Microplastics Induce Structural Color Deterioration in Fish Poecilia reticulata Mediated by Oxidative Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Design
2.3. Sample Collection
2.4. Body Color Measurement
2.5. Accumulation of MPs/NPs in Fish Gill and Gut
2.6. Quality Assurance and Quality Control
2.7. Measurement of Antioxidant Capacity
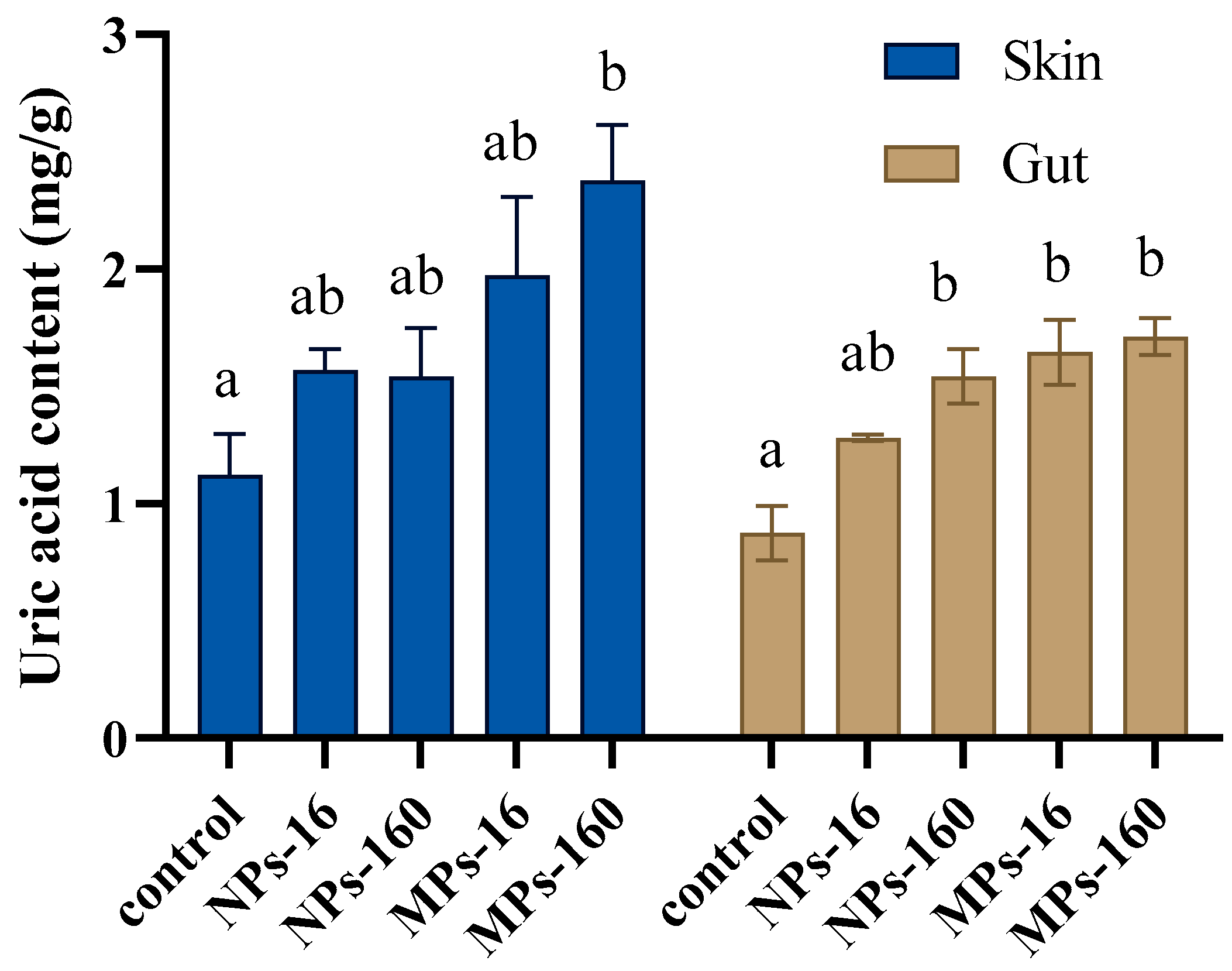
2.8. Determination of Uric Acid
2.9. Data Analysis
3. Results
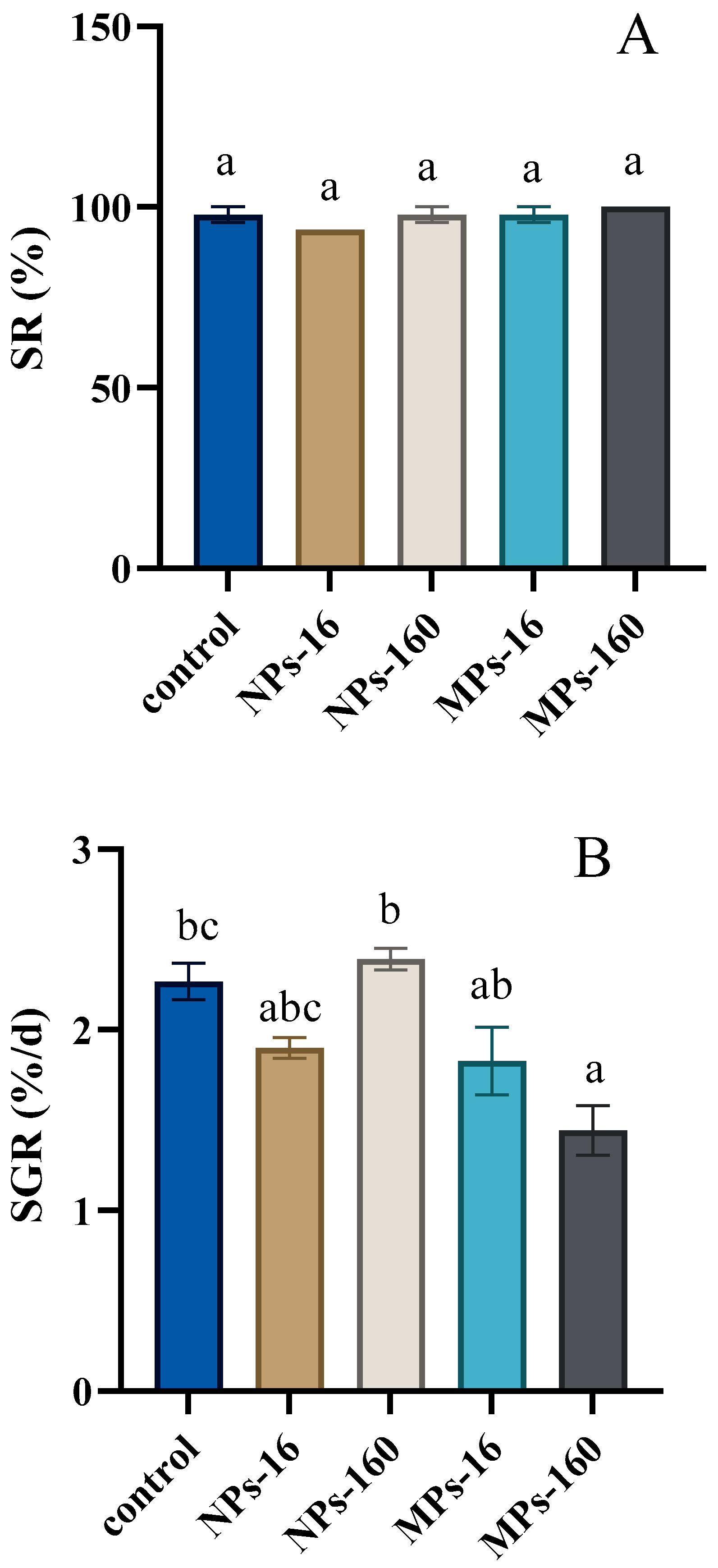
3.1. Growth Performance
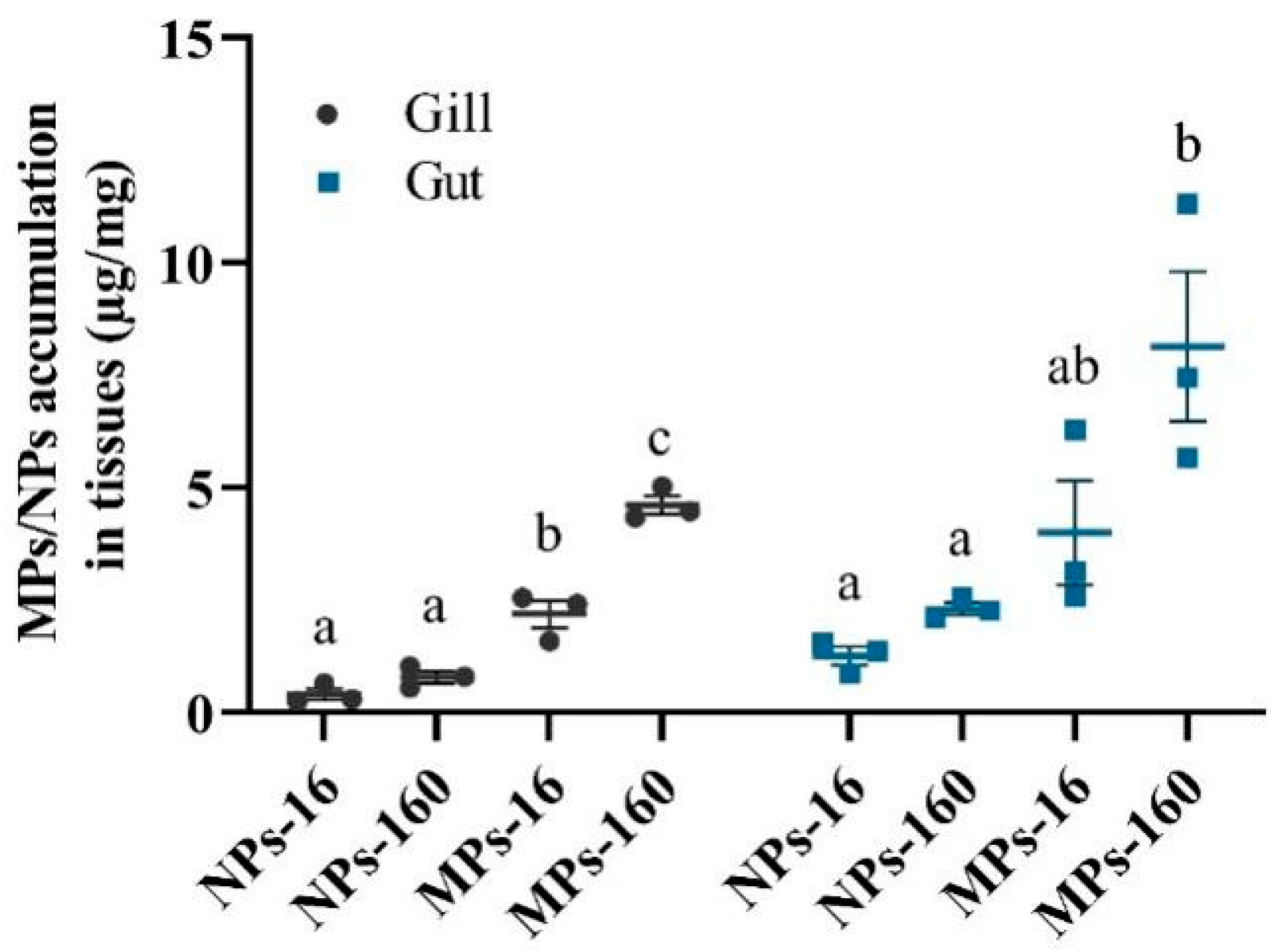
3.2. MPs and NPs Accumulation
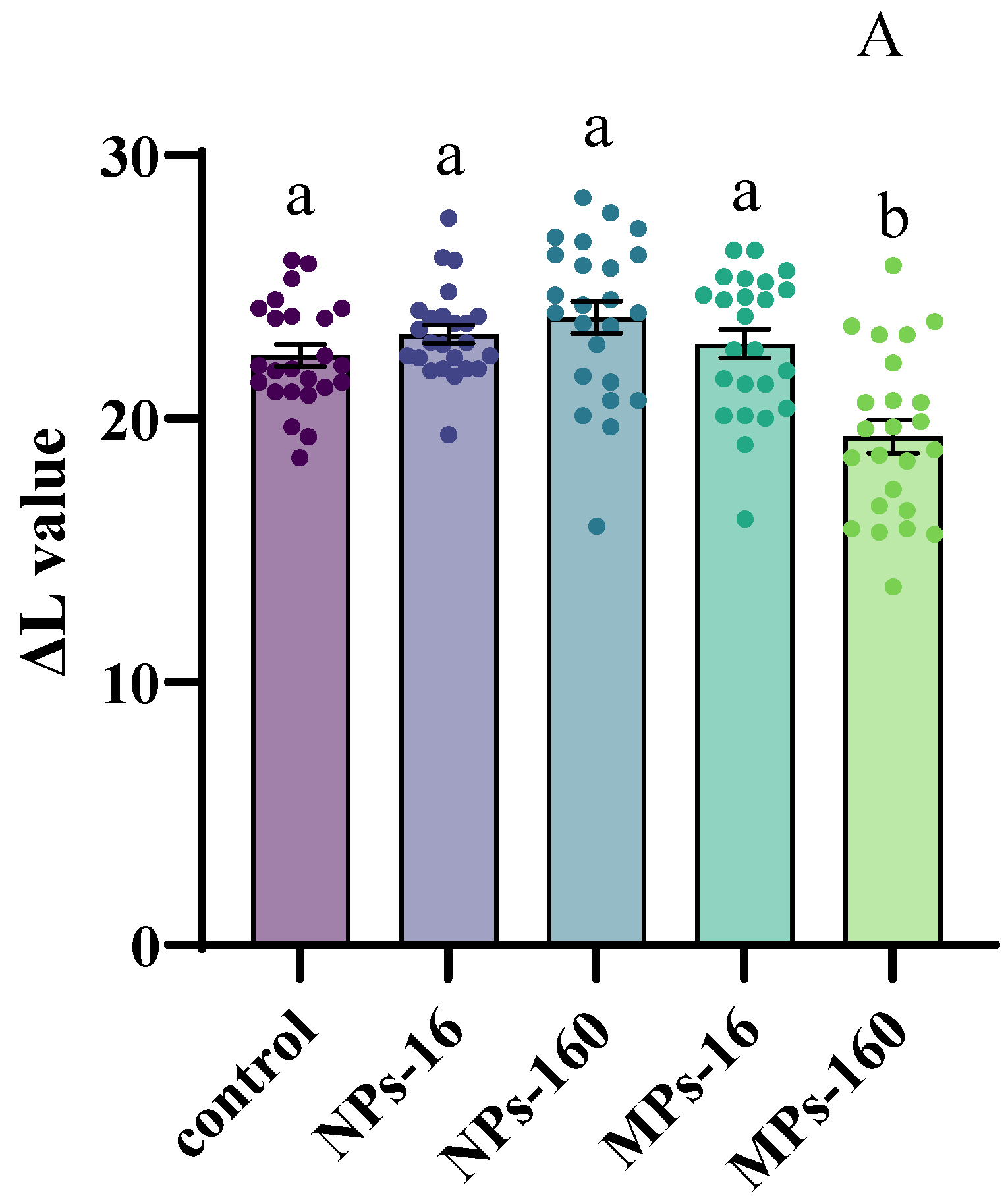
3.3. Change of Body Color
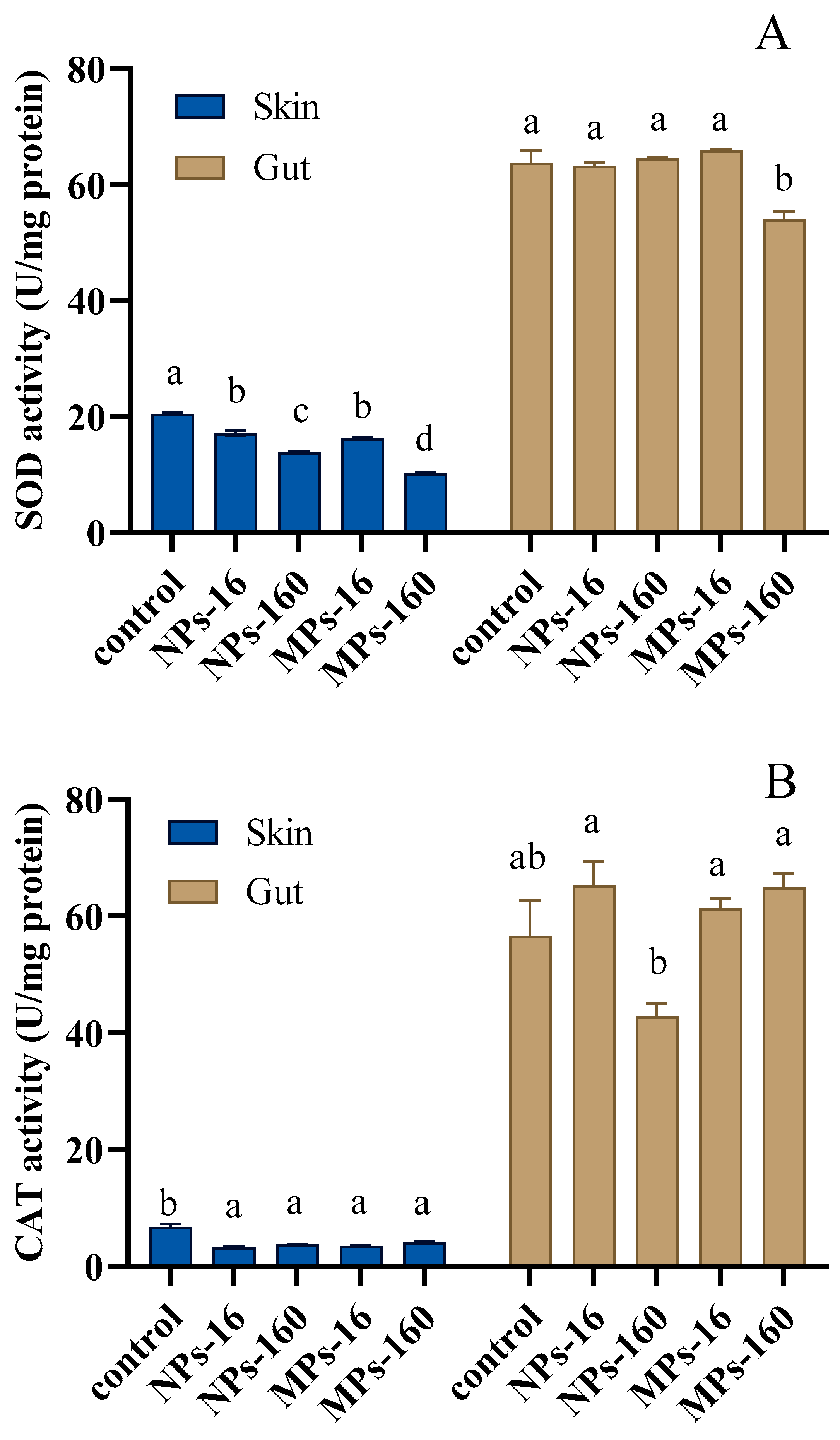
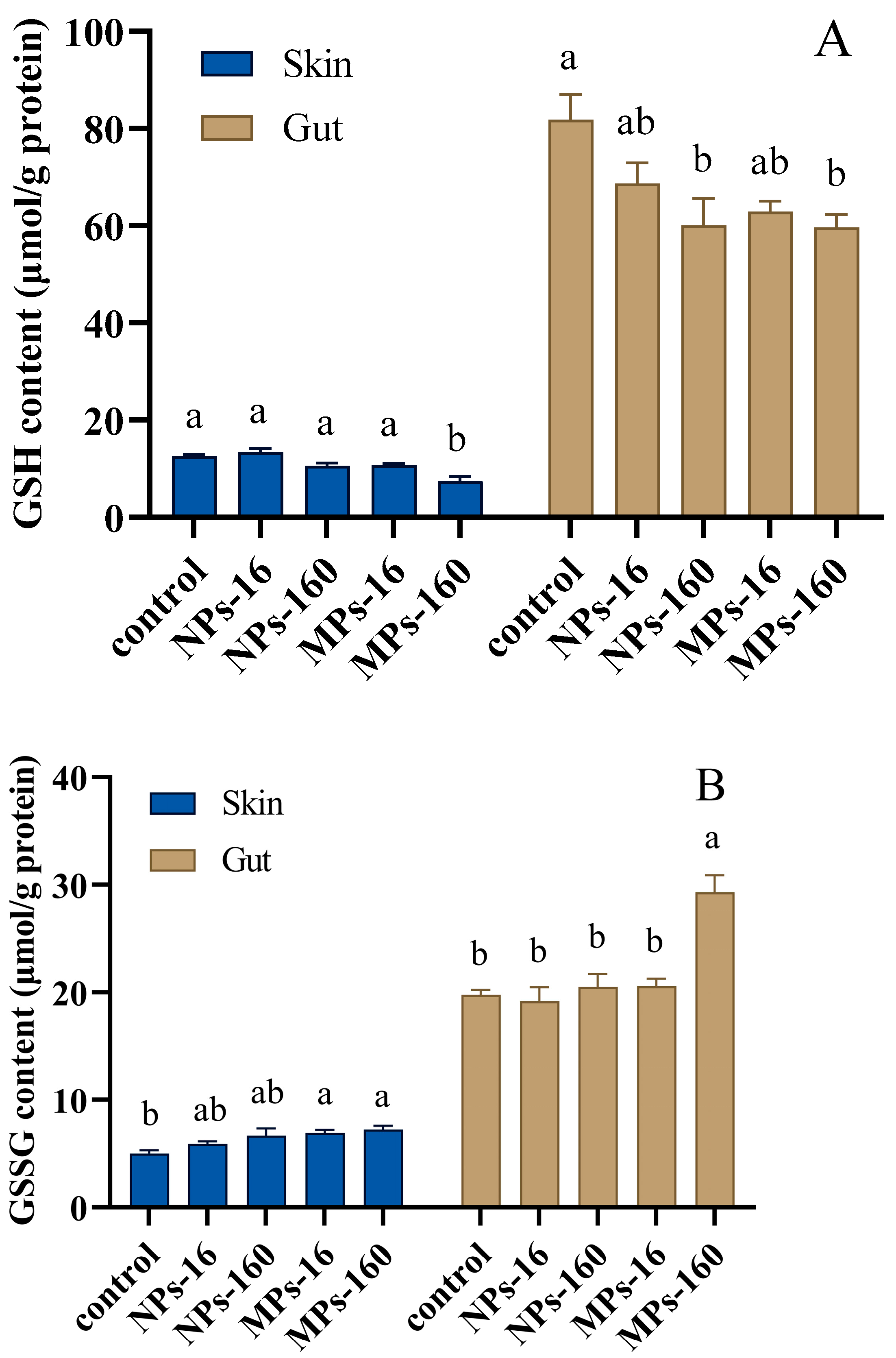
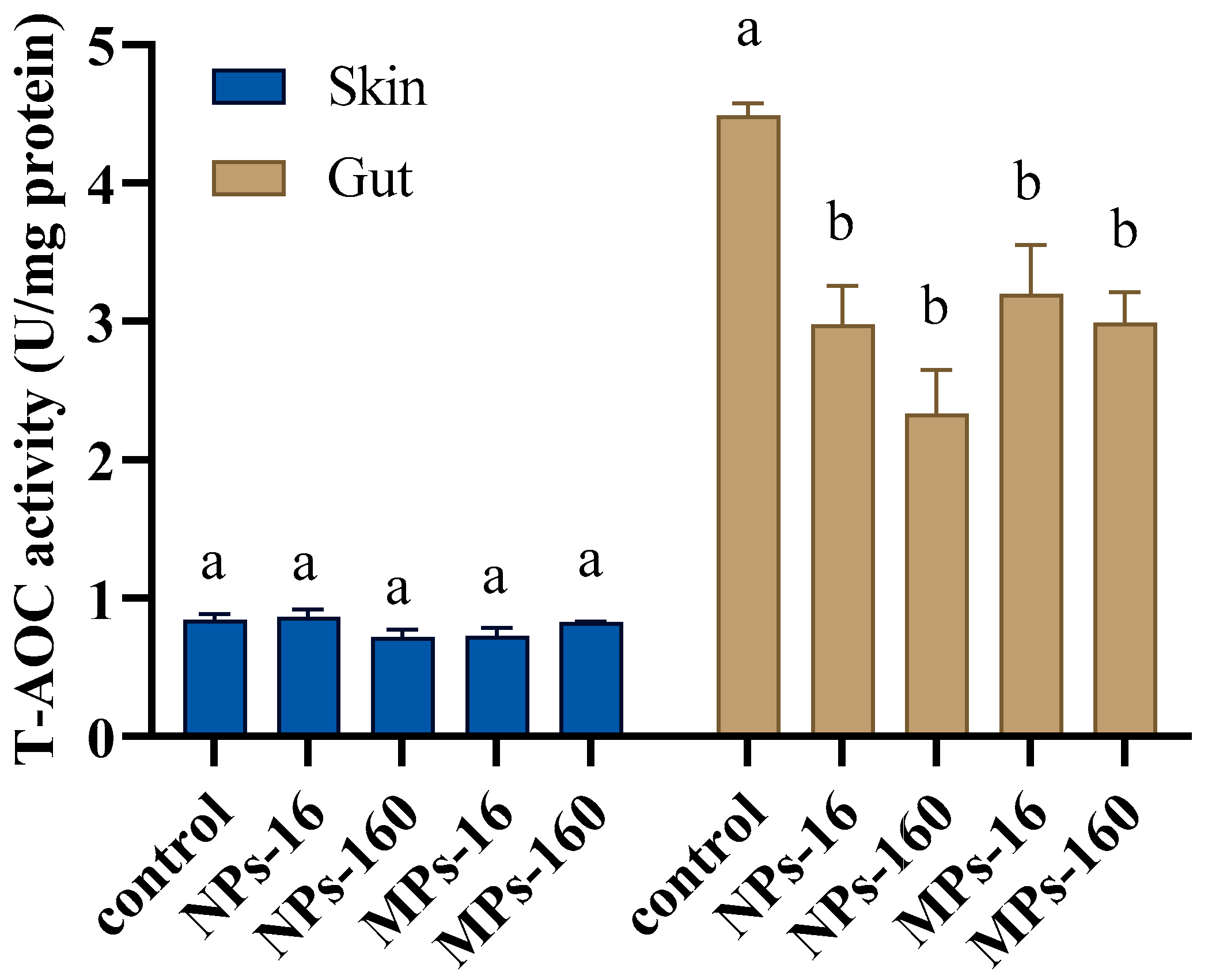
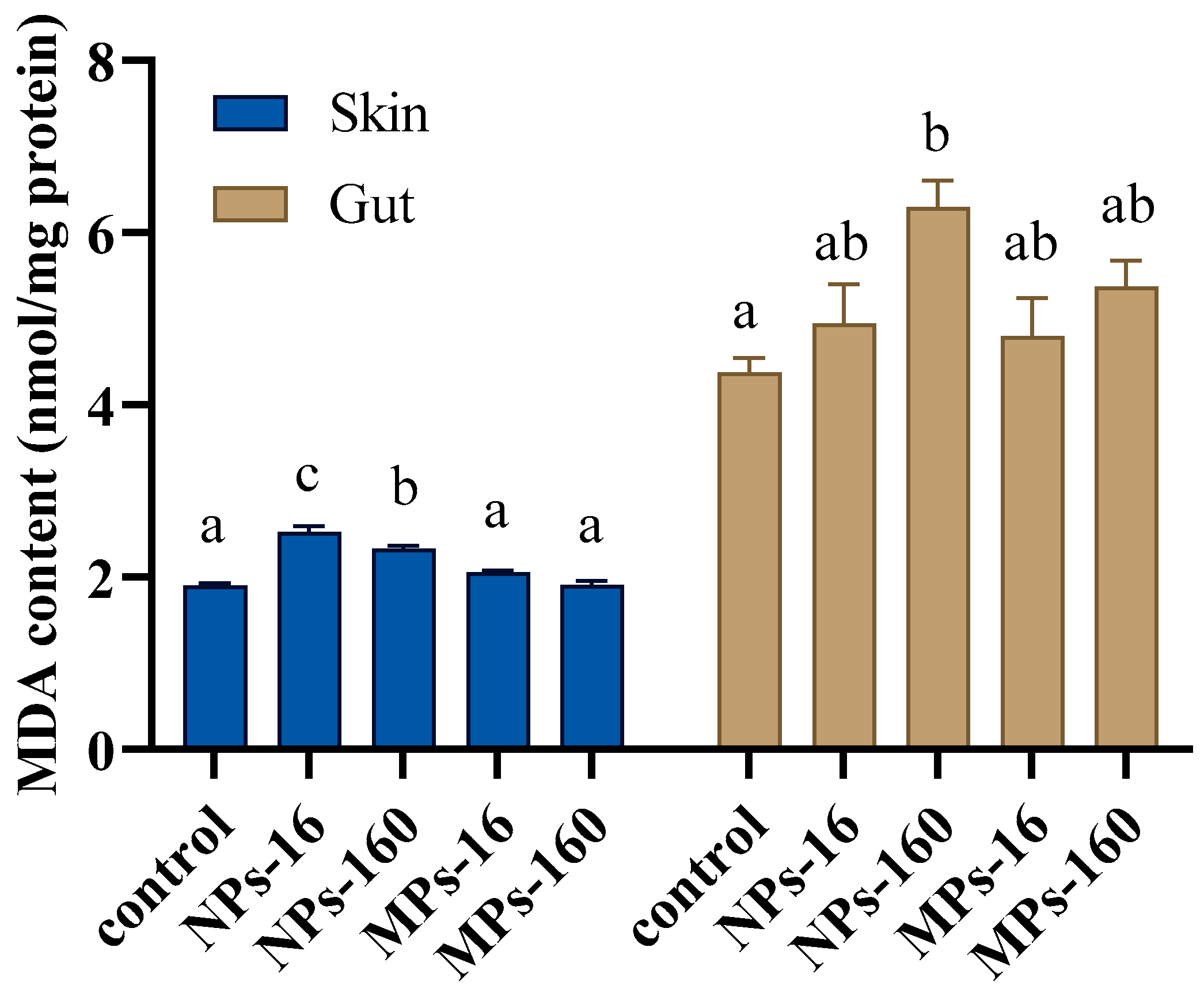
3.4. Antioxidant Enzyme Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cuthill, I.C.; Allen, W.L.; Arbuckle, K.; Caspers, B.; Chaplin, G.; Hauber, M.E.; Hill, G.E.; Jablonski, N.G.; Jiggins, C.D.; Kelber, A.; et al. The biology of color. Science 2017, 357, eaan0221. [Google Scholar] [CrossRef] [PubMed]
- Schartl, M.; Larue, L.; Goda, M.; Bosenberg, M.W.; Hashimoto, H.; Kelsh, R.N. What is a vertebrate pigment cell? Pigment Cell Melanoma Res. 2016, 29, 8–14. [Google Scholar] [CrossRef]
- Braasch, I.; Schartl, M.; Volff, J.N. Evolution of pigment synthesis pathways by gene and genome duplication in fish. BMC Evol. Biol. 2007, 7, 74. [Google Scholar] [CrossRef]
- Nilsson Skold, H.; Aspengren, S.; Wallin, M. Rapid color change in fish and amphibians—function, regulation, and emerging applications. Pigment Cell Melanoma Res. 2013, 26, 29–38. [Google Scholar] [CrossRef]
