Fecundity Study and Histological Analysis of the Gonads of the Sea Cucumber Holothuria tubulosa (Echinodermata: Holothuroidea) in the Central Aegean Sea, Greece: Insights into Reproductive Biology
Abstract
1. Introduction
2. Material and Methods
2.1. Study Area and Sampling
2.2. Biometric Measurements
2.3. Fecundity
2.4. Histological Analysis
2.5. Data Analysis
3. Results
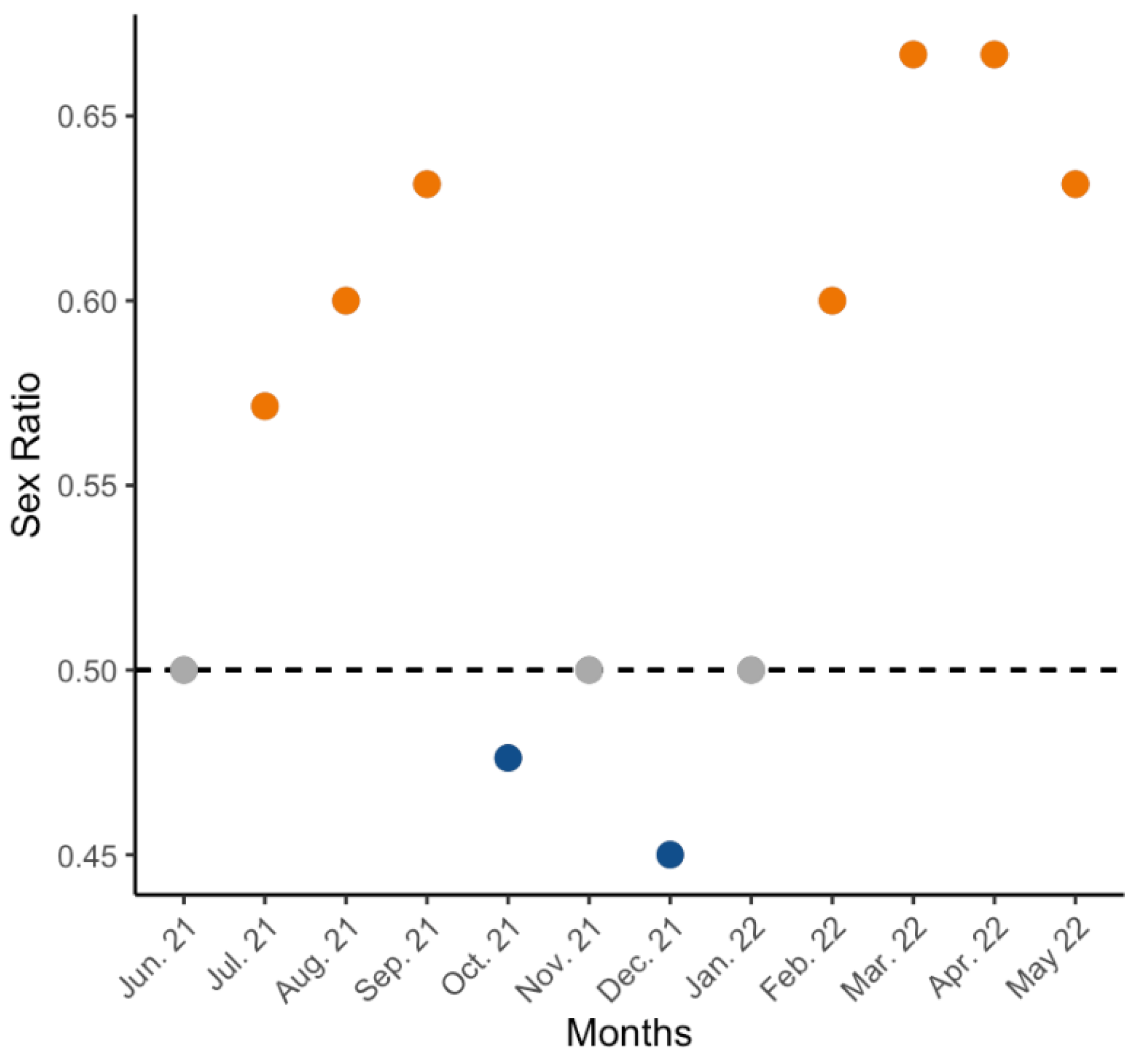
3.1. Sex Ratio
3.2. Reproductive Cycle
3.2.1. Microscopic Description
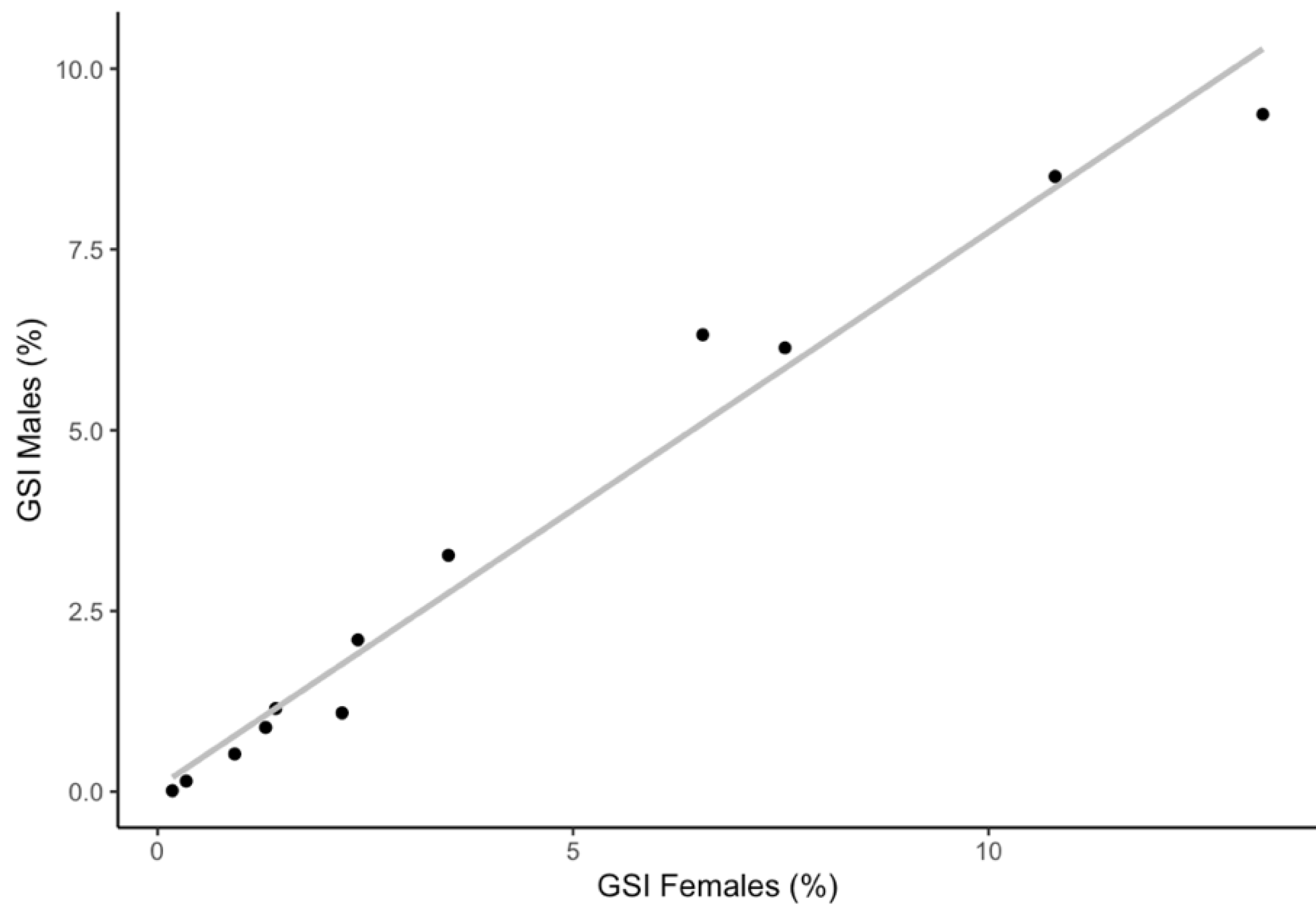
3.2.2. Gonadosomatic Index and Monthly Variability of Sexual Maturity Stages
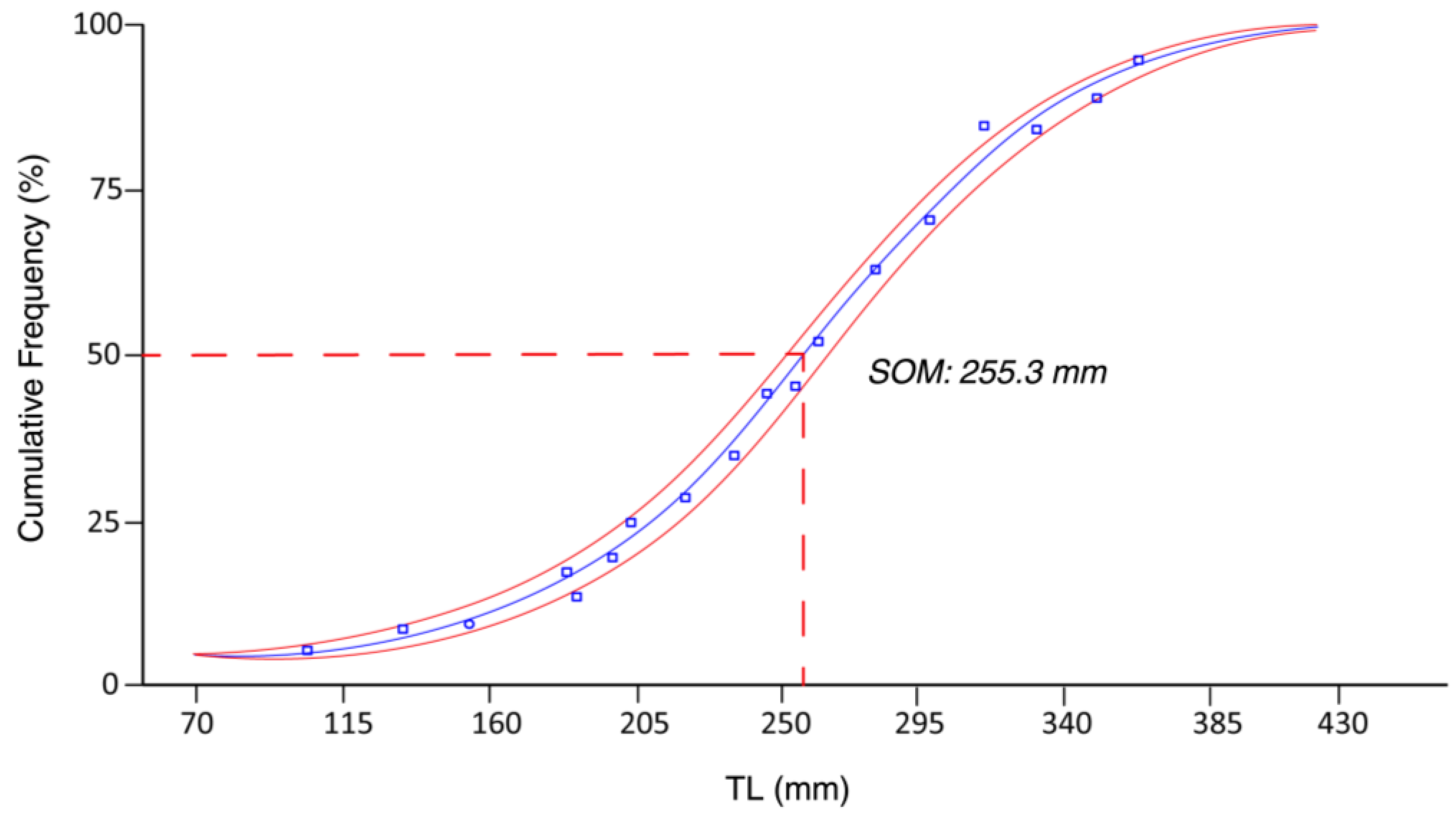
3.3. Size at First Sexual Maturity
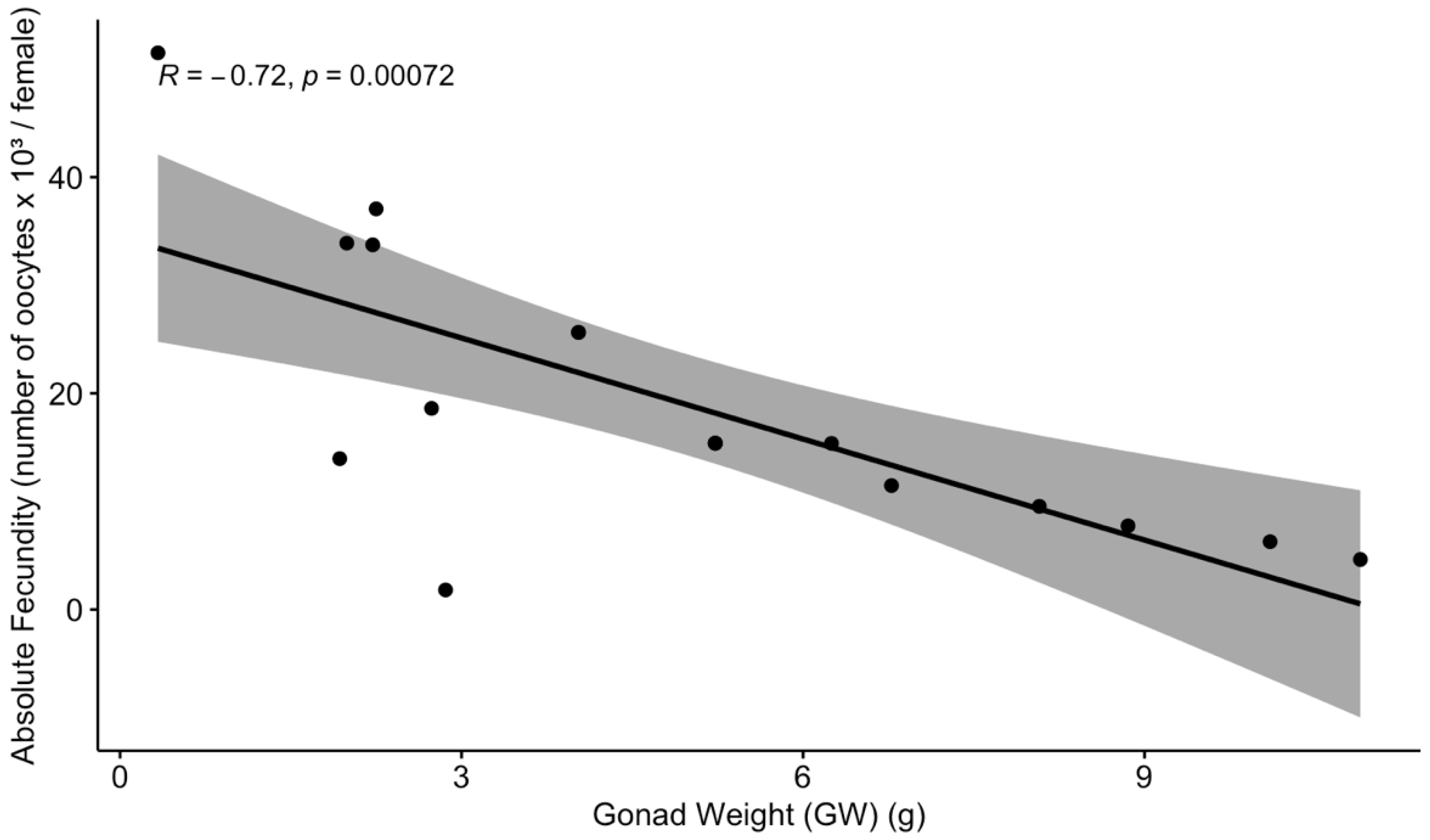
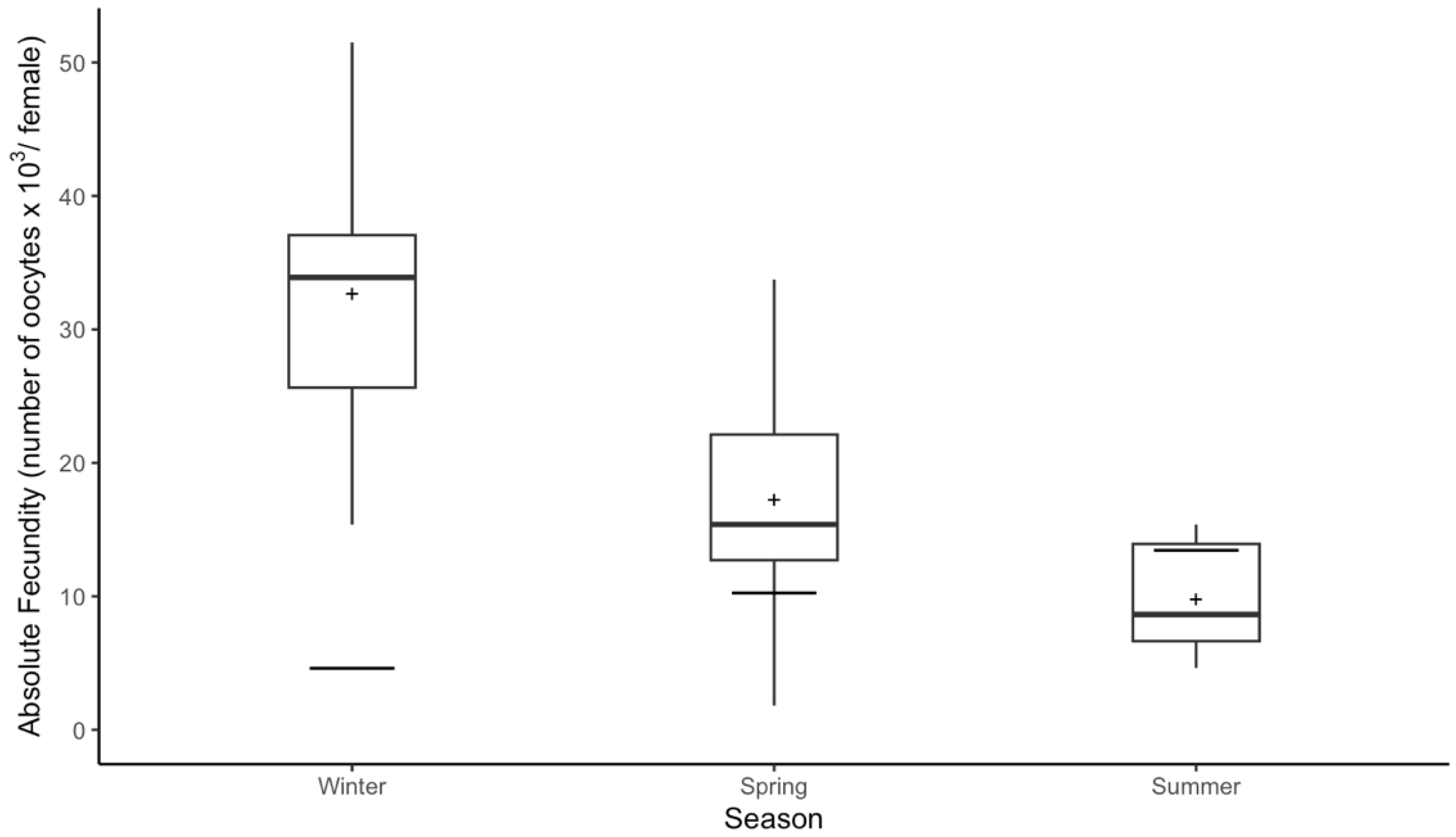
3.4. Absolute Fecundity and Seasonal Variation
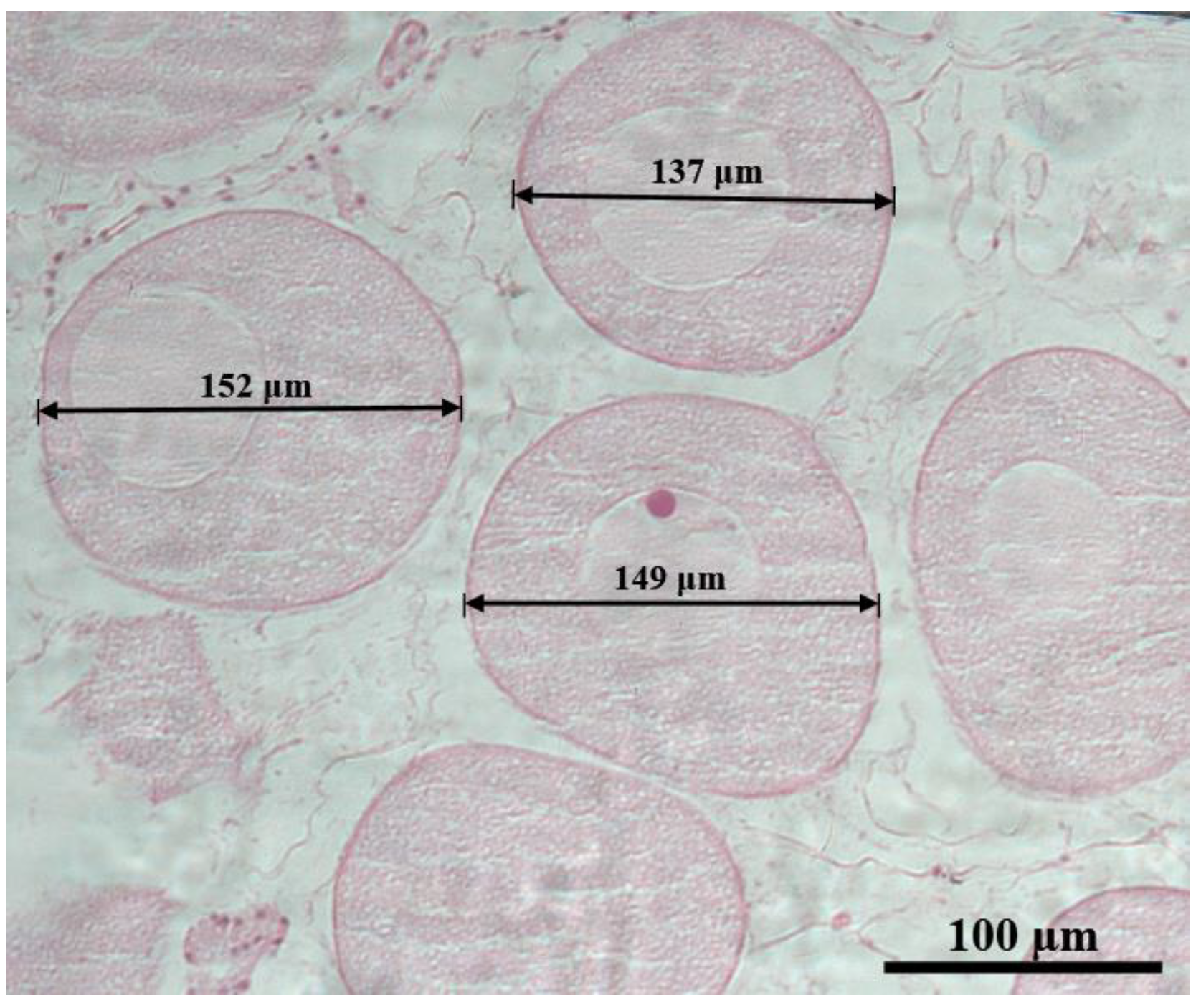
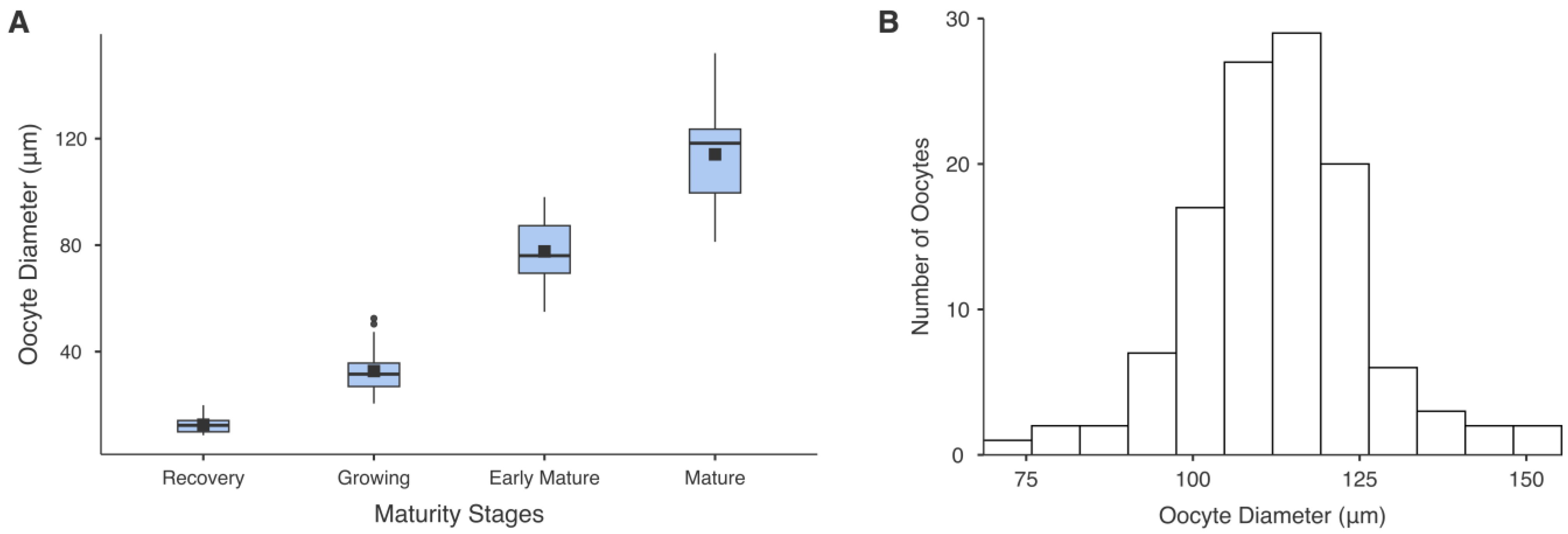
3.5. Oocyte Diameter
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Purcell, S.W.; Conand, C.; Uthicke, S.; Byrne, M. Ecological roles of exploited sea cucumbers. In Oceanography and Marine Biology: An Annual Review; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Pierrat, J.; Bédier, A.; Eeckhaut, I.; Magalon, H.; Frouin, P. Sophistication in a seemingly simple creature: A review of wild holothurian nutrition in marine ecosystems. Biol. Rev. 2022, 97, 273–298. [Google Scholar] [CrossRef] [PubMed]
- Skordas, K.; Georgiou, K.; Kinigopoulou, V.; Kelepertzis, E.; Apostologamvrou, C.; Lolas, A.; Petrotou, A.; Neofitou, N.; Vafidis, D. Potentially toxic element assessment and biological accumulation in two sea cucumbers species Holothuria poli and Holothuria tubulosa. Reg. Stud. Mar. Sci. 2024, 70, 103370. [Google Scholar] [CrossRef]
- Vafidis, D.; Antoniadou, C. Holothurian Fisheries in the Hellenic Seas: Seeking for Sustainability. Sustainability 2023, 15, 9799. [Google Scholar] [CrossRef]
