Smaller Body Size and Warmer Water Improve Two Temperate Fishes’ Upstream Passage over Wetted Ramps
Abstract
1. Introduction
- To quantify the effect of fish size on swimming performance on a wetted ramp (Experiment I);
- To explore the effect of temperature on swimming performance on a wetted ramp (Experiment II).
2. Materials and Methods
2.1. Collection Methods
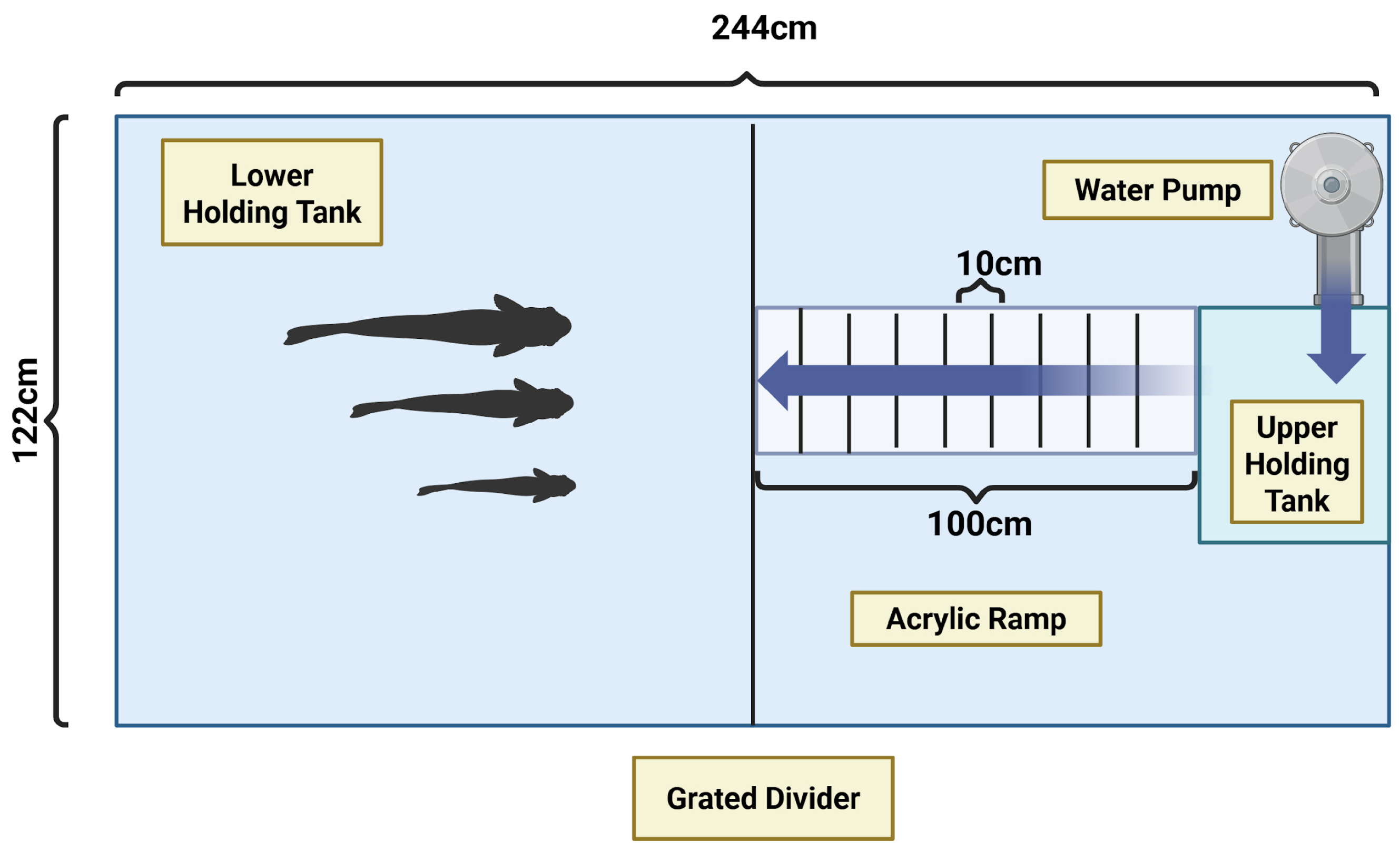
2.2. Experimental Setup
2.3. Experimental Procedure
2.4. Data Analysis
3. Results
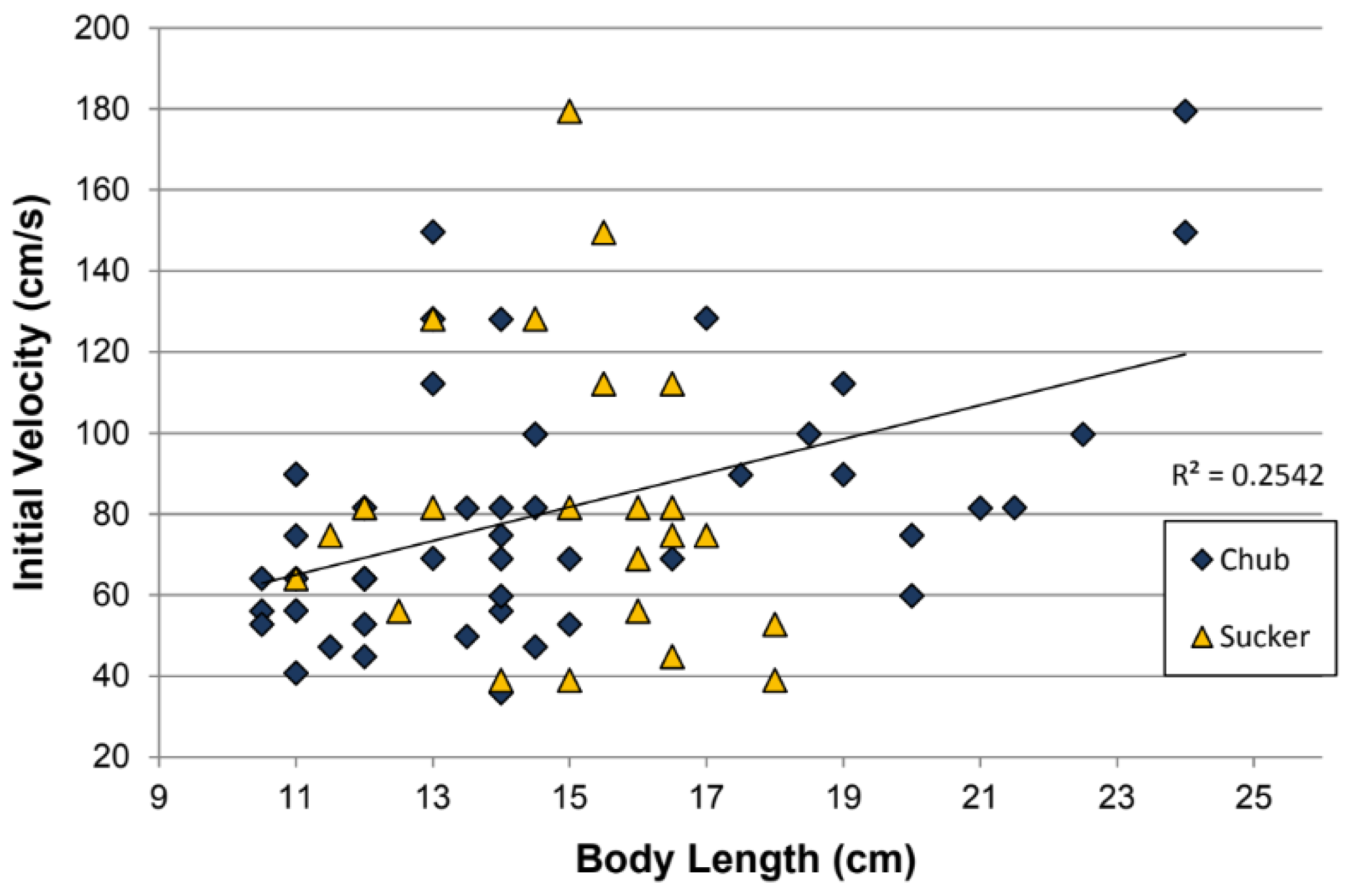
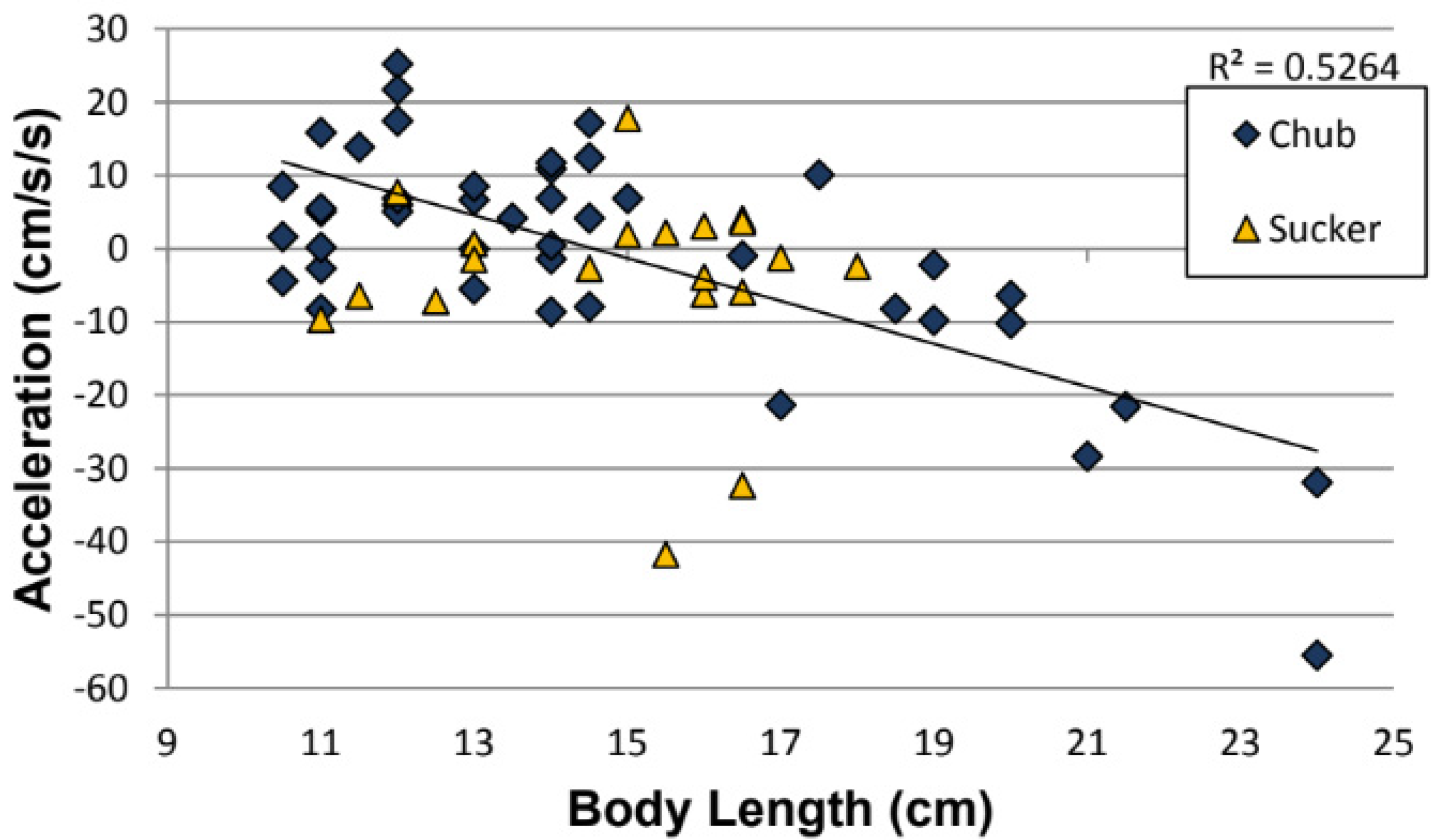
3.1. Effect of Fish Length on Distance Traveled, Initial Velocity, and On-Ramp Acceleration
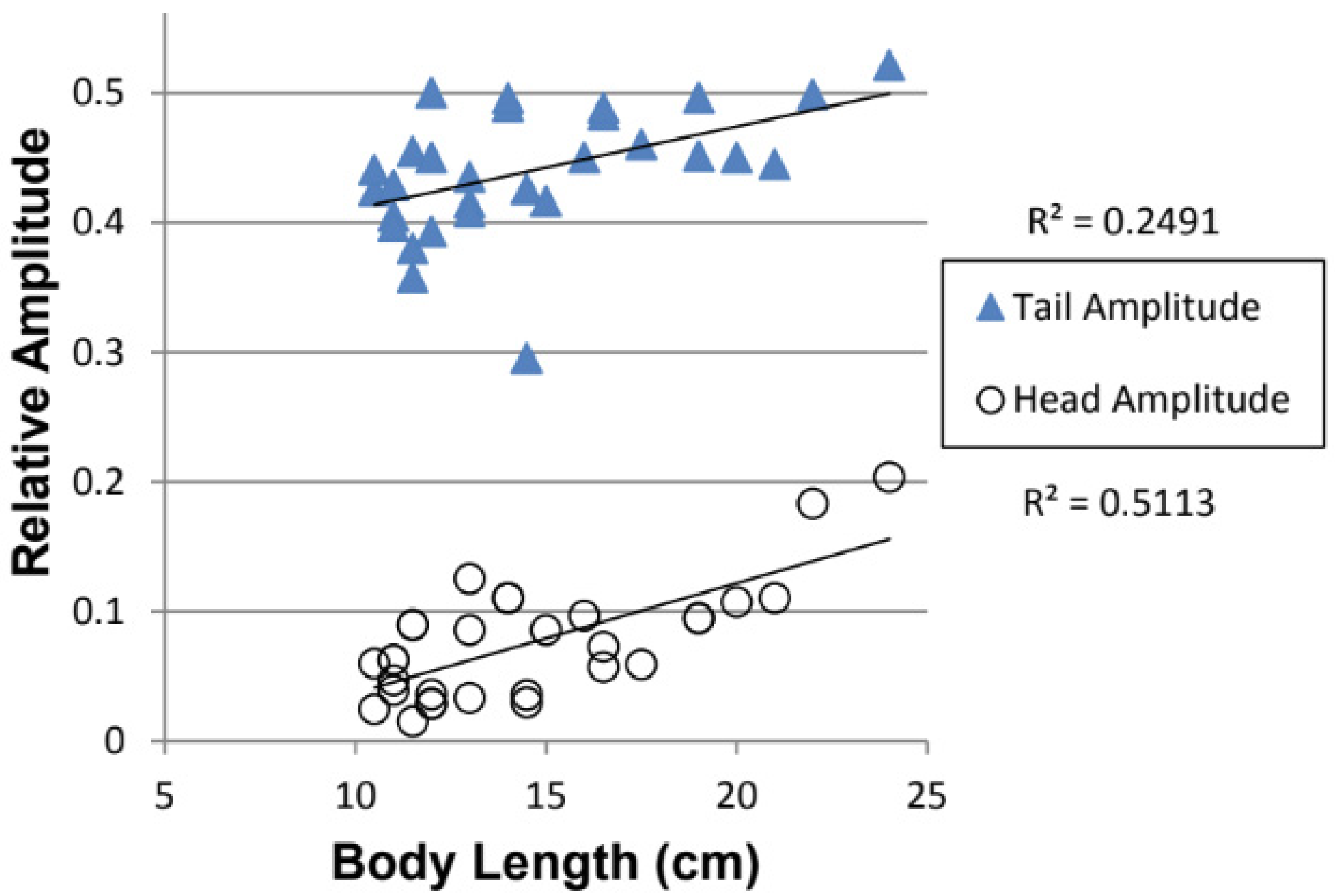
