current study, 1–100 μM rutin significantly increased T-AOC in ZFL and was proportional to rutin concentration. After the preincubation of rutin, CAT and SOD were increased to varying degrees. The above results indicated that rutin could increase the level of antioxidant enzymes in ZFL after histamine stimulation. Combined with our previous experimental results [44], it was found that rutin can improve the antioxidant capacity of the liver after histamine stimulation both in vivo and in vitro.
The Nrf2 signaling pathway is one of the most classical antioxidant signaling pathways. Oluranti et al. [45] reported that the molecular mechanism of the neuroprotective properties of rutin may be related to the activation of Nrf2 and the inhibition of the NF-κB signaling pathway. Nrf2 is a transcription factor that plays a crucial role in the cellular defense system, especially in response to oxidative stress or inflammation [46]. When a cell is under oxidative or electrophile stress, Nrf2 shifts to the nucleus and binds to antioxidant response elements (ARE) located upstream of genes involved in antioxidant and detoxifying pathways. These genes include HO-1 and GPx4, which help clear harmful factors from cells [42,47]. The current study detected the key protein of Nrf2 signaling pathway, and the results showed that 10 μM rutin significantly promoted the expression of Nrf2 protein, and HO-1 protein was significantly up-regulated in the 0.1–10 μM rutin treatment group, indicating that rutin can activate the Nrf2 signaling pathway to a certain extent and promote the expression of antioxidant enzymes. This result was also confirmed in rat studies. Caglayan et al. [48] found that rutin significantly inhibited oxidative stress in diabetic rats, decreased ROS and MDA levels in nervous tissues, and found that rutin significantly up-regulated Nrf2 and HO-1 in DRG neurons.
In addition to its antioxidant properties, rutin has been shown to have powerful anti-inflammatory properties. Studies have shown that rutin can reduce inflammation by reducing levels of typical pro-inflammatory factors such as il-6, il-1β, and tnf-α in the spleen of silver catfish [49], all of which are effector factors downstream of the NF-κB signaling pathway. The abnormal activation of the NF-κB signaling pathway was closely related to the pathogenesis and pathological progression of liver injury [50]. The effect of rutin on the NF-κB signaling pathway has been reported. Rutin can reduce histamine release in mast cells, inhibit the activation and nuclear translocation of nf-κb, and inhibit the release of pro-inflammatory factors [51]. Rutin alleviated CCL4-induced liver inflammation in mice, a mechanism that may be related to the TLR4/MyD88/NF-κB inflammatory signaling pathway [52]. In the current study, histamine activated the NF-κB p65 signaling pathway, promoted the expression of il-1β, and induced inflammation within ZFL. NF-κB p65 protein was significantly down-regulated after 0.1 μM and 1 μM preincubation of rutin, and IL1-β protein levels were significantly down-regulated after 0.1–10 μM preincubation of rutin, suggesting that rutin could inhibit histamine-induced inflammatory response in ZFL by inhibiting the activation of NF-κB. Zheng et al. [53] also found in the tilapia study that the transcriptions of pro-inflammatory cytokines tnf-α and il1-β in the liver of tilapia in the 0.3 g/kg rutin group were significantly reduced. These results prove that rutin plays an important role in anti-inflammation.
In addition, there may be a relationship between the production of ROS, the Nrf2 signaling pathway, and the NF-κB signaling pathway. In the resting state, inhibitory κB kinase (IκB) binds to NF-κB and inhibits the activation of NF-κB. Poli et al. [54] indicated that ROS can activate IκB and lead to phosphorylation of IκB. This releases the free NF-κB dimer and enables it to cross the nuclear membrane and bind to specific regions in the gene, thereby enhancing the production of inflammatory cytokines such as tnf-α and il-1β. In addition, the activation of NF-κB also inhibits the expression of antioxidant genes by down-regulating the Nrf2 pathway, thereby indirectly weakening innate antioxidant defenses and increasing ROS production [55]. According to the current research results, rutin can inhibit the accumulation of ROS in ZFL after histamine stimulation and can play an antioxidant and anti-inflammatory role by activating the Nrf2 signaling pathway and inhibiting the NF-κB signaling pathway. Therefore, this mechanism of rutin may also be the joint result of inhibiting ROS, activating the Nrf2 signaling pathway, and inhibiting the NF-κB signaling pathway.
