Microecological Preparations as Antibiotic Alternatives in Cyprinid Aquaculture
Abstract
1. Introduction
| Side Effect | Description | Reference |
|---|---|---|
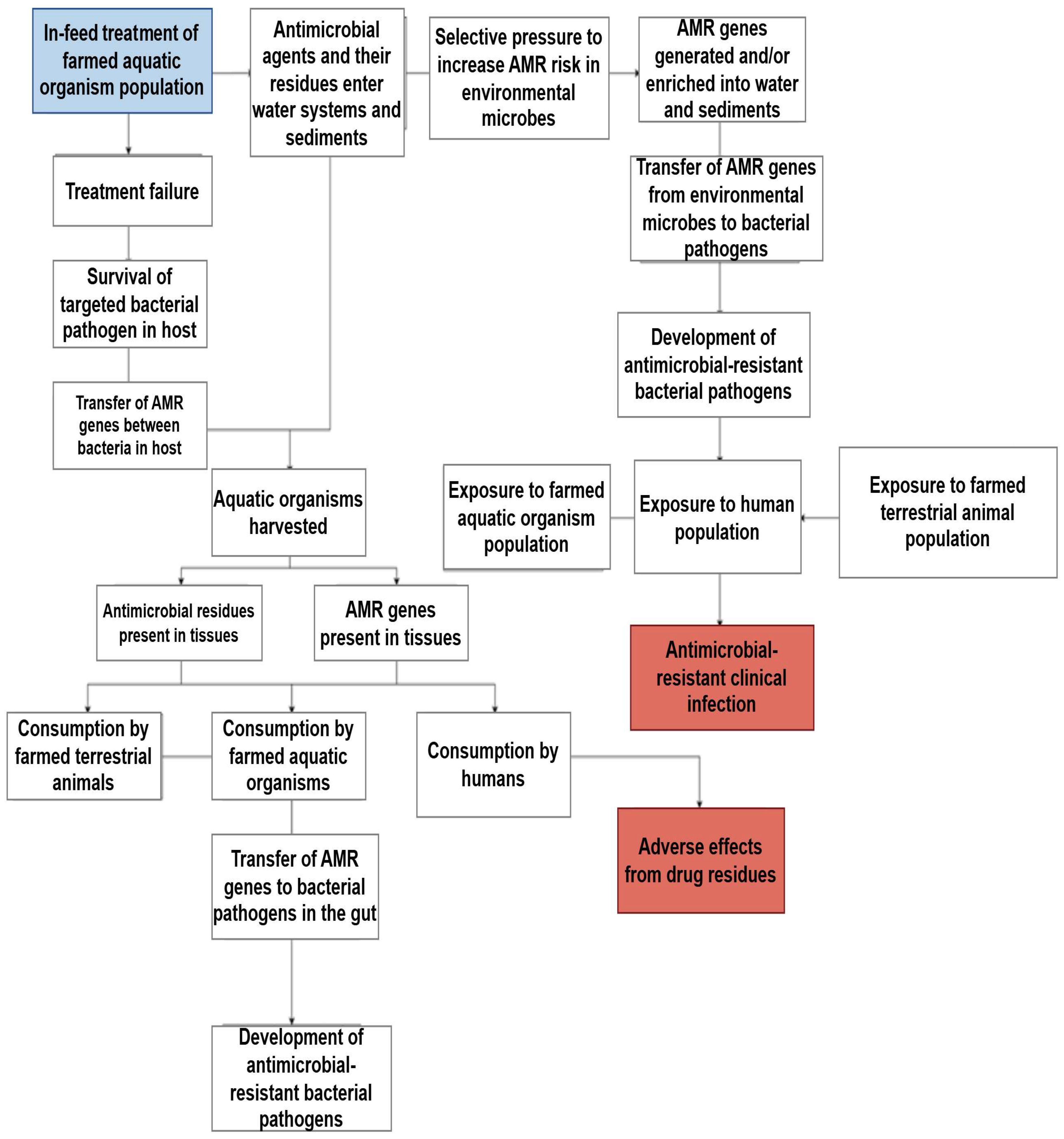
| AMR | The residues in aquaculture products can lead to the emergence of antibiotic-resistant bacteria. This will reduce the effectiveness of the antibiotics used to treat human infections, leading to prolonged disease progression and increased healthcare costs. | [11] |
| Residue accumulation | The continued consumption of residues in fish products can lead to biological accumulation in the human body, which, over time, may increase the risk of chronic health problems, such as organ damage and endocrine disorders. | [12] |
| Allergic reactions | Some people may experience allergic reactions to antibiotic residues, ranging from mild symptoms, such as rash or urticaria, to more severe reactions, such as allergic reactions. Specific antibiotics, such as penicillin, are more likely to trigger allergic reactions. | [13,14] |
| Toxicity | Long-term exposure to antibiotic residues, especially those that have not been fully metabolized, may have toxic effects on human organs and systems. For example, certain antibiotics, such as tetracyclines and sulfonamides, can cause kidney or liver damage if consumed in large quantities. | [15,16] |
| Disruption of gut microbiota | Antibiotic residues can alter the natural balance of gut bacteria, potentially leading to digestive problems, weakened immune responses, and even increased susceptibility to infections. This kind of destruction is particularly concerning in children, who heavily rely on healthy gut microbiota for normal development. | [17] |
| Potential carcinogenicity | Some antibiotics used in aquaculture, such as nitrofurans and quinolones, have been shown to have carcinogenic properties. These antibiotics may cause DNA damage or mutations, thereby disrupting normal cell growth. For example, nitrofuran can form reactive intermediates that bind to cellular macromolecules, leading to potential cancer development. Similarly, it has been found that certain quinolone drugs increase oxidative stress and interact with enzymes involved in DNA replication, which may lead to chromosomal damage. | [18,19,20,21,22] |
| Endocrine disruption | Some antibiotics, such as fluoroquinolones and tetracyclines, can act as endocrine disruptors. Their residues can interfere with hormone regulation and may affect reproductive health, growth, and development. This kind of destruction may have a significant impact on human fertility and development. | [23] |
| Drug interactions | Antibiotic residues in food can interact with drugs taken by humans, reducing their effectiveness or causing adverse reactions. For example, residual tetracycline may reduce the efficacy of certain anticoagulant drugs, thereby increasing the risk of blood clotting. | [24] |
2. The Advantages and Disadvantages of Different Antibiotic Alternatives
- The efficacy of bacteriophages must be supported by in vivo experiments.
- Ensuring the safety of bacteriophages for humans and aquatic animals is non-negotiable.
- Phage management should be economically feasible.
| Vaccine Type | Administration Method | Advantages | Disadvantages | Application Examples (Pathogens/Diseases) | Host Fish | Significant Effect | Reference |
|---|---|---|---|---|---|---|---|
| Inactivated vaccine | Injection, oral administration. | High safety. Easy production and storage. | Multiple vaccinations are required. Short duration of immunity. | Salmon Aeromonas and Streptococcus infections. | Salmon and tilapia. | ++ | [45,46,47] |
| Attenuated live vaccine | Injection and soaking. | Long lasting immunity. Single vaccination is effective. | Potential risk of toxicity reversal. Poor thermal stability. | Koi herpesvirus, Edwardsiella late-onset. | Carp, sea bream. | +++ | [48,49,50] |
| Subunit vaccine | Oral administration and injection. | High purity and safety. Targeted key antigens. | Adjuvant is needed to enhance the effect. Weak immunogenicity. | Fish respiratory enterovirus (PRV), rhabdovirus. | Atlantic salmon. | ++ | [51,52] |
| DNA vaccine | Intamuscular injection. | Inducing cellular immunity. Rapid production. | Potential risks of genome integration. Local absorption limitation. | IHNV, SVCV Bullet virus. | Salmonidae fish. | +++ | [53,54,55] |
| Vector vaccine | Injection, oral administration. | Multivalent antigen delivery. Mucosal immune activation. | Risk of carrier autoimmune response. | Recombinant Delayed Edwardsiella (RAEV). | Zebrafish, salmon. | ++ | [6,56] |
| Nanoparticle vaccine | Oral administration, soaking. | Enhance antigen stability. Targeted delivery. | Long-term toxicity unknown. High cost. | Virus-like particles (VLPs), ISCOM. | Various freshwater fish. | ++ | [57,58] |
| Pathogen | Disease | Animal or Seafood Species | Name of Phage (Morphology) 1 | Method of Application 2 | Outcome | Reference |
|---|---|---|---|---|---|---|
| Aeromonas hydrophila | Septicemia | Catfish (Pangasianodon hypophthalmus) | φ2 and φ5 (Myoviridae) | I.P. injection | Improved survival rates from 18.3% to 100%. | [59] |
| Loach (Misgurnus anguillicaudatus) | Akh-2 (Siphoviridae) | Immersion | Reduced cumulative mortality rates from 100% to 56.67%. | [60] | ||
| Loach (Misgurnus anguillicaudatu) | AH1 (n.d.) | Infection with phage before injection in fish | Pathogenicity eliminated following phage infection. | [61] | ||
| Loach (Misgurnus anguillicaudatu) | pAh1-C, pAh6-C (Myoviridae) | I.P. injection and oral administration via feeding | Reduced cumulative mortality rates. I.P. injection: from 100% to 43.33% using pAh1-C and to 16.67% using pAh6-C. Oral administration: from 95.83% to 46.67% with pAh1-C and to 26.67% with pAh6-C. | [62] | ||
| A. salmonicida | Furunculosis | Rainbow trout (Oncorhynchus mykiss) | PAS-1 (Myoviridae) | I.M. injection | Increase in survival rates from 0% to 26.7%. | [62] |
| Senegalese sole (Solea senegalensis) | AS-A (Myoviridae) | Immersion | Decrease in cumulative mortality rates from 36% to 0%. | [63] | ||
| Brook trout (Salvelinus fontinalis) | HER 110 (Myoviridae) | Immersion | Decrease in total mortality rates from 100% to 10%. | [64] | ||
| Edwardsiella tarda | Edwardsiellosis | Zebrafish (Danio rerio) | ETP-1 (Podoviridae) | Immersion prior to bacterial challenge | Survival rates improved from 18% to 68%. | [65] |
| Flavobacterium columnare | Columnaris disease | Rainbow trout and zebrafish | FCL-2 (Myoviridae) | Immersion | Improved survival rates. Rainbow trout: increased from 8.3% to 50%. Zebrafish: increased from 0% to 60%. | [66] |
| F. psychrophilum | Rainbow trout fry syndrome and cold water disease | Rainbow trout and Atlantic salmon (Salmo salar) | 1H, 6H (Siphoviridae) | I.P. injection | Reduced cumulative mortality rates. Trout: decreased from 80% to 67% (1H), 47% (6H). Salmon: decreased from 13% to 0% (1H), 6% (6H). | [67] |
