A Kinetic Study on the Accumulation of No.0 Fuel Oil and Pinghu Crude Oil Water-Accommodated Fraction in Exopalaemon carinicauda
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.1.1. Experimental Animals
2.1.2. Experimental Reagents and Containers
2.2. Preparation of Experimental Water-Accommodated Fraction (WAF)
2.3. Experimental Methods
2.4. Sample Analysis
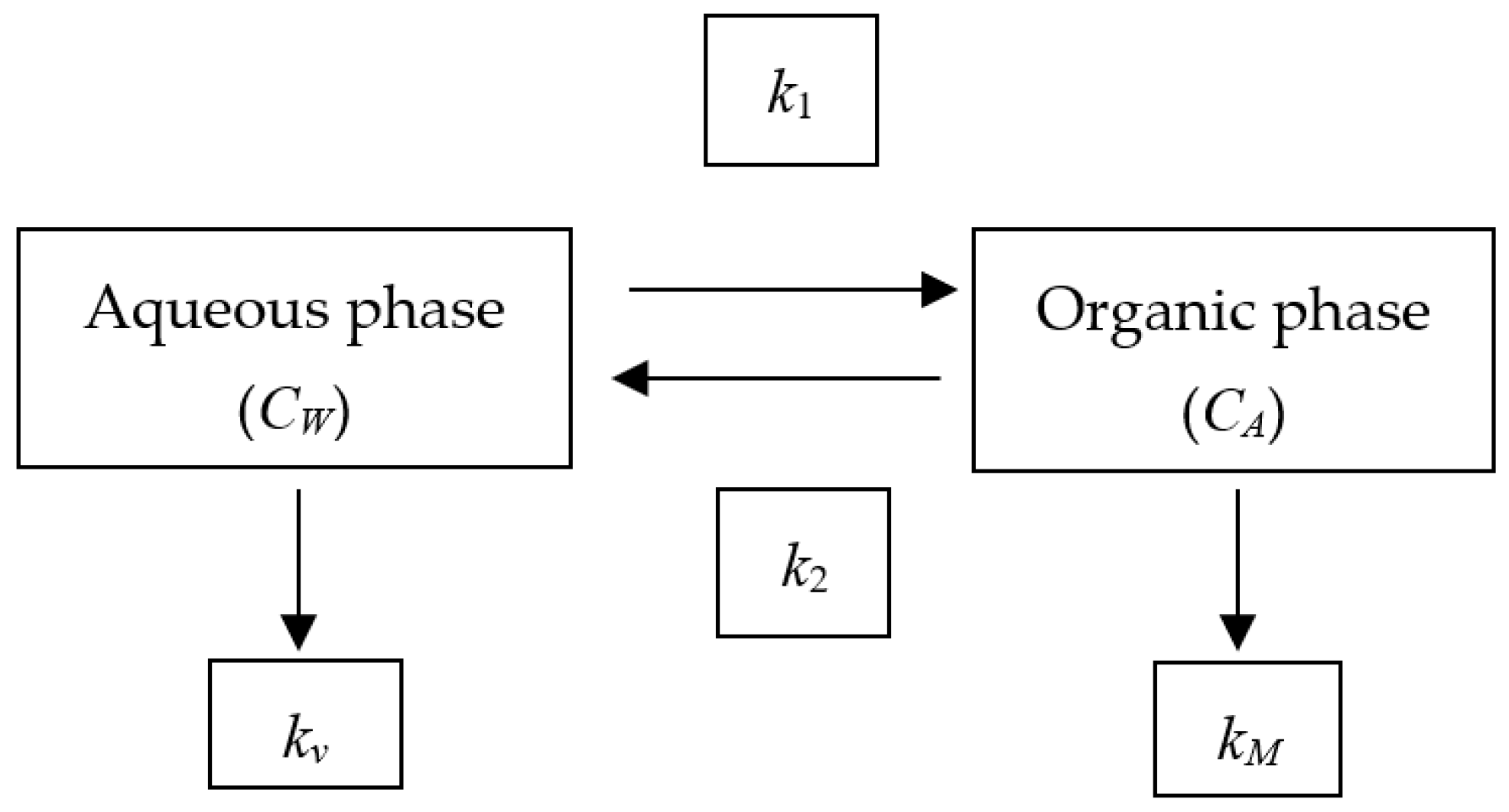
2.5. Semi-Static Two-Compartment Kinetic Model
2.6. Data Processing and Goodness-of-Fit
3. Results
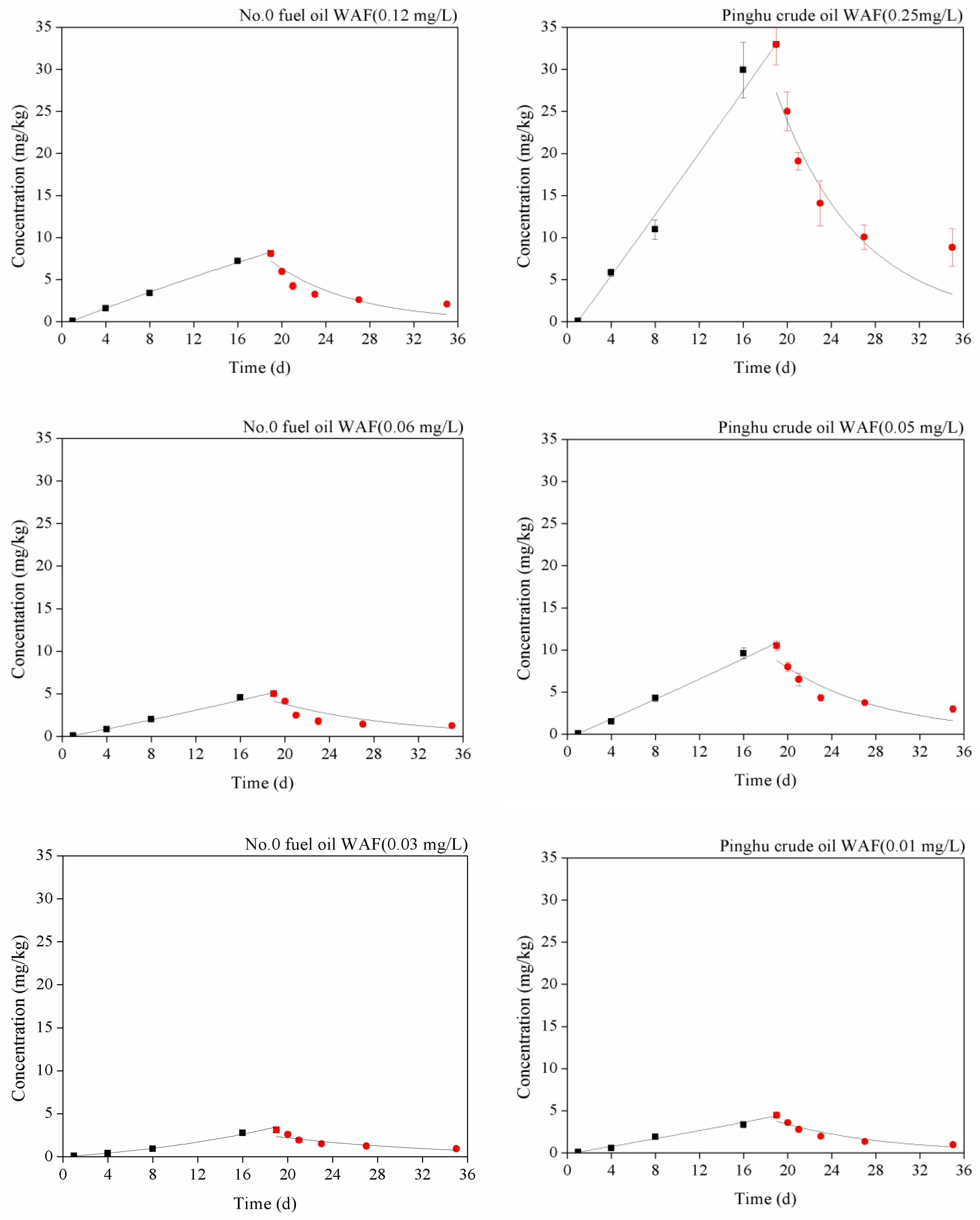
3.1. Results and Analysis
3.2. Enrichment Kinetic Parameters of No.0 Fuel Oil and Crude Oil WAF at Different Concentrations in Exopalaemon Carinicauda
3.3. Goodness-of-Fit Test of the Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zakaria, M.P.; Horinouchi, A.; Tsutsumi, S.; Takada, H.; Tanabe, S.; Ismail, A. Oil Pollution in the Straits of Malacca, Malaysia: Application of Molecular Markers for Source Identification. Environ. Sci. Technol. 2000, 34, 1189–1196. [Google Scholar] [CrossRef]
- Ding, H.; Ma, Q.; Ren, J.; Liu, X.; Sun, F. Study on the characteristic parameters of n-alkanes, steranes and terpanes in the surface seawater of Zhoushan Sea Area. Mar. Environ. Sci. 2018, 37, 239–244. [Google Scholar]
- Kingston, P.F.; Runciman, D.; McDougall, J. Oil contamination of sedimentary shores of the Galápagos Islands following the wreck of the Jessica. Mar. Pollut. Bull. 2003, 47, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Awewomom, J.; Benyade, B.K.; Osei, F.E. A review of health hazards associated with exposure to galamsey-related pollutants. Health Sci. Investig. J. 2024, 5, 726–734. [Google Scholar]
- Gilde, K.; Pinckney, J.L. Sublethal Effects of Crude Oil on the Community Structure of Estuarine Phytoplankton. Estuaries Coasts 2012, 35, 853–861. [Google Scholar] [CrossRef]
- Bejarano, A.C.; Chandler, G.T.; He, L.; Coull, B.C. Individual to population level effects of South Louisiana crude oil water accommodated hydrocarbon fraction (WAF) on a marine meiobenthic copepod. J. Exp. Mar. Biol. Ecol. 2006, 332, 49–59. [Google Scholar] [CrossRef]