3.2. Effect of Fish Length on Relative Tail and Head Amplitude
3.3. Effect of Temperature on Distance Traveled
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANCOVA | Analysis of Covariance |
| UCrit | Maximum aerobic swim speed |
References
- Bednarek, A.T. Undamming Rivers: A review of the ecological impacts of dam removal. Environ. Manag. 2001, 27, 803–814. [Google Scholar] [CrossRef]
- Pringle, C.M. Hydrologic connectivity and the management of biological reserves: A global perspective. Ecol. Appl. 2001, 11, 981–998. [Google Scholar] [CrossRef]
- Cushman, S.A. Effects of habitat loss and fragmentation on amphibians: A review and prospectus. Biol. Conserv. 2006, 128, 231–240. [Google Scholar] [CrossRef]
- Fahrig, L. Effects of habitat fragmentation on biodiversity. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 487–515. [Google Scholar] [CrossRef]
- Roni, P.; Hanson, K.; Beechie, T. Global review of the physical and biological effectiveness of stream habitat rehabilitation techniques. N. Am. J. Fish Manag. 2008, 28, 856–890. [Google Scholar] [CrossRef]
- Knapp, M.; Haxton, T.; Chu, C. Fish passage hydrodynamics: Insights into overcoming migration challenges for small-bodied fish. J. Ecohydraul. 2019, 4, 43–55. [Google Scholar] [CrossRef]
- Akanyeti, O.; Liao, J.C. The effect of flow speed and body size on Kármán gait kinematics in rainbow trout. J. Exp. Biol. 2013, 216, 3442–3449. [Google Scholar] [CrossRef] [PubMed]