| Lactococcus garvieae | Lactococcosis | Yellowtail (Seriola quinqueradiata) | PLgY-16 (Siphoviridae) | I.P. injection and oral administration (feeding) | I.P. injection: improved survival rates from 45% to 90%. Oral administration: reduced cumulative mortality from 65% to 10%. | [68] |
| Pseudomonas plecoglossicida | Bacterial hemorrhagic ascites disease | Ayu (Plecoglossus altivelis) | PPpW-3 (Myoviridae), PPpW-4 (Podoviridae) | Oral administration (feeding) | Reduced cumulative mortality rates from 93.3% to 53.3% for PPpW-3, 40.0% for PPpW-4, and 20.0% for PPpW-3/W-4. | [69] |
| Streptococcus agalactiae | Streptococcosis | Nile tilapia (Oreochromis niloticus) | HN48 (n.d.) | I.P. injection | Improved survival rates from 0% to 60%. | [55] |
| S. iniae | Streptococcosis | Japanese flounder (Paralichthys olivaceus) | PSiJ31, 32, 41, 42 (Siphoviridae) | I.P. injection | Improved survival rates from 0% to 28 or 33% (combined usage of PSiJ31 and 32); from 0% to 48, 70, or 90% (combined usage of PSiJ31, 32, 41, and 42). | [70] |
| Vibrio anguillarum | Hemorrhagic septicemia | Atlantic salmon | CHOED (n.d.) | Immersion | Survival rates improved from less than 10% to 100%. | [71] |
| Vibriosis | Zebrafish larvae | VP-2 (n.d.) | Immersion | Cumulative larval mortality rates reduced from 17% to 2%. | [72] | |
| V. harveyi | Luminescent vibriosis | Shrimp (Penaeus monodon) larvae | A (Siphoviridae) | Immersion | Larval survival rates improved from 17% to 86%. | [73] |
| Shrimp larvae | VHM1, VHM2 (Myoviridae) VHS1 (Siphoviridae) | Immersion | Larval survival rates improved from 26.6% to 86.6%. | [74] | ||
| Abalone (Haliotis laevigata) | vB_VhaS-tm (Siphoviridae) | Immersion | Larval survival rates improved from 0% to 70%. | [75] | ||
| Black tiger shrimp (Litopenaeus monodon) larvae | VHP6b (Siphoviridae) | Immersion | Cumulative larval mortality rates reduced from 70% to 20%. | [76] | ||
| Shrimp larvae | Viha10, Viha8 (Siphoviridae) | Immersion | Larval survival rates improved from 65% to 88%. | [77] | ||
| V. parahaemolyticus | Blue mussel (Mytilus edulus) | VP10 (n.d.) | Immersion | Reduction in bacterial growth to undetectable levels. | [59] | |
| Oyster (Crassostrea gigas) | pVp-1 (Siphoviridae) | Immersion, surface inoculation | Reduction in bacterial growth from 106 CFU/g to 10 CFU/g. | [78] | ||
| Acute hepatopancreatic necrosis disease | Marine shrimp (P. vannamei) | pVp-1 (Siphoviridae) | Immersion, oral administration | Reduced cumulative mortality rates from 100% to 0%. | [79] | |
| V. splendidus | Vibriosis | Sea cucumber (Apostichopus japonicus) | PVS-1, PVS-2 (Myoviridae) PVS-3 (Siphoviridae) | Oral administration (feeding) and coelomic injection | Survival rates increased from 18% to 82% through feeding and from 20% to 80% via coelomic injection. | [60] |
3. Microecological Agents
3.1. Probiotics, Prebiotic, Synbiotics, and Postbiotics
3.1.1. Probiotics
- Improving Water Quality: they aid in nitrification, denitrification, water quality management, and pathogen control.
- Enhancing the Intestinal Microenvironment.
- The synthesis of antimicrobial metabolites.
- Competitive exclusion (nutrient/attachment site competition).
- The suppression of virulence-related gene expression.
- Quorum-sensing interference.
- Water quality modulation.
- Immune function enhancement.
- The provision of essential nutrients.
- The facilitation of digestive enzyme activity [91].
- Host species specificity.
- Non-pathogenicity and genomic stability.
- Antimicrobial compound production capacity.
- Immunomodulatory competence.
- (1)
- IgM-mediated pathogen recognition;
- (2)
- LYZ-driven nonspecific immunity;
- (3)
- AKP-SOD antioxidant synergy against oxidative stress.
| Description | Reference | |
|---|---|---|
| 1. | Probiotics compete for resources and receptor binding sites, making it difficult for harmful microorganisms to survive in the intestine. They produce peptides, including anti-microbial peptides, viz., bacteriocins and β-defensins, to limit the pathogenic growth within the host species. | [106,107] |
| 2. | Probiotics synthesize anti-bacterial substances, such as short chain fatty acids (SCFAs), e.g., propionate, butyrate, acetate, and H2O2. They also produce organic acids that lower the pH of the GIT, preventing harmful microorganisms from growing and supporting the propagation of probiotics. | [107] |
| 3. | Probiotics improve intestinal barrier function by regulating the expressivity of tight junction proteins, including occludin, zonula occludens, and claudin, promoting defensin and mucin formation and regulating the immune function in the intestine. | [108] |
| 4. | They affect both innate and adaptive immunity by modulating B- and T-cell, lysozyme, dendritic cell, complement and macrophage activities. Probiotics interact with intestinal epithelial cells, attracting macrophages and mononuclear cells, leading to the increased production of anti-inflammatory cytokines. They also inhibit pro-inhibitory markers by regulating the cytokines. | [91] |
| 5. | Probiotics have the ability to produce neurotransmitters in the intestine through the gut–brain axis. Some strains can change the amounts of GABA, serotonin, and dopamine, which can have a positive effect on gastrointestinal motility and stress, as well as mood- and behavior-related pathways | [109] |
| 6. | Probiotics alter the microbiome of the GIT through boosting the diversity of healthy microbes and decreasing the number of harmful ones. | [110] |
| Empty Cell | Probiotics | Species | References |
|---|---|---|---|
| Aeromonas | Aeromonas veronii | Grass carp (Ctenopharyngodon idellus) | [111] |
| Common carp (Cyprinus carpio) | [112] | ||
| Bacillus | Bacillus amyloliquefaciens | Common carp (Cyprinus carpio) | [113] |
| Bacillus coagulans SCC-19 | Common carp (Cyprinus carpio) | [114] | |
| Bacillus subtilis B. velezensis R-71003 | Grass carp (Ctenopharyngodon idellus) | [115] | |
| Common carp (Cyprinus carpio) | [116] | ||
| Carnobacterium | C. divergens | Common carp (Cyprinus carpio) | [117] |
| Enterococcus | Enterococcus faecium L6 | Grass carp (Ctenopharyngodon idellus) | [118] |
| Flavobacterium | Flavobacterium sasangense BA-3 | Common carp (Cyprinus carpio) | [112] |
| Lactobacillus | Lactobacillus acidophilus | Goldfish (Carassius auratus gibelio) | [119] |
| Lactobacillus delbrueckii | Cyprinus carpio Huanghe | [120] | |
| Lactobacillus casei | Common carp (Cyprinus carpio) | [121] | |
| Lactobacillus sakei | Common carp (Cyprinus carpio) | [122] | |
| Common carp (Cyprinus carpio) | [123] | ||
| Pediococcus | Pediococcus acidilactici MA18/5M | Common carp (Cyprinus carpio) | [124] |
| Pseudomonas | Pseudomonas aeruginosa VSG2 | Common carp (Cyprinus carpio) | [125] |
| Saccharomyces | Saccharomyces cerevisiae | ||
| Common carp (Cyprinus carpio) | [126] | ||
| Shewanella xiamenensis | Grass carp (Ctenopharyngodon idellus) | [127] | |
| Vibrio | Vibrio lentus BA-2 | Common carp (Cyprinus carpio) | [112] |
- Ecological impacts on aquatic microbiomes.
- Host-specific isolation hurdles from mucosal tissues [104].
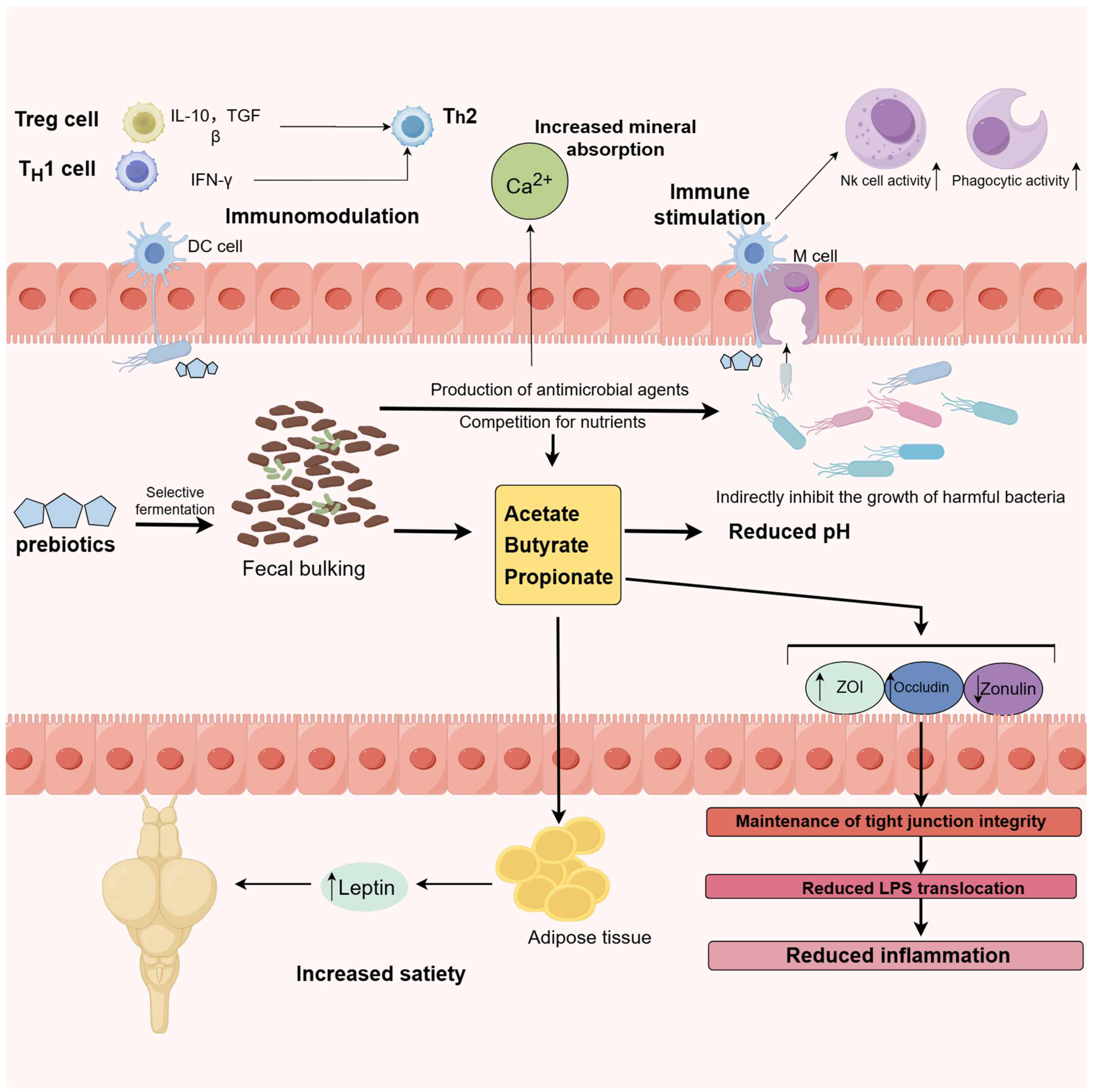
3.1.2. Prebiotic
- Regulating the gut microbiota.