5. Conclusions
In summary, this study revealed the inhibitory effect of exogenous rutin addition on histamine-induced ZFL cytotoxicity. The results showed that 0.1–100 μM rutin can significantly improve the survival rate of ZFL after histamine stimulation and protect cell morphology. Rutin inhibits the negative effects of histamine on ZFL by clearing or inhibiting the accumulation of ROS, H2O2, and MDA, and increasing the activities of SOD, CAT, and T-AOC, which may be accomplished by activating the Nrf2 antioxidant signaling pathway and inhibiting the NF-κB inflammatory signaling pathway (Figure 6C). Within the experimental concentration range, 10 μM rutin has the best inhibitory effects on histamine-induced ZFL death and oxidative stress. The results of this study provide theoretical analysis and data support for evaluating the feasibility of rutin as a green aquatic feed additive. However, this study was limited to the cellular level. In the future, more in-depth research should be conducted on farmed fish.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fishes10080408/s1. Figure S1: Pre-experiment on incubation concentration of rutin and exposure concentration of histamine; Figure S2: The structural formulas of rutin and histamine. Reference [56] is cited in the supplementary materials.
Author Contributions
Conceptualization, H.W.; methodology, K.C., D.P., L.D. and Y.L. (Yangyang Liu); validation, K.C. and A.L.; resources, J.T. and Y.L. (Yongju Luo); data curation, K.C.; writing—original draft preparation, K.C.; writing—review and editing, M.J.; project administration, M.J.; funding acquisition, Z.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by an earmarked fund for the open fund of China (Guangxi)-ASEAN Key Laboratory of Comprehensive Exploitation and Utilization of Aquatic Germplasm Resources, Key Laboratory of Aquaculture Genetics and Breeding, and Healthy Aquaculture of Guangxi Academy of Fishery Sciences. Funder: Z.G., grant number “GXKEYLA-2023-01-12” and China Agriculture Research System (No. CARS-46), funder: Z.G. The APC was funded by CARS-46.
Institutional Review Board Statement
According to Paragraph 4 of Article 32 of the Measures for the Ethical Review of Life Science and Medical Research Involving Humans (2023) of the People’s Republic of China, ethical review is exempted.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available from the corresponding author by request.
Conflicts of Interest
The author Apeng Liu was employed by Shenzhen Aohua Group Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ROS | Reactive oxygen species |
| ZFL | Zebrafish liver cells |
| TEM | Transmission electron microscopy |
| MDA | Malondialdehyde |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| T-AOC | Total antioxidant capacity |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| GPx4 | Glutathione peroxidase 4 |
| HO-1 | Heme oxygenase-1 |
| NF-κB p65 | Nuclear factor kappa-B p65 |
| TNF-α | Tumor necrosis factor-α |
| IL-1β | Interleukin-1β |
| GST | Glutathione S-transferase |
| ARE | Antioxidant response elements |
| IκB | Inhibitory κB kinase |
References
- Ma, D.; Cai, P.; Zhai, S.; Chen, X. Effect of dietary histamine on growth performance, digestive enzyme activities and antioxidant indices in intestine of juvenile American eels (Anguilla rostrata). Feed Res. 2020, 2, 42–45. [Google Scholar] [CrossRef]
