Ageing Mediterranean Bluefin Tuna: A Comparative Study Between Dorsal Fin Spines and Vertebrae
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
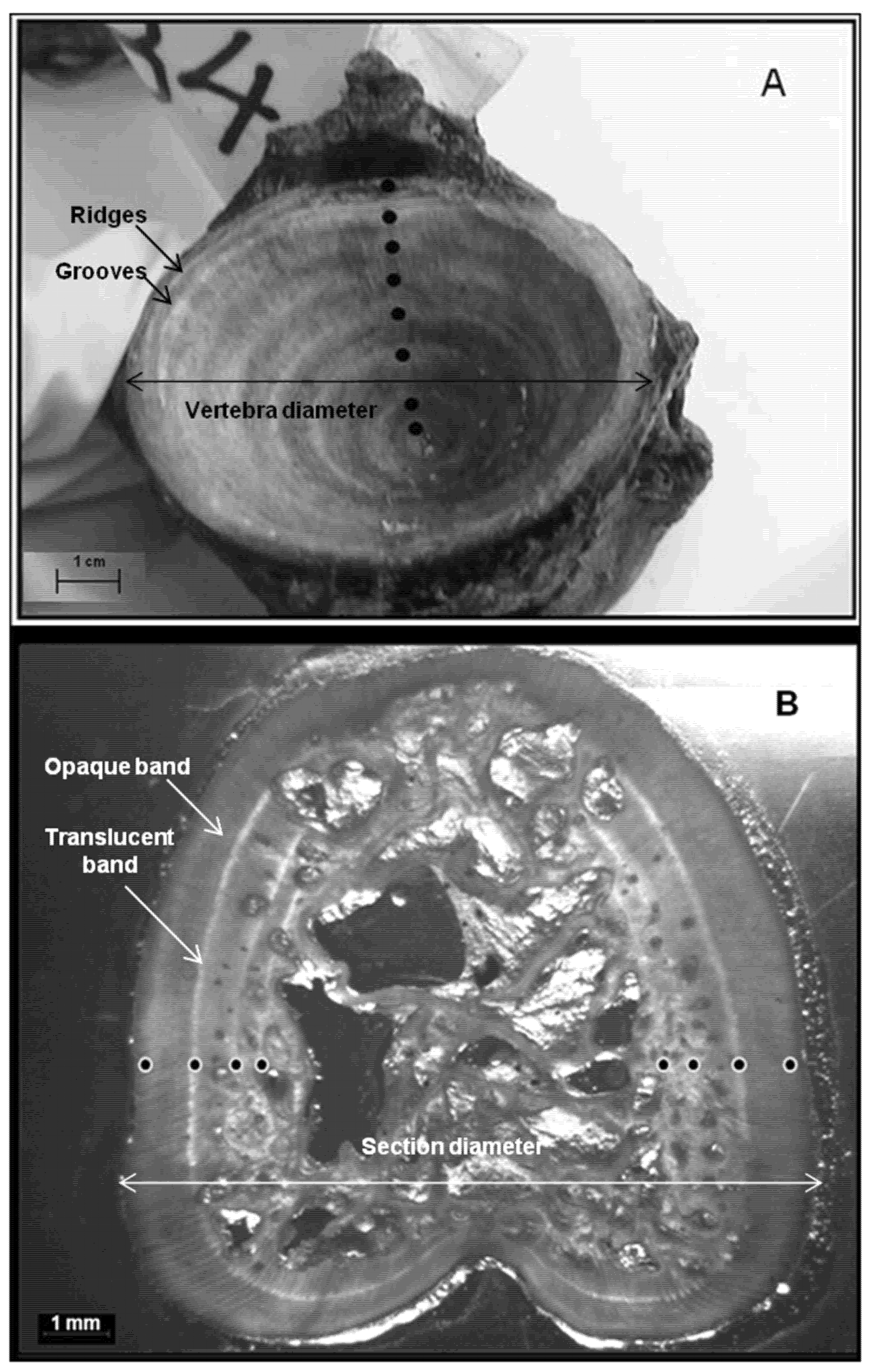
2.2. Measurements of the Calcified Structures
