Abstract
Photobacterium damselae subsp. damselae is a well-recognized marine animal pathogen. Herein, 70 P. damselae subsp. damselae isolates were investigated for quinolone susceptibility and prevalence of the genes including quinolone resistance-determining regions (QRDRs) and plasmid-mediated quinolone resistance (PMQR) genes. A total of 18/70 isolates exhibited high-level resistance, and 23/70 isolates exhibited moderate resistance according to the MIC values. QRDR analysis showed that double mutants in both GyrA (Ser83Ile) and ParC (6/17 Ser80Phe or 11/17 Ser80Tyr) were detected in 94.4% (17/18) high-level quinolone resistance P. damselae subsp. damselae strains. PMQR detection showed that 60.0% (42/70) carried at least one PMQR (1/42 qnrB coexistence with aac(6′)-Ib-cr, 1/42 qnrS coexistence with aac(6′)-Ib-cr, 44/46 qnrS). QnrA, QnrC, qnrD and qepA were not detected in all strains. Among the 42 PMQR-positive strains, 24 showed fluoroquinolones MICs ≤ 0.5 mg/L and 13 MICs ≥ 2 mg/L, all carrying QRDR mutations. For the twenty-eight non-PMQR strains, twenty-three showed fluoroquinolone MICs ≤ 0.5 mg/L without QRDR mutations, and five MICs ≥ 2 mg/L carrying QRDR mutations. In conclusion, qnrS (qnrS2 allele) is the major PMQR widespread in P. damselae subsp. damselae isolated from eastern China; however, QRDR mutation plays a marked role in mediating fluoroquinolone resistance.
Keywords:
Photobacterium damselae subsp. damselae; quinolones; quinolone resistance-determining region (QRDR); plasmid-mediated quinolone resistance (PMQR) Key Contribution:
This study provides new insights into the prevalence and role of the quinolone resistance genes in Photobacterium damselae subsp. Damselae and it will allow the improvement of the rational use of quinolone drugs in aquaculture.
1. Introduction
Photobacterium damselae is a marine Gram-negative bacterium of the family Vibrionaceae, which includes two subspecies, damselae (formerly Vibrio damsela) and piscicida (previously known as Pasteurella piscicida) [1]. Although both subspecies are widespread in aquatic ecosystems, they have pathogenic potential for a variety of marine animals. P. damselae subsp. piscicida is the pathogen of fish pasteurellosis (also called pseudotuberculosis), characterized by the formation of granulomatous lesions in different organs of fish [1]. P. damselae subsp. damselae is considered a primary pathogen of wild and cultivated marine animals, including crustaceans, molluscs, fish, dolphins, and turtles [1,2]. Typical signs of P. damselae subsp. damselae infection in fish includes external skin ulceration that may progress to hemorrhagic septicemia, and petechiae or lesions in distinct internal organs [1,3]. In addition, P. damselae subsp. damselae is considered a zoonotic pathogen that can cause wound infections and fatal necrotizing fasciitis in humans due to exposure to marine fish, seawater, or raw seafood [1,3,4].
In aquaculture, intensification of existing farming systems delivers both higher production and greater risk of disease outbreaks [5]. Some emerging bacterial diseases, especially those caused by Vibrio spp., Aeromonas spp. and Pseudomonas spp., have been frequently reported in aquaculture, causing significant economic losses [6,7,8]. In China, the largest aquaculture production is accompanied by a large amount of antibiotic consumption [9]. Quinolones are synthetic antimicrobial agents that are widely used in aquaculture for the prevention and treatment of bacterial infections [10,11]. However, misuse of these antibiotics usually happens in some aquaculture systems due to high stocking densities and poor culture management and conditions [11,12]. Moreover, oral administration, as the main drug administration route in aquaculture, has many drawbacks such as the inability to precisely control the dosage, poor absorption, and easy spread into the environment, which will increase the antibiotic resistance in bacteria accumulated in cultured animals and environments around farms, and decrease the efficiency of the antibiotics and exacerbate their misuse [13,14]. Quinolone resistance has been reported in several aquatic pathogens including Aeromonas spp. [15] and Vibrio spp. [16,17]. Moreover, quinolone resistance genes have been reported in P. damselae subsp. piscicida strains [18]; however, this has not been studied in P. damselae subsp. damselae.
Several mechanisms are involved in quinolone resistance, such as chromosomal mutations on some quinolone target proteins and plasmid-mediated quinolone resistance genes (PMQRs) that protect bacteria from the effects of quinolones. Bacterial DNA gyrase and topoisomerase IV are the targets of quinolones, and some mutations associated with drug-binding domains in these proteins have been designated as quinolone resistance determining regions (QRDRs) [19]. Bacterial DNA gyrase and topoisomerase IV, both heteromeric tetramer proteins, were encoded by the gyrA and gyrB genes, and parC and parE genes, respectively. It has been demonstrated that the most frequent resistance-associated mutations occurred in the gyrA gene and parC gene, and substantially less commonly occurred in the gyrB gene and parE gene. Meanwhile, different types of PMQRs have been reported including target protection proteins (qnrs genes including qnrS, qnrA, qnrB, qnrC, and qnrD), drug modification enzymes (aac(6′)-Ib-cr), and active efflux pumps, including qepA and oqxAB [20]. In this study, we reported the quinolone resistance level and the presence of QRDRs and PMQRs in P. damselae subsp. damselae isolates recovered from diseased fish in eastern China.