- Taylor, S.L.; Stratton, J.E.; Nordlee, J.A. Histamine poisoning (scombroid fish poisoning): An allergy like intoxication. Clin. Toxicol. 1989, 27, 225–240. [Google Scholar] [CrossRef]
- Maintz, L.; Novak, N. Histamine and histamine intolerance. Am. J. Clin. Nutr. 2007, 85, 1185–1196. [Google Scholar] [CrossRef]
- Wang, Y.T.; Fang, F.D.; Liu, X.J. Targeting histamine in metabolic syndrome: Insights and therapeutic potential. Life Sci. 2024, 358, 123172. [Google Scholar] [CrossRef] [PubMed]
- Shiozaki, K.; Nakano, T.; Yamaguchi, T.; Sato, A. Metabolism of exogenous histamine in rainbow trout (Oncorhynchus mykiss). Fish Physiol. Biochem. 2003, 29, 289–295. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Yang, H.L.; Hu, L.H.; Yang, W.; Ai, C.; Sun, Y.Z. Autochthonous probiotics alleviate the adverse effects of dietary histamine in juvenile grouper (Epinephelus coioides). Front. Microbiol. 2021, 12, 79271. [Google Scholar] [CrossRef]
- Li, W.; Liu, B.; Liu, Z.; Yin, Y.; Xu, G.; Han, M.; Xie, L. Effect of dietary histamine on intestinal morphology, inflammatory status, and gut microbiota in yellow catfish (Pelteobagrus fulvidraco). Fish Shellfish Immun. 2021, 117, 95–103. [Google Scholar] [CrossRef]
- Lin, C.; Yan, P.; Lou, Z.; Shi, X.; Zhao, Q.; Li, E. Effects of histamine on the neuroendocrine-immune regulatory network in the Pacific white shrimp, Litopenaeus vannamei. Aquaculture 2022, 554, 738156. [Google Scholar] [CrossRef]
- Yang, X.; Wang, J.; Zhao, L.; Fan, P.; Wu, X.; Cheng, Y.; Zeng, C. Effects of elevated ambient histamine level on survival, growth, sexual maturity and tissue histamine accumulation of the mysis Neomysis awatschensis and Neomysis japonica Nakazawa. Aquac. Int. 2012, 20, 347–356. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, Y.; Cheng, W.; Li, W.; Chen, J.; Xie, L.; Xu, G. Effect of dietary histamine levels on growth performance and body pigmentation of Pelteobagrus fulvidraco. Freshw. Fish 2017, 47, 79–84. [Google Scholar]
- Cheng, Y.B.; Li, W.; Han, M.L.; Xu, G.H.; Xie, L.W.; Yin, Y.L.; Liang, J.Q. The enterohepatic protection of Pelteobagrus fulvidraco adding Lactobacillus reuteri induced by histamine. Acta Hydrobiol. Sin. 2019, 43, 94–101. [Google Scholar] [CrossRef]
- Shiozaki, K.; Nakano, T.; Yamaguchi, T.; Sato, M.; Sato, N. The protective effect of stevia extract on the gastric mucosa of rainbow trout Oncorhynchus mykiss (Walbaum) fed dietary histamine. Aquac. Res. 2004, 35, 1421–1428. [Google Scholar] [CrossRef]
- Lu, W.Q.; He, Y.Z.; Liang, Y.; Chen, X.H.; Zhai, S.W. Effects of grape seed proanthocyanidins supplementation on parameters related to liver health of American eels (Anguilla rostrata) exposed to dietary histamine stress. Feed Ind. 2020, 41, 56–60. [Google Scholar] [CrossRef]
- Matsuo, N.; Yamada, K.; Yamashita, K.; Shoji, K.; Mori, M.; Sugano, M. Inhibitory effect of tea polyphenols on histamine and leukotriene B4 release from rat peritoneal exudate cells. Vitr. Cell Dev. Biol. Anim. 1996, 32, 340–344. [Google Scholar] [CrossRef]