- Uthicke, S.; Karez, R. Sediment patch selectivity in tropical sea cucumbers (Holothurioidea: Aspidochirotida) analysed with multiple choice experiments. J. Exp. Mar. Biol. Ecol. 1999, 236, 69–87. [Google Scholar] [CrossRef]
- Rahman, M.A.; Chowdhury, S.H.; Hasan, J.; Rahman, H.; Yeasmin, S.M.; Farjana, N.; Molla, H.R.; Parvez, S. Status, Prospects and Market Potentials of the Sea Cucumber Fisheries with Special Reference on Their Proper Utilization and Trade. Annu. Res. Rev. Biol. 2020, 35, 84–101. [Google Scholar] [CrossRef]
- Sroyraya, M.; Hanna, P.J.; Siangcham, T.; Tinikul, R.; Jattujan, P.; Poomtong, T.; Sobhon, P. Nutritional components of the sea cucumber Holothuria scabra. Funct. Foods Health Dis. 2017, 7, 168. [Google Scholar] [CrossRef]
- Roggatz, C.C.; González-Wangüemert, M.; Pereira, H.; Rodrigues, M.J.; da Silva, M.M.; Barreira, L.; Varela, J.; Custódio, L. First report of the nutritional profile and antioxidant potential of Holothuria arguinensis, a new resource for aquaculture in Europe. Nat. Prod. Res. 2016, 30, 2034–2040. [Google Scholar] [CrossRef] [PubMed]
- Pangestuti, R.; Arifin, Z. Medicinal and health benefit effects of functional sea cucumbers. J. Tradit. Complement. Med. 2018, 8, 341–351. [Google Scholar] [CrossRef]
- Bordbar, S.; Anwar, F.; Saari, N. High-value components and bioactives from sea cucumbers for functional foods—A review. Mar. Drugs 2011, 9, 1761–1805. [Google Scholar] [CrossRef]
- Shi, S.; Feng, W.; Hu, S.; Liang, S.; An, N.; Mao, Y. Bioactive compounds of sea cucumbers and their therapeutic effects. Chin. J. Oceanol. Limnol. 2016, 34, 549–558. [Google Scholar] [CrossRef]
- Ming, L.C.; Manan, W.Z.W.; Mahalingam, S.R.; Arshad, K.; Bukhari, S. Safety and efficacy of sea cucumber containing products. Arch. Pharm. Pract. 2016, 7, 48. [Google Scholar] [CrossRef]
- Mohsen, M.; Yang, H. Sea Cucumbers Research in the Mediterranean and the Red Seas. In The Sea Cucumber Apostichopus Japonicus: History, Biology and Aquaculture; Hasan, M., Yang, H., Zhou, Z., Eds.; Academic Press: Oxford, UK, 2021; pp. 61–101. [Google Scholar] [CrossRef]