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
A total of 70 P. damselae subsp. damselae isolates were previously isolated from diseased marine fish including Lateolabrax japonicus, Larimichthys crocea, and Pampus argenteus from some cage fish culture farms in Xiangshan and Yinzhou region, Ningbo, Zhejiang province, China (Supplementary Table S1). All isolates were identified by 16S rRNA gene sequencing and specific polymerase chain reaction (PCR) detection, as previously described [21]. Moreover, these bacterial strains were also cultivated in thiosulfate citrate bile salts sucrose agar (TCBS, HopebioTM, Qingdao, China) and blood agar (trypticase soy agar supplemented with 5% defatted sheep blood, HopebioTM, Qingdao, China) at 28 °C for phenotypic characteristics detection. All bacterial strains were stored frozen at −80 °C for future use. Escherichia coli ATCC 25922 was purchased from American Type Culture Collection and used as the reference strain for antibiotic susceptibility testing [22,23].
2.2. Minimum Inhibitory Concentration Test
The antibiotic agents included nalidixic acid (NA), pipemidic acid (PIP), norfloxacin (NFX), ciprofloxacin (CIP), gatifloxacin (GAT), and moxifloxacin (MXF). The minimum inhibitory concentrations (MICs) of antibiotic agents for P. damselae subsp. damselae isolates were performed visually and by optical density measurements, and a suddenly clear culture or decreasing OD value was interpreted as the drug MIC. In detail, bacterial cell suspensions were prepared in Mueller–Hinton broth (MHB, Oxoid) and the cell optical density was adjusted to 0.5 McFarland. Antibiotic reference standards including NA, PIP, NFX, CIP, GAT, and MXF (Dr. EhrenstorferTM, Augsburg, Germany) were prepared as per the method described by the Clinical and Laboratory Standards Institute (CLSI) [24]. All agents were two-fold diluted from an initial concentration of 1024 μg/mL in MHB, and 50 μL was added into the wells of 96-well plates. Then, 50 μL of P. damselae subsp. damselae suspensions was added into the wells and incubated at 28 °C for 24 h. Each plate included a quality control well, positive control well (bacterial suspension in MH broth without antimicrobials), and a negative control well (MH broth without bacteria), and all tests were carried out in triplicate. Escherichia coli ATCC 25922 was used as the quality control strain to assure the reliability of the MIC results. To verify the results, the Graphpad Prism 8 and TB tools software 8.0 was used to draw statistical maps.
2.3. Bacterial DNA Extraction
P. damselae subsp. damselae isolates were inoculated on TSA plates and incubated at 28 °C for 24 h, respectively. A single colony of the P. damselae subsp. damselae isolates were inoculated into 1 mL of TSB and incubated for 12 h respectively. Then, the bacterial cultures were centrifuged at 10,000× g for 2 min, and the bacterial pellets were washed twice with PBS and resuspended with 200 μL double-distilled water. These bacterial suspensions were treated in a boiling water bath for 5 min, and then placed into an ice bath for 5 min. The suspensions were centrifuged at 12,000× g for 5 min, and the supernatant was transferred to the sterilized centrifuge tube for antibiotic-resistant gene detection.
2.4. Amplified and Sequence Analysis of QRDRs
The complete sequences of gyrA, gyrB, parC, and parE in P. damselae subsp. damselae isolates were amplified by super-fidelity DNA polymerase (Vazyme, Nanjing China) using the templates prepared above and the primer sets were listed in Table 1. The polymerase chain reaction (PCR) was performed as follows: pre-denaturation at 98 °C for 30 s followed by 35 cycles of 98 °C for 10 s, 60 °C for 10 s, and 72 °C for 30 s. Then the PCR products were purified and sequenced for analysis. Each DNA sequence of the gyrA, gyrB, parC, and parE genes from different P. damselae subsp. damselae isolates were translated to an amino acid sequence by the ExPASy Translate tool (https://www.expasy.org/, accessed on 15 July 2024). The predicted amino acid sequences were blasted against the NCBI database using the BLASTP program (http://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 15 July 2024) to identify amino acid substitutions in GyrA, GyrB, ParC, and ParE, respectively.

Table 1.
Primers of resistance gene amplification for PCR.
2.5. Detection and Sequence Analysis of PMQRs
The presence of PMQR genes including qnrA, qnrB, qnrS, qnrC, qnrD, aac(6′)-Ib-cr, and qepA was also detected in P. damselae subsp. damselae isolates using the primer sets listed in Table 1. The PCR for detection was performed as follows: pre-denaturation at 98 °C for 30 s followed by 35 cycles of 98 °C for 10 s, 55–60 °C for 10 s, and 72 °C for 10 s. All PCR amplicons were checked by agarose gel electrophoresis and the PCR products were then subcloned into plasmid vector pMD19-T simple (Takara, Shiga, Japan) and transformed into competent E. coli DH5α cells for sequencing and alignments. Phylogenetic analyses of QnrB and QnrS were conducted on amino acids using the MEGA 11 program.
