Enhancing African Catfish (Clarias gariepinus) Aquaculture in Uganda: Insights into Hatchery Propagation, Population Suitability, and Broodstock Management
Abstract
1. Introduction
2. Materials and Methods
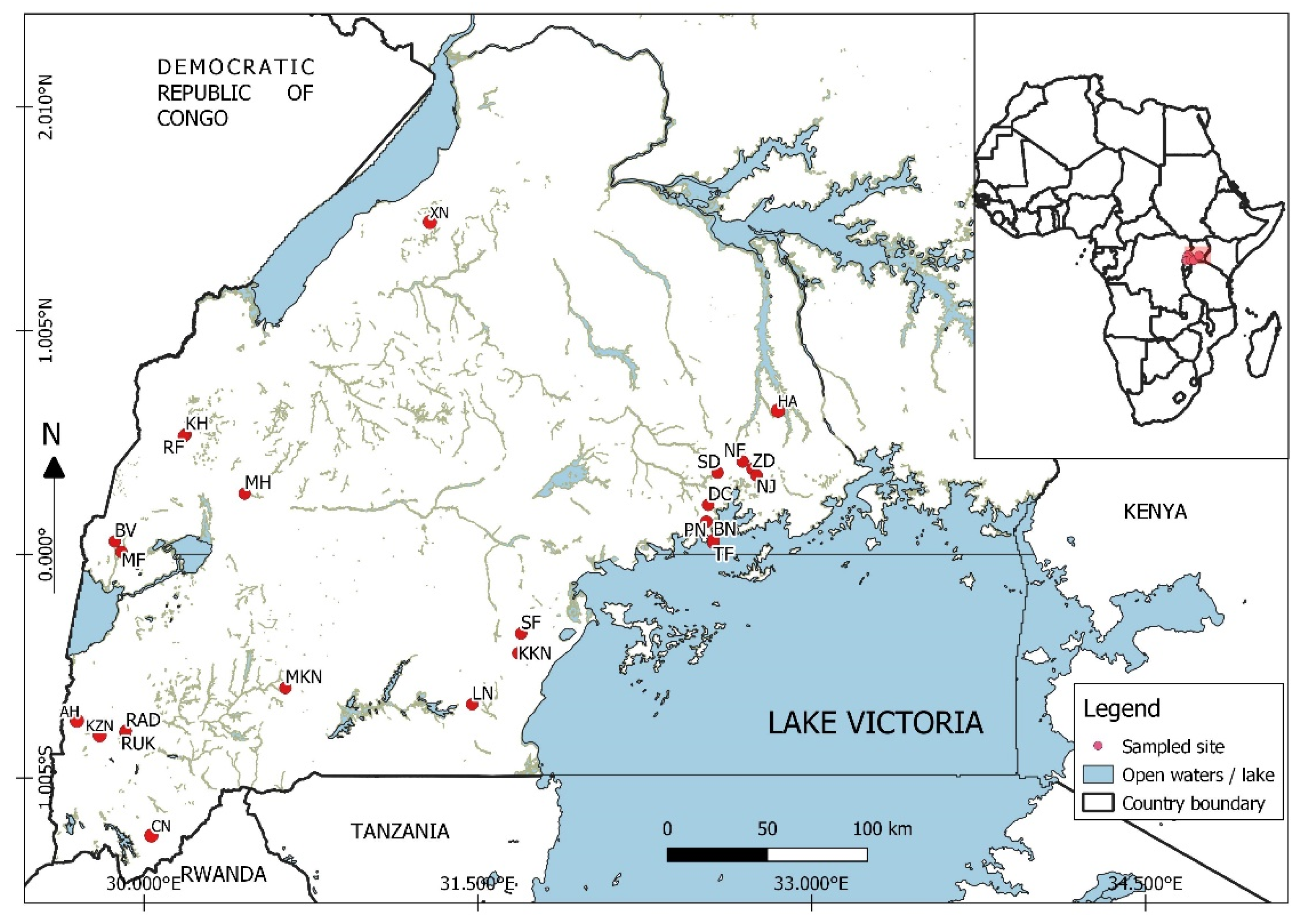
2.1. Description of the Study Area
2.2. Field Survey
2.3. Sampling Technique
2.4. Quality Check
2.5. Ethical Approach
2.6. Potential Biases and Bias Control Measures (BCM)
2.7. Statistical Analysis
2.8. Determination of Effective Breeding Number (Ne) and Average Inbreeding Coefficient (ΔF)
3. Result
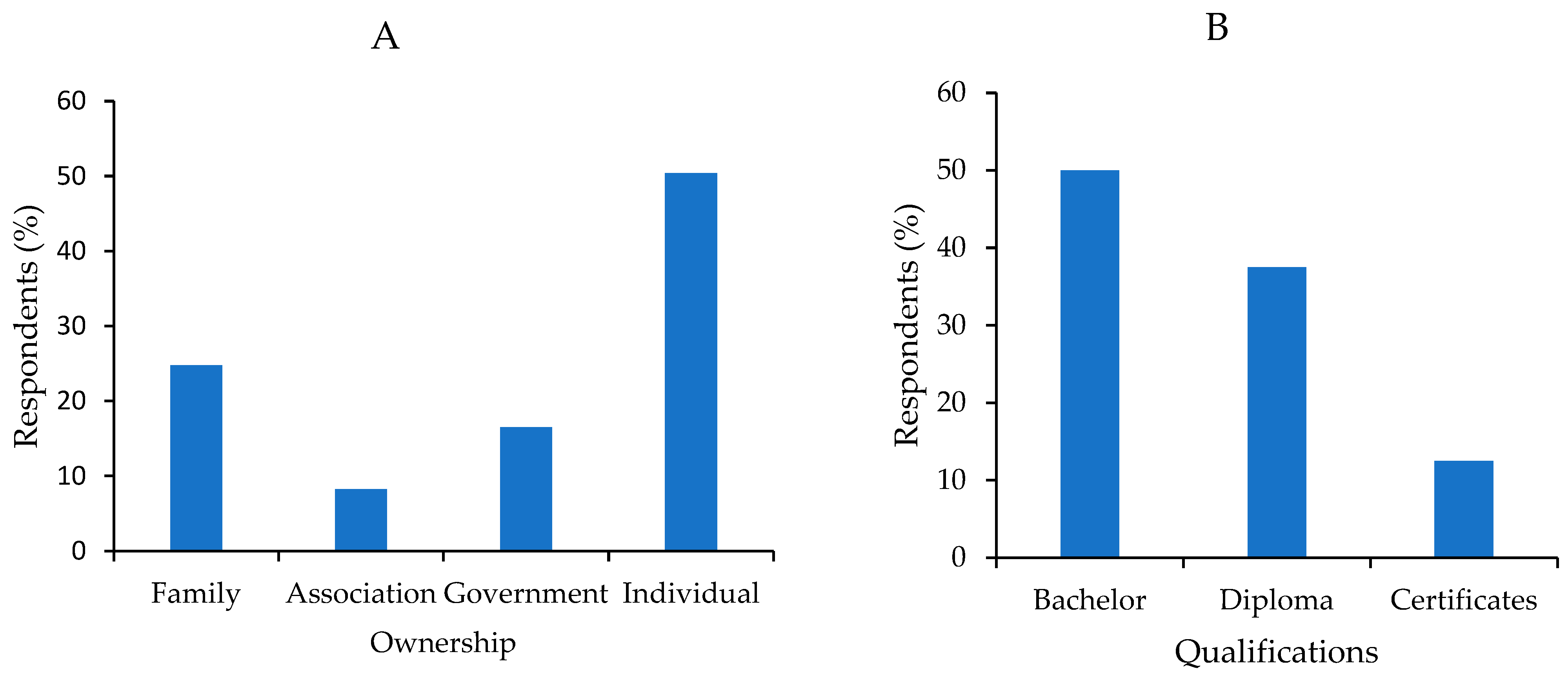
3.1. Broodstock Source and Production Systems
3.2. Broodstock Evaluation and Replacement Strategies
3.3. Effective Breeding Number (Ne) and Average Inbreeding Coefficient (F)
3.4. Induction and Spawning of the Broodstock
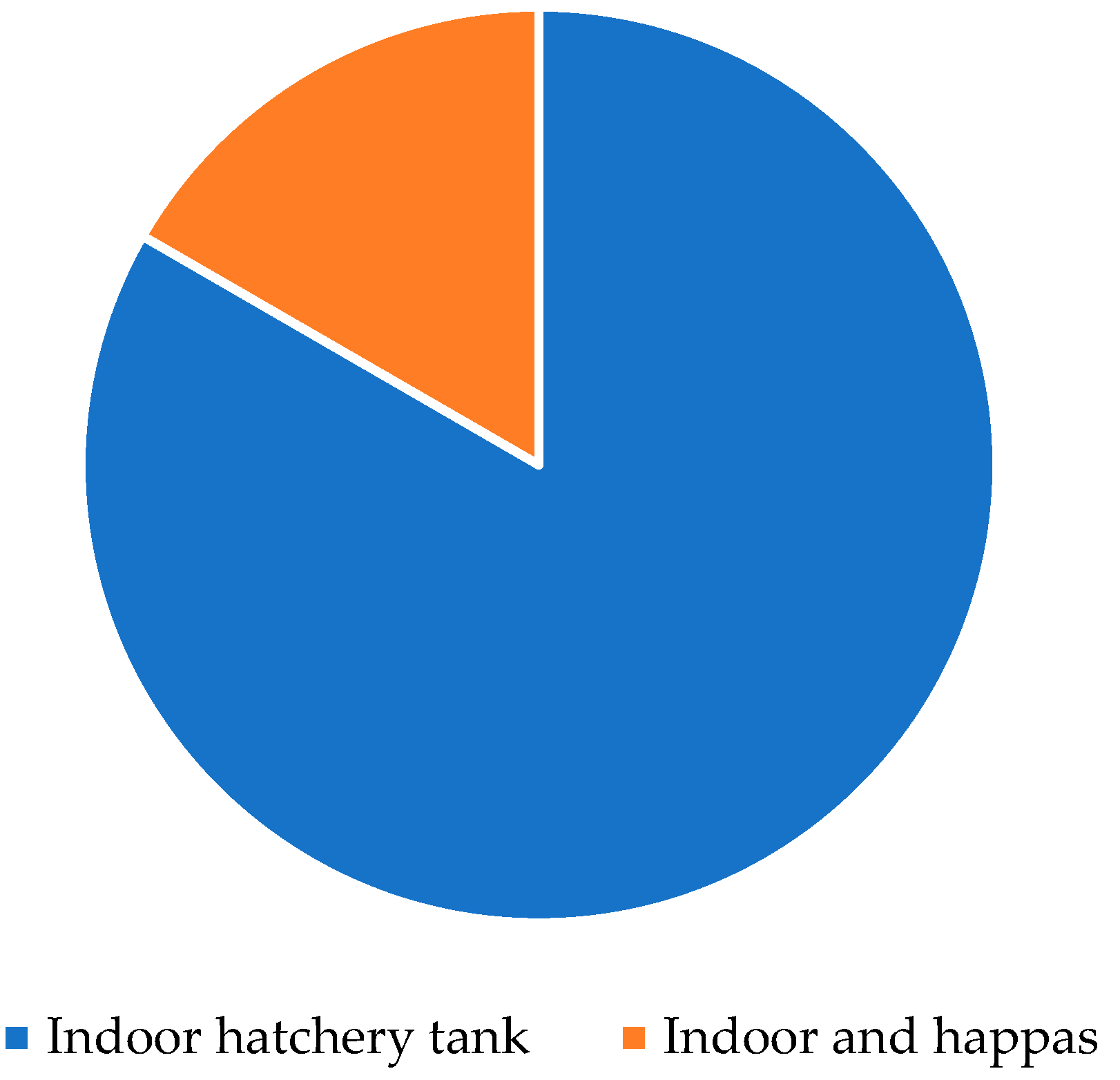