- Yang, B.T.; Wen, B.; Ji, Y.; Wang, Q.; Zhang, H.R.; Zhang, Y.; Gao, J.Z.; Chen, Z.Z. Comparative metabolomics analysis of pigmentary and structural coloration in discus fish (Symphysodon haraldi). J. Proteom. 2021, 233, 104085. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, I. The pteridine pathway in zebrafish: Regulation and specification during the determination of neural crest cell-fate. Pigment Cell Res. 2003, 16, 172–182. [Google Scholar] [CrossRef]
- Maan, M.E.; Sefc, K.M. Colour variation in cichlid fish: Developmental mechanisms, selective pressures and evolutionary consequences. Semin. Cell. Dev. Biol. 2013, 24, 516–528. [Google Scholar] [CrossRef]
- Kaur, R.; Dua, A. Colour changes in Labeo rohita (Ham.) due to pigment translocation in melanophores, on exposure to municipal wastewater of Tung Dhab drain, Amritsar, India. Environ. Toxicol. Pharmacol. 2015, 39, 747–757. [Google Scholar] [CrossRef]
- Lifshitz, N.; St Clair, C.C. Coloured ornamental traits could be effective and non-invasive indicators of pollution exposure for wildlife. Conserv. Physiol. 2016, 4, cow028. [Google Scholar] [CrossRef]
- Parolini, M.; Iacobuzio, R.; Bassano, B.; Pennati, R.; Saino, N. Melanin-Based Skin Coloration Predicts Antioxidant Capacity in the Brown Trout (Salmo trutta). Physiol. Biochem. Zool. 2018, 91, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Li, Y.; Wang, W.; Wu, P.; Ru, S. Exposure to monocrotophos pesticide during sexual development causes the feminization/demasculinization of the reproductive traits and a reduction in the reproductive success of male guppies (Poecilia reticulata). Toxicol. Appl. Pharmacol. 2012, 263, 163–170. [Google Scholar] [CrossRef]