3. Results
3.1. Phenotypic Characteristics of the P. damselae subsp. damselae Isolates
Seventy P. damselae subsp. damselae strains were isolated from the diseased culture of marine fish including L. japonicus, L. crocea, and P. argenteus. In the blood plates, most of the isolates (59/70) showed significant hemolytic activity, some isolates (5/70) showed weak hemolytic activity, and some isolates (6/70) exhibited no hemolytic activity. Moreover, these P. damselae subsp. damselae strains showed different colors grown in TCBS plates: 11/70 showed yellow colonies in TCBS and 59/70 showed green colonies in TCBS (Supplementary Table S1).
3.2. Antibiotic Resistance Profiles
The MIC values for the bacterial strains are shown in Figure 1 and Table 2. According to the MIC values, 25.17% (18/70) and 25.71% (18/70) of the isolates showed high-level resistance to early quinolones NAL and PIP, and the MIC values ranged from 128 mg/L to greater than 512 mg/L and 64 mg/L to greater than 512 mg/L, respectively. A total of 18.57% (15/70) and 5.71% (4/70) of the isolates showed high-level resistance to third-generation quinolones CIP and NOR, with the MIC values ranging from 4 mg/L to 32 mg/L and 16 mg/L to 32 mg/L, respectively. It was shown that 21.42% (15/70) and 5.71% (4/70) of the isolates were highly resistant to fourth-generation quinolones MOX and GAT, with the MIC values ranging from 8 mg/L to 32 mg/L and 8 mg/L to 16 mg/L, respectively (Table 2). Moreover, there were 23 other isolates that showed moderate resistance to at least one quinolone or/and fluoroquinolone drug (more than 4-fold MIC values) (Table 2). In these moderate resistance strains, eight isolates showed resistance to both quinolones and fluoroquinolones, and fifteen isolates showed only resistance to at least one fluoroquinolone drug (Figure 2).
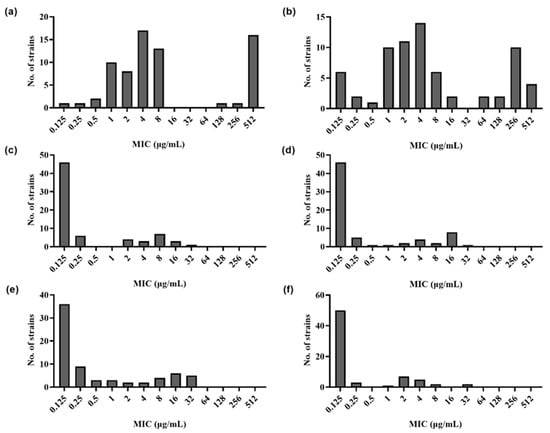
Figure 1.
MICs of 70 P. damselae subsp. damselae isolates against (a) nalidixic acid, (b) pipemidic acid, (c) norfloxacin, (d) ciprofloxacin, (e) moxifloxacin, and (f) gatifloxacin.

Table 2.
Amino acid substitution in GyrA, GyrB, and ParC and the detection of PMQRs and MICs in 70 Photobacterium damselae subsp. damselae isolates.
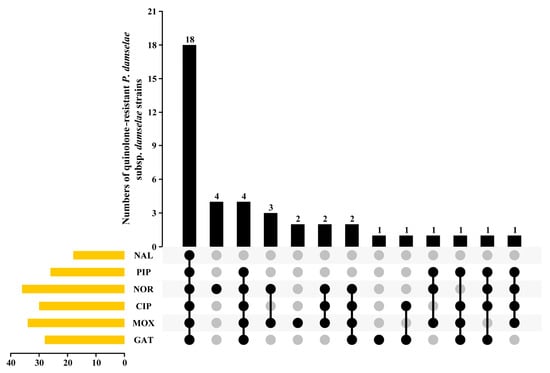
Figure 2.
Drug resistance spectrum of 41 P. damselae subsp. damselae strains to 6 quinolone or fluoroquinolone drugs. NAL, nalidixic acid; PIP, pipemidic acid; NOR, norfloxacin; CIP, ciprofloxacin; MOX, moxifloxacin; The round dots represent each quinolone antibiotic drug, the grey dots represent no drug resistance, and the black dots represent drug resistance.
3.3. Mutations in Genes That Encode the Quinolone Target Enzymes
Sequences analysis of the genes that encode quinolone target enzymes showed that at least one mutation was found in gyrA, gyrB, or parC, but no mutation was detected in parE of the P. damselae subsp. damselae isolates. As shown in Table 1, some GyrA substitution strains were detected in these isolates, including fifteen single-GyrA substitutions, Ser83Ile (14/18) and Ser224Asn (1/18), two double-GyrA substitutions, Ser83Ile plus His154Gln (2/18), and one triple substitution Ser83Ile, Asp141Glu plus His154Gln (1/18). The Ser83Ile mutation was found in all high-level quinolone resistance strains; the Asp141Glu and His154Gln mutations which were found were accompanied by the Ser83Ile mutation in some high-level quinolone resistance strains. The only Ser224Asn mutation was found in a moderate resistance strain. Sequence analysis showed five GyrB substitution strains including four single substitutions—Gly585Ser (1/5), Pro613Leu (1/5), Ala717Val (2/5) and a double mutation, Val630Ala plus Ser655Asn (1/5). Nineteen ParC substitution strains were detected in this study, including the single mutation Ser80Phe (4/19), Ser80Tyr (8/19), Asp475Asn (1/19), Ala697Ser (1/19), and the double mutations Ser80Phe plus Thr459Ser (1/19), Ser80Phe plus Glu556Lys (1/19) and Ser80Phe plus Ala697Ser (2/19). Likewise, the Ser80Phe or Ser80Tyr mutation was found in all high-level quinolone resistance strains. In these strains, a single ParC substitution Asp475Asn was an exception, because it showed high-level quinolone resistance even though the single mutation was not within the QRDR of ParC according to current reports.