- Costa, V.; Mazzola, A.; Vizzini, S. Holothuria tubulosa Gmelin 1791 (Holothuroidea, Echinodermata) enhances organic matter recycling in Posidonia oceanica meadows. J. Exp. Mar. Biol. Ecol. 2014, 461, 226–232. [Google Scholar] [CrossRef]
- Boncagni, P.; Rakaj, A.; Fianchini, A.; Vizzini, S. Preferential assimilation of seagrass detritus by two coexisting Mediterranean sea cucumbers: Holothuria polii and Holothuria tubulosa. Estuar. Coast. Shelf Sci. 2019, 231, 106464. [Google Scholar] [CrossRef]
- Kazanidis, G.; Antoniadou, C.; Lolas, A.P.; Neofitou, N.; Vafidis, D.; Chintiroglou, C.; Neofitou, C. Population dynamics and reproduction of Holothuria tubulosa (Holothuroidea: Echinodermata) in the Aegean Sea. J. Mar. Biol. Assoc. U. K. 2010, 90, 895–901. [Google Scholar] [CrossRef]
- Mezali, K.; Zupo, V.; Francour, P. Population dynamics of Holothuria (Holothuria) tubulosa and Holothuria (Lessonothuria) polii of an Algerian Posidonia oceanica meadow. Biol Mar Med. 2006, 13, 158–161. [Google Scholar]
- Aydın, M. Density and Biomass of Commercial Sea Cucumber Species Relative to Depth in the Northern Aegean Sea. Thalass. Int. J. Mar. Sci. 2019, 35, 541–550. [Google Scholar] [CrossRef]
- Bulteel, P.; Jangoux, M.; Coulon, P. Biometry, Bathymetric Distribution, and Reproductive Cycle of the Holothuroid Holothuria tubulosa (Echinodermata) from Mediterranean Sea grass Beds. Mar. Ecol. 1992, 13, 53–62. [Google Scholar] [CrossRef]
- Aydin, M.; Erkan, S. Identification and some biological characteristics of commercial sea cucumber in the Turkey coast waters. Int. J. Fish. Aquat. Stud. 2015, 3, 260–265. [Google Scholar]
- Luparello, C.; Ragona, D.; Asaro, D.M.L.; Lazzara, V.; Affranchi, F.; Celi, M.; Arizza, V.; Vazzana, M. Cytotoxic potential of the coelomic fluid extracted from the sea cucumber Holothuria tubulosa against triple-negative MDA-MB231 breast cancer cells. Biology 2019, 8, 76. [Google Scholar] [CrossRef]