- Alves, R.N.; Mariz, C.F., Jr.; de Paulo, D.V. Toxicity of effluents from gasoline stations oil-water separators to early life stages of zebrafish Danio rerio. Chemosphere 2017, 178, 224–230. [Google Scholar] [CrossRef]
- Mariz, C.F., Jr.; Nascimento, J.V.G.; Morais, B.S.; Alves, M.K.M.; Rojas, L.A.V.; Zanardi-Lamardo, E.; Carvalho, P.S. Toxicity of the oil spilled on the Brazilian coast at different degrees of natural weathering to early life stages of the zebrafish Danio rerio. Mar. Pollut. Bull. 2024, 207, 116816–116820. [Google Scholar] [CrossRef]
- De Souza, J.V.N.; Vieira, M.L.M.; De Assis, J.E.; Zanardi-Lamardo, E.; Gomes, P.B.; De Souza, J.R.B. Responses of functional traits of macrobenthic communities on the presence of Polycyclic Aromatic Hydrocarbons contamination in three tropical estuaries. Estuar. Coast. Shelf Sci. 2021, 250, 107103–107109. [Google Scholar]
- Pacheco, M.; Santos, M.A. Biotransformation, Endocrine, and Genetic Responses of Anguilla anguilla L. to Petroleum Distillate Products and Environmentally Contaminated Waters. Ecotoxicol. Environ. Saf. 2001, 49, 64–75. [Google Scholar] [CrossRef]
- Keramea, P.; Spanoudaki, K.; Zodiatis, G. Oil spill modeling: A critical review on current trends, perspectives, and challenges. J. Mar. Sci. Eng. 2021, 9, 181. [Google Scholar] [CrossRef]
- Monteiro, C.B.; Oleinik, P.H.; Leal, T.F.; Marques, W.C.; Nicolodi, J.L.; Lopes, B.D.C.F.L. Integrated environmental vulnerability to oil spills in sensitive areas. Environ. Pollut. 2020, 267, 115234–115240. [Google Scholar] [CrossRef] [PubMed]
- Yakan, S.D.; Focks, A.; Klasmeier, J. Numerical evaluation of bioaccumulation and depuration kinetics of PAHs in Mytilus galloprovincialis. Environ. Pollut. 2017, 220, 1244–1250. [Google Scholar] [CrossRef] [PubMed]
- Gan, N.; Martin, L.; Xu, W. Impact of polycyclic aromatic hydrocarbon accumulation on oyster health. Front. Physiol. 2021, 12, 734461–734465. [Google Scholar] [CrossRef]