3.4. Prevalence of PMQR Genes
Detection of PMQR genes showed that 42/70 of P. damselae subsp. damselae strains carried at least one PMQR gene (1/42 qnrB plus aac(6′)-Ib-cr, 1/42 qnrS plus aac(6′)-Ib-cr, 40/42 qnrS). Other PMQR genes including qnrA, qnrC, qnrD, and qepA were not detected in these strains (Table 2). Among these PMQR-positive strains, 24 showed fluoroquinolone MICs ≤ 0.5 mg/L and 13 MICs ≥ 2 mg/L, all carrying mutations in quinolone target enzymes. For the twenty-eight non-PMQR strains, twenty-three showed fluoroquinolone MICs ≤ 0.5 mg/L without QRDR mutations, and five MICs ≥ 2 mg/L carrying QRDR mutations. Sequence similarity analysis showed 100% homology among the qnrS from different P. damselae subsp. damselae strains. The phylogenetic trees constructed using different bacterial QnrS and QnrB sequences showed that P. damselae subsp. damselae QnrS was grouped with QnrS2 in V. alginolyticus, and the P. damselae subsp. damselae QnrB was grouped with QnrB7 in E. coli (Figure 3).
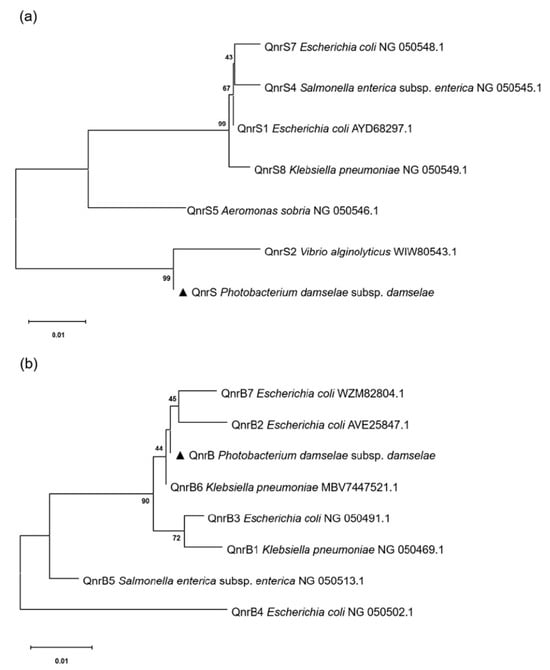
Figure 3.
Phylogenetic tree of QnrS (a) and QnrB (b) proteins. QnrS and QnrB of P. damselae subsp. damselae detected in the present study were marked by triangles. The rooted trees were constructed via the neighbor-joining method and bootstrapped 1000 times using MEGA 8.0 software.
4. Discussion
Antibiotics, especially quinolones, are most extensively used for preventing and treating Gram-negative bacteria infections in aquaculture; however, the natural aquatic environment is a significant contributor to the dissemination of the quinolone resistance determinants [31,32]. One study reported that the E. coli strains isolated from imported shrimp (Litopenaeus vannamei) in the USA exhibited high quinolone resistance rates—52.4% (55/105) were resistant to nalidixic acid, 34.5% (19/55) of which were resistant to ciprofloxacin and ofloxacin [33]. Another report showed that 44.1% (15/34) of Aeromonas spp. strains showed resistance to quinolones in South Africa [15]. In recent years, P. damselae subsp. damselae has been frequently reported and caused high mortality in some aquatic animals in China, such as some marine fish and crustacean species including Pampus argenteus [21], Litopenaeus vannamei [34], and Scylla paramamosain [35]. However, quinolone resistance and prevalence of the related genes in P. damselae subsp. damselae have not been monitored and reported in China. P. damselae subsp. damselae, a commensal bacterium in aquatic ecosystems and a multiple zoonotic pathogen, showed a considerable proportion of high-level quinolone and fluoroquinolone resistance in our study. These results indicated the effect of quinolone drugs would not be good when they were used for treatment of P. damselae subsp. damselae infections in aquaculture. Moreover, the results also showed a higher fluoroquinolone resistance rate than quinolone resistance rate in these P. damselae subsp. damselae strains. In China, enrofloxacin was the only quinolone drug that was permitted to be used for the prevention and treatment of bacterial infections in aquaculture [36], and the higher fluoroquinolone resistance rate might be due to the frequent use of enrofloxacin.