- Ward, J.L.; Blum, M.J. Exposure to an environmental estrogen breaks down sexual isolation between native and invasive species. Evol. Appl. 2012, 5, 901–912. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.L.; Zhang, C.N.; Li, E.C.; Jin, M.M.; Huang, M.X.; Cui, W.; Lin, Y.Y.; Shi, Y.J. Triphenyltin exposure affects mating behaviors and attractiveness to females during mating in male guppies (Poecilia reticulata). Ecotoxicol. Environ. Saf. 2019, 169, 76–84. [Google Scholar] [CrossRef]
- Lennquist, A.; Lindblad, L.G.E.M.; Hedberg, D.; Kristiansson, E.; Forlin, L. Colour and melanophore function in rainbow trout after long term exposure to the new antifoulant medetomidine. Chemosphere 2010, 80, 1050–1055. [Google Scholar] [CrossRef]
- Cahn, M.D.; Brown, A.C.; Clotfelter, E.D. Guanine-based structural coloration as an indicator of oxidative stress in a cichlid fish. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2015, 323, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, M.C.; Rosenberg, M.; Cheng, L.N. Increased oceanic microplastic debris enhances oviposition in an endemic pelagic insect. Biol. Lett. 2012, 8, 817–820. [Google Scholar] [CrossRef]
- Zhao, S.Y.; Zhu, L.X.; Wang, T.; Li, D.J. Suspended microplastics in the surface water of the Yangtze Estuary System, China: First observations on occurrence, distribution. Mar. Pollut. Bull. 2014, 86, 562–568. [Google Scholar] [CrossRef]
- Luo, W.Y.; Su, L.; Craig, N.J.; Du, F.N.; Wu, C.X.; Shi, H.H. Comparison of microplastic pollution in different water bodies from urban creeks to coastal waters. Environ. Pollut. 2019, 246, 174–182. [Google Scholar] [CrossRef]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef]
- Huang, J.N.; Wen, B.; Meng, L.J.; Li, X.X.; Wang, M.H.; Gao, J.Z.; Chen, Z.Z. Integrated response of growth, antioxidant defense and isotopic composition to microplastics in juvenile guppy (Poecilia reticulata). J. Hazard. Mater. 2020, 399, 10. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Wu, S.; Lu, S.; Liu, M.; Song, Y.; Fu, Z.; Shi, H.; Raley-Susman, K.M.; He, D. Microplastic particles cause intestinal damage and other adverse effects in zebrafish Danio rerio and nematode Caenorhabditis elegans. Sci. Total Environ. 2018, 619–620, 1–8. [Google Scholar] [CrossRef]
- Besseling, E.; Wang, B.; Lurling, M.; Koelmans, A.A. Nanoplastic affects growth of S. obliquus and reproduction of D. magna. Environ. Sci. Technol. 2014, 48, 12336–12343. [Google Scholar] [CrossRef]
- Huang, J.N.; Zhang, Y.; Xu, L.; He, K.X.; Wen, B.; Yang, P.W.; Ding, J.Y.; Li, J.Z.; Ma, H.C.; Gao, J.Z.; et al. Microplastics: A tissue-specific threat to microbial community and biomarkers of discus fish (Symphysodon aequifasciatus). J. Hazard. Mater. 2022, 424, 14. [Google Scholar] [CrossRef]
- Huang, J.N.; Wen, B.; Li, X.X.; Xu, L.; Gao, J.Z.; Chen, Z.Z. Astaxanthin mitigates oxidative stress caused by microplastics at the expense of reduced skin pigmentation in discus fish. Sci. Total Environ. 2023, 874, 162494. [Google Scholar] [CrossRef]
- Brown, G.B.; Roll, P.M. The utilization of adenine for nucleic acid synthesis and as a precursor of guanine. J. Biol. Chem. 1948, 172, 469–484. [Google Scholar] [CrossRef] [PubMed]
- San-Jose, L.M.; Granado-Lorencio, F.; Sinervo, B.; Fitze, P.S. Iridophores and Not Carotenoids Account for Chromatic Variation of Carotenoid-Based Coloration in Common Lizards (Lacerta vivipara). Am. Nat. 2013, 181, 396–409. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, M.Y.; Feng, X.S.; Li, X.X.; Wen, B.; Liu, J.H.; Huang, J.N.; Gao, J.Z.; Chen, Z.Z. Microplastics intake and excretion: Resilience of the intestinal microbiota but residual growth inhibition in common carp. Chemosphere 2021, 276, 130144. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Niu, X.; Zhang, D.; Lu, L.; Ye, X.; Deng, W.; Li, Y.; Lin, Z. High levels of microplastic pollution in aquaculture water of fish ponds in the Pearl River Estuary of Guangzhou, China. Sci. Total Environ. 2020, 744, 140679. [Google Scholar] [CrossRef]