- Jiang, C.; Qiao, Q.; Cai, Y.; Xu, J. Kinetic features and threshold value of petroleum hydrocarbons for Ruditapes philippinarum. Mar. Fish. 2006, 28, 314–320. [Google Scholar]
- Liu, B.; Lv, L.; Ding, L. Comparison of phthalate esters (PAEs) in freshwater and marine food webs: Occurrence, bioaccumulation, and trophodynamics. J. Hazard. Mater. 2024, 466, 133532–133534. [Google Scholar] [CrossRef]
- Keitel-Gröner, F.; Bamber, S.; Bechmann, R.K. Effects of chronic exposure to the water-soluble fraction of crude oil and in situ burn residue of oil on egg-bearing Northern shrimp (Pandalus borealis). Ecotoxicol. Environ. Saf. 2021, 228, 113011–113014. [Google Scholar] [CrossRef]
- Jin, F.; Wang, Y.; Yu, F. Acute and chronic effects of crude oil water-accommodated fractions on the early life stages of marine medaka (Oryzias melastigma, McClelland, 1839). Toxics 2023, 11, 236. [Google Scholar] [CrossRef] [PubMed]
- Carro, N.; Cobas, J.; García, I.; Ignacio, M.; Mouteira, A.; Miranda, M.; Picado, L. Organochlorine compounds and polycyclic aromatic hydrocarbons in mussels from Ria de Vigo (the Northern Spanish coast). Current levels and long—Term trends (2010–2019). Relationship with human pressures. Reg. Stud. Mar. Sci. 2021, 44, 101740–101746. [Google Scholar] [CrossRef]
- Ding, G.; Zhang, S. Ecological characteristics and the causes of Karenia mikimotoi bloom in the Sansha Bay in 2012. Haiyang Xuebao 2018, 40, 104–112. [Google Scholar]
- Moore, M.N.; Livingstone, D.R.; Widdows, J. Hydrocarbons in marine mollusks: Biological effects and ecological consequences/Metabolism of polycyclic aromatic hydrocarbons in the aquatic environment. CRC Press. 2024, 16, 291–328. [Google Scholar]
- Xue, Q.; Sun, Y.; Wang, X.; Zhang, J. Determination of kinetic parameters for bioconcentration of petroleum hydrocarbons by Mytilus edulis. Chin. Mar. Fish Res. 2001, 22, 32–36. [Google Scholar]