- Strengthening the intestinal mucosal barrier.
- Directly activating the immune system.
3.1.3. Synbiotics
- Compatibility: prebiotic physicochemical properties (e.g., molecular weight, solubility) must align with antigen delivery systems to prevent epitope masking [148].
- Dose Optimization: immunomodulatory effects follow U-shaped dose-response curves, requiring empirical determination to avoid immunosuppression (low dose) or inflammatory cascades (high dose) [147].
- Safety Profiling: chronic exposure risks include microbiota dysbiosis and hepatic xenobiotic metabolism alterations, necessitating longitudinal toxicity assessments [149].
- Adaptive Immunity: enhance T-cell and B-cell responses, bolstering mucosal and systemic antibody production, thereby improving disease resistance in fish.
3.1.4. Postbiotics
- Structural cell elements: e.g., peptidoglycan, teichoic acids, and cellular debris from lysed probiotics.
- Metabolic products such as short-chain fatty acids (SCFAs: acetate, propionate, butyrate), organic acids (e.g., lactate), enzymes (proteases, lipases), antimicrobial agents (e.g., bacteriocins), vitamins (B-complex, vitamin K), and antioxidants (catalase, superoxide dismutase).
- Other bioactive molecules, like nucleotides and their derivatives.
- Defined Chemical Structure and Stability: Postbiotics have a clear chemical composition, greater stability, and a longer shelf life [164];
- High Safety Profile: Since postbiotics are non-living, they cannot acquire virulence genes or transmit antibiotic resistance, reducing the risk of producing harmful metabolites. This makes them safer for consumption, and they are effective at lower doses [165].
- Lower Risk for Immunocompromised Hosts: Active probiotics require large doses to be effective, which may cause adverse reactions in immunocompromised individuals. Postbiotics, however, are safer and cause minimal impact even in higher amounts [163].
- Ease of Storage and Transport: Postbiotics do not need to maintain viability, allowing them to remain stable across a wide range of temperatures and pH levels. This makes them easier to store and transport [166].
- Environmental Resilience: Postbiotics retain their beneficial effects under extreme environmental conditions, such as high temperatures or exposure to digestive enzymes [167].
3.2. Antimicrobial Peptides
4. Conclusions and Future Perspective
- Precision nutrition.
- Biosecurity-enhanced management.
- Water quality optimization.
- Preventive health protocols.
- Cost–benefit analyses.
- Dose–response relationships.
- Environmental fate assessments.
- Smallholder applicability.
- Evaluating the efficacy and safety of feed additives under varying environmental conditions.
- Assessing the environmental impact and alternative methods for antimicrobial agents.
- Implementing the active and passive monitoring of drug withdrawal periods, wastewater treatment, residues, and AMR detection.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tan, M.; Armbruster, J.W. Phylogenetic classification of extant genera of fishes of the order Cypriniformes (Teleostei: Ostariophysi). Zootaxa 2018, 4476, 6–39. [Google Scholar] [CrossRef] [PubMed]
- Mulchandani, R.; Wang, Y.; Gilbert, M.; Van Boeckel, T.P. Global trends in antimicrobial use in food-producing animals: 2020 to 2030. PLOS Glob. Public Health 2023, 3, e0001305. [Google Scholar] [CrossRef] [PubMed]
- Wenning, R. The state of world fisheries and aquaculture (sofia) 2020 report. Integr. Environ. Assess. Manag. 2020, 16, 800–801. [Google Scholar]
- Atterbury, R.J.; Tyson, J. Predatory bacteria as living antibiotics—Where are we now? Microbiology 2021, 167, 001025. [Google Scholar] [CrossRef]
- Hou, Y.H.; Cao, Y.L. Discussion on several common diseases of carp and their prevention and treatment techniques. China Fish. 2023, 79–82. [Google Scholar]
- Irshath, A.A.; Rajan, A.P.; Vimal, S.; Prabhakaran, V.-S.; Ganesan, R. Bacterial pathogenesis in various fish diseases: Recent advances and specific challenges in vaccine development. Vaccines 2023, 11, 470. [Google Scholar] [CrossRef]
- Rodriguez-Mozaz, S.; Vaz-Moreira, I.; Giustina, S.V.D.; Llorca, M.; Barceló, D.; Schubert, S.; Berendonk, T.U.; Michael-Kordatou, I.; Fatta-Kassinos, D.; Martinez, J.L.; et al. Antibiotic residues in final effluents of European wastewater treatment plants and their impact on the aquatic environment. Environ. Int. 2020, 140, 105733. [Google Scholar] [CrossRef]
- Okocha, R.C.; Olatoye, I.O.; Adedeji, O.B. Food safety impacts of antimicrobial use and their residues in aquaculture. Public Health Rev. 2018, 39, 1–22. [Google Scholar] [CrossRef]
- Chen, J.; Sun, R.; Pan, C.; Sun, Y.; Mai, B.; Li, Q.X. Antibiotics and food safety in aquaculture. J. Agric. Food Chem. 2020, 68, 11908–11919. [Google Scholar] [CrossRef]
- Joseph, A.M.M.; Linda, D.A.K.; Irina, V.P.; Larissa, A.S.; Souadkia, S.; Ibrahim, K.; Milana, S.D. The public health issue of antibiotic residues in food and feed: Causes, consequences, and potential solutions. Vet. World 2022, 15, 662–671. [Google Scholar]
- Ben, Y.; Fu, C.; Hu, M.; Liu, L.; Wong, M.H.; Zheng, C. Human health risk assessment of antibiotic resistance associated with antibiotic residues in the environment: A review. Environ. Res. 2018, 169, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Puvača, N.; Vapa-Tankosić, J.; Ignjatijević, S.; Carić, M.; Soleša, D.; Soleša, K. Consumer awareness of antimicrobal residues in drinking Water. Ekon. Misao I Praksa-Econ. Thought Pract. 2023, 16, 40–56. [Google Scholar] [CrossRef]
- Mirakian, R.; Leech, S.C.; Krishna, M.T.; Richter, A.G.; Huber, P.A.J.; Farooque, S.; Khan, N.; Pirmohamed, M.; Clark, A.T.; Nasser, S.M. Management of allergy to penicillins and other beta-lactams. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2015, 45, 300–327. [Google Scholar] [CrossRef] [PubMed]
- Del Pozzo-Magaña, B.R.; Liy-Wong, C. Drugs and the Skin: A concise review of cutaneous adverse drug reactions. Br. J. Clin. Pharmacol. 2024, 90, 1838–1855. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, Y.; Chen, S.; Guan, X.; Zhong, Y.; Yang, Q. Occurrence, risk assessment, and in vitro and in vivo toxicity of antibiotics in surface water in China. Ecotoxicol. Environ. Saf. 2023, 255, 114817. [Google Scholar] [CrossRef]
- Seo, J.; Kloprogge, F.; Smith, A.M.; Karu, K.; Ciric, L. Antibiotic residues in UK Foods: Exploring the exposure pathways and associated health risks. Toxics 2024, 12, 174. [Google Scholar] [CrossRef]
- Ribeiro, C.F.A.; Silveira, G.G.D.O.S.; Cândido, E.D.S.; Cardoso, M.H.; Espínola Carvalho, C.M.; Franco, O.L. Effects of antibiotic treatment on gut microbiota and how to overcome its negative impacts on human health. Acs Infect. Dis. 2020, 6, 2544–2559. [Google Scholar] [CrossRef]
- O’Neill, A.J.; Chopra, I. Preclinical evaluation of novel antibacterial agents by microbiological and molecular techniques. Expert Opin. Investig. Drugs 2005, 13, 1045–1063. [Google Scholar] [CrossRef]
- Bao, R.; Yang, Y.; Chen, H.; Li, Y. Occurrence, distribution and health risk assessment of quinolone residues in cultured fish in southeast China. J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 2024, 59, 714–724. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on nitrofurans and their metabolites in food. EFSA J. 2015, 13, 4140. [Google Scholar]
- Bush, N.G.; Diez-Santos, I.; Abbott, L.R.; Maxwell, A. Quinolones: Mechanism, Lethality and Their Contributions to antibiotic resistance. Molecules 2020, 25, 5662. [Google Scholar] [CrossRef] [PubMed]
- Drlica, K.; Zhao, X. Bacterial death from treatment with fluoroquinolones and other lethal stressors. Expert Rev. Anti-Infect. Ther. 2020, 19, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Yun, C.; Pang, Y.; Qiao, J. The Impact of the Gut Microbiota on the Reproductive and Metabolic Endocrine System. Gut Microbes 2021, 13, 1894070. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ying, G.-G.; Deng, W.J. Antibiotic Residues in Food: Extraction, Analysis, and Human Health Concerns. J. Agric. Food Chem. 2019, 67, 7569–7586. [Google Scholar] [CrossRef]