- Huang, J.-N.; Xu, L.; Wen, B.; Gao, J.Z.; Chen, Z.Z. Characteristics and risks of microplastic contamination in aquaculture ponds near the Yangtze Estuary, China. Environ. Pollut. 2024, 343, 123288. [Google Scholar] [CrossRef]
- Materic, D.; Kasper-Giebl, A.; Kau, D.; Anten, M.; Greilinger, M.; Ludewig, E.; van Sebille, E.; Rockmann, T.; Holzinger, R. Micro- and Nanoplastics in Alpine Snow: A New Method for Chemical Identification and (Semi)Quantification in the Nanogram Range. Environ. Sci. Technol. 2020, 54, 2353–2359. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, Y.; Jiao, Y.; Chen, Q.; Wu, D.; Yu, P.; Li, Y.; Cai, M.; Zhao, Y. Polystyrene nanoplastic induces ROS production and affects the MAPK-HIF-1/NFkB-mediated antioxidant system in Daphnia pulex. Aquat. Toxicol. 2020, 220, 105420. [Google Scholar] [CrossRef]
- Zuo, J.; Huo, T.; Du, X.; Yang, Q.; Wu, Q.; Shen, J.; Liu, C.; Hung, T.-C.; Yan, W.; Li, G. The joint effect of parental exposure to microcystin-LR and polystyrene nanoplastics on the growth of zebrafish offspring. J. Hazard. Mater. 2021, 410, 124677. [Google Scholar] [CrossRef]
- Huang, J.N.; Wen, B.; Xu, L.; Ma, H.C.; Li, X.X.; Gao, J.Z.; Chen, Z.Z. Micro/nano-plastics cause neurobehavioral toxicity in discus fish (Symphysodon aequifasciatus): Insight from brain-gut-microbiota axis. J. Hazard. Mater. 2022, 421, 126830. [Google Scholar] [CrossRef]
- Gómez-Polo, C.; Muñoz, M.P.; Lorenzo Luengo, M.C.; Vicente, P.; Galindo, P.; Martín Casado, A.M. Comparison of the CIELab and CIEDE2000 color difference formulas. J. Prosthet. Dent. 2016, 115, 65–70. [Google Scholar] [CrossRef]