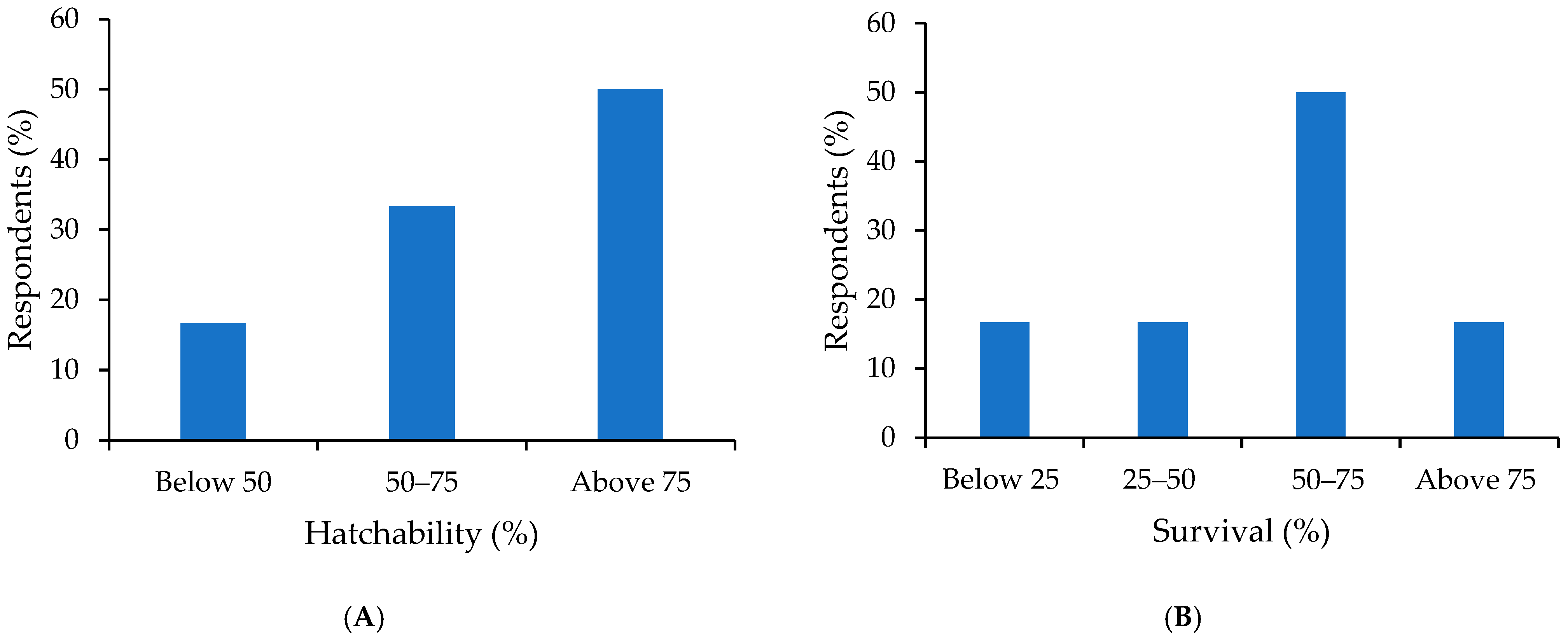
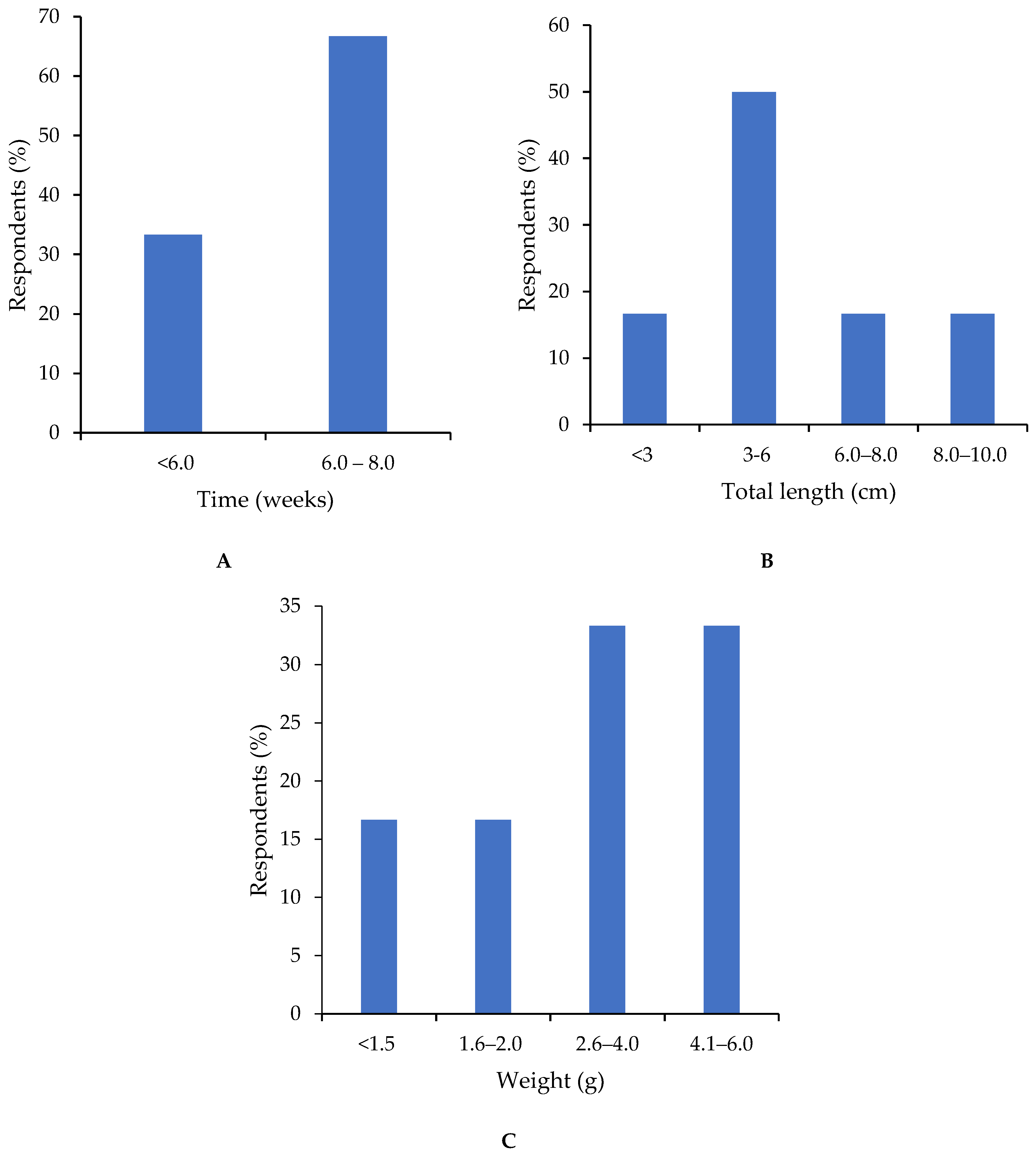
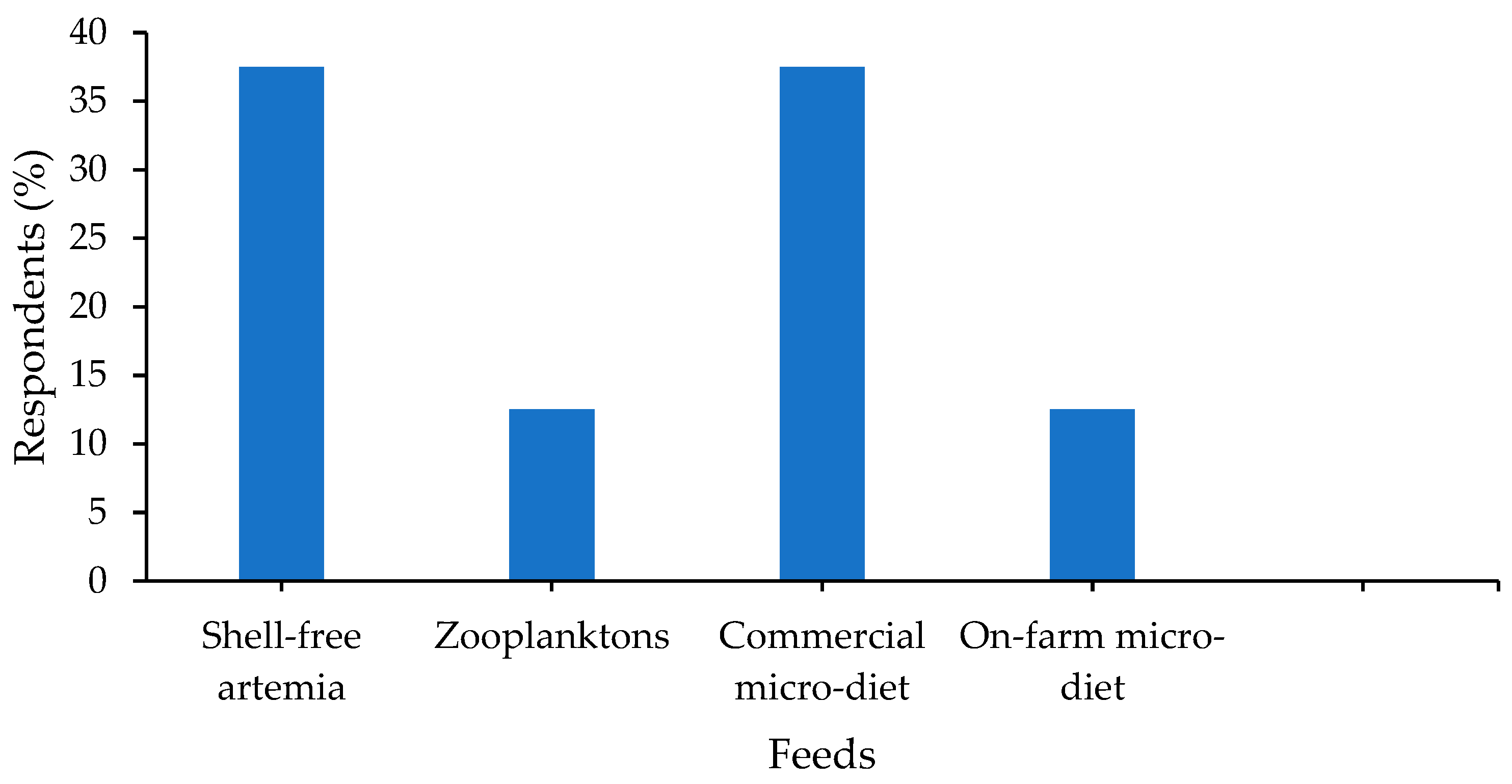
3.5. Hatchery Management
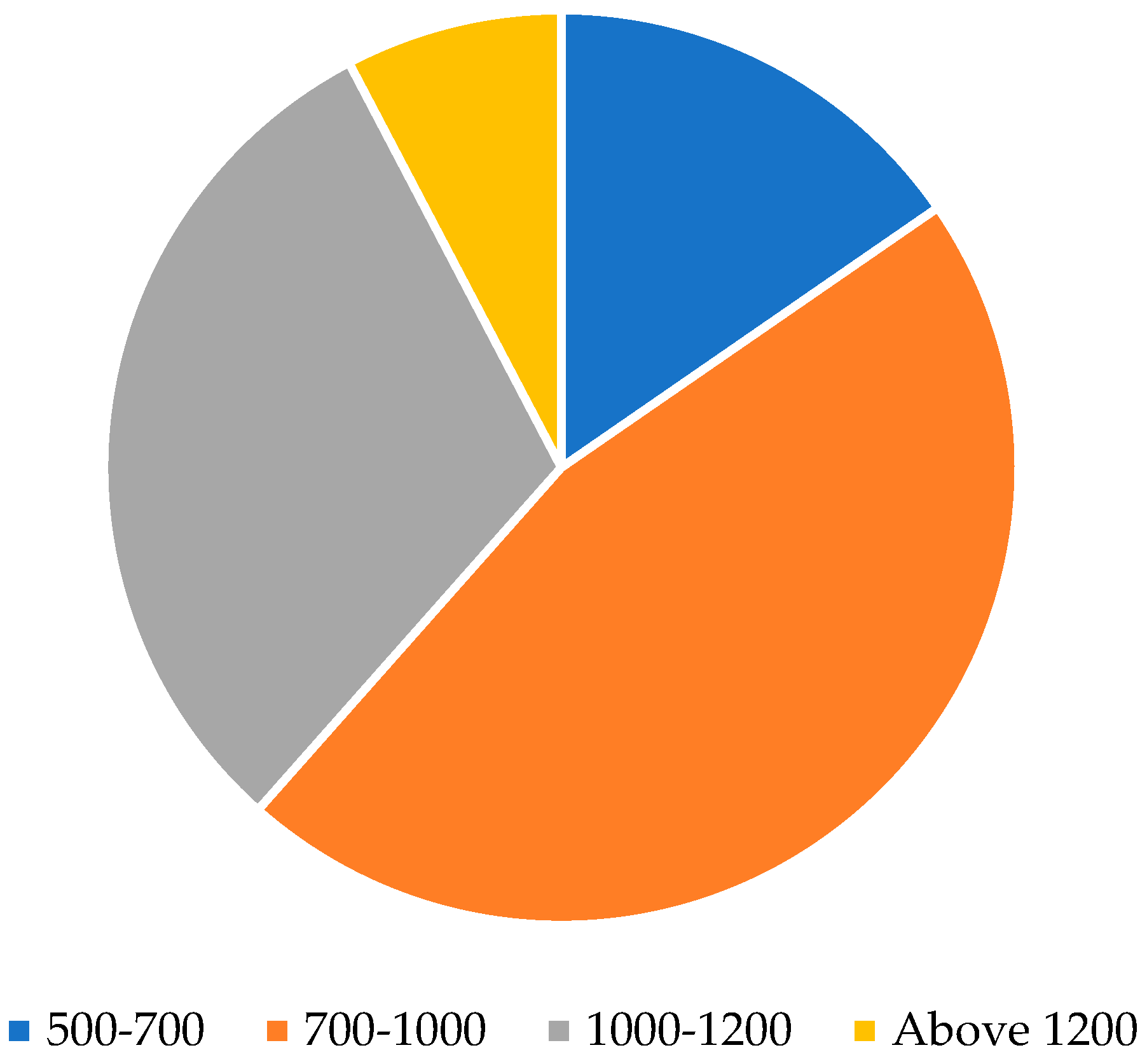
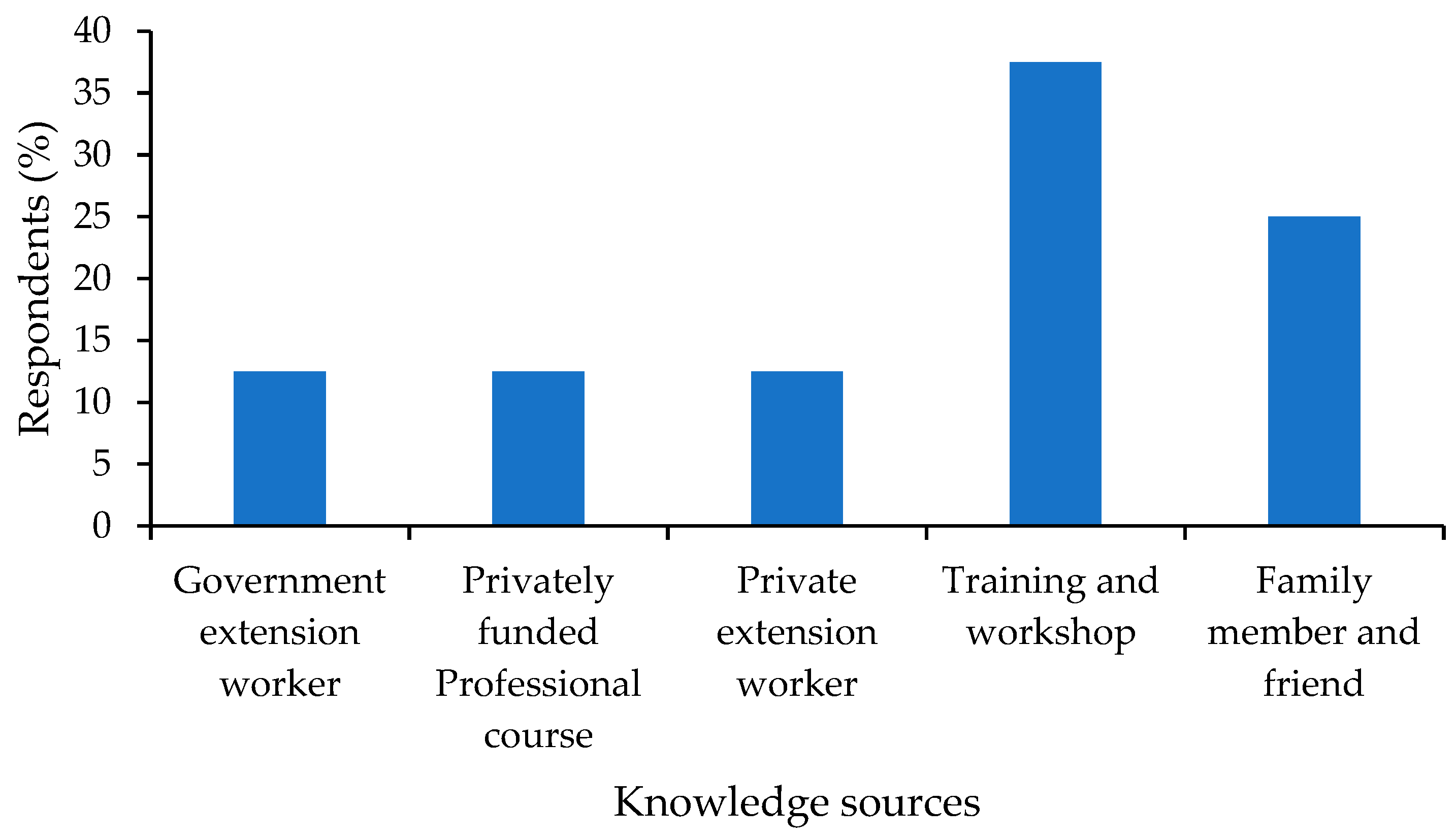
3.6. Demographics
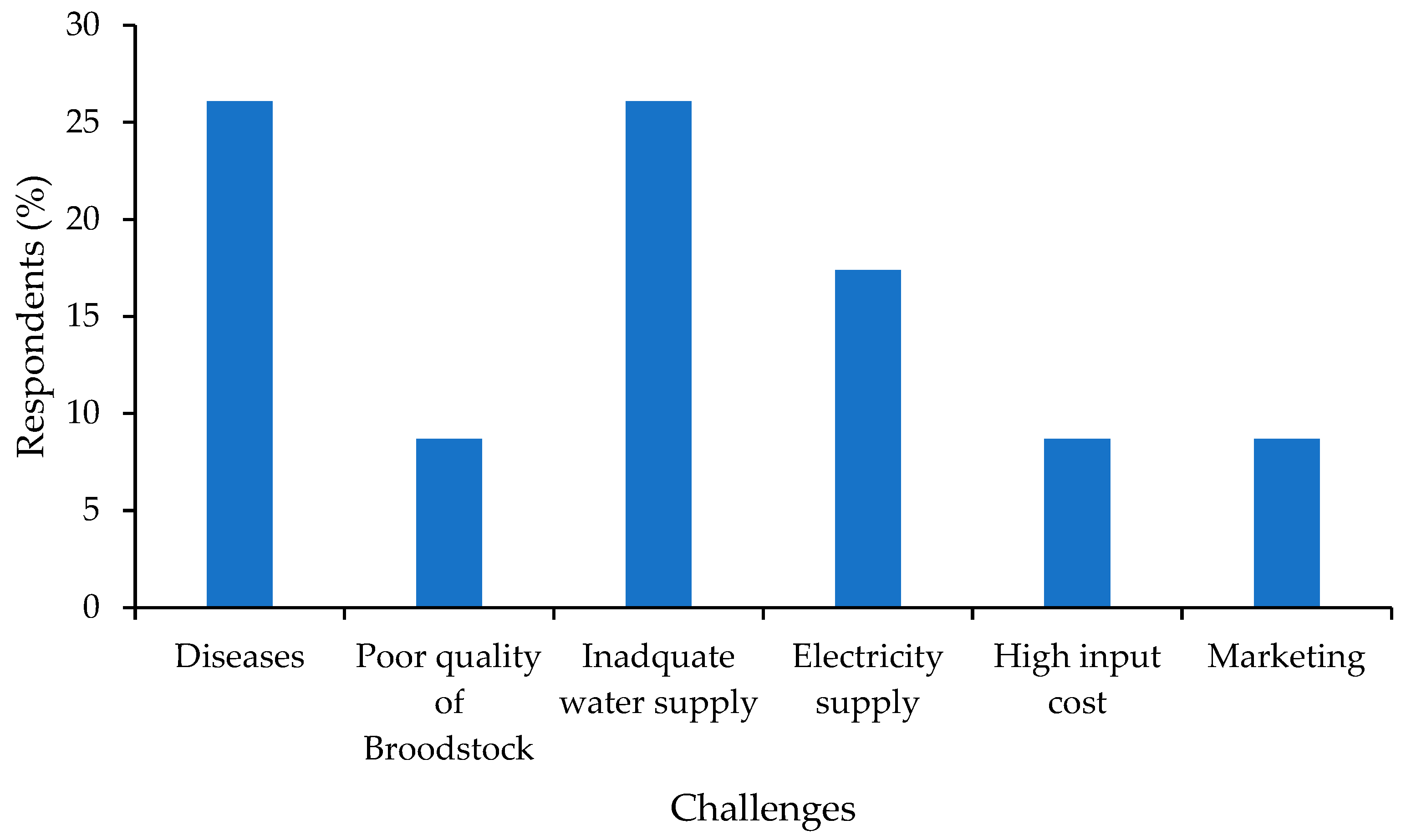
3.7. Main Obstacles in Hatchery Operation
4. Discussion
4.1. Broodstock Source
4.2. Use of Shooters and Their Implications
4.3. Broodstock Production, Evaluation, and Replacement Strategies
4.4. Effective Breeding Number (Ne) and Average Inbreeding Coefficient (F) and Its Implications on C. gariepinus Broodstock Management
4.5. Hatchery Operations and Fry Production
4.6. Demographics and Their Implications on Broodstock Management and Hatchery Practices