- Kumar, A.; Pal, D. Antibiotic resistance and wastewater: Correlation, impact and critical human health challenges. J. Environ. Chem. Eng. 2018, 6, 52–58. [Google Scholar] [CrossRef]
- Wang, X.; Li, F.; Hu, X.; Hua, T. Electrochemical advanced oxidation processes coupled with membrane filtration for degrading antibiotic residues: A review on its potential applications, advances, and challenges. Sci. Total Environ. 2021, 784, 146912. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Z.; Zhao, W.; Liu, C.; Qian, X.; Zhang, M.; Wei, G.; Khan, E.; Ng, Y.H.; Ok, Y.S. Recent advances in photodegradation of antibiotic residues in water. Chem. Eng. J. 2021, 405, 126806. [Google Scholar] [CrossRef]
- Farid, M.U.; Choi, P.J.; Kharraz, J.A.; Lao, J.-Y.; St-Hilaire, S.; Ruan, Y.; Lam, P.K.S.; An, A.K. Hybrid nanobubble-forward osmosis system for aquaculture wastewater treatment and reuse. Chem. Eng. J. 2022, 435, 135164. [Google Scholar] [CrossRef]
- Melchiorri, D.; Rocke, T.; Alm, R.A.; Cameron, A.M.; Gigante, V. Addressing urgent priorities in antibiotic development: Insights from WHO 2023 antibacterial clinical pipeline analyses. Lancet. Microbe 2024, 6, 100992. [Google Scholar] [CrossRef]
- He, W.; Martin, J.H.; Shaw, P.N.; Lu, X.; Walpole, E.T.; Dimeski, G. A Simple and sensitive LC-MS/MS method for the simultaneous determination of cyclophosphamide and doxorubicin concentrations in human plasma. Curr. Pharm. Anal. 2017, 14, 53–59. [Google Scholar] [CrossRef]
- Yu, X.; Liu, H.; Pu, C.; Chen, J.; Sun, Y.; Hu, L. Determination of multiple antibiotics in leafy vegetables using QuEChERS-UHPLC-MS/MS. J. Sep. Sci. 2018, 41, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, J.; Li, Y.; Yang, J.; Jin, J.; Shah, S.M.; Chen, J. Novel dummy molecularly imprinted polymers for matrix solid-phase dispersion extraction of eight fluoroquinolones from fish samples. J. Chromatogr. A 2014, 1359, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, V.; Rubies, A.; Centrich, F.; Companyó, R.; Guiteras, J. Development and validation of a multiclass method for the analysis of antibiotic residues in eggs by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2011, 1218, 1443–1451. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Zhao, P.; Dai, X.; Hou, X.; Zhao, L.; Liang, N. Trace determination of antibacterial pharmaceuticals in fishes by microwave-assisted extraction and solid-phase purification combined with dispersive liquid–liquid microextraction followed by ultra-high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2016, 1011, 136–144. [Google Scholar]
- Dai, T.; Duan, J.; Li, X.; Xu, X.; Shi, H.; Kang, W.; Huang, K. Determination of sulfonamide residues in food by capillary zone electrophoresis with on-line chemiluminescence detection based on an Ag(III) complex. Int. J. Mol. Sci. 2017, 18, 1286. [Google Scholar] [CrossRef]
- Desimoni, E. About CCα and CCβ as introduced by the commission decision of 12 August 2002 implementing Council Directive 96/23/EC. Accredit. Qual. Assur. 2004, 9, 724–725. [Google Scholar] [CrossRef]
- Liu, K.J. Research on the detection method of residual antibiotics in food. Mod. Food 2024, 30, 95–97. [Google Scholar]
- Costa, L.F.; da Silva, G.S.; Freitas, A.S.; da Silva, G.S.; de Sousa, E.R. Application of dispersive liquid-liquid microextraction technique for the analysis of norfloxacin antibiotic in chicken breast (pectoralis major) by high performance liquid chromatography with fluorescence detector. Rev. Virtual Química 2020, 12, 681–692. [Google Scholar] [CrossRef]
- Gunning, D.; Maguire, J.; Burnell, G. The Development of sustainable saltwater-based food production systems: A review of established and novel concepts. Water 2016, 8, 598. [Google Scholar] [CrossRef]
- Murray, K.N.; Clark, T.S.; Kebus, M.J.; Kent, M.L. Specific Pathogen Free – A review of strategies in agriculture, aquaculture, and laboratory mammals and how they inform new recommendations for laboratory zebrafish. Res. Vet. Sci. 2022, 142, 78–93. [Google Scholar] [CrossRef]
- Hai, V.N. The use of medicinal plants as immunostimulants in aquaculture: A review. Aquaculture 2015, 44, 688–696. [Google Scholar]
- Marnis, H.; Syahputra, K. Advancing fish disease research through CRISPR-Cas genome editing: Recent developments and future perspectives. Fish Shellfish Immunol. 2025, 160, 110–220. [Google Scholar] [CrossRef] [PubMed]
- Brudeseth, B.E.; Wiulsrød, R.; Fredriksen, B.N.; Lindmo, K.; Løkling, K.-E.; Bordevik, M.; Steine, N.; Klevan, A.; Gravningen, K. Status and future perspectives of vaccines for industrialised fin-fish farming. Fish Shellfish Immunol. 2013, 35, 1759–1768. [Google Scholar] [CrossRef] [PubMed]
- Seed, K.D. Battling Phages: How bacteria defend against viral attack? PLoS Pathog. 2015, 11, e1004847. [Google Scholar] [CrossRef]
- Ma, J.; Bruce, T.J.; Jones, E.M.; Cain, K.D. A review of fish vaccine development strategies: Conventional methods and modern biotechnological approaches. Microorganisms 2019, 7, 569. [Google Scholar] [CrossRef]
- Hølvold, L.B.; Myhr, A.I.; Dalmo, R.A. Strategies and hurdles using DNA vaccines to fish. Vet. Res. 2014, 45, 21. [Google Scholar] [CrossRef]
- Subasinghe, R.P.; Curry, D.; McGladdery, S.E.; Bartley, D. Recent technological innovations in aquaculture. Rev. State World Aquac. FAO Fish. Circ. 2003, 886, 59–74. [Google Scholar]
- Triet, T.H.; Tinh, B.T.T.; Hau, L.V.; Huong, T.V.; Binh, N.-Q. Development and potential use of an Edwardsiella ictaluri wzz mutant as a live attenuated vaccine against enteric septicemia in Pangasius hypophthalmus (Tra catfish). Fish Shellfish Immunol. 2019, 87, 87–95. [Google Scholar] [CrossRef]
- Minor, P.D. Live attenuated vaccines: Historical successes and current challenges. Virology 2015, 479–480, 379–392. [Google Scholar] [CrossRef]
- Ruiz de Ybáñez, M.R.; Laura, R.; César, F.; Pilar, M.; Eduardo, B.; Silvia, R.; Carlos, M. Monitoring for Anguillicoloides crassus, Anguillid herpesvirus 1, aquabirnavirus EVE and rhabdovirus EVEX in the European eel population of southern Spain. J. Fish Dis. 2023, 46, 417–431. [Google Scholar] [CrossRef]
- Björklund, H.V.; Higman, K.H.; Kurath, G. The glycoprotein genes and gene junctions of the fish rhabdoviruses spring viremia of carp virus and hirame rhabdovirus: Analysis of relationships with other rhabdoviruses. Virus Res. 1996, 42, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Siwicki, A.K.; Pozet, F.; Morand, M.; Kazuń, B.; Trapkowska, S.; Małaczewska, J. Influence of methisoprinol on the replication of rhabdoviruses isolated from carp (Cyprinus carpio) and catfish (Ictalurus melas): In vitro study. Pol. J. Vet. Sci. 2003, 6, 47–50. [Google Scholar] [PubMed]
- Stone, D.M.; Ahne, W.; Denham, K.L.; Dixon, P.F.; Liu, C.Y.; Sheppard, A.M.; Taylor, G.R.; Way, K. Nucleotide sequence analysis of the glycoprotein gene of putative spring viraemia of carp virus and pike fry rhabdovirus isolates reveals four genogroups. Dis. Aquat. Org. 2003, 53, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Coll, J.M. Herpesvirus Infection induces both specific and heterologous antiviral antibodies in carp. Front. Immunol. 2018, 9, 39. [Google Scholar] [CrossRef]
- Anna, S.; Patrycja, R.; Piotr, K.; Anna, P.; Anna, G.-S.; Grzegorz, K.; Agnieszka, S. Codon optimization of antigen coding sequences improves the immune potential of DNA vaccines against avian influenza virus H5N1 in mice and chickens. Virol. J. 2016, 13, 143. [Google Scholar]
- Swain, B.; Powell, C.T.; Curtiss, R., 3rd. Construction and evaluation of recombinant attenuated Edwardsiella piscicida vaccine (raev) vector system encoding Ichthyophthirius multifiliis (Ich) Antigen IAG52B. Front. Immunol. 2022, 12, 802760. [Google Scholar] [CrossRef]
- Vinay, T.N.; Bhat, S.; Choudhury, T.G.; Paria, A.; Jung, M.-H.; Kallappa, G.S.; Jung, S.-J. Recent Advances in Application of Nanoparticles in Fish Vaccine Delivery. Rev. Fish. Sci. Aquac. 2018, 26, 29–41. [Google Scholar] [CrossRef]