Chromosomal mutations in the quinolone target enzymes were recognized to be the major mechanisms of quinolone resistance in many Gram-negative bacteria [37,38]. In some Enterobacteriaceae species, quinolone resistance is mainly mediated by mutations in gyrA and parC [20,39]. It was found that the QRDR of GyrA spans 67 to 107 amino acids in its catalytic core and the most common alternation region in GyrA was Ser83 followed by Asp87; the QRDR of ParC contains the residues of 63 to 102 amino acids and the most common mutations were substitutions at positions Ser80 and Glu84 [20,39]. Moreover, the Ser83 substitution in gyrA was also the most commonly detected mutation in Aeromonas spp. [32,33,34]. An earlier study found that all quinolone-resistant P. damselae subsp. piscicida strains in Japan carried the single substitution Ser83Ile in the QRDR of GyrA [18]. In our study, it showed that the double substitutions Ser83Ile in GyrA QRDR and Ser80 Tyr or Ser80Phe in ParC QRDR were detected in 17/18 of high-level quinolone resistance P. damselae subsp. damselae strains isolated from diseased fish in eastern China. Meanwhile, some novel amino acid substitutions not within the QRDR of the quinolone target enzymes were also detected in these high-level quinolone resistance strains, such as Asp141Glu and His154Gln in gyrA, Ser655Asn, Pro613Leu, Val630Ala in gyrB, Thr459Ser, Glu556Lys in parC. Some amino acid substitutions were also detected in moderate resistance strains, such as Ser224Asn substitution in gyrA and the Gly585Ser in gyrB. Two amino acid substitutions, Ala717Val in gyrB and Ala697Ser in parC, were detected both in a quinolone-sensitive strain and resistance strain, which suggested these mutations are not associated with quinolone resistance in P. damselae subsp. damselae. Interestingly, a P. damselae subsp. damselae strain contains a novel single amino acid substitution, Asp475Asn, not within the parC QRDR, which showed high-level quinolone resistance in this study; however, whether this novel mutation was associated with quinolone resistance remains to be confirmed by further experiments.
Commonly, the PMQR genes could reduce bacterial susceptibility to quinolones, but not always mediate high-level drug resistance [32]. Notwithstanding the foregoing, PMQR genes have shown significant effects on quinolone resistance, because they make a significant supplement to chromosomal mutations on quinolone resistance and spread quickly among bacteria via conjunction or other routes [20,40]. Therefore, the PMQR-mediated quinolone resistance in bacteria has been paid much attention worldwide. Prevalence of PMQR genes has been reported in many environmental or/and pathogenic bacteria, such as E. coli [16], Klebsiella pneumoniae [41], Pseudomonas aeruginosa [42], and V. cholerae [43]. The studies have shown that the prevalence of PMQR genes were different along with different bacterial species and ecoregions. The qnrS genes seem to be the most commonly identified acquired qnr genes in the environment and have been mainly identified from waterborne species, such as Aeromonas spp. [15,44] and V. parahaemolyticus [16]. In the present study, it has been shown that the qnrS2 gene was the most the most frequently detected PMQR gene (41/70) in P. damselae subsp. damselae, which suggested qnrS (qnrS2 allele) is the major PMQR and widespread in P. damselae subsp. damselae strains in eastern China. Comparatively, a recent report showed that only one strain carried qnrS in V. alginolyticus isolates from aquatic environments in costal mariculture areas in China [45]. This was consistent with another report that a total of 34/1811 (1.88%) bacteria were found to harbor the qnrS gene in foodborne Vibrio isolates [46]. The qnrB was one of the most frequently detected qnr genes in E. coli and K. pneumoniae [47], but rarely detected in commensal bacteria in aquatic ecosystems [48]. In our study, the qnrB was detected in one P. damselae subsp. damselae strain, and it was grouped with QnrB7 in E. coli, which suggested that it may uptake it from other bacteria species in environment. Moreover, two P. damselae subsp. damselae isolates harboring aac(6′)-Ib-cr were detected in coexistence with qnrS and qnrB, respectively, and they showed higher quinolone and fluroquinolone resistance levels in aac(6′)-Ib-cr coexistence with the qnrS strain according to the MIC values.
In sum, the P. damselae subsp. damselae isolates used in this study exhibited high diversity based on information on the origin host, time of isolation, bacterial colony color, hemolytic characteristics, sequence analysis of QRDRs, and detection of PMQR genes. However, this study showed that all high-level resistance P. damselae subsp. damselae strains were isolated from the Xiangshan region, and some strains showed similar colony color, hemolytic characteristics, and sequence analysis of QRDRs (showed in Table 2 and Supplementary Table S1), suggesting the existence of clone spreading among those isolates. Moreover, qnrB and aac(6′)-Ib-cr were only detected in P. damselae subsp. damselae strains isolated from the Yinzhou region but were not detected in Xiangshan isolates, which suggested these two PMQRs might be acquired from the surrounding water environment or other bacteria. The qnrS2 was the major PMQR and was detected in bacterial strains isolated from both regions, which demonstrated the ubiquity of qnrS2 in the aquatic environment of these two regions. Given the high-level quinolone or/and fluoroquinolone resistance found in P. damselae subsp. damselae, other antibiotics or microorganism composites rather than enrofloxacin would be better for treatment of P. damselae subsp. damselae infections in fish.