4.7. Main Challenges of Hatchery Operations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Munguti, J.; Muthoka, M.; Mboya, J.B.; Kyule, D.; Meenakshisundaram, M.; Tanga, C.M. Unraveling the Potential of Black Soldier Fly Larvae as a Sustainable Protein Source for Nile Tilapia Production in Diverse Aquaculture Systems. Aquac. Nutr. 2025, 2025, 3598843. [Google Scholar] [CrossRef] [PubMed]
- Subasinghe, R.; Soto, D.; Jia, J. Global aquaculture and its role in sustainable development. Rev. Aquac. 2009, 1, 2–9. [Google Scholar] [CrossRef]
- Saidu, M. Contributions of Fisheries and Aquaculture to Food Security in Africa. In Food Security, Nutrition and Sustainability Through Aquaculture Technologies; Springer: Berlin/Heidelberg, Germany, 2025; pp. 493–502. [Google Scholar]
- The State of World Fisheries and Aquaculture. In The State of World Fisheries and Aquaculture; Blue Transformation in Action; FAO: Rome, Italy, 2024.
- Adeleke, B.; Robertson-Andersson, D.; Moodley, G.; Taylor, S. Aquaculture in Africa: A comparative review of Egypt, Nigeria, and Uganda vis-a-vis South Africa. Rev. Fish. Sci. Aquac. 2020, 29, 167–197. [Google Scholar] [CrossRef]
- MAAIF. The National Fisheries and Aquaculture Policy Implementation Strategy and Action Plan; MAAIF: Entebbe, Uganda, 2018; p. 53. [Google Scholar]
- Aanyu, M.; Denis, O.; Cassius, A.; Gertrude, A. Potential for enhancing and sustaining commercial aquaculture in Uganda: Producer organizations, contract farming schemes and public-private partnerships. Int. J. Fish. Aquat. Stud. 2020, 8, 258–264. [Google Scholar]
- Rutaisire, J.; Nandi, S.; Sundaray, J.K. A review of Uganda and India’s freshwater aquaculture: Key practices and experience from each country. J. Ecol. Nat. Environ. 2017, 9, 15–29. [Google Scholar]
- Safina, N.; Gertrude, A.; Lawrance, O.; Ronald, W.; Alphonse, C.; Samuel, O.; Izaara, A.A. Profitability and viability analysis of aquaculture production in Central Uganda: A case of urban and peri-urban areas. Asian J. Agric. Ext. Econ. Soc. 2018, 22, 1–11. [Google Scholar] [CrossRef]
- Aruho, C.; Mwanja, M.T.; Bugenyi, F.; Rutaisire, J. Effectiveness of African catfish pituitary extracts, dagin and water flow for optimising egg production, fertilisation and hatchability in artificial spawning of Barbus altianalis. Uganda J. Agric. Sci. 2016, 17, 183–195. [Google Scholar] [CrossRef]
- MAAIF. Ministry of Agriculture, Animal Industry and Fisheries, Uganda. Agriculture Sector Strategic Plan (2020/21–2024/25) ASSP III; Fish Thematic Area. 2025, p. 50. Available online: https://www.agriculture.go.ug/agriculture-sector-strategic-plan-assp/ (accessed on 11 June 2025).