- Shahin, K.; Pirezan, F.; Rogge, M.; Lafrentz, B.R.; Shrestha, R.P.; Hildebrand, M.; Lu, F.; Hogenesch, H.; Soto, E. Development of Ig1c and Groel Recombinant Vaccines for Francisellosis In Nile Tilapia, Oreochromis niloticus. Fish Shellfish Immunol. 2020, 105, 341–349. [Google Scholar] [CrossRef]
- Onarinde, B.A.; Dixon, R.A. Prospects for biocontrol of Vibrio parahaemolyticus contamination in blue mussels (Mytilus edulus)—A Year-Long Study. Front. Microbiol. 2018, 9, 1043. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.; Zhang, J.; Wang, X.; Wang, L.; Cao, Z.; Xu, Y. Use of phages to control Vibrio splendidus infection in the juvenile sea cucumber Apostichopus japonicus. Fish Shellfish Immunol. 2016, 54, 302–311. [Google Scholar] [CrossRef]
- Le, T.S.; Nguyen, T.H.; Vo, H.P.; Doan, V.C.; Nguyen, H.L.; Tran, M.T.; Tran, T.T.; Southgate, P.C.; Kurtböke, D.I. Protective effects of bacteriophages against Aeromonas hydrophila causing motile Aeromonas septicemia (MAS) in striped catfish. Antibiotics 2018, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Akmal, M.; Rahimi-Midani, A.; Hafeez-ur-Rehman, M.; Hussain, A.; Choi, T. Isolation, Characterization, and Application of a Bacteriophage Infecting the Fish Pathogen Aeromonas hydrophila. Pathogens 2020, 9, 215. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.L.; Lin, H.M.; Jan, L.; Hsu, Y.L.; Chang, L.H. Biological Control of Fish Bacterial Pathogen, Aeromonas hydrophila, by Bacteriophage AH 1. Fish Pathol. 1981, 15, 271–276. [Google Scholar] [CrossRef]
- Jun, J.W.; Kim, J.H.; Shin, S.P.; Han, J.E.; Chai, J.Y.; Park, S.C. Protective Effects of the Aeromonas Phages PAh1-C and PAh6-C against Mass Mortality of the Cyprinid Loach (Misgurnus anguillicaudatus) Caused by Aeromonas hydrophila. Aquaculture 2013, 416, 289–295. [Google Scholar] [CrossRef]
- Kim, J.H.; Choresca, C.H.; Shin, S.P.; Han, J.E.; Jun, J.W.; Park, S.C. Biological control of Aeromonas salmonicida subsp. salmonicida infection in rainbow trout (Oncorhynchus mykiss) using Aeromonas phage PAS-1. Transbound. Emerg. Dis. 2015, 62, 81–86. [Google Scholar]
- Silva, Y.J.; Moreirinha, C.; Pereira, C.; Costa, L.; Rocha, R.J.; Cunha, Â.; Gomes, N.C.; Calado, R.; Almeida, A. Biological Control of Aeromonas salmonicida Infection in Juvenile Senegalese Sole (Solea senegalensis) with Phage AS-A. Aquaculture 2016, 450, 225–233. [Google Scholar] [CrossRef]
- Imbeault, S.; Parent, S.; Lagacé, M.; Uhland, C.F.; Blais, J.-F. Using Bacteriophages to Prevent Furunculosis Caused by Aeromonas salmonicida in Farmed Brook Trout. J. Aquat. Anim. Health 2006, 18, 203–214. [Google Scholar] [CrossRef]
- Nikapitiya, C.; Chandrarathna, H.; Dananjaya, S.; De Zoysa, M.; Lee, J. Isolation and characterization of Phage (ETP-1) Specific to multidrug resistant pathogenic Edwardsiella tarda and its in vivo biocontrol efficacy in zebrafish (Danio rerio). Biologicals 2020, 63, 14–23. [Google Scholar] [CrossRef]
- Laanto, E.; Bamford, J.K.; Ravantti, J.J.; Sundberg, L.R. The Use of Phage FCL-2 as an Alternative to Chemotherapy Against Columnaris Disease in Aquaculture. Front. Microbiol. 2015, 6, 829. [Google Scholar] [CrossRef]
- Castillo, D.; Higuera, G.; Villa, M.; Middelboe, M.; Dalsgaard, I.; Madsen, L.; Espejo, R.T. Diversity of Flavobacterium psychrophilum and the potential use of its phages for protection against bacterial cold water disease in salmonids. Fish Dis. 2012, 35, 193–201. [Google Scholar] [CrossRef]
- Nakai, T.; Sugimoto, R.; Park, K.H.; Matsuoka, S.; Mori, K.; Nishioka, T.; Maruyama, K. Protective effects of bacteriophage on experimental Lactococcus garvieae infection in yellowtail. Dis. Aquat. Org. 1999, 37, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Park, S.C.; Nakai, T. Bacteriophage control of Pseudomonas plecoglossicida infection in ayu Plecoglossus altivelis. Dis. Aquat. Org. 2003, 53, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Liao, G.; Liu, C.; Jiang, X.; Lin, M.; Zhao, C.; Tao, J.; Huang, Z. Characterization of bacteriophage HN 48 and its protective effects in nile tilapia Oreochromis niloticus against Streptococcus agalactiae infections. Fish Dis. 2018, 41, 1477–1484. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, S.; Hashizume, T.; Kanzaki, H.; Iwamoto, E.; Park, S.C.; Yoshida, T.; Nakai, T. Phage therapy against beta-hemolytic streptococcicosis of Japanese flounder Paralichthys olivaceus. Fish Pathol. 2007, 42, 181–189. [Google Scholar] [CrossRef]
- Higuera, G.; Bastías, R.; Tsertsvadze, G.; Romero, J.; Espejo, R.T. Recently discovered vibrio anguillarum phages can protect against experimentally induced vibriosis in atlantic salmon, Salmo salar. Aquaculture 2013, 392–395, 128–133. [Google Scholar] [CrossRef]
- Silva, Y.J.; Costa, L.; Pereira, C.; Mateus, C.; Cunha, A.; Calado, R.; Gomes, N.C.; Pardo, M.A.; Hernandez, I.; Almeida, A. Phage therapy as an approach to prevent Vibrio anguillarum infections in fish larvae production. PLoS ONE 2014, 9, e114197. [Google Scholar] [CrossRef]
- Vinod, M.; Shivu, M.; Umesha, K.; Rajeeva, B.; Krohne, G.; Karunasagar, I.; Karunasagar, I. Isolation of Vibrio harveyi bacteriophage with a potential for biocontrol of luminous vibriosis in hatchery environments. Aquaculture 2006, 255, 117–124. [Google Scholar] [CrossRef]
- Stalin, N.; Srinivasan, P. Efficacy of potential phage cocktails against Vibrio harveyi and closely related Vibrio species isolated from shrimp aquaculture environment in the South East coast of India. Vet. Microbiol. 2017, 207, 83–96. [Google Scholar] [CrossRef]
- Wang, Y.; Barton, M.; Elliott, L.; Li, X.; Abraham, S.; O’Dea, M.; Munro, J. Bacteriophage therapy for the control of Vibrio harveyi in greenlip abalone (Haliotis laevigata). Aquaculture 2017, 473, 251–258. [Google Scholar] [CrossRef]
- Patil, J.R.; Desai, S.N.; Roy, P.; Durgaiah, M.; Saravanan, R.S.; Vipra, A. Simulated hatchery system to assess bacteriophage efficacy against Vibrio harveyi. Dis. Aquat. Org. 2014, 112, 113–119. [Google Scholar] [CrossRef]
- Karunasagar, I.; Shivu, M.; Girisha, S.; Krohne, G.; Karunasagar, I. Biocontrol of pathogens in shrimp hatcheries using bacteriophages. Aquaculture 2007, 268, 288–292. [Google Scholar] [CrossRef]
- Jun, J.W.; Kim, H.J.; Yun, S.K.; Chai, J.Y.; Park, S.C. Eating oysters without risk of vibriosis: Application of a bacteriophage against Vibrio parahaemolyticus in oysters. Int. J. Food Microbiol. 2014, 188, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.W.; Han, J.E.; Giri, S.S.; Tang, K.F.J.; Zhou, X.; Aranguren, L.F.; Kim, H.J.; Yun, S.; Chi, C.; Kim, S.G.; et al. Phage application for the protection from acute hepatopancreatic necrosis disease (AHPND) in Penaeus vannamei. Indian J. Microbiol. 2018, 58, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Nakai, T.; Park, S.C. Bacteriophage therapy of infectious diseases in aquaculture. Res. Microbiol. 2002, 153, 13–18. [Google Scholar] [CrossRef]
- Choudhury, T.G.; Nagaraju, V.T.; Gita, S.; Paria, A.; Parhi, J. Advances in bacteriophage research for bacterial disease control in aquaculture. Rev. Fish. Sci. Aquac. 2017, 25, 113–125. [Google Scholar] [CrossRef]
- Colombo, S.; Arioli, S.; Guglielmetti, S.; Lunelli, F.; Mora, D. Virome-associated antibiotic-resistance genes in an experimental aquaculture facility. FEMS Microbiol. Ecol. 2016, 92, fiw003. [Google Scholar] [CrossRef]
- Skliros, D.; Kalatzis, P.G.; Katharios, P.; Flemetakis, E. Comparative functional genomic analysis of two vibrio phages reveals complex metabolic interactions with the host cell. Front. Microbiol. 2016, 7, 1807. [Google Scholar] [CrossRef]
- Hill, C.; Mills, S.; Ross, R.P. Phages & antibiotic resistance: Are the most abundant entities on earth ready for a comeback? Future Microbiol. 2018, 13, 711–726. [Google Scholar]
- Labrie, S.J.; Samson, J.E.; Moineau, S. Bacteriophage resistance mechanisms. Nat. Rev. Microbiol. 2010, 8, 317–327. [Google Scholar] [CrossRef]