- Mezali, K.; Thandar, A.S.; Khodja, I. On the taxonomic status of Holothuria (Holothuria) tubulosa (s.s.) from the Algerian coast with the description of a new Mediterranean species, Holothuria (Holothuria) algeriensis n. sp. (Echinodermata: Holothuroidea: Holothuriidae). Zootaxa 2021, 4981, 89–106. [Google Scholar] [CrossRef]
- Mansouri, T.; Mezali, K. Taxonomic status and phylogenetic relationship of some Algerian shallow-water sea cucumber species (Holothuroidea: Echinodermata) deduced from mitochondrial DNA sequences. In Proceedings of the Bioinformatics and Data Analysis Workshop, Tangier, Morocco, 12–13 March 2018. [Google Scholar]
- Gkafas, G.A.; Sarantopoulou, J.; Apostologamvrou, C.; Antoniadou, C.; Exadactylos, A.; Fleris, G.; Vafidis, D. Admixture of Holothurian Species in the Hellenic Seas (Eastern Mediterranean) as Revealed by RADseq. Sustainability 2023, 15, 11493. [Google Scholar] [CrossRef]
- Tolon, T.; Emiroğlu, D.; Günay, D.; Hancı, B. Effect of stocking density on growth performance of juvenile sea cucumber Holothuria tubulosa (Gmelin, 1788). Aquac. Res. 2016, 48, 4124–4131. [Google Scholar] [CrossRef]
- Rakaj, A.; Fianchini, A.; Boncagni, P.; Lovatelli, A.; Scardi, M.; Cataudella, S. Spawning and rearing of Holothuria tubulosa: A new candidate for aquaculture in the Mediterranean region. Aquac. Res. 2018, 49, 557–568. [Google Scholar] [CrossRef]
- Despalatović, M.; Grubelić, I.; Šimunović, A.; Antolić, B.; Žuljević, A. Reproductive biology of the holothurian Holothuria tubulosa (Echinodermata) in the Adriatic Sea. J. Mar. Biol. Assoc. U. K. 2004, 84, 409–414. [Google Scholar] [CrossRef]
- Kazanidis, G.; Lolas, A.; Vafidis, D. Reproductive cycle of the traditionally exploited sea cucumber Holothuria tubulosa (Holothuroidea: Aspidochirotida) in Pagasitikos Gulf, western Aegean Sea, Greece. Turk. J. Zool. 2014, 38, 306–315. [Google Scholar] [CrossRef]