- Abaho, I.; Kwikiriza, G.; Atukwatse, F.; Izaara, A.A.; Ekwangu, J.; Baguma, S.D.; Kasozi, N. Selective Breeding for Genetic Improvement of Nile tilapia (Oreochromis niloticus Linnaeus, 1758) in Uganda: Current Status, Challenges, and Future Perspectives. Animals 2025, 15, 142. [Google Scholar] [CrossRef]
- Matthew, M.; Justus, R.; Constantine, O.; Richard, D.; Casius, A. Current fish hatchery practices in Uganda: The potential for future investment. Int. J. Fish. Aquat. Stud. 2015, 2, 224–232. [Google Scholar]
- Mwanja, M.; Ondhoro, C.; Sserwada, M.; Achieng, P.; Ddungu, R.; Mwanja, W. Morphological variation of Nile tilapia populations from major water bodies of Uganda. Uganda J. Agric. Sci. 2016, 17, 21–32. [Google Scholar] [CrossRef]
- Kwikiriza, G.; Yegon, M.J.; Byamugisha, N.; Beingana, A.; Atukwatse, F.; Barekye, A.; Nattabi, J.K.; Meimberg, H. Morphometric variations of Nile tilapia (Oreochromis niloticus) (Linnaeus, 1758) local strains collected from different fish farms in South Western Highland Agro-Ecological Zone (SWHAEZ), Uganda: Screening strains for aquaculture. Fishes 2023, 8, 217. [Google Scholar] [CrossRef]
- Nabulime, M.; Rutaisire, J.; Ssekaayi, J.; Smith, D.; Kabasa, J.D. Development of fry diets for African catfish (C. gariepinus) larvae in Uganda. J. Fish. Aqua. Sci. 2015, 10, 45. [Google Scholar] [CrossRef]
- Beingana, A.; Kwikiriza, G.; Bwanika, G.; Abaho, I.; Izaara, A.A. Performance of African Catfish Clarias gariepinus (Clariidae) fry fed on live rotifers (Brachionus calyciflorus), formulated diet, and a mixture of rotifers and formulated diet. Int. J. Fish. Aquat. Stud. 2016, 4, 11–15. [Google Scholar]
- Langi, S.; Maulu, S.; Hasimuna, O.J.; Kaleinasho Kapula, V.; Tjipute, M. Nutritional requirements and effect of culture conditions on the performance of the African catfish (Clarias gariepinus): A review. Cogent Food Agric. 2024, 10, 2302642. [Google Scholar] [CrossRef]
- Kemigabo, C.; Abdel-Tawwab, M.; Lazaro, J.W.; Sikawa, D.; Masembe, C.; Kang’Ombe, J. Combined effect of dietary protein and phytase levels on growth performance, feed utilization, and nutrients digestibility of African catfish, Clarias gariepinus (B.), reared in earthen ponds. J. Appl. Aquac. 2018, 30, 211–226. [Google Scholar] [CrossRef]
- Tibihika, P.D.M.; Barekye, A.; Byakora, E. Critical analysis of the growth performance of three fish species: Mirror carp (Cyprinus carpio), Nile tilapia (Oreochromis niloticus), and Sharp-toothed African catfish (Clarias gariepinus), cultured in earthen ponds in Kigezi region, Southwestern Uganda. Int. J. Fish. Aqua. Stud. 2015, 2, 63–73. [Google Scholar]
- Kayima, P.; Karasiewicz, T. Harnessing Geo-Diversity: Geosites and Geotourism in Uganda’s Albertine Region. Geoheritage 2025, 17, 1–21. [Google Scholar] [CrossRef]
- Awange, J. Lake Victoria Monitored from Space; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–321. [Google Scholar]
- Odada, E.O.; Olago, D.O.; Kulindwa, K.; Ntiba, M.; Wandiga, S. Mitigation of environmental problems in Lake Victoria, East Africa: Causal chain and policy options analyses. Ambio.J. Hum. Environ. 2004, 33, 13–23. [Google Scholar] [CrossRef]
- Mugo, R.; Waswa, R.; Nyaga, J.W.; Ndubi, A.; Adams, E.C.; Flores-Anderson, A.I. Quantifying land use land cover changes in the Lake Victoria basin using satellite remote sensing: The trends and drivers between 1985 and 2014. Remote Sens. 2020, 12, 2829. [Google Scholar] [CrossRef]
- Kwikiriza, G.; Tibihika, P.D.; Abaho, I.; Nattabi, J.K.; Vijayan, T.; Rupprecht, C.; Curto, M.; Melcher, A.; Meimberg, H. Genetic characterization and interspecies gene flow among the Oreochromis species in the Lake Victoria and Kyoga basins, Uganda: Implications for conservation. J. Great Lakes Res. 2025, 51, 102535. [Google Scholar] [CrossRef]
- Crow, J.F.; Denniston, C. Inbreeding and variance effective population numbers. Evolution 1988, 42, 482–495. [Google Scholar] [CrossRef] [PubMed]
- Adewumi, A.A.; Olaleye, V.F. Catfish culture in Nigeria: Progress, prospects and problems. Afr. J. Agric. Res. 2011, 6, 1281–1285. [Google Scholar]
- Isa, S.I. Development of Genetic Improvement in the African Catfish (Clarias Gariepinus, Burchell, 1822). Ph.D. thesis, Institute of Aquaculture, Faculty of Natural Sciences, University of Stirling, Scotland, UK, 2019. [Google Scholar]
- Ojiambo, D. Population Genetic Structure of Wild and Domesticated African Catfish (Clarias Gariepinus) in Victoria and Albertine Drainage Basins. Master’s thesis, Makerere University, Kampala, Uganda, 2015. [Google Scholar]
- Barasa, J.E.; Abila, R.; Grobler, J.P.; Dangasuk, O.G.; Njahira, M.N.; Kaunda-Arara, B. Genetic diversity and gene flow in Clarias gariepinus from Lakes Victoria and Kanyaboli, Kenya. Afr. J. Aquat. Sci. 2014, 39, 287–293. [Google Scholar] [CrossRef]
- Kebtieneh, N.; Alemayehu, K.; Tilahun, G. Genetic Diversity and Population Structure of African Catfish (Clarias gariepinus) Species: Implications for Selection and Sustainable Genetic Improvement. A Review. J. Aquac. Res. Dev. 2024, 15, 828. [Google Scholar]
- Sanda, M.K.; Metcalfe, N.B.; Capstick, M.; Nichols, J.; Mable, B.K. Genetic diversity, population structure, and differentiation of farmed and wild African catfish (Clarias gariepinus) in Nigeria. bioRxiv 2024, 2024-10. [Google Scholar] [CrossRef]