5. Conclusions
This study showed a considerable proportion of quinolone or/and fluoroquinolone resistance in P. damselae subsp. damselae recovered from diseased fish in eastern China. The high-level quinolone or/and fluoroquinolone resistance was mainly mediated by the double substitutions Ser83Ile in GyrA QRDR and Ser80 Tyr or Ser80Phe in ParC QRDR. PMQR detection showed the qnrS (qnrS2 allele) was the most prevalent qnr in P. damselae subsp. damselae isolated in eastern China. Moreover, based on integrated information on the bacterial isolates, it is suggested that the quinolone or/and fluoroquinolone resistance demonstrates clone spreading among P. damselae subsp. damselae isolates.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fishes10060280/s1, Table S1: Phenotypic and isolation data for 70 Photobacterium damselae subsp. damselae isolates from diseased marine fish in Zhejiang province, China.
Author Contributions
X.Y.: Methodology; C.S.: Methodology; S.Z.: Designed study, Writing—original draft, Funding acquisition; L.J.: Data curation; Y.W.: Resources, Writing—review and editing; F.Y.: Writing—review and editing, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Zhejiang Provincial Natural Science Foundation (LTGN24C190002).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rivas, A.J.; Lemos, M.L.; Osorio, C.R. Photobacterium damselae subsp. damselae, a bacterium pathogenic for marine animals and humans. Front. Microbiol. 2013, 4, 283. [Google Scholar] [CrossRef]
- Andreoni, F.; Magnani, M. Photobacteriosis: Prevention and diagnosis. J. Immunol. Res. 2014, 2014, 793817. [Google Scholar] [CrossRef]
- Yamane, K.; Asato, J.; Kawade, N.; Takahashi, H.; Kimura, B.; Arakawa, Y. Two cases of fatal necrotizing fasciitis caused by Photobacterium damsela in Japan. J. Clin. Microbiol. 2004, 42, 1370–1372. [Google Scholar] [CrossRef]
- Osorio, C.R.; Vences, A.; Matanza, X.M.; Terceti, M.S. Photobacterium damselae subsp. damselae, a generalist pathogen with unique virulence factors and high genetic diversity. J. Bacteriol. 2018, 200, e00002–e000018. [Google Scholar] [CrossRef] [PubMed]
- Samsing, F.; Barnes, A.C. The rise of the opportunists: What are the drivers of the increase in infectious diseases caused by environmental and commensal bacteria? Rev. Aquacult. 2024, 16, 1787–1797. [Google Scholar] [CrossRef]
- Sanches-Fernandes, G.M.M.; Sá-Correia, I.; Costa, R. Vibriosis outbreaks in aquaculture: Addressing environmental and public health concerns and preventive therapies using gilthead seabream farming as a model system. Front. Microbiol. 2022, 13, 904815. [Google Scholar] [CrossRef]
- Igbinosa, I.H.; Igumbor, E.U.; Aghdasi, F.; Tom, M.; Okoh, A.I. Emerging Aeromonas species infections and their significance in public health. Sci. World J. 2012, 13, 625023. [Google Scholar] [CrossRef]
- Duman, M.; Mulet, M.; Altun, S.; Saticioglu, I.B.; Ozdemir, B.; Ajmi, N.; Lalucat, J.; Elena García-Valdés, E. The diversity of Pseudomonas species isolated from fish farms in Turkey. Aquaculture 2021, 535, 736369. [Google Scholar] [CrossRef]
- Wang, C.; Liu, X.; Yang, Y.; Wang, Z. Antibiotic and antibiotic resistance genes in freshwater aquaculture ponds in China: A meta-analysis and assessment. J. Clean. Prod. 2021, 329, 129719. [Google Scholar] [CrossRef]
- Samuelsen, O.B. Pharmacokinetics of quinolones in fish: A review. Aquaculture 2006, 255, 55–75. [Google Scholar] [CrossRef]
- Shao, G.J.; Pan, X.D.; Han, J.L. Antibiotic residues in commercial freshwater fish from southeast China: Distribution and human health risk assessment. Environ. Sci. Pollut. Res. 2024, 31, 23780–23789. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Wang, Y.; Yuan, Y.; Xie, Y. A systematic review on antibiotics misuse in livestock and aquaculture and regulation implications in China. Sci. Total Environ. 2021, 798, 149205. [Google Scholar] [CrossRef]
- Singh, M.; Singh, P.; Singh, M.; Singh, P. Drugs and chemicals applied in aquaculture industry: A review of commercial availability, recommended dosage and mode of application. J. Entomol. Zool. Stud. 2018, 6, 903–907. [Google Scholar]
- Ljubojević Pelić, D.; Radosavljević, V.; Pelić, M.; Živkov Baloš, M.; Puvača, N.; Jug-Dujaković, J.; Gavrilović, A. Antibiotic residues in cultured fish: Implications for food safety and regulatory concerns. Fishes 2024, 9, 484. [Google Scholar] [CrossRef]
- Chenia, H.Y. Prevalence and characterization of plasmid-mediated quinolone resistance genes in Aeromonas spp. isolated from South African freshwater fish. Int. J. Food Microbiol. 2016, 231, 26–32. [Google Scholar] [CrossRef]
- Aedo, S.; Ivanova, L.; Tomova, A.; Cabello, F.C. Plasmid-related quinolone resistance determinants in epidemic Vibrio parahaemolyticus, uropathogenic escherichia coli, and marine bacteria from an aquaculture area in Chile. Microb. Ecol. 2014, 68, 324–328. [Google Scholar] [CrossRef]