- Pasquini, V.; Porcu, C.; Marongiu, M.F.; Follesa, M.C.; Giglioli, A.A.; Addis, P. New insights upon the reproductive biology of the sea cucumber Holothuria tubulosa (Echinodermata, Holothuroidea) in the Mediterranean: Implications for management and domestication. Front. Mar. Sci. 2022, 9, 1029147. [Google Scholar] [CrossRef]
- Tahri, Y.; Dermeche, S.; Chahrour, F.; Bouderbala, M. The reproduction cycle of the sea cucumber Holothuria (Holothuria) tubulosa Gmelin, 1791 (Echinodermata Holo-thuroidea Holothuriidae) in Oran coast, Algeria. Biodivers. J. 2019, 10, 159–172. [Google Scholar] [CrossRef]
- Dereli, H.; Çulha, S.T.; Çulha, M.; Özalp, B.H.; Tekinay, A.A. Reproduction and population structure of the sea cucumber Holothuria tubulosa in the Dardanelles Strait, Turkey. Mediterr. Mar. Sci. 2016, 17, 47. [Google Scholar] [CrossRef]
- Ocaña, A.; Sanchez-Tocino, L. Spawning of Holothuria tubulosa (Holothurioidea, Echinodermata) in the Alboran Sea (Mediterranean Sea). Zool. Baetica 2005, 16, 147–150. [Google Scholar]
- Aydin, M. The commercial sea cucumber fishery in Turkey. SPC Beche Mer Inf. Bull. 2008, 28, 40–41. [Google Scholar]
- Çakli, Ş.; Cadun, A.; Kişla, D.; Dinçer, T. Determination of quality characteristics of Holothuria tubulosa, (Gmelin, 1788) in Turkish sea (Aegean Region) depending on sun drying process step used in Turkey. J. Aquat. Food Prod. Technol. 2004, 13, 69–78. [Google Scholar] [CrossRef]
- González-Wangüemert, M.; Aydin, M.; Conand, C. Assessment of sea cucumber populations from the Aegean Sea (Turkey): First insights to sustainable management of new fisheries. Ocean Coast. Manag. 2014, 92, 87–94. [Google Scholar] [CrossRef]
- González-Wangüemert, M.; Valente, S.; Aydin, M. Effects of fishery protection on biometry and genetic structure of two target sea cucumber species from the Mediterranean Sea. Hydrobiologia 2015, 743, 65–74. [Google Scholar] [CrossRef]
- Rakaj, A.; Fianchini, A.; Boncagni, P.; Scardi, M.; Cataudella, S. Artificial reproduction of Holothuria polii: A new candidate for aquaculture. Aquaculture 2019, 498, 444–453. [Google Scholar] [CrossRef]