- Niyibizi, L.; Vidakovic, A.; Norman Haldén, A.; Rukera Tabaro, S.; Lundh, T. Aquaculture and aquafeed in Rwanda: Current status and perspectives. Appl. Aquac. 2023, 35, 743–764. [Google Scholar] [CrossRef]
- Kwikiriza, G.; Abaho, I.; Tibihika, P.D.; Izaara, A.A.; Atukwatse, F.; Omara, T.; Nattabi, J.K.; Kasozi, N.; Curto, M.; Melcher, A.; et al. Genetic Diversity and Population Differentiation of Farmed Nile Tilapia (Oreochromis niloticus Linnaeus, 1758) to Advance Selective Breeding in Uganda. Diversity 2025, 17, 128. [Google Scholar] [CrossRef]
- Geletu, T.T.; Tang, S.; Zhao, J. Genetic diversity and differentiation of cultured Nile tilapia populations from Ethiopia revealed by ddRAD-seq: Implications for better hatchery management. Aquat. Living Resour. 2025, 38, 2. [Google Scholar] [CrossRef]
- Fregene, B.T.; Bolorunduro, P.; Yossa, R.; Karisa, H.C.; Olaniyi, A.; Ajose, I. Extension manual on the production of quality catfish seed. Gates Open Res. 2024, 8, 68. [Google Scholar]
- Abdulraheem, I.; Ojelade, O.C.; Idi-Ogede, A.M.; Onimisi, H.U.; Tokede, O.E. Emergence of new shooters from the populations of selected shooters of African mudcatfish Clarias gariepinus (BURCHELL, 1822). Ife J. Agric. 2019, 31, 3. [Google Scholar]
- Srimai, W.; Koonawootrittriron, S.; Manee-Aphai, W.; Chatchaiphan, S.; Koolboon, U.; Na-Nakorn, U. Genetic parameters and genotype-environment interaction for growth traits of North African catfish, Clarias gariepinus (Burchell, 1822). Aquaculture 2019, 501, 104–110. [Google Scholar] [CrossRef]
- Tave, D. Growth of triploid and diploid bighead carp, Hypophthalmichthys nobilis. J. Appl. Aquac. 1993, 2, 13–26. [Google Scholar] [CrossRef]
- Beardmore, J.A.; Mair, G.C.; Lewis, R.I. Monosex male production in finfish as exemplified by tilapia: Applications, problems, and prospects. Aquaculture 2001, 197, 283–301. [Google Scholar] [CrossRef]
- Chaivichoo, P.; Sukhavachana, S.; Khumthong, R.; Srisapoome, P.; Chatchaiphan, S.; Na-Nakorn, U. Genome–wide association study and genomic prediction of growth traits in bighead catfish (Clarias macrocephalus Günther, 1864). Aquaculture 2023, 562, 738–748. [Google Scholar] [CrossRef]
- Nguyen, D.H.M.; Ponjarat, J.; Laopichienpong, N.; Panthum, T.; Singchat, W.; Ahmad, S.F.; Kraichak, E.; Muangmai, N.; Duengkae, P.; Peyachoknagul, S.; et al. Genome-wide SNP analysis of hybrid clariid fish reflects the existence of polygenic sex-determination in the lineage. Front. Genet. 2022, 13, 789573. [Google Scholar] [CrossRef]
- Miller, J.W.; Atanda, T. The rise of peri-urban aquaculture in Nigeria. Int. J. Agric. Sustain. 2011, 9, 274–281. [Google Scholar] [CrossRef]
- Mafwila Kinkela, P.; Kambashi Mutiaka, B.; Dochain, D.; Rollin, X.; Mafwila, J.; Bindelle, J. Smallholders’ practices of integrated agriculture aquaculture system in peri-urban and rural areas in Sub-Saharan Africa. Tropicultura 2019, 37. [Google Scholar] [CrossRef]
- Amoah, K.; Adu-Asiamah, P.; Dong, X.H.; Ampofo-Yeboah, A.; Abarike, E.D. A comparative study on the hatchability and survival rate of African catfish, Clarias gariepinus (Burchell, 1822), induced with catfish’s pituitary gland hormone from farmed and wild sources. Aquac. Int. 2020, 28, 2221–2234. [Google Scholar] [CrossRef]
- Esa, Y.B.; Dadile, A.M.; Syukri, F.; Christianus, A.; Diyaware, M.Y. Evaluation of fecundity, fertilization, hatching, and gonadosomatic index of exotic Clarias gariepinus (Burchell, 1822) and native Clarias macromystax (Gunther, 1864) under semi-arid conditions of Nigeria. Animals 2023, 13, 1723. [Google Scholar] [CrossRef]
- Sriphairoj, K.; Kamonrat, W.; Na-Nakorn, U. Genetic aspect in broodstock management of the critically endangered Mekong giant catfish, Pangasianodon gigas in Thailand. Aquaculture 2007, 264, 36–46. [Google Scholar] [CrossRef]
- Opiyo, M.A.; Orina, P.; Charo-Karisa, H. Fecundity, growth parameters, and survival rate of three African catfish (Clarias gariepinus) strains under hatchery conditions. J. Aquac. Eng. Fish. Res. 2017, 3, 75–81. [Google Scholar] [CrossRef]
- Ferosekhan, S.; Sahoo, S.K.; Giri, S.S.; Das, B.K.; Pillai, B.R.; Das, P.C. Broodstock development, captive breeding and seed production of bagrid catfish, Mahanadi rita, Rita chrysea (Day, 1877). Aquaculture 2019, 503, 339–346. [Google Scholar] [CrossRef]
- Imron, I.; Iswanto, B.; Suparapto, R.; Marnis, H. Development of genetically improved farmed African catfish, Clarias gariepinus; A review and lessons learned from Indonesian fish breeding program. IOP Conf. Ser. Earth Environ. Sci. 2020, 593, 012032. [Google Scholar] [CrossRef]
- Tine, M.; Ndiaye, F.; Bale, K.; Magblenou, L.D.; Sene, M.A. Effects of inbreeding depression on the success of artificial reproduction in the African catfish Clarias gariepinus (Burchell, 1822). Int. J. Aquac. Fish. Sci. 2022, 8, 045–053. [Google Scholar]
- Niu, J.S.; Wang, T.; Li, Z.; Wang, Z.W.; Ding, M.; Wang, M.T.; Li, X.Y. Efficient breeding and growth advantage of all-male population in Lanzhou catfish (Silurus lanzhouensis). Aquaculture 2024, 578, 740023. [Google Scholar] [CrossRef]
- Dupont-Nivet, M.; Vandeputte, M.; Haffray, P.; Chevassus, B. Effect of different mating designs on inbreeding, genetic variance and response to selection when applying individual selection in fish breeding programs. Aquaculture 2006, 252, 161–170. [Google Scholar] [CrossRef]