- Castro-Santos, T. Quantifying the combined effects of attempt rate and swimming capacity on passage through velocity barriers. Can. J. Fish. Aquat. Sci. 2004, 61, 1602–1615. [Google Scholar] [CrossRef]
- Haro, A.; Castro-Santos, T.; Noreika, J.; Odeh, M. Swimming performance of upstream migrant fishes in open-channel flow: A new approach to predicting passage through velocity barriers. Can. J. Fish. Aquat. Sci. 2004, 61, 1590–1601. [Google Scholar] [CrossRef]
- Baker, C.F.; Boubée, J.A.T. Upstream passage of inanga Galaxias maculatus and redfin bullies Gobiomorphus huttoni over artificial ramps. J. Fish Biol. 2006, 69, 668–681. [Google Scholar] [CrossRef]
- Calles, E.O.; Greenberg, L.A. Evaluation of nature-like fishways for re-establishing connectivity in fragmented salmonid populations in the river Emån. Riv. Res. Appl. 2005, 21, 951–960. [Google Scholar] [CrossRef]
- Noonan, M.J.; Grant, J.W.A.; Jackson, C.D. A quantitative assessment of fish passage efficiency. Fish Fish. 2012, 13, 450–464. [Google Scholar] [CrossRef]
- Cuenco, M.L.; McCullough, D.A. Framework for Estimating Salmon Survival as a Function of Habitat Conditions; revised ed.; Columbia River Inter-Tribal Fish Commission Technical Report; Columbia River Inter-Tribal Fish Commission: Portland, OR, USA, 1997; Volume 96, 119p.