- Karami, A.; Golieskardi, A.; Choo, C.K.; Romano, N.; Ho, Y.B.; Salamatinia, B. A high-performance protocol for extraction of microplastics in fish. Sci. Total Environ. 2017, 578, 485–494. [Google Scholar] [CrossRef]
- Sinha Ray, S.; Zumr, D.; Wilken, F.; Dostál, T.; Fiener, P. A cost-effective protocol for detecting fluorescent microplastics in arable soils to study redistribution processes. Polym. Test. 2025, 147, 108824. [Google Scholar] [CrossRef]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef] [PubMed]
- van Franeker, J.A.; Blaize, C.; Danielsen, J.; Fairclough, K.; Gollan, J.; Guse, N.; Hansen, P.L.; Heubeck, M.; Jensen, J.K.; Le Guillou, G.; et al. Monitoring plastic ingestion by the northern fulmar Fulmarus glacialis in the North Sea. Environ. Pollut. 2011, 159, 2609–2615. [Google Scholar] [CrossRef]
- Ghosh, T. Microplastics bioaccumulation in fish: Its potential toxic effects on hematology, immune response, neurotoxicity, oxidative stress, growth, and reproductive dysfunction. Toxicol. Rep. 2025, 14, 101854. [Google Scholar] [CrossRef]
- Nuamah, F.; Tulashie, S.K.; Debrah, J.S.; Pèlèbè, R.O.E. Microplastics in the Gulf of Guinea: An analysis of concentrations and distribution in sediments, gills, and guts of fish collected off the coast of Ghana. Environ. Res. 2023, 234, 9. [Google Scholar] [CrossRef] [PubMed]
- Sohal, R.S.; Weindruch, R. Oxidative stress, caloric restriction, and aging. Science 1996, 273, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Qiao, R.; Deng, Y.; Zhang, S.; Wolosker, M.B.; Zhu, Q.; Ren, H.; Zhang, Y. Accumulation of different shapes of microplastics initiates intestinal injury and gut microbiota dysbiosis in the gut of zebrafish. Chemosphere 2019, 236, 124334. [Google Scholar] [CrossRef]