- Busack, C.; Knudsen, C.M. Using factorial mating designs to increase the effective number of breeders in fish hatcheries. Aquaculture 2007, 273, 24–32. [Google Scholar] [CrossRef]
- Kánainé Sipos, D.; Bakos, K.; Ősz, Á.; Hegyi, Á.; Müller, T.; Urbányi, B.; Kovács, B. Development and characterization of 49 novel microsatellite markers in the African catfish, Clarias gariepinus (Burchell, 1822). Mol. Biol. Rep. 2019, 46, 6599–6608. [Google Scholar] [CrossRef]
- Zamri, A.S.; Zulperi, Z.; Esa, Y.; Syukri, F. Hormone Application for Artificial Breeding Towards Sustainable Aquaculture–A Review. Pertanika J. Trop. Agric. Sci. 2022, 45. [Google Scholar] [CrossRef]
- Ataguba, G.A.; Kwala, G.J.; Abum, T. The Effect of Diluted Doses of GnRHa (Ovulin) Supplemented with Buserelin Acetate-GnRHa (Suprecur) on Final Ova Maturation and Breeding of the African Catfish (Clarias gariepinus). Aqua. Sci. Fish Res. 2023, 4, 55–63. [Google Scholar] [CrossRef]
- Toko, I.; Fiogbe, E.D.; Koukpode, B.; Kestemont, P. Rearing of African catfish (Clarias gariepinus) and vundu catfish (Heterobranchus longifilis) in traditional fish ponds (whedos): Effect of stocking density on growth, production, and body composition. Aquaculture 2007, 262, 65–72. [Google Scholar] [CrossRef]
- Brown, T.W.; Mischke, C.C.; Roy, L.A.; Li, M.H. A length–weight relationship for pond-raised hybrid catfish fingerlings. J. World Aquac. Soc. 2016, 47, 93–96. [Google Scholar] [CrossRef]
- Pita, C.; Eleftheriou, M.; Fernández-Borrás, J.; Gonçalves, S.; Mente, E.; Santos, M.B.; Seixas, S.; Pierce, G.J. Generic skills needs for graduate employment in the aquaculture, fisheries, and related sectors in Europe. Aquac. Int. 2015, 23, 767–786. [Google Scholar] [CrossRef]
- Khademi-Vidra, A.; Urbányi, B.; Bakos, I.M. Educational and training innovation opportunities in the aquaculture and fisheries sector of Hungarian secondary agricultural education. Aquac. Int. 2024, 32, 5437–5459. [Google Scholar] [CrossRef]
- Boyd, C.E.; D’Abramo, L.R.; Glencross, B.D.; Huyben, D.C.; Juarez, L.M.; Lockwood, G.S.; McNevin, A.A.; Tacon, A.G.; Teletchea, F.; Tomasso, J.R., Jr.; et al. Achieving sustainable aquaculture: Historical and current perspectives and future needs and challenges. J. World Aquac. Soc. 2020, 51, 578–633. [Google Scholar] [CrossRef]
- Nkambo, M.; Mwanja, M.; Balirwa, J.S.; Nayiga, S.; Bugenyi, F.W. Hatchability of Selected Commercial Artemia Strains using waters from selected saline crater lakes of Western Uganda. J. Nat. Sci. Res. 2019, 9, 37–42. [Google Scholar]
- Nyonje, B.M.; Opiyo, M.A.; Orina, P.S.; Abwao, J.; Wainaina, M.; Charo-Karisa, H. Current status of freshwater fish hatcheries, broodstock management, and fingerling production in the Kenya aquaculture sector. Livestock Res. Rural Dev. 2018, 30, 1–15. [Google Scholar]
- Mugyenyi, J.; Kinhonhi, I.V.; Modi, V. Electricity outages in Uganda: Causes, trends and regional disparities. In Proceedings of the 2024 IEEE PES/IAS PowerAfrica, Johannesburg, South Africa, 7–11 October 2024; IEEE: Piscataway, NJ, USA, 2024; pp. 1–5. [Google Scholar]
- Migisha, A.G.; Ntayi, J.M.; Adaramola, M.S.; Buyinza, F.; Senyonga, L.; Abaliwano, J. Effects of technical and security factors on grid electricity reliability: Evidence from Uganda national electricity grid network. Technol. Sustain. 2024, 3, 41–67. [Google Scholar] [CrossRef]
- Tumwesigye, Z.; Tumwesigye, W.; Opio, F.; Kemigabo, C.; Mujuni, B. The effect of water quality on aquaculture productivity in Ibanda District, Uganda. Aquac. J. 2022, 2, 23–36. [Google Scholar] [CrossRef]
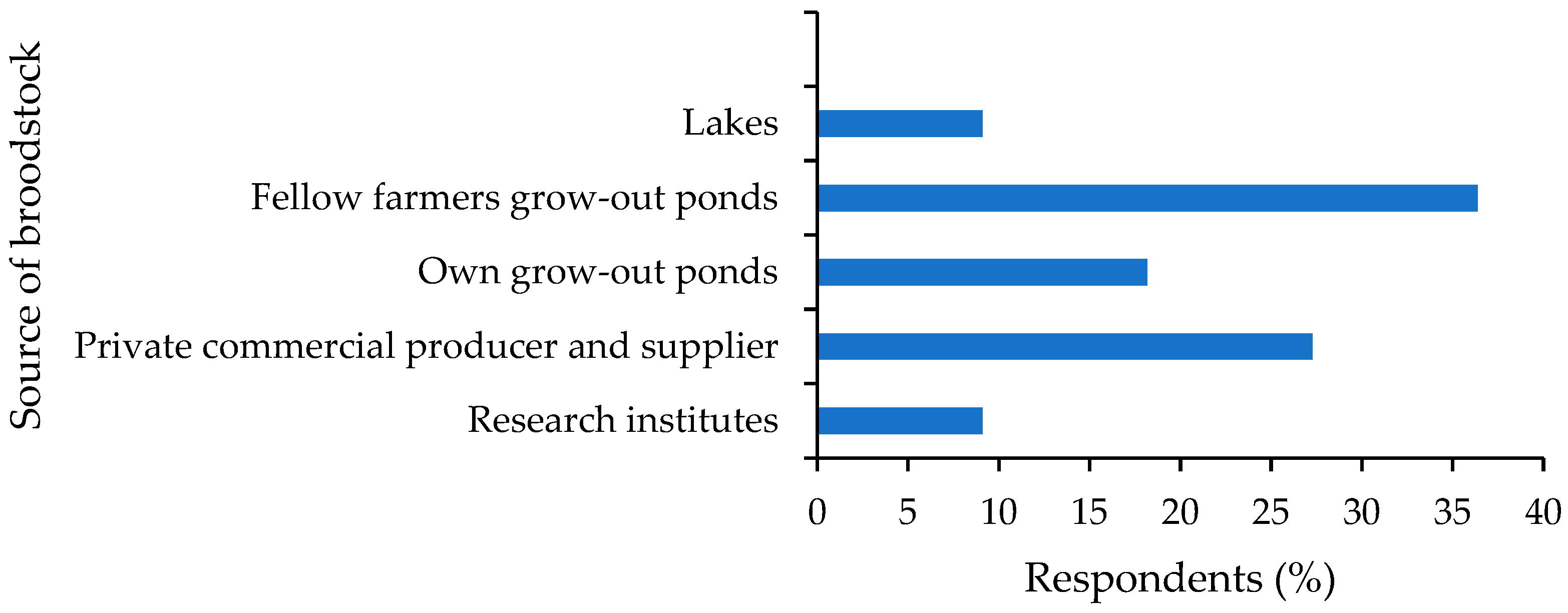


| Mating Ratios | Ne | F | F (%) |
|---|---|---|---|
| 1 male–1 female | 400 | 0.001 | 0.1 |
| 2 males–2 females | 200 | 0.002 | 0.2 |
| 1 male–3 females | 178 | 0.003 | 0.3 |
| 1 male–2 females | 133 | 0.004 | 0.4 |
| 1 male–4 females | 128 | 0.004 | 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwikiriza, G.; Tebitendwa, S.M.; Rwezawula, P.; Mwanja, W.W.; Abaho, I.; Meimberg, H. Enhancing African Catfish (Clarias gariepinus) Aquaculture in Uganda: Insights into Hatchery Propagation, Population Suitability, and Broodstock Management. Fishes 2025, 10, 290. https://doi.org/10.3390/fishes10060290
Kwikiriza G, Tebitendwa SM, Rwezawula P, Mwanja WW, Abaho I, Meimberg H. Enhancing African Catfish (Clarias gariepinus) Aquaculture in Uganda: Insights into Hatchery Propagation, Population Suitability, and Broodstock Management. Fishes. 2025; 10(6):290. https://doi.org/10.3390/fishes10060290
Chicago/Turabian StyleKwikiriza, Gerald, Sylvie Muwanga Tebitendwa, Philip Rwezawula, Waiswa Wilson Mwanja, Ivan Abaho, and Harald Meimberg. 2025. "Enhancing African Catfish (Clarias gariepinus) Aquaculture in Uganda: Insights into Hatchery Propagation, Population Suitability, and Broodstock Management" Fishes 10, no. 6: 290. https://doi.org/10.3390/fishes10060290
APA StyleKwikiriza, G., Tebitendwa, S. M., Rwezawula, P., Mwanja, W. W., Abaho, I., & Meimberg, H. (2025). Enhancing African Catfish (Clarias gariepinus) Aquaculture in Uganda: Insights into Hatchery Propagation, Population Suitability, and Broodstock Management. Fishes, 10(6), 290. https://doi.org/10.3390/fishes10060290