- Laganà, P.; Caruso, G.; Minutoli, E.; Zaccone, R.; Santi, D. Susceptibility to antibiotics of Vibrio spp. and Photobacterium damsela spp. piscicida strains isolated from italian aquaculture farms. New Microbiol. 2011, 34, 53. [Google Scholar] [CrossRef]
- Kim, M.; Hirono, I.; Aoki, T. Detection of quinolone-resistance genes in Photobacterium damselae subsp. piscicida strains by targeting-induced local lesions in genomes. J. Fish Dis. 2005, 28, 463–471. [Google Scholar] [CrossRef]
- Hooper, D.C. Bacterial topoisomerases, anti-topoisomerases, and anti-topoisomerase resistance. Clin. Infect. Dis. 1998, 27 (Suppl. S1), 54–63. [Google Scholar] [CrossRef]
- Hooper, D.C.; Jacoby, G.A. Mechanisms of drug resistance: Quinolone resistance. Ann. N. Y. Acad. Sci. 2015, 1354, 12–31. [Google Scholar] [CrossRef]
- Tao, Z.; Shen, C.; Zhou, S.M.; Yang, N.; Wang, G.L.; Wang, Y.J.; Xu, S.L. An outbreak of Photobacterium damselae subsp. damselae infection in cultured silver pomfret Pampus argenteus in Eastern China. Aquaculture 2018, 492, 201–205. [Google Scholar] [CrossRef]
- Lin, D.; Chen, K.; Wai-Chi Chan, E.; Chen, S. Increasing prevalence of ciprofloxacin-resistant food-borne Salmonella strains harboring multiple PMQR elements but not target gene mutations. Sci. Rep. 2015, 5, 14754. [Google Scholar] [CrossRef]
- Gomaa Elsayed, A.; Fahmy, E.M.; Abdellatif Alsayed, M.; Ahmed, M.E.; El Sayed Zaki, M.; Mofreh Mohamed, M. Study of plasmid mediated quinolone resistance genes among Escherichia coli and Klebsiella pneumoniae isolated from pediatric patients with sepsis. Sci. Rep. 2024, 14, 11849. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 28th ed.; CLSI Supplement M100; Clinical and Laboratory Standards Institute: Berwyn, PA, USA, 2018. [Google Scholar]
- Robicsek, A.; Strahilevitz, J.; Sahm, D.F.; Jacoby, G.A.; Hooper, D.C. Qnr prevalence in ceftazidime-resistant enterobacteriaceae isolates from the United States. Antimicrob. Agents Chemother. 2006, 50, 2872–2874. [Google Scholar] [CrossRef]
- Gay, K.; Robicsek, A.; Strahilevitz, J.; Park, C.H.; Jacoby, G.; Barrett, T.J.; Medalla, F.; Chiller, T.M.; Hooper, D.C. Plasmid-mediated quinolone resistance in non-typhi serotypes of Salmonella enterica. Clin. Infect. Dis. 2006, 43, 297–304. [Google Scholar] [CrossRef]
- Wang, M.; Guo, Q.; Xu, X.; Wang, X.; Ye, X.; Wu, S.; Hooper, D.C.; Wang, M. New plasmid-mediated quinolone resistance gene, qnrC, found in a clinical isolate of Proteus mirabilis. Antimicrob. Agents Chemother. 2009, 53, 1892–1897. [Google Scholar] [CrossRef] [PubMed]
- Cavaco, L.M.; Hasman, H.; Xia, S.; Aarestrup, F.M. qnrD, A novel gene conferring transferable quinolone resistance in Salmonella enterica serovar kentucky and bovismorbificans strains of human origin. Antimicrob. Agents Chemother. 2009, 53, 603–608. [Google Scholar] [CrossRef]
- Park, C.H.; Robicsek, A.; Jacoby, G.A.; Sahm, D.; Hooper, D.C. Prevalence in the United States of aac(6′)-Ib-cr encoding a ciprofloxacin-modifying enzyme. Antimicrob. Agents Chemother. 2006, 50, 3953–3955. [Google Scholar] [CrossRef]
- Kim, E.S.; Jeong, J.Y.; Choi, S.-H.; Lee, S.O.; Kim, S.H.; Kim, M.N.; Woo, J.H.; Kim, Y.S. Plasmid-mediated fluoroquinolone efflux pump gene, qepA, in Escherichia coli clinical isolates in Korea. Diagn. Microbiol. Infect. Dis. 2009, 65, 335–338. [Google Scholar] [CrossRef]
- Kamruzzaman, M.; Shoma, S.; Bari, S.M.N.; Ginn, A.N.; Wiklendt, A.M.; Partridge, S.R.; Faruque, S.M.; Iredell, J.R. Genetic diversity and antibiotic resistance in Escherichia coli from environmental surface water in Dhaka City, Bangladesh. Diagn. Microbiol. Infect. Dis. 2013, 76, 222–226. [Google Scholar] [CrossRef]
- Miranda, C.D.; Concha, C.; Godoy, F.A.; Lee, M.R. Aquatic environments as hotspots of transferable low-level quinolone resistance and their potential contribution to high-level quinolone resistance. Antibiotics 2022, 11, 1487. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.; Sung, K.; Kweon, O.; Khan, S.; Nawaz, S.; Steele, R. Characterisation of novel mutations involved in quinolone resistance in Escherichia coli isolated from imported shrimp. Int. J. Antimicrob. Agents 2015, 45, 471–476. [Google Scholar] [CrossRef]
- Wang, Z.; Shi, C.; Wang, H.; Wan, X.; Zhang, Q.; Song, X.; Li, G.; Gong, M.; Ye, S.; Xie, G.; et al. A novel research on isolation and characterization of Photobacterium damselae subsp. damselae from pacific white shrimp, Penaeus vannamei, displaying black gill disease cultured in China. J. Fish Dis. 2020, 43, 551–559. [Google Scholar] [CrossRef]
- Xie, J.; Mei, H.; Jin, S.; Bu, L.; Wang, X.; Wang, C.; Zhao, Q.; Ma, R.; Zhou, S. First report of Photobacterium damselae subsp. damselae infection in the mud crab Scylla paramamosain cultured in China. Aquaculture 2021, 530, 735880. [Google Scholar] [CrossRef]
- MAPRC. Decision on the Prohibition of Four Veterinary Drugs (Lomefloxacin, Pefloxacin, Ofloxacin and Norfloxacin) in Food Animals; Bulletin No. 2292; MAPRC: Beijing, China, 2015.