- Honda, M.; Suzuki, N. Toxicities of polycyclic aromatic hydrocarbons for aquatic animals. Int. J. Environ. Res. Public Health 2020, 17, 1363. [Google Scholar] [CrossRef]
- Viñas, L.; Franco, M.; Soriano, J.; González, J.; Ortiz, L.; Bayona, J.; Albaigés, J. Accumulation trends of petroleum hydrocarbons in commercial shellfish from the Galician coast (NW Spain) affected by the Prestige oil spill. Chemosphere 2009, 75, 534–541. [Google Scholar] [CrossRef]
- Vijayanand, M.; Ramakrishnan, A.; Subramanian, R. Polyaromatic hydrocarbons (PAHs) in the water environment: A review on toxicity, microbial biodegradation, systematic biological advancements, and environmental fate. Environ. Res. 2023, 227, 115714–115717. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.; Gagnon, M.M.; Nugegoda, D. Alterations of metabolic enzymes in Australian bass, Macquaria novemaculeata, after exposure to petroleum hydrocarbons. Arch. Environ. Contam. Toxicol. 2005, 49, 200–205. [Google Scholar] [CrossRef]
- Mai, Y.; Wang, Y.; Geng, T. A systematic toxicologic study of polycyclic aromatic hydrocarbons on aquatic organisms via food-web bioaccumulation. Sci. Total Environ. 2024, 929, 172360–172364. [Google Scholar] [CrossRef]
- Ruiz-Frau, A.; Gelcich, S.; Hendriks, I.E. Current state of seagrass ecosystem services: Research and policy integration. Ocean Coast. Manag. 2017, 149, 107–115. [Google Scholar] [CrossRef]
- Cohen, A.; Nugegoda, D.; Gagnon, M.M. The effect of different oil spill remediation techniques on petroleum hydrocarbon elimination in Australian bass (Macquaria novemaculeata). Arch. Environ. Contam. Toxicol. 2001, 40, 264–270. [Google Scholar] [CrossRef] [PubMed]
| Oils | Concentration (mg/L) | k1 | k2 | BCF | CAmax(mg/kg) | B1/2(d) |
|---|---|---|---|---|---|---|
| No.0 Fuel Oil WAF | 0.12 | 4.16 ± 0.32 a | 0.1333 ± 0.010 b | 31.21 ± 2.15 a | 3.75 ± 0.28 a | 5.20 ± 0.31 a |
| No.0 Fuel Oil WAF | 0.06 | 5.35 ± 0.41 b | 0.0985 ± 0.008 a | 54.29 ± 3.62 b | 3.26 ± 0.21 a | 7.03 ± 0.45 b |
| No.0 Fuel Oil WAF | 0.03 | 6.12 ± 0.38 b | 0.0997 ± 0.007 a | 61.42 ± 4.05 b | 1.84 ± 0.15 a | 6.95 ± 0.38 b |