- Webb, P.W. The effect of size on the fast-start performance of rainbow trout Salmo gairdneri, and a consideration of piscivorous predator-prey interactions. J. Exp. Biol. 1976, 65, 157–177. [Google Scholar] [CrossRef]
- Froese, R. Cube law, condition factor and weight-length relationships: History, meta-analysis and recommendations. J. Appl. Ichthyol. 2006, 22, 241–253. [Google Scholar] [CrossRef]
- Moutopoulos, D.K.; Stergiou, K.I. Length-weight and length-length relationships of fish species from the Aegean Sea (Greece). J. Appl. Ichthyol. 2002, 18, 200–203. [Google Scholar] [CrossRef]
- Amaral, S.D.; Branco, P.; Katopodis, C.; Ferreira, M.T.; Pinheiro, A.N.; Santos, J.M. Passage performance of potamodromous cyprinids over an experimental low-head ramped weir: The effect of ramp length and slope. Sustainability 2019, 11, 1456. [Google Scholar] [CrossRef]
- Sherburne, S.; Reinhardt, U.G. First test of a species-selective adult sea lamprey migration barrier. J. Great Lakes Res. 2016, 42, 893–898. [Google Scholar] [CrossRef]
- Reinhardt, U.G.; Corniuk, J. Wetted ramps selectively block upstream passage of adult sea lampreys. Fishes 2024, 9, 293. [Google Scholar] [CrossRef]
- Cowan, W.R.; Rankin, D.E.; Gard, M. Evaluation of Central Valley spring-run Chinook salmon passage through lower Butte Creek using hydraulic modelling techniques. River Res. Appl. 2017, 33, 328–340. [Google Scholar] [CrossRef]
- Myrick, C.A.; Cech Jr, J.J. Swimming performances of four California stream fishes: Temperature effects. Environ. Biol. Fishes 2000, 58, 289–295. [Google Scholar] [CrossRef]
- Randall, D.J.; Brauner, C.J. Effects of environmental factors on exercise in fish. J. Exp. Biol. 1991, 160, 113–126. [Google Scholar] [CrossRef]
- Wardle, C.S.; Videler, J.J. Fish Swimming. In Aspects of Animal Movement; Elder, H.Y., Trueman, E.R., Eds.; Cambridge University Press: Cambridge, UK, 1980; pp. 113–136. [Google Scholar]
- Brett, J.R. The respiratory metabolism and swimming performance of young sockeye salmon. J. Fish. Res. Board Can. 1964, 21, 1183–1226. [Google Scholar] [CrossRef]
- Riyanto, M.; Yanase, K.; Arimoto, T. Temperature and fatigue effect on the maximum swimming speed of jack mackerel Trachurus japonicus. Fish. Sci. 2014, 80, 53–59. [Google Scholar] [CrossRef]
- Wardle, C.S. Limit of fish swimming speed. Nature 1975, 255, 725–727. [Google Scholar] [CrossRef]
- Webb, P.W. The swimming energetics of trout. I. thrust and power output at cruising speeds. J. Exp. Biol. 1971, 55, 489–520. [Google Scholar] [CrossRef] [PubMed]
- Kane, T.R. Length–weight relationship of hatchery-reared Atlantic salmon. Prog. Fish-Cult. 1988, 50, 28–30. [Google Scholar] [CrossRef]
- Tucker, C.S.; Sommerville, C.; Wootten, R. Does size really matter? effects of fish surface area on the settlement and initial survival of Lepeophtheirus salmonis, an ectoparasite of Atlantic salmon Salmo salar. Dis. Aquat. Organ. 2002, 49, 145–152. [Google Scholar] [CrossRef]
- Jones, R.E.; Petrell, R.J.; Pauly, D. Using modified length–weight relationships to assess the condition of fish. Aquacult. Eng. 1999, 20, 261–276. [Google Scholar] [CrossRef]
- Nagtegaal, D.A.; Candy, J.R.; Riddell, B. A preliminary report on the chinook productivity study conducted on the Cowichan River during 1993. In Canadian Manuscript Report of Fisheries and Aquatic Sciences 2315; Fisheries and Oceans Canada: Ottawa, ON, Canada, 1995; 84p. [Google Scholar]
- Vadas, R.L., Jr. Instream-flow needs for anadromous salmonids and lamprey on the Pacific coast, with special reference to the Pacific Southwest. Environ. Monit. Assess. 2000, 64, 331–358. [Google Scholar] [CrossRef]
- Vadas, R.L., Jr.; Beecher, H.A.; Boessow, S.N.; Kohr, H. Coastal Cutthroat Trout redd counts impacted by natural water supply variations. N. Am. J. Fish. Manag. 2016, 36, 900–912. [Google Scholar] [CrossRef]