- Clerici, L.; Bottari, D.; Bottari, B. Gut Microbiome, diet and depression: Literature review of microbiological, nutritional and neuroscientific aspects. Curr. Nutr. Rep. 2025, 14, 30. [Google Scholar] [CrossRef]
- Shen, X.; Jin, H.; Zhao, F.; Kwok, L.Y.; Zhao, Z.; Sun, Z. Short-term probiotic supplementation affects the diversity, genetics, growth, and interactions of the native gut microbiome. iMeta 2024, 3, e253. [Google Scholar] [CrossRef] [PubMed]
- Merrifield, D.L.; Dimitroglou, A.; Foey, A.; Davies, S.J.; Baker, R.T.M.; Bøgwald, J.; Castex, M.; Ringø, E. The current status and future focus of probiotic and prebiotic applications for salmonids. Aquaculture 2010, 302, 1–18. [Google Scholar] [CrossRef]
- Zhang, M.L.; Dong, W.X.; Du, Z.Y. The effect of probiotics on the growth and development of young fish and its regulation mechanism. J. Fish. 2024, 48, 19–27. [Google Scholar]
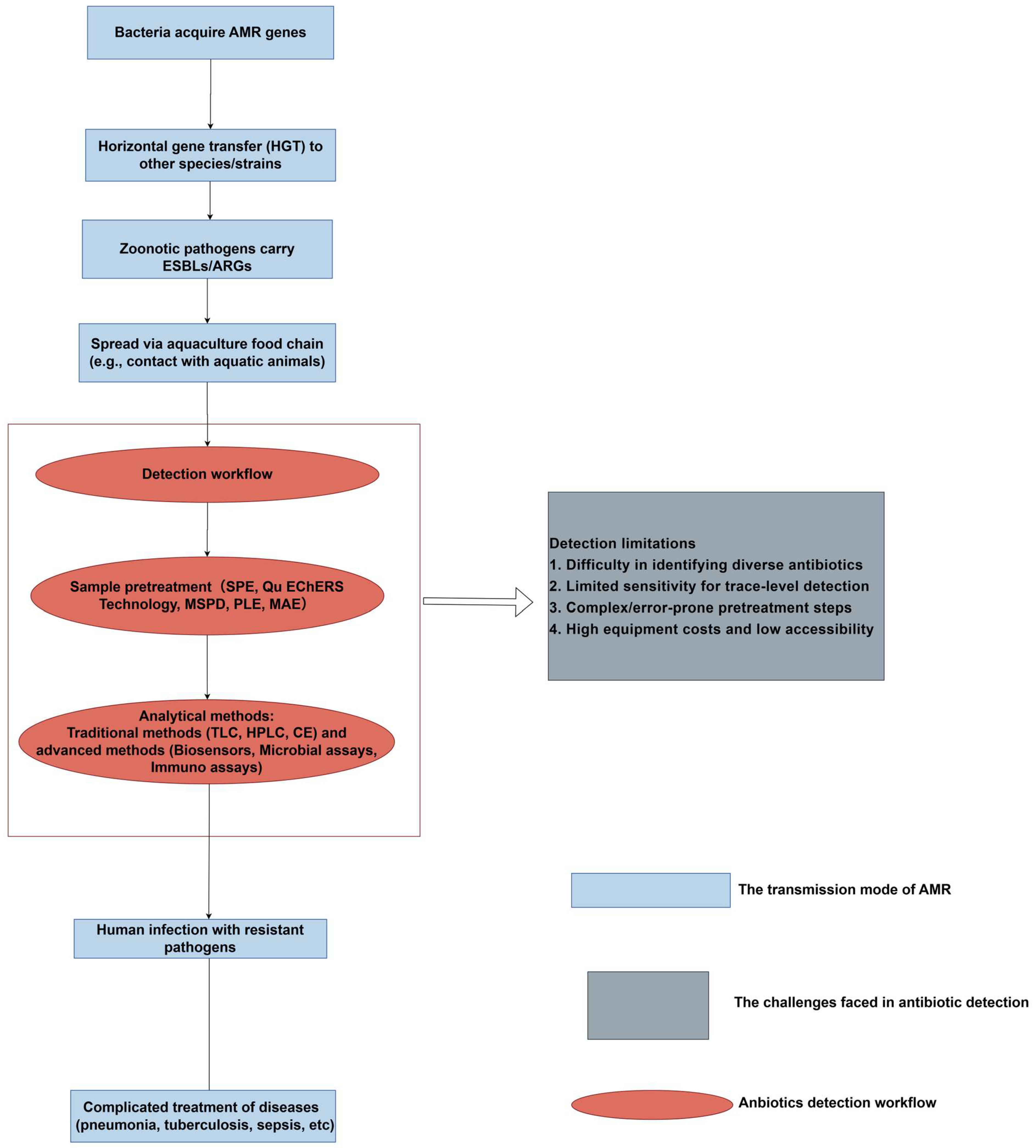
- Yuan, X.; Lv, Z.; Zhang, Z.; Han, Y.; Liu, Z.; Zhang, H. A Review of antibiotics, antibiotic resistant bacteria, and resistance genes in aquaculture: Occurrence, contamination, and transmission. Toxics 2023, 11, 420. [Google Scholar] [CrossRef]
- Buckner, M.M.C.; Ciusa, M.L.; Piddock, L.J.V. Strategies to combat antimicrobial resistance: Anti-plasmid and plasmid curing. Fems Microbiol. Rev. 2018, 42, 781–804. [Google Scholar] [CrossRef]
- Kesarcodi-Watson, A.; Kaspar, H.; Lategan, M.J.; Gibson, L. Probiotics in aquaculture: The need, principles and mechanisms of action and screening processes. Aquaculture 2008, 274, 1–14. [Google Scholar] [CrossRef]
- Qi, Z.; Zhang, X.-H.; Boon, N.; Bossier, P. Probiotics in aquaculture of China—Current state, problems and prospect. Aquaculture 2009, 290, 15–21. [Google Scholar] [CrossRef]
- Wang, A.; Ran, C.; Wang, Y.; Zhang, Z.; Ding, Q.; Yang, Y.; Olsen, R.E.; Ringø, E.; Bindelle, J.; Zhou, Z. Use of probiotics in aquaculture of China—A review of the past decade. Fish Shellfish Immunol. 2019, 86, 734–755. [Google Scholar] [CrossRef]
- Ringø, E. Probiotics in shellfish aquaculture. Aquac. Fish. 2020, 5, 1–27. [Google Scholar] [CrossRef]
- Cerrato, R.M. What Fish Biologists Should Know About Bivalve Shells. Fish. Res. 2000, 46, 39–49. [Google Scholar] [CrossRef]
- Li, R.; Zhang, M.; Zhou, Y.; Wei, D.; Yang, Y.; Gao, D.; Shan, X.; Sun, W.; Dong, H.; Wang, G. Fish-derived lactic acid bacteria supplementation enhanced the immunity and resistance in Crucian carp (Carassius auratus). Aquac. Rep. 2024, 36, 102037. [Google Scholar] [CrossRef]
- Li, M.; Liang, H.; Xie, J.; Chao, W.; Zou, F.; Ge, X.; Ren, M. Diet supplemented with a novel Clostridium autoethanogenum protein have a positive effect on the growth performance, antioxidant status and immunity in juvenile Jian carp (Cyprinus carpio var. Jian). Aquac. Rep. 2021, 19, 100572. [Google Scholar] [CrossRef]
- Yang, D.; Wang, Z.; Dai, X.; Liu, M.; Zhang, D.; Zeng, Y.; Zeng, D.; Ni, X.; Pan, K. Addition of Brevibacillus laterosporus to the rearing water enhances the water quality, growth performance, antioxidant capacity, and digestive enzyme activity of crucian carp Carassius auratus. Fish Sci. 2023, 89, 659–670. [Google Scholar] [CrossRef]
- Rahayu, S.; Amoah, K.; Huang, Y.; Cai, J.; Wang, B.; Shija, V.M.; Jin, X.; Anokyewaa, M.A.; Jiang, M. Probiotics application in aquaculture: Its potential effects, current status in China and future prospects. Front. Mar. Sci. 2024, 11, 1455905. [Google Scholar] [CrossRef]
- Ghosh, S.; Ringø, E.; Deborah, G.; Rahiman, K.M.; Hatha, A. Enterobacter hormaechei bac 1010 from the gut of flathead grey mullet as probable aquaculture probiont. Nat. Sustain. 2011, 5, 189. [Google Scholar]
- Food and Agriculture Organization; World Health Organization. Probiotics in Food: Health and Nutritional Properties and Guidelines for Evaluation; World Health Organization: Rome, Italy, 2006. [Google Scholar]
- Bui, N.M.N.; Heyse, J.; Delamare-Deboutteville, J.; Defoirdt, T.; Props, R.; Shelley, C. Bacterial and microalgal communities in carp polyculture systems: Composition, affecting factors and further perspectives. Aquaculture 2024, 582, 46213. [Google Scholar] [CrossRef]
- Pelić, D.L.; Radosavljević, V.; Pelić, M.; Baloš, M.Ž.; Puvača, N.; Dujaković, J.J.; Gavrilović, A. Antibiotic residues in cultured fish: Implications for food safety and regulatory concerns. Fishes 2024, 9, 484. [Google Scholar] [CrossRef]
- Ma, T.; Shen, X.; Shi, X.; Sakandar, H.A.; Quan, K.; Li, Y.; Jin, H.; Kwok, L.Y.; Zhang, H.; Sun, Z. Targeting gut microbiota and metabolism as the major probiotic mechanism—An evidence-based review. Trends Food Sci. Technol. 2023, 138, 178–198. [Google Scholar] [CrossRef]
- Abbaszadeh, S.H.; Hosseini, S.R.A.; Mahmoodpoor, A.; Yousefi, M.; Dizaji, L.L.; Mameghani, M.E. Investigating the role of probiotics in modulating T cells and the immune response: A systematic review. Indian J. Microbiol. 2024, 1–13. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.; Wu, Z.B.; Qu, S.Y.; Wang, G.X.; Wei, D.D.; Li, P.F.; Ling, F. Enterobacter asburiae E7, a Novel potential probiotic, enhances resistance to Aeromonas veronii infection via stimulating the immune response in common carp (Cyprinus carpio). Microbiol. Spectr. 2023, 11, 0427322. [Google Scholar] [CrossRef]
- Wu, Z.-Q.; Jiang, C.; Ling, F.; Wang, G.-X. Effects of dietary supplementation of intestinal autochthonous bacteria on the innate immunity and disease resistance of grass carp (Ctenopharyngodon idellus). Aquaculture 2015, 438, 105–114. [Google Scholar] [CrossRef]
- Chi, C.; Jiang, B.; Yu, X.-B.; Liu, T.-Q.; Xia, L.; Wang, G.-X. Effects of three strains of intestinal autochthonous bacteria and their extracellular products on the immune response and disease resistance of common carp, Cyprinus carpio. Fish Shellfish Immunol. 2014, 36, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Ran, C.; He, S.; Ren, P.; Hu, J.; Zhao, X.; Zhou, Z. Effects of dietary Saccharomyces cerevisiae culture or live cells with Bacillus amyloliquefaciens spores on growth performance, gut mucosal morphology, hsp70 gene expression, and disease resistance of juvenile common carp (Cyprinus carpio). Aquaculture 2015, 438, 33–38. [Google Scholar]
- Chang, X.; Kang, M.; Shen, Y.; Yun, L.; Yang, G.; Zhu, L.; Meng, X.; Zhang, J.; Su, X. Bacillus coagulans SCC-19 maintains intestinal health in cadmium-exposed common carp (Cyprinus carpio L.) by strengthening the gut barriers, relieving oxidative stress and modulating the intestinal microflora. Ecotoxicol. Environ. Saf. 2021, 228, 112977. [Google Scholar] [CrossRef]
- Jiang, H.; Chen, T.; Sun, H.; Tang, Z.; Yu, J.; Lin, Z.; Ren, P.; Zhou, X.; Huang, Y.; Li, X.; et al. Immune response induced by oral delivery of Bacillus subtilis spores expressing enolase of Clonorchis sinensis in grass carps (Ctenopharyngodon idellus). Fish Shellfish Immunol. 2017, 60, 318–325. [Google Scholar] [CrossRef]
- Kang, M.; Su, X.; Yun, L.; Shen, Y.; Feng, J.; Yang, G.; Meng, X.; Zhang, J.; Chang, X. Evaluation of probiotic characteristics and whole genome analysis of Bacillus velezensis R-71003 isolated from the intestine of common carp (Cyprinus carpio L.) for its use as a probiotic in aquaculture. Aquac. Rep. 2022, 25. [Google Scholar]
- Mazurkiewicz, J.; Przyby, A.; Sip, A.; Grajek, W. Effect of Carnobacterium divergens and Enterococcus hirae as probiotic bacteria in feed for common carp, Cyprinus carpio L. Arch. Pol. Fish. 2007, 15, 93–102. [Google Scholar]
- Sun, X.; Xu, H.; Song, Y.; Long, J.; Yan, C.; Qi, X.; Wang, L.; Jin, Y.; Liu, H. Effect of host-derived Enterococcus faecium L6 on growth performance, intestinal health and antibacterial activities of juvenile grass carp. Aquaculture 2025, 596, 741879. [Google Scholar] [CrossRef]
- Hosseini, M.; Miandare, H.K.; Hoseinifar, S.H.; Yarahmadi, P. Dietary Lactobacillus acidophilus modulated skin mucus protein profile, immune and appetite genes expression in gold fish (Carassius auratus gibelio). Fish Shellfish Immunol. 2016, 59, 149–154. [Google Scholar] [CrossRef]