- Apostologamvrou, C.; Hatziioannou, M.; Exadactylos, A.; Vafidis, D. Reproductive biology of the commercial sea cucumber Holothuria (Roweothuria) poli, in the Central Aegean Sea, Greece. Fish. Res. 2024, 279, 107157. [Google Scholar] [CrossRef]
- Harms, C.A. ECHINODERMS. In Invertebrate Medicine [Internet]; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2022; pp. 579–598. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/9781119569831.ch23 (accessed on 31 May 2025).
- Purcell, S.; Samyn, Y.; Conand, C. Commercially Important Sea Cucumbers of the World; Food & Agriculture Organization of the United Nations: Rome, Italy, 2012; Volume 6, pp. 1–150. [Google Scholar]
- Jakobsen, T.; Fogarty, M.; Megrey, B. Fish Reproductive Biology: Implications for Assessment and Management; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 1–8. [Google Scholar]
- Conand, C. Sexual cycle of three commercially important holothurian species (Echinodermata) from the lagoon of New Caledonia. Bull. Mar. Sci. 1981, 31, 523–543. [Google Scholar]
- Muthiga, N.; Kawaka, J.; Ndirangu, S. The timing and reproductive output of the commercial sea cucumber Holothuria scabra on the Kenyan coast. Estuarine, Coast. Shelf Sci. 2009, 84, 353–360. [Google Scholar] [CrossRef]
- Apostologamvrou, C.; Vlachou, M.; Theocharis, A.; Ntavaros, C.; Klaoudatos, D. Reproductive aspects of European hake, (Merluccius merluccius, Linnaeus, 1758) based on histological depiction of both sexes in the Eastern Mediterranean (Aegean Sea). Reg. Stud. Mar. Sci. 2023, 68, 103281. [Google Scholar] [CrossRef]
- Slimane-Tamacha, F.; Soualili, D.L.; Mezali, K. Reproductive biology of Holothuria (Roweothuria) poli (Holothuroidea: Echinodermata) from Oran Bay, Algeria. SPC Beche-De-Mer Inf. Bull. 2019, 39, 47–53. Available online: https://api.semanticscholar.org/CorpusID:164791495 (accessed on 1 July 2023).