- Ghosh, A.S.; Ahamed, J.; Chauhan, K.K.; Kundu, M. Involvement of an efflux system in high-level fluoroquinolone resistance of Shigella dysenteriae. Biochem. Biophys. Res. Commun. 1998, 242, 54–56. [Google Scholar] [CrossRef]
- Wolfson, J.S.; Hooper, D.C. Fluoroquinolone antimicrobial agents. Clin. Microbiol. Rev. 1989, 2, 378–424. [Google Scholar] [CrossRef]
- Bhatnagar, K.; Wong, A. The mutational landscape of quinolone resistance in Escherichia coli. PLoS ONE 2019, 14, e0224650. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Martínez, J.M.; Machuca, J.; Cano, M.E.; Calvo, J.; Martínez-Martínez, L.; Pascual, A. Plasmid-mediated quinolone resistance: Two decades on. Drug Resist. Updat. 2016, 29, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Kareem, S.M.; Al-kadmy, I.M.; Kazaal, S.S.; Ali, A.N.M.; Aziz, S.N.; Makharita, R.R.; Algammal, A.M.; Al-Rejaie, S.; Behl, T.; Batiha, G.E.-S. Detection of gyrA and parC mutations and prevalence of plasmid-mediated quinolone resistance genes in Klebsiella pneumoniae. Infect. Drug Resist. 2021, 14, 555–563. [Google Scholar] [CrossRef]
- Saki, M.; Farajzadeh Sheikh, A.; Seyed-Mohammadi, S.; Asareh Zadegan Dezfuli, A.; Shahin, M.; Tabasi, M.; Veisi, H.; Keshavarzi, R.; Khani, P. Occurrence of plasmid-mediated quinolone resistance genes in Pseudomonas aeruginosa strains isolated from clinical specimens in Southwest Iran: A multicentral study. Sci. Rep. 2022, 12, 2296. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Ma, L.Y.; Yu, L.; Lu, X.; Liang, W.L.; Kan, B.; Su, J.R. Quinolone resistance genes and their contribution to resistance in Vibrio cholerae serogroup O139. Antibiotics 2023, 12, 416. [Google Scholar] [CrossRef] [PubMed]
- Dobiasova, H.; Kutilova, I.; Piackova, V.; Vesely, T.; Cizek, A.; Dolejska, M. Ornamental fish as a source of plasmid-mediated quinolone resistance genes and antibiotic resistance plasmids. Vet. Microbiol. 2014, 171, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Li, H.; Wang, Y.; Zhang, Z.; Liao, M.; Rong, X.; Li, B.; Wang, C.; Ge, J.; Zhang, X. Antibiotic resistance, virulence and genetic characteristics of Vibrio alginolyticus isolates from aquatic environment in costal mariculture areas in China. Mar. Pollut. Bull. 2022, 185, 114219. [Google Scholar] [CrossRef]
- Xu, Y.; Zheng, Z.; Ye, L.; Chan, E.W.C.; Chen, S. Identification and genetic characterization of conjugative plasmids encoding coresistance to ciprofloxacin and cephalosporin in foodborne Vibrio spp. Microbiol. Spectrum 2023, 11, e01032-23. [Google Scholar] [CrossRef]
- Shin, J.H.; Jung, H.J.; Lee, J.Y.; Kim, H.R.; Lee, J.N.; Chang, C.L. High rates of plasmid-mediated quinolone resistance qnrB variants among ciprofloxacin-resistant Escherichia coli and Klebsiella pneumoniae from urinary tract infections in Korea. Microb. Drug Resist. 2008, 14, 221–226. [Google Scholar] [CrossRef]
- Yan, L.; Liu, D.; Wang, X.H.; Wang, Y.; Zhang, B.; Wang, M.; Xu, H. Bacterial plasmid-mediated quinolone resistance genes in aquatic environments in China. Sci. Rep. 2017, 7, 40610. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).