- Zhang, C.; Pu, C.; Li, S.; Xu, R.; Qi, Q.; Du, J. Lactobacillus delbrueckii ameliorates Aeromonas hydrophila-induced oxidative stress, inflammation, and immunosuppression of Cyprinus carpio huanghe var NF-κB/Nrf2 signaling pathway. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2024, 285, 110000. [Google Scholar] [CrossRef]
- Mohammadian, T.; Nasirpour, M.; Tabandeh, M.R.; Mesbah, M. Synbiotic effects of β-glucan, mannan oligosaccharide and Lactobacillus casei on growth performance, intestine enzymes activities, immune-hematological parameters and immune-related gene expression in common carp, Cyprinus carpio: An experimental infection with Aeromonas hydrophila. Aquaculture 2019, 511, 634197. [Google Scholar]
- Salih, A.M.; Patra, I.; Sivaraman, R.; Alhamzawi, R.; Khalikov, K.M.; Al-Qaim, Z.H.; Golgouneh, S.; Jawad, M.A.; Adhab, A.H.; Vázquez-Cárdenas, A.L.; et al. The probiotic Lactobacillus sakei subsp. Sakei and hawthorn extract supplements improved growth performance, digestive enzymes, immunity, and resistance to the pesticide acetamiprid in common carp (Cyprinus carpio). Aquac. Nutr. 2023, 2023, 8506738. [Google Scholar]
- Hoseinifar, S.H.; Hosseini, M.; Paknejad, H.; Safari, R.; Jafar, A.; Yousefi, M.; Doan, H.V.; Mozanzadeh, M.T. Enhanced mucosal immune responses, immune related genes and growth performance in common carp (Cyprinus carpio) juveniles fed dietary Pediococcus acidilactici MA18/5M and raffinose. Dev. Comp. Immunol. 2019, 94, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Fatemeh, H.; Roghieh, S.; Ali, S.; Hossein, H.S.; Hadi, G.; Bahareh, S.; Rahamat, U.M.; Siddik, M.A.B. The effects of combined or singular administration of formic acid and Pediococcus acidilactici on stress resistance, growth performance, immune responses and related genes expression in common carp, Cyprinus carpio. Aquac. Rep. 2023, 29, 101474. [Google Scholar]
- Giri, S.S.; Jun, J.W.; Yun, S.; Kim, H.J.; Kim, S.G.; Kim, S.W.; Woo, K.J.; Han, S.J.; Oh, W.T.; Kwon, J.; et al. Effects of dietary heat-killed Pseudomonas aeruginosa strain VSG2 on immune functions, antioxidant efficacy, and disease resistance in Cyprinus carpio. Aquaculture 2020, 514, 734489. [Google Scholar] [CrossRef]
- Francisco, V.-A.; Rafael, M.-C.L.; Adrián, H.-M.; Francesco, C.; Asunción, L.-L.; Marcel, M.-P. Therapeutic modulation of fish gut microbiota, a feasible strategy for aquaculture? Aquaculture 2021, 544, 737050. [Google Scholar]
- Singh, A.; Chouhan, N.; Chauhan, V.; Choudhary, B. Challenges and contemplations of using probiotics in aquaculture. Aquac. Mag. 2024, 7, 1–10. [Google Scholar]
- Jiajia, Z.; Yunsheng, C.; Kálmán, I.; Damla, A.A.; Ramazan, I.F.; Yuwen, F.; Gaspar, R.; Kui, Z.; Ulas, A. Mechanisms of probiotic Bacillus against enteric bacterial infections. One Health Adv. 2023, 1, 21. [Google Scholar]
- Wang, J.; He, M.; Yang, M.; Ai, X. Gut microbiota as a key regulator of intestinal mucosal immunity. Life Sci. 2024, 345, 122612. [Google Scholar] [CrossRef]
- Joanna, J.-F.; Krystyna, S.; Justyna, B.; Daniel, W.; Agnieszka, Ż.-S.; Mariusz, S.P. The promises and risks of probiotic Bacillus species. Acta Biochim. Pol. 2018, 65, 509–519. [Google Scholar]
- Wang, D.; Xu, R.; Liu, S.; Sun, X.; Zhang, T.; Shi, L.; Wang, Y. Enhancing the application of probiotics in probiotic food products from the perspective of improving stress resistance by regulating cell physiological function: A review. Food Res. Int. 2025, 199, 115369. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Yang, W.; Du, X.; Chen, N.; Xu, Q.; Wei, M.; Zhang, C. High-level and -yield production of L-leucine in engineered Escherichia coli by multistep metabolic engineering. Metab. Eng. 2023, 78, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Yook, G.; Nam, J.; Jo, Y.; Yoon, H.; Yang, D. Metabolic engineering approaches for the biosynthesis of antibiotics. Microb. Cell Fact. 2025, 24, 35. [Google Scholar] [CrossRef] [PubMed]
- Hoseinifar, S.H.; Faheem, M.; Liaqat, I.; Doan, H.V.; Ghosh, K.; Ringø, E. Promising Probiotic Candidates for Sustainable Aquaculture: An Updated Review. Animals 2024, 14, 3644. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.H.; Jia, J.B. Effects of probiotics diet fed myxcobacteria (jch-04) on survival, growth, digestive enzymes activities and aquatic environment in grass carp (Ctenopharyngodon idellus). In Proceedings of the 2nd Annual International Conference on Advanced Material Engineering (AME 2016), Wuhan, China, 15–17 April 2016. [Google Scholar]
- Hoseinifar, S.H.; Sun, Y.Z.; Caipang, C.M. Short-chain fatty acids as feed supplements for sustainable aquaculture: An updated view. Aquac. Res. 2017, 48, 1380–1391. [Google Scholar] [CrossRef]
- Dimitroglou, A.; Merrifield, D.L.; Moate, R.; Davies, S.J.; Spring, P.; Sweetman, J.; Bradley, G. Dietary mannan oligosaccharide supplementation modulates intestinal microbial ecology and improves gut morphology of rainbow trout, Oncorhynchus mykiss (Walbaum). J. Anim. Sci. 2009, 87, 3226–3234. [Google Scholar] [CrossRef]
- Rodriguez-Estrada, U.; Satoh, S.; Haga, Y.; Fushimi, H.; Sweetman, J. Effects of inactivated enterococcus faecalis and mannan oligosaccharide and their combination on growth, immunity, and disease protection in rainbow trout. N. Am. J. Aquac. 2013, 75, 416–428. [Google Scholar] [CrossRef]
- Wollowski, I.; Rechkemmer, G.; Pool-Zobel, B.L. Protective role of probiotics and prebiotics in colon cancer. Am. J. Clin. Nutr. 2001, 73, 451S–455S. [Google Scholar] [CrossRef]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef]
- Yadav, M.; Sehrawat, N.; Sharma, A.K.; Kumar, S.; Singh, R.; Kumar, A.; Kumar, A. Synbiotics as potent functional food: Recent updates on therapeutic potential and mechanistic insight. J. Food Sci. Technol. 2024, 61, 11–15. [Google Scholar] [CrossRef]
- Doe, J.; Smith, J. Probiotics, Prebiotics, and Synbiotics in Aquaculture: A review. Biology 2023, 12, 123–135. [Google Scholar]
- Doe, J.; Smith, J.; Johnson, R. The synergistic effects of synbiotics on microbiota regulation and immune enhancement in aquaculture. Biology 2024, 13, 567–585. [Google Scholar]
- Vine, J.; Doe, J.; Smith, E. Immunomodulatory Properties of Probiotics and Their interactions with host immunity. Biology 2024, 13, 4726. [Google Scholar]
- Ning, D.; Huang, Y.; Zhou, Y.; Zhao, H.; Nie, W.; Zheng, Y.; Huang, X. Evaluation of the immunomodulatory effects of a novel synbiotic made of combined use of probiotic-prebiotic-Chinese traditional herbs. J. Agric. Food Res. 2024, 18, 101497. [Google Scholar] [CrossRef]
- Stagg, A.J.; Hart, A.L.; Knight, S.C.; Kamm, M.A. Microbialgut interactions in health and disease. Interactions between dendritic cells and bacteria in the regulation of intestinal immunity. Best Pract. Res. Clin. Gastroenterol. 2004, 18, 255–270. [Google Scholar] [CrossRef]
- Wee, W.; Abdul Hamid, N.K.; Mat, K.; Khalif, R.I.A.R.; Rusli, N.D.; Rahman, M.M.; Kabir, M.A.; Wei, L.S. The effects of mixed prebiotics in aquaculture: A review. Aquac. Fish. 2024, 9, 28–34. [Google Scholar] [CrossRef]
- Giampietro, P.D.; Lorenzo, M. Probiotics as adjuvants in vaccine strategy: Is there more room for improvement? Vaccines 2021, 9, 811. [Google Scholar] [CrossRef]
- Soltani, M.; Lymbery, A.; Song, K.S.; Shekarabi, P.H. Adjuvant effects of medicinal herbs and probiotics for fish vaccines. Rev. Aquac. 2019, 11, 1325–1341. [Google Scholar] [CrossRef]
- Liu, J.; Wang, B.; Lai, Q.; Lu, Y.; Li, L.; Li, Y.; Liu, S. Boosted growth performance, immunity, antioxidant capacity and disease resistance of crucian carp (Carassius auratus) by single or in combination dietary Bacillus subtilis and xylo-oligosaccharides. Comp. Biochem. Physiol. C-Toxicol. Pharmacol. 2022, 256, 109296. [Google Scholar] [CrossRef]
- Wang, J.L.; Shan, J.F.; Zhu, H.Y.; Chun, W.U.; Nie, G.X. Effects of dietary xylo-oligosaccharides(XOS) on immunity and growth performance in common carp, Cyprinus carpio. Fish Sci. 2014, 33, 611–615. [Google Scholar]