- Hung, L.D.; Hori, K.; Nang, H.Q.; Kha, T.; Hoa, L.T. Seasonal changes in growth rate, carrageenan yield and lectin content in the red alga Kappaphycus alvarezii cultivated in Camranh Bay, Vietnam. J. Appl. Phycol. 2009, 21, 265–272. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, K.W.; Kim, D.Y.; Park, H.H.; Kwon, I.B.; Lee, H.J. Optimal recovery of high-purity rutin crystals from the whole plant of Fagopyrum esculentum Moench (buckwheat) by extraction, fractionation, and recrystallization. Bioresour. Technol. 2005, 96, 170–912. [Google Scholar] [CrossRef]
- Afanas’ev, I.B.; Ostrachovitch, E.A.; Abramova, N.E.; Korkina, L.G. Different antioxidant activities of bioflavonoid rutin in normal and iron-overloading rats. Biochem. Pharmacol. 1995, 50, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Guo, J.; Yuan, J. In vitro antioxidant properties of rutin. LWT-Food Sci. Technol. 2008, 41, 1060–1066. [Google Scholar] [CrossRef]
- Xu, Z.; Yang, H.; Poolsawat, L.; Rahman, M.M.; Xu, X.Y.; Jiang, X.R.; Li, X.Q.; Tan, H.X.; Leng, X.J. Flavonoid-enriched diets improved the growth and flesh quality of grass carp (Ctenopharyngodon idellus) based on metabolomics. Aquacult. Nutr. 2021, 27, 2514–2528. [Google Scholar] [CrossRef]
- Zhang, C.M.; Wang, S. Molecular mechanisms of neuroprotective effect of rutin. Front. Pharmacol. 2025, 16, 1599167. [Google Scholar] [CrossRef]
- Kobayashi, M.; Yamamoto, M. Nrf2–Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv. Enzym. Regul. 2006, 46, 113–140. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, P.; Aggarwal, A. NF-kB transcription factor: A key player in the generation of immune response. Curr. Sci. Bangal. 2006, 90, 519–531. [Google Scholar]
- Hu, Y.; Jia, K.; Zhou, Y.T.; Chen, L.X.; Wang, F.; Yi, X.K.; Huang, Y.; Ge, Y.R.; Chen, X.M.; Liao, D.L.; et al. Rutin hydrate relieves neuroinflammation in zebrafish models: Involvement of NF-κB pathway as a central network. Fish Shellfish Immunol. 2023, 141, 109062. [Google Scholar] [CrossRef]
- Liu, A.P.; Lu, X.; Ji, Z.H.; Dong, L.X.; Jiang, J.Y.; Tian, J.; Wen, H.; Xu, Z.; Xu, G.H.; Jiang, M. Preliminary Study to Assess the Impact of Dietary Rutin on Growth, Antioxidant Capacity, and Intestinal Health of Yellow Catfish, Pelteobagrus fulvidraco. Animals 2023, 13, 3386. [Google Scholar] [CrossRef]
- Cheng, K.; Huang, Y.Q.; Wang, C.F. 1,25(OH)2D3 Inhibited Ferroptosis in Zebrafish Liver Cells (ZFL) by Regulating Keap1-Nrf2-GPx4 and NF-κB-hepcidin Axis. Int. J. Mol. Sci. 2020, 22, 11334. [Google Scholar] [CrossRef]
- Muvhulawa, N.; Dludla, P.V.; Ziqubu, K.; Mthembu, S.X.H.; Mthiyane, F.; Nkambule, B.B.; Mazibuko-Mbeje, S.E. Rutin ameliorates inflammation and improves metabolic function: A comprehensive analysis of scientific literature. Pharm. Res. 2022, 178, 106163. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Q.; Liu, K.; Zhu, Q.; Deng, H.; Le, Y.; Ouyang, W.; Yan, X.; Zhou, W.; Tong, J. Brain-penetration and neuron-targeting DNA nanoflowers Co-delivering miR- 124 and rutin for synergistic therapy of Alzheimer’s disease. Small 2022, 18, 2107534. [Google Scholar] [CrossRef]
- Li, F.; Zhang, L.; Zhang, X.X.; Fang, Q.M.; Xu, Y.S.; Wang, H. Rutin alleviates Pb-induced oxidative stress, inflammation and cell death via activating Nrf2/ARE system in SH-SY5Y cells. Neuro 2024, 104, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.H.; Wang, H.; Liu, B.; Shi, W.H.; Shi, J.Z.; Zhang, Z.; Xing, J.P. Rutin attenuates H2O2-induced oxidation damage and apoptosis in Leydig cells by activating PI3K/Akt signal pathways. Biomed. Pharmacother. 2017, 88, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Dubey, V.; Meena, A.; Siddiqui, L.; Maurya, A.K.; Luqman, S. Rutin restricts hydrogen peroxide-induced alterations by up-regulating the redox-system: An in vitro, in vivo and in silico study. Eur. J. Pharmacol. 2018, 835, 115–125. [Google Scholar] [CrossRef]