- Hertel, H. Structure, Form, Movement; Reinhold Publishing Corporation: New York, NY, USA, 1966; 251p. [Google Scholar]
- Liu, Y.; Jiang, H. Research development on fish swimming. Chin. J. Mech. Eng. 2022, 35, 114. [Google Scholar] [CrossRef]
- Sobenes, C.; Link, O.; Habit, E.; Górski, K.; Tonkin, J.D.; Monsalve, A.; Arismendi, I.; Laborde, A. Critical swimming speed at different temperatures for small-bodied freshwater native riverine fish species. Sci. Rep. 2024, 14, 5355. [Google Scholar] [CrossRef] [PubMed]
- Claireaux, G.; Couturier, C.; Groison, A.L. effect of temperature on maximum swimming speed and cost of transport in juvenile European sea bass (Dicentrarchus labrax). J. Exp. Biol. 2006, 209, 3420–3428. [Google Scholar] [CrossRef] [PubMed]
- Johansen, J.L.; Jones, G.P. Increasing ocean temperature reduces the metabolic performance and swimming ability of coral reef damselfishes. Glob. Change Biol. 2011, 17, 2971–2979. [Google Scholar] [CrossRef]
- Koumoundouros, G.; Sfakianakis, D.G.; Divanach, P.; Kentouri, M. Effect of temperature on swimming performance of sea bass juveniles. J. Fish Biol. 2002, 60, 923–932. [Google Scholar] [CrossRef]
- Reinhardt, U.G.; Binder, T.R.; McDonald, D.G. Ability of adult sea lamprey to climb inclined surfaces. Am. Fish. Soc. Symp. 2009, 72, 125–138. [Google Scholar] [CrossRef]
- Reinhardt, U.G.; Eidietis, L.; Friedl, S.E.; Moser, M. Pacific lamprey climbing behavior. Can. J. Zool. 2008, 86, 1264–1272. [Google Scholar] [CrossRef]
- Great Lakes Fisheries Commission: Fish Pass. Available online: https://www.glfc.org/fishpass.php (accessed on 15 April 2025).
- Kivari, L. The Wetted Ramp as a Useful Tool to Service Smaller-Bodied Finfishes at Low-Head Aquatic Barriers. Master’s Thesis, Eastern Michigan University, Ypsilanti, MI, USA, 2016; 68p. Available online: https://commons.emich.edu/theses/833 (accessed on 20 April 2025).



| Measurement | Definition |
|---|---|
| Distance traveled | The greatest distance the fish’s snout traveled on the ramp plane during one attempt. One replicate in the analysis represents the mean of all observed attempts for an individual fish. |
| Fish Size | Fork length of the fish. |
| Entry Velocity | The number of video frames recorded for travel between two successive 10 cm lines on the ramp, measured between 10 and 20 cm above the water line. |
| Acceleration | Difference in ground speed between successive 10 cm intervals on the ramps. |
| Head Amplitude | The total lateral distance the head moved in one direction plus the total lateral distance moved in the opposite direction during a swim stroke [25]. |
| Tail Amplitude | The total lateral distance the tail moved in one direction plus the total lateral distance moved in the opposite direction during a swim stroke. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reinhardt, U.; Halcrow, G.S. Smaller Body Size and Warmer Water Improve Two Temperate Fishes’ Upstream Passage over Wetted Ramps. Fishes 2025, 10, 401. https://doi.org/10.3390/fishes10080401
Reinhardt U, Halcrow GS. Smaller Body Size and Warmer Water Improve Two Temperate Fishes’ Upstream Passage over Wetted Ramps. Fishes. 2025; 10(8):401. https://doi.org/10.3390/fishes10080401
Chicago/Turabian StyleReinhardt, Uli, and Grace Scott Halcrow. 2025. "Smaller Body Size and Warmer Water Improve Two Temperate Fishes’ Upstream Passage over Wetted Ramps" Fishes 10, no. 8: 401. https://doi.org/10.3390/fishes10080401
APA StyleReinhardt, U., & Halcrow, G. S. (2025). Smaller Body Size and Warmer Water Improve Two Temperate Fishes’ Upstream Passage over Wetted Ramps. Fishes, 10(8), 401. https://doi.org/10.3390/fishes10080401