- Liu, J.; Yao, B.; Sun, J.; Bi, C.; Lu, Y.; Yan, Z.; Li, Y.; Lv, W. Bacillus subtilis and xylo-oligosaccharide ameliorates NaHCO3-induced intestinal barrier dysfunction and autophagy by regulating intestinal microflora and PI3K/Akt pathway of crucian carp (Carassius auratus). Aquac. Rep. 2024, 36, 102048. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Chen, M.; Xie, S.W.; Chen, X.Q.; Niu, J. Effects of dietary xylooligosaccharide on growth performance, enzyme activity and immunity of juvenile grass carp, Ctenopharyngodon idellus. Aquac. Rep. 2020, 18, 100519. [Google Scholar] [CrossRef]
- Katerina, T.; Theolis, B.; Giuseppe, P.; Flavio, C.; Angelica, S.; Giuseppe, V.; Maria, R. Probiotic and postbiotic activity in health and disease: Comparison on a novel polarised ex-vivo organ culture model. Gut 2012, 61, 1007–1015. [Google Scholar]
- Pérez-Sánchez, T.; Ruiz-Zarzuela, I.; Blas, I.; Balcázar, J.L. Probiotics in aquaculture: A current assessment. Rev. Aquac. 2014, 6, 133–146. [Google Scholar] [CrossRef]
- Leser, T.; Baker, A. Molecular mechanisms of Lacticaseibacillus rhamnosus GG in probiotic function. Microorganisms 2024, 12, 794. [Google Scholar]
- Post, S.E.; Brito, I.L. Structural insight into protein–protein interactions between intestinal microbiome and host. Curr. Opin. Struct. Biol. 2022, 74, 102354. [Google Scholar] [CrossRef]
- Smith, J.; Doe, A. Probiotic Gastrointestinal Transit and Colonization: Adhesion Mechanisms. Front. Cell. Infect. Microbiol. 2021, 11, 609722. [Google Scholar]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. Author Correction: The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nature reviews. Gastroenterol. Hepatol. 2022, 19, 551. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, S.; Li, S.; Jiang, W.; Wang, J.; Xiao, J.; Chen, T.; Ma, J.; Khan, M.Z.; Wang, W.; et al. Unlocking the power of postbiotics: A revolutionary approach to nutrition for humans and animals. Cell Metab. 2024, 36, 725–744.7. [Google Scholar] [CrossRef]
- Gao, J.; Li, Y.; Wan, Y.; Hu, T.; Liu, L.; Yang, S.; Gong, Z.; Zeng, Q.; Wei, Y.; Yang, W.; et al. A Novel Postbiotic From Lactobacillus rhamnosus GG With a Beneficial Effect on Intestinal Barrier Function. Front. Microbiol. 2019, 10, 477. [Google Scholar] [CrossRef]
- Shigwedha, N.; Sichel, L.; Jia, L.; Zhang, L. Probiotical Cell Fragments (PCFs) as “Novel Nutraceutical Ingredients”. J. Biosci. Med. 2014, 2, 43–55. [Google Scholar] [CrossRef]
- Wegh, C.A.M.; Geerlings, S.Y.; Knol, J.; Roeselers, G.; Belzer, C. Postbiotics and Their Potential Applications in Early Life Nutrition and Beyond. Int. J. Mol. Sci. 2019, 20, 4673. [Google Scholar] [CrossRef] [PubMed]
- Niamh, H.; David, C.; Igor, F.; Pierre-Alain, G.; Valérie, L.; Alexandre, R.; Nicolas, M. Epigenetic regulatory elements: Recent advances in understanding their mode of action and use for recombinant protein production in mammalian cells. Biotechnol. J. 2015, 10, 967–978. [Google Scholar]
- Dawood, M.A.O.; Koshio, S.; Ishikawa, M.; Yokoyama, S. Effects of heat killed Lactobacillus plantarum (LP20) supplemental diets on growth performance, stress resistance and immune response of red sea bream, Pagrus major. Aquaculture 2015, 442, 29–36. [Google Scholar] [CrossRef]
- Zhu, X.; Cao, M. Research progress on inactivated probiotics. Chin. J. Microbiol. 2010, 22, 175–178. [Google Scholar]
- Demin, C.; Meijuan, T.; Dan, W. Editorial: The actions of trace element metabolism and epigenetics on animal health and disease. Front. Vet. Sci. 2022, 9, 1086322. [Google Scholar]
- Banan Khojasteh, S.M. The morphology of the post-gastric alimentary canal in teleost fishes: A brief review. Int. J. Aquat. Sci. 2020, 3, 70–88. [Google Scholar]
- Estrada, U.R.; Satoh, S.; Haga, Y.; Fushimi, H.; Sweetman, J. Effects of single and combined supplementation of enterococcus faecalis, mannan oligosaccharide and polyhydroxybutyrate acid on growth performance and immune response of rainbow trout Oncorhynchus mykiss. Aquac. Sci. 2009, 57, 609–617. [Google Scholar]
- Yu, Z.; Hao, Q.; Liu, S.B.; Zhang, Q.S.; Chen, X.Y.; Li, S.H.; Ran, C.; Yang, Y.L.; Teame, T.; Zhang, Z.; et al. The positive effects of postbiotic (SWF concentration®) supplemented diet on skin mucus, liver, gut health, the structure and function of gut microbiota of common carp (Cyprinus carpio) fed with high-fat diet. Fish Shellfish Immunol. 2023, 135, 108681. [Google Scholar] [CrossRef]
- Bahabadi, M.A.; Shekarbi, S.P.H.; Sharifinia, M.; Khanjani, M.H. Exploring fish antimicrobial peptides (AMPs): Classification, biological activities, and mechanisms of action. Int. J. Pept. Res. Ther. 2024, 30, 633. [Google Scholar]
- Rodrigues, T.; Guardiola, F.A.; Almeida, D.; Antunes, A. Aquatic invertebrate antimicrobial peptides in the fight against aquaculture pathogens. Microorganisms 2025, 13, 156. [Google Scholar] [CrossRef] [PubMed]
- Huan, Y.C.; Kong, Q.; Mou, H.J.; Yi, H.X. Antimicrobial peptides: Classification, design, application and research progress in multiple fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef] [PubMed]
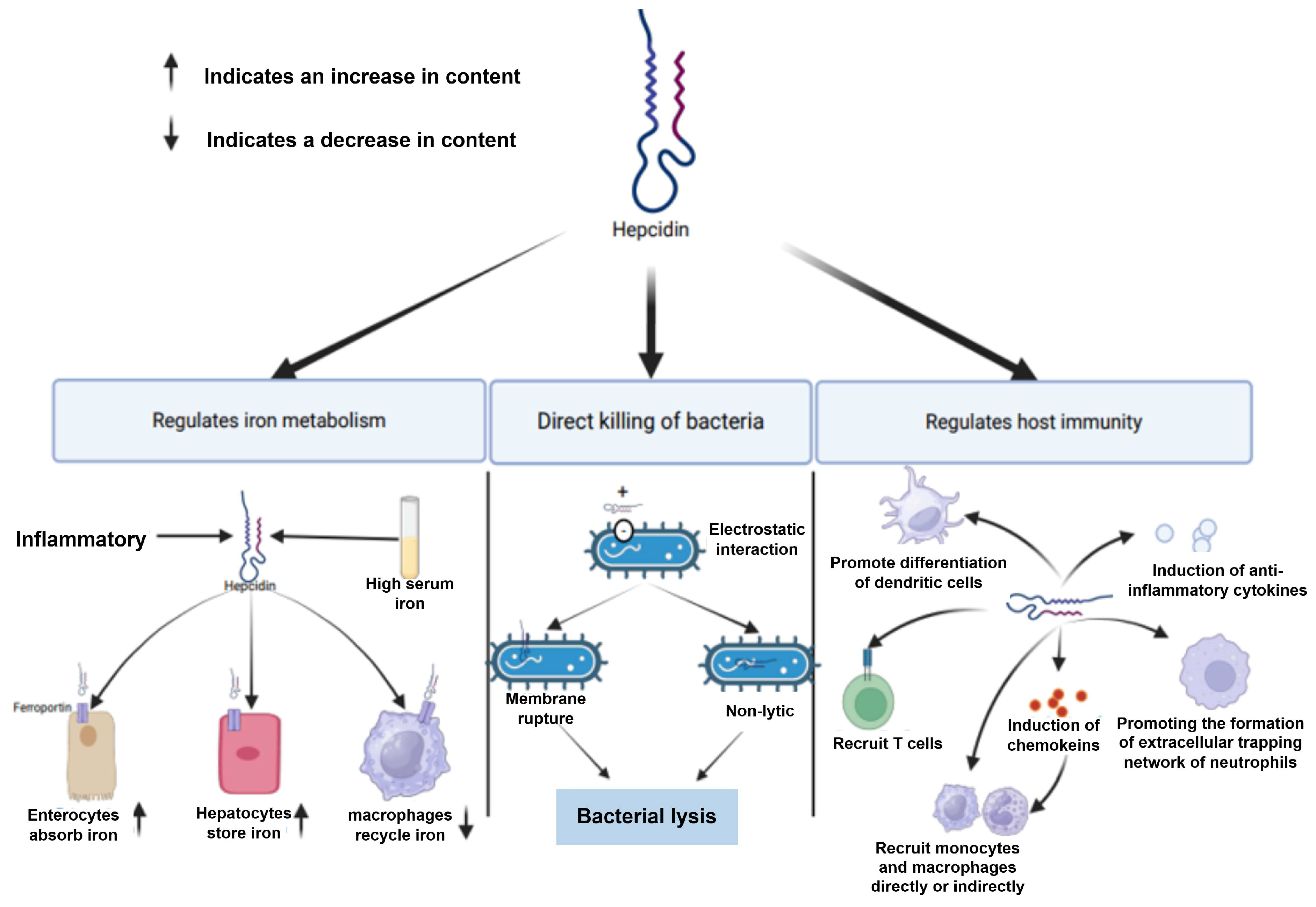
- Feng, W.; Hu, X.Q.; Wang, F.C.; Huang, F.; Liu, L.; Li, H.; Liu, H.M.; Yang, W.M. Effect of dietary iron levels on growth, iron concentration in tissues, and blood concentration levels of transferrin and hepcidin in bighead carp (Aristichthys nobilis). Aquac. Res. 2020, 51, 1113–1119. [Google Scholar] [CrossRef]
- Liu, H.; Wang, S.; Zhang, Z.; Yan, H.; He, T.; Wei, X.; Shi, Y.; Chen, Y.; Wang, W.; Li, X. Nanopore-based full-length transcriptome sequencing of the skin in Pseudopleuronectes yokohamae identifies novel antimicrobial peptide genes. Fish Shellfish Immunol. 2024, 154, 109957. [Google Scholar] [CrossRef]
- Zhang, A.; Chen, D.; Wei, H.; Du, L.; Zhao, T.; Wang, X.; Zhou, H. Functional characterization of TNF-alpha in grass carp head kidney leukocytes: Induction and involvement in the regulation of NF-kappaB signaling. Fish Shellfish Immunol. 2012, 33, 1123–1132. [Google Scholar] [CrossRef]
- Yan, Y.; Li, X.D.; Pei, C. Research progress on the biological function and in vitro expression of fish ferritin. Aquat. Sci. 2023, 42. [Google Scholar] [CrossRef]
- Jiang, Y.Y.; Chen, Y.; Song, Z.Y.; Tan, Z.; Cheng, J. Recent advantageous in design of antimicrobial peptides and poly-peptide stoward clinical translation. Adv. Drug Deliv. Rev. 2021, 170, 261–280. [Google Scholar] [CrossRef]
- Yan, J.; Wang, K.; Dang, W.; Chen, R.; Xie, J.; Zhang, B.; Song, J.; Wang, R. Two hits are better than one: Membrane-active and DNA binding-related double-action mechanism of NK-18, a novel antimicrobial peptide derived from mammalian NK-lysin. Antimicrob. Agents Chemother. 2013, 57, 220–228. [Google Scholar] [CrossRef]
- Ko, S.J.; Kang, N.H.; Kim, M.K.; Park, J.; Park, E.; Park, G.H.; Kang, T.W.; Na, D.E.; Park, J.B.; Yi, Y.E.; et al. Antibacterial and anti-biofilm activity, and mechanism of action of pleurocidin against drug resistant Staphylococcus aureus. Microb. Pathog. 2018, 127, 70–78. [Google Scholar] [CrossRef]
- Cohen, P.A. Probiotic Safety—No Guarantees. JAMA Intern. Med. 2018, 178, 1577–1578. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, R.; Wu, H.; Gaafar, A.Y.; Younes, A.M.; Cao, Q. Microecological Preparations as Antibiotic Alternatives in Cyprinid Aquaculture. Fishes 2025, 10, 263. https://doi.org/10.3390/fishes10060263
Qu R, Wu H, Gaafar AY, Younes AM, Cao Q. Microecological Preparations as Antibiotic Alternatives in Cyprinid Aquaculture. Fishes. 2025; 10(6):263. https://doi.org/10.3390/fishes10060263
Chicago/Turabian StyleQu, Ruiheng, Hao Wu, Alkhateib Y. Gaafar, Abdelgayed Metwaly Younes, and Quanquan Cao. 2025. "Microecological Preparations as Antibiotic Alternatives in Cyprinid Aquaculture" Fishes 10, no. 6: 263. https://doi.org/10.3390/fishes10060263
APA StyleQu, R., Wu, H., Gaafar, A. Y., Younes, A. M., & Cao, Q. (2025). Microecological Preparations as Antibiotic Alternatives in Cyprinid Aquaculture. Fishes, 10(6), 263. https://doi.org/10.3390/fishes10060263