- Polster, B.M.; Fiskum, G. Mitochondrial mechanisms of neural cell apoptosis. J. Neurochem. 2004, 90, 1281–1289. [Google Scholar] [CrossRef]
- Ishikawa, K.; Takenaga, K.; Akimoto, M.; Koshikawa, N.; Yamaguchi, A.; Imanishi, H.; Nakada, K.; Honma, Y.; Hayashi, J.I. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science 2008, 320, 661–664. [Google Scholar] [CrossRef]
- Suleiman, M.S.; Halestrap, A.P.; Griffiths, E.J. Mitochondria: A target for myocardial protection. Pharmacol. Ther. 2001, 89, 29–46. [Google Scholar] [CrossRef]
- Huang, W.W.; Zhou, X.Y. Anti-histamine effects of dipotassium glycyrrhizinate on lung fibroblasts, implicating its therapeutic mechanism for pulmonary fibrosis. J. Pharm. Pharmacol. 2022, 74, 1241–1250. [Google Scholar] [CrossRef]
- Danial, N.N.; Korsmeyer, S.J. Cell death: Critical control points. Cell 2004, 116, 205–219. [Google Scholar] [CrossRef]
- Freitas, P.A.; Oliveira, K.A.; Magalhães, L.A.; Neves, R.D.; Maia, C.S.; Silveira, L.; Lima, T.T.; Vasconcelos, R.P.; Brito, L.C.; Torres–Leal, F.L.; et al. Improvement of 2,2’-azobis(2-methylpropionamidine) dihydrochloride-induced hepatic redox imbalance in Swiss mice and hepG2 cells by rutin. J. Med. Food 2022, 25, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qian, Y.; Zhang, M.; Qiao, W. Repairing of rutin to the toxicity of combined F-53B and chromium pollution on the biofilm formed by Pseudomonas aeruginosa. J. Environ. Sci. 2023, 127, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Hanasaki, Y.; Ogawa, S.; Fukui, S. The correlation between active oxygens scavenging and antioxidative effects of flavonoids. Free Radic. Biol. Med. 1994, 16, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Enogieru, A.B.; Haylett, W.; Hiss, D.C.; Bardien, S.; Ekpo, O.E. Rutin as a potent antioxidant: Implications for neurodegenerative disorders. Oxid. Med. Cell. Longev. 2018, 2018, 6241017. [Google Scholar] [CrossRef]
- Zaghloul, R.A.; Abdelghany, A.M.; Samra, Y.A. Rutin and selenium nanoparticles protected against STZ-induced diabetic nephropathy in rats through downregulating Jak-2/Stat3 pathway and upregulating Nrf-2/HO-1 pathway. Eur. J. Pharm. 2022, 933, 175289. [Google Scholar] [CrossRef]
- Adefegha, S.A.; Leal, D.B.R.; de Oliveira, J.S.; Manzoni, A.G.; Bremm, J.M. Modulation of reactive oxygen species production, apoptosis and cell cycle in pleural exudate cells of carrageenan-induced acute inflammation in rats by rutin. Food Funct. 2017, 8, 4459–4468. [Google Scholar] [CrossRef]
- Singh, S.; Singh, D.K.; Meena, A.; Dubey, V.; Masood, N.; Luqman, S. Rutin protects t-butyl hydroperoxide-induced oxidative impairment via modulating the Nrf2 and iNOS activity. Phytomedicine 2019, 55, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Pês, T.S.; Saccol, E.M.; Ourique, G.M.; Londero, E.P.; Gressler, L.T.; Finamor, I.A.; Rotili, D.A.; Golombieski, J.I.; Glanzner, W.G.; Llesuy, S.F.; et al. Effect of diets enriched with rutin on blood parameters, oxidative biomarkers and pituitary hormone expression in silver catfish (Rhamdia quelen). Fish Physiol. Biochem. 2016, 42, 321–333. [Google Scholar] [CrossRef]