- Zar, J.H. Biostatistical Analysis., 2nd ed.; Prentice-Hall: Englewood Cliffs, NJ, USA, 1984. [Google Scholar]
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio, PBC: Boston, MA, USA, 2020; Available online: http://www.rstudio.com (accessed on 31 May 2025).
- Lolas, A.; Vafidis, D. Population dynamics, fishery, and exploitation status of norway lobster (Nephrops norvegicus) in eastern mediterranean. Water 2021, 13, 289. [Google Scholar] [CrossRef]
- Leite-Castro, L.V.; Junior, J.d.S.; Salmito-Vanderley, C.S.B.; Nunes, J.F.; Hamel, J.-F.; Mercier, A. Reproductive biology of the sea cucumber Holothuria grisea in Brazil: Importance of social and environmental factors in breeding coordination. Mar. Biol. 2016, 163, 67. [Google Scholar] [CrossRef]
- Navarro, P.G.; García-Sanz, S.; Tuya, F. Reproductive biology of the sea cucumber Holothuria sanctori (Echinodermata: Holothuroidea). Sci. Mar. 2012, 76, 741–752. [Google Scholar] [CrossRef]
- Singh, R.; Macdonald, B.A.; Lawton, P.; Thomas, M.L. The reproductive biology of the dendrochirote sea cucumber Cucumaria frondosa (Echinodermata: Holothuriodea) using new quantitative methods. Invertebr. Reprod. Dev. 2001, 40, 125–141. [Google Scholar] [CrossRef]
- Guzmán, H.; Guevara, C.; Hernández, I. Reproductive cycle of two commercial species of sea cucumber (Echinodermata: Holothuroidea) from Caribbean Panama. Mar. Biol. 2003, 142, 271–279. [Google Scholar] [CrossRef]
- Ramofafia, C.; Battaglene, S.C.; Bell, J.D.; Byrne, M. Reproductive biology of the commercial sea cucumber Holothuria fuscogilva in the Solomon Islands. Mar. Biol. 2000, 136, 1045–1056. [Google Scholar] [CrossRef]
- Shiell, G.R.; Knott, B. Diurnal observations of sheltering behaviour in the coral reef sea cucumber Holothuria whitmaei. Fish. Res. 2008, 91, 112–117. [Google Scholar] [CrossRef]
- Mercier, A.; Hamel, J.F. Endogenous and exogenous control of gametogenesis and spawning in echinoderms. Adv. Mar. Biol. 2009, 55, 1–291. [Google Scholar] [CrossRef]
- Santos, R.; Dias, E.; Tecelão, C.; Pedrosa, R.; Pombo, A. Reproductive biological characteristics and fatty acid profile of Holothuria mammata (Grube, 1840). SPC Beche-De-Mer Inf. Bull. 2017, 37, 57–64. [Google Scholar]
- Venâncio, E.; Félix, P.M.; Brito, A.C.; e Silva, F.A.; Simões, T.; Sousa, J.; Mendes, S.; Pombo, A. Reproductive Biology of the Sea Cucumber Holothuria mammata (Echinodermata: Holothuroidea). Biology 2022, 11, 622. [Google Scholar] [CrossRef]
- Shiell, G.R.; Uthicke, S. Reproduction of the commercial sea cucumber Holothuria whitmaei [Holothuroidea: Aspidochirotida] in the Indian and Pacific Ocean regions of Australia. Mar. Biol. 2006, 148, 973–986. [Google Scholar] [CrossRef]
- Gaudron, S.M.; Kohler, S.A.; Conand, C. Reproduction of the sea cucumber Holothuria leucospilota in the Western Indian Ocean: Biological and ecological aspects. Invertebr. Reprod. Dev. 2008, 51, 19–31. [Google Scholar] [CrossRef]
- Ramos-Miranda, J.; del Río-Rodríguez, R.; Flores-Hernández, D.; Rojas-González, R.I.; Gómez-Solano, M.; Cu-Escamilla, A.D.; Gómez-Criollo, F.; Sosa-López, A.; Torres-Rojas, Y.E.; Juárez-Camargo, P. Reproductive cycle of the sea cucumber Holothuria floridana in the littorals of Campeche, Mexico. Fish. Sci. 2017, 83, 699–714. [Google Scholar] [CrossRef]
- Sewell, M.A.; Tyler, P.A.; Young, C.M.; Conand, C. Ovarian development in the class holothuroidea: A reassessment of the “tubule recruitment model”. Biol. Bull. 1997, 192, 17–26. [Google Scholar] [CrossRef]
- Dolmatov, I.Y. Asexual reproduction in holothurians. Sci. World J. 2014, 2014, 527234. [Google Scholar] [CrossRef]
- Micael, J.; Alves, M.J.; Costa, A.C.; Jones, M.B. Exploitation and conservation of echinoderms. In Oceanography and Marine Biology; CRC Press: Boca Raton, FL, USA, 2009; Volume 47. [Google Scholar]
- Mercier, A.; Battaglene, S.C.; Hamel, J.-F. Periodic movement, recruitment and size-related distribution of the sea cucumber Holothuria scabra in Solomon Islands. Hydrobiologia 2000, 440, 81–100. [Google Scholar] [CrossRef]
- Antoniadou, C.; Vafidis, D. Population structure of the traditionally exploited holothurian Holothuria tubulosa in the south Aegena Sea. Cah. Biol. Mar. 2011, 52, 171–175. [Google Scholar]
- Despalatović, M.; Grubelić, I.; Šimunović, A.; Antolić, B.; Žuljević, A. New data about reproduction of the holothurian Holothuria forskali (Echinodermata) living in geographically different places. Fresenius Environ. Bull. 2003, 12, 1345–1347. [Google Scholar]
- Acosta, E.J.; Rodríguez-Forero, A.; Werding, B.; Kunzmann, A. Ecological and reproductive characteristics of holothuroids Isostichopus badionotus and Isostichopus sp. in Colombia. PLoS ONE 2021, 16, e0247158. [Google Scholar] [CrossRef]
- Tuwo, A.; Conand, C. Reproductive biology of the holothurian Holothuria forskali (Echinodermata). J. Mar. Biol. Assoc. U. K. 1992, 72, 745–758. [Google Scholar] [CrossRef]
- Tuwo, A.; Conand, C. Fécondité de trois holothuries tempérées à développement pélagique. In Echinoderms Through Time; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Hopper, D.R.; Hunter, C.L.; Richmond, R.H. Sexual reproduction of the tropical sea cucumber, Actinopyga mauritiana (Echinodermata: Holothuroidea), in Guam. Bull. Mar. Sci. 1998, 63, 1–9. [Google Scholar]
- Costelloe, J. The annual reproductive cycle of the holothurian Aslia lefevrei (Dendrochirota: Echinodermata). Mar. Biol. 1985, 88, 155–165. [Google Scholar] [CrossRef]