2.3. Ageing Procedure Using Dorsal Fin Spines
2.4. Ageing Procedure Using Vertebrae
2.5. Precision of Age Estimations
2.6. Estimation of Growth Parameters
2.7. Statistical Analysis
3. Results
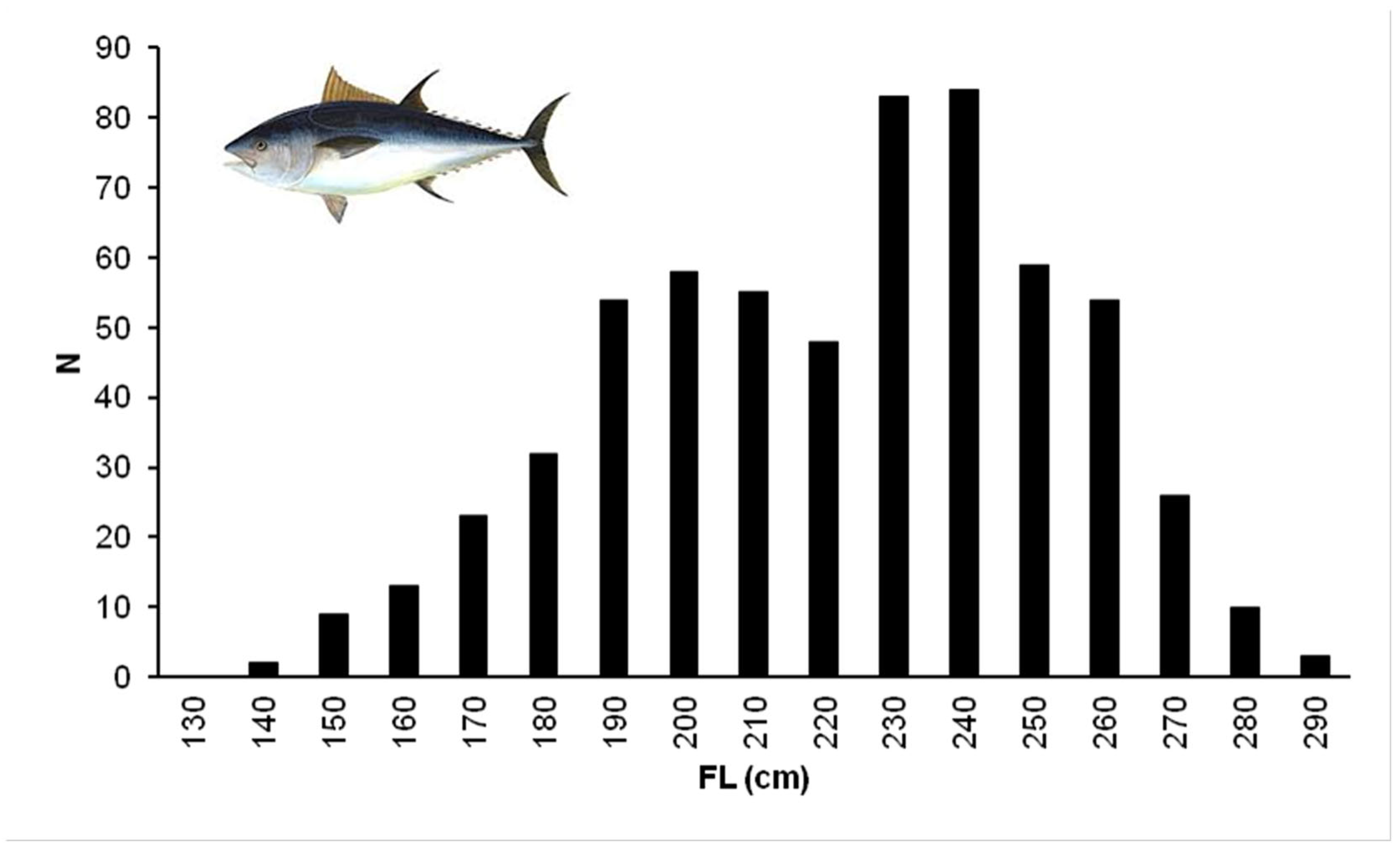