- Liu, S.J.; Tian, F.; Qi, D.L.; Qi, H.F.; Wang, Y.; Xu, S.X.; Zhao, K. Physiological, metabolomic, and transcriptomic reveal metabolic pathway alterations in Gymnocypris przewalskii due to cold exposure. BMC Genom. 2023, 24, 545. [Google Scholar] [CrossRef]
- Oluranti, O.I.; Alabi, B.A.; Michael, O.S.; Ojo, A.O.; Fatokun, B.P. Rutin prevents cardiac oxidative stress and inflammation induced by bisphenol A and dibutyl phthalate exposure via NRF-2/NF-kappaB pathway. Life Sci. 2021, 284, 119878. [Google Scholar] [CrossRef]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharm. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Lai, X.; Zhang, Y.; Wu, J.; Shen, M.; Yin, S.; Yan, J. Rutin attenuates oxidative stress Via PHB2-mediated mitophagy in MPP (+)-Induced SH-SY5Y cells. Neurotox. Res. 2023, 41, 242–255. [Google Scholar] [CrossRef]
- Caglayan, C.; Kandemir, F.M.; Darendelioglu, E.; Yildirim, S.; Kucukler, S.; Dortbudak, M.B. Rutin ameliorates mercuric chloride-induced hepatotoxicity in rats via interfering with oxidativestress, inflammation and apoptosis. J. Trace. Elem. Med. Biol. 2019, 56, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Baldissera, M.D.; Souza, C.F.; Viana, A.R.; da Silva, A.S.; Baldisserotto, B. Protective role of rutin dietary supplementation mediated by purinergic signaling in spleen of silver catfish Rhamdia quelen exposed to organophosphate pesticide trichlorfon. Comp. Biochem. Physiol. C 2021, 244, 109006. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Chi, X.; Jin, Y.; Wang, Y.; Huang, P.; Wu, S.; Xia, Z.; Cai, J. Dexmedetomidine inhibits TLR4/NF-kB activation and reduces acute kidney injury after orthotopic autologous liver transplantation in rats. Sci. Rep. 2015, 5, 16849. [Google Scholar] [CrossRef] [PubMed]
- Park, H.H.; Lee, S.; Son, H.Y.; Park, S.B.; Kim, M.S.; Choi, E.J.; Singh, T.S.K.; Ha, J.H.; Lee, M.G.; Kim, J.E. Flavonoids inhibit histamine release and expression of proinflammatory cytokines in mast cells. Arch. Pharm. Res. 2008, 31, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- He, Q.Y.; Hao, H.; Zhao, K.K. Investigating the anti-inflammatory effects of rutin in carbon tetrachloride-induced hepatotoxicity: Role of TLR4/MyD88/NFκB signaling pathway modulation. Pharmacogn. Mag. 2024, 20, 107–115. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhao, Z.X.; Fan, L.M.; Meng, S.L.; Song, C.; Qiu, L.P.; Xu, P.; Chen, J.Z. Dietary supplementation with rutin has pro-anti-inflammatory effects in the liver of juvenile GIFT tilapia, Oreochromis niloticus. Fish Shellfish Immunol. 2017, 64, 49–55. [Google Scholar] [CrossRef]
- Poli, G.; Leonarduzzi, G.; Biasi, F.; Chiarpotto, E. Oxidative stress and cell signalling. Curr. Med. Chem. 2004, 11, 1163–1182. [Google Scholar] [CrossRef]
- Yerra, G.V.; Negi, G.; Sharma, S.S.; Kumar, A. Potential therapeutic effects of the simultaneous targeting of the Nrf2 and NF-κB pathways in diabetic neuropathy. Redox Biol. 2013, 1, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Vijay, M.; Sivagami, G.; Thayalan, K.; Nalini, N. Radiosensitizing potential of rutin against human colon adenocarcinoma HT-29 cells. Bratisl Lek Listy. 2016, 117, 171–178. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).