- Dissanayake, D.C.; Stefansson, G. Abundance and distribution of commercial sea cucumber species in the coastal waters of Sri Lanka. Aquat. Living Resour. 2010, 23, 303–313. [Google Scholar] [CrossRef]
- Abdel-Razek, F.A.; Abdel-Rahmen, S.H.; El-Shumy, N.A.; Omar, H.A. Reproductive biology of the tropical sea cucumber Holothuria atra (Echinodermata:Holothuroidea) in the Red Sea coast of Egypt. Egypt J. Aquat. Res. 2005, 31, 383–402. [Google Scholar]
- Muthiga, N.A. The reproductive biology of a new species of sea cucumber, Holothuria (Mertensiothuria) arenacava in a Kenyan marine protected area: The possible role of light and temperature on gametogenesis and spawning. Mar. Biol. 2006, 149, 585–593. [Google Scholar] [CrossRef]
- Marquet, N.; Conand, C.; Power, D.M.; Canário, A.V.; González-Wangüemert, M. Sea cucumbers, Holothuria arguinensis and H. mammata, from the southern Iberian Peninsula: Variation in reproductive activity between populations from different habitats. Fish. Res. 2017, 191, 120–130. [Google Scholar] [CrossRef]
- Benítez-Villalobos, F.; Avila-Poveda, O.H.; Gutiérrez-Méndez, I.S. Reproductive biology of Holothuria fuscocinerea (Echinodermata: Holothuroidea) from Oaxaca, Mexico. Sex. Early Dev. Aquat. Org. 2013, 1, 13–24. [Google Scholar] [CrossRef]
- Battaglene, S.C.; Seymour, J.; Ramofafia, C.; Lane, I. Spawning induction of three tropical sea cucumbers, Holothuria scabra, H. fuscogilva and Actinopyga mauritiana. Aquaculture 2002, 207, 29–47. [Google Scholar] [CrossRef]
- Hamel, J.F.; Himmelman, J.H.; Dufresne, L. Gametogenesis and Spawning of the Sea Cucumber Psolus fabricii (Duben and Koren). Biol. Bull. 1993, 184, 125–143. [Google Scholar] [CrossRef]
- Cameron, J.L.; Fankboner, P.V. Reproductive biology of the commercial sea cucumber Parastichopus californicus (Stimpson) (Echinodermata: Holothuroidea). I. Reproductive periodicity and spawning behavior. Can. J. Zool. 1986, 64, 168–175. [Google Scholar] [CrossRef]
- Vergara-Chen, C.; González-Wangüemert, M.; Marcos, C.; Pérez-Ruzafa, Á. Genetic diversity and connectivity remain high in Holothuria polii (Delle Chiaje 1823) across a coastal lagoon-open sea environmental gradient. Genetica 2010, 138, 895–906. [Google Scholar] [CrossRef]



| Stage | Macroscopic Description | Microscopic Description | |
|---|---|---|---|
| Males | Females | ||
| Recovery (I) | Short and thin blind-ended tubules. Color: Translucent to whitish. | Thick gonadal walls (gw) with developing spermatocytes (sc) and oocytes (do) (12.6 ± 3.39 μm) lining the germinal epithelium (ge). Empty lumen. | |
| Growing (II) | Thick and long tubules. Color: Whitish in males, light pink in females. | Spermatocytes lining the numerous invaginations of the germinal epithelium, and increasing of spermatozoa in the lumen. | Developing oocytes lining the germinal epithelium, with pre-vitellogenic oocytes (32.7 ± 8.04 μm) progressively occupying the lumen of the tubule. |
| Early mature (III) | Long, numerous, elongated, and branching tubules. Color: Cream in males, light pink to orange in females. | Spermatogenesis advances, and spermatocytes are present along the germinal epithelium. Spermatozoa centrally located in the lumen. | Presence of oocytes at various stages of vitellogenesis (77.6 ± 2.158 μm). With the progression of vitellogenesis, fully developed oocytes, surrounded by follicular cells, commence occupying a central position in the lumen. |
| Mature (IV) | Maximum length and width of blind-ended tubules with thin walls. Color: Cream to beige in males, orange to reddish orange in females. | Lumen completely filled with densely packed mature spermatozoa. | Lumen completely filled with mature oocytes, surrounded by the follicle membrane, reach their maximum size (114.0 ± 18.1 μm). |
| Spent (V) | Shrunken gonadal walls. Color: Medium-brown to semitransparent with brownish blotches. | Presence of phagocytes and relict gametes within the lumen. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balatsou, A.; Apostologamvrou, C.; Vafidis, D. Fecundity Study and Histological Analysis of the Gonads of the Sea Cucumber Holothuria tubulosa (Echinodermata: Holothuroidea) in the Central Aegean Sea, Greece: Insights into Reproductive Biology. Fishes 2025, 10, 283. https://doi.org/10.3390/fishes10060283
Balatsou A, Apostologamvrou C, Vafidis D. Fecundity Study and Histological Analysis of the Gonads of the Sea Cucumber Holothuria tubulosa (Echinodermata: Holothuroidea) in the Central Aegean Sea, Greece: Insights into Reproductive Biology. Fishes. 2025; 10(6):283. https://doi.org/10.3390/fishes10060283
Chicago/Turabian StyleBalatsou, Athina, Chrysoula Apostologamvrou, and Dimitris Vafidis. 2025. "Fecundity Study and Histological Analysis of the Gonads of the Sea Cucumber Holothuria tubulosa (Echinodermata: Holothuroidea) in the Central Aegean Sea, Greece: Insights into Reproductive Biology" Fishes 10, no. 6: 283. https://doi.org/10.3390/fishes10060283
APA StyleBalatsou, A., Apostologamvrou, C., & Vafidis, D. (2025). Fecundity Study and Histological Analysis of the Gonads of the Sea Cucumber Holothuria tubulosa (Echinodermata: Holothuroidea) in the Central Aegean Sea, Greece: Insights into Reproductive Biology. Fishes, 10(6), 283. https://doi.org/10.3390/fishes10060283