- Wang, X.; Huang, W.; Wei, S.; Shang, Y.; Gu, H.; Wu, F.; Lan, Z.; Hu, M.; Shi, H.; Wang, Y. Microplastics impair digestive performance but show little effects on antioxidant activity in mussels under low pH conditions. Environ. Pollut. 2020, 258, 113691. [Google Scholar] [CrossRef]
- Pandey, S.; Parvez, S.; Sayeed, I.; Haque, R.; Bin-Hafeez, B.; Raisuddin, S. Biomarkers of oxidative stress: A comparative study of river Yamuna fish Wallago attu (Bl. & Schn.). Sci. Total Environ. 2003, 309, 105–115. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Kono, Y. Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch. Biochem. Biophys. 1978, 186, 189–195. [Google Scholar] [CrossRef]
- Kono, Y.; Fridovich, I. Superoxide radical inhibits catalase. J. Biol. Chem. 1982, 257, 5751–5754. [Google Scholar] [CrossRef]
- Jones, D.P. [11] Redox potential of GSH/GSSG couple: Assay and biological significance. In Methods in Enzymology; Sies, H., Packer, L., Eds.; Academic Press: Cambridge, MA, USA, 2002; Volume 348, pp. 93–112. [Google Scholar]
- Jia, R.; Cao, L.-P.; Du, J.-L.; Wang, J.-H.; Liu, Y.-J.; Jeney, G.; Xu, P.; Yin, G.-J. Effects of carbon tetrachloride on oxidative stress, inflammatory response and hepatocyte apoptosis in common carp (Cyprinus carpio). Aquat. Toxicol. 2014, 152, 11–19. [Google Scholar] [CrossRef]
- Ziajahromi, S.; Kumar, A.; Neale, P.A.; Leusch, F.D.L. Environmentally relevant concentrations of polyethylene microplastics negatively impact the survival, growth and emergence of sediment-dwelling invertebrates. Environ. Pollut. 2018, 236, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, W.M.; Bolan, N.S.; Tsang, D.C.W.; Li, Y.; Qin, M.; Hou, D. Environmental fate, toxicity and risk management strategies of nanoplastics in the environment: Current status and future perspectives. J. Hazard. Mater. 2021, 401, 123415. [Google Scholar] [CrossRef] [PubMed]