| Crude Oil WAF | 0.25 | 8.14 ± 0.52 c | 0.1025 ± 0.009 a | 79.37 ± 5.21 c | 19.84 ± 1.32 c | 6.76 ± 0.29 a |
| Crude Oil WAF | 0.05 | 9.16 ± 0.63 d | 0.1059 ± 0.006 a | 86.47 ± 4.89 d | 4.32 ± 0.33 a | 6.54 ± 0.32 a |
| Crude Oil WAF | 0.01 | 13.47 ± 0.75 e | 0.1047 ± 0.008 a | 128.61 ± 6.93 e | 1.29 ± 0.11 a | 6.62 ± 0.27 a |
| Oils | Concentration (mg/L) | R2 | F-Test p-Value | t-Test p-Value |
|---|---|---|---|---|
| No.0 Fuel Oil WAF | 0.12 | 0.9978 | 0.0275 | 0.3957 |
| No.0 Fuel Oil WAF | 0.06 | 0.9883 | 0.0391 | 0.4015 |
| No.0 Fuel Oil WAF | 0.03 | 0.9783 | 0.0297 | 0.4186 |
| Crude Oil WAF | 0.25 | 0.9892 | 0.0324 | 0.3944 |
| Crude Oil WAF | 0.05 | 0.9862 | 0.0366 | 0.3929 |
| Crude Oil WAF | 0.01 | 0.8331 | 0.0113 | 0.4222 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Zhang, Y.; Wei, L.; Lin, A.; Cai, J.; Wei, Z.; Wu, Q.; Niu, J.; Sui, Y.; Jiang, M. A Kinetic Study on the Accumulation of No.0 Fuel Oil and Pinghu Crude Oil Water-Accommodated Fraction in Exopalaemon carinicauda. Fishes 2025, 10, 403. https://doi.org/10.3390/fishes10080403
Li L, Zhang Y, Wei L, Lin A, Cai J, Wei Z, Wu Q, Niu J, Sui Y, Jiang M. A Kinetic Study on the Accumulation of No.0 Fuel Oil and Pinghu Crude Oil Water-Accommodated Fraction in Exopalaemon carinicauda. Fishes. 2025; 10(8):403. https://doi.org/10.3390/fishes10080403
Chicago/Turabian StyleLi, Lei, Yiyun Zhang, Li Wei, Aijia Lin, Jiaying Cai, Zengqiao Wei, Qingyuan Wu, Junxiang Niu, Yanming Sui, and Mei Jiang. 2025. "A Kinetic Study on the Accumulation of No.0 Fuel Oil and Pinghu Crude Oil Water-Accommodated Fraction in Exopalaemon carinicauda" Fishes 10, no. 8: 403. https://doi.org/10.3390/fishes10080403
APA StyleLi, L., Zhang, Y., Wei, L., Lin, A., Cai, J., Wei, Z., Wu, Q., Niu, J., Sui, Y., & Jiang, M. (2025). A Kinetic Study on the Accumulation of No.0 Fuel Oil and Pinghu Crude Oil Water-Accommodated Fraction in Exopalaemon carinicauda. Fishes, 10(8), 403. https://doi.org/10.3390/fishes10080403