- Leibovitz, B.E.; Siegel, B.V. Aspects of Free Radical Reactions in Biological Systems: Aging1. J. Gerontol. 1980, 35, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Sautin, Y.Y.; Johnson, R.J. Uric Acid: The Oxidant-Antioxidant Paradox. Nucleosides Nucleotides Nucleic Acids 2008, 27, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Ciereszko, A.; Dabrowski, K.; Kucharczyk, D.; Dobosz, S.; Goryczko, K.; Glogowski, J. The presence of uric acid, an antioxidantive substance, in fish seminal plasma. Fish Physiol. Biochem. 1999, 21, 313–315. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, H.-Y.; Ma, H.-C.; He, R.-P.; Gao, C.-C.; Wen, B.; Gao, J.-Z.; Chen, Z.-Z. Microplastics Induce Structural Color Deterioration in Fish Poecilia reticulata Mediated by Oxidative Stress. Fishes 2025, 10, 382. https://doi.org/10.3390/fishes10080382
Ren H-Y, Ma H-C, He R-P, Gao C-C, Wen B, Gao J-Z, Chen Z-Z. Microplastics Induce Structural Color Deterioration in Fish Poecilia reticulata Mediated by Oxidative Stress. Fishes. 2025; 10(8):382. https://doi.org/10.3390/fishes10080382
Chicago/Turabian StyleRen, Hong-Yu, Huan-Chao Ma, Rui-Peng He, Cong-Cong Gao, Bin Wen, Jian-Zhong Gao, and Zai-Zhong Chen. 2025. "Microplastics Induce Structural Color Deterioration in Fish Poecilia reticulata Mediated by Oxidative Stress" Fishes 10, no. 8: 382. https://doi.org/10.3390/fishes10080382
APA StyleRen, H.-Y., Ma, H.-C., He, R.-P., Gao, C.-C., Wen, B., Gao, J.-Z., & Chen, Z.-Z. (2025). Microplastics Induce Structural Color Deterioration in Fish Poecilia reticulata Mediated by Oxidative Stress. Fishes, 10(8), 382. https://doi.org/10.3390/fishes10080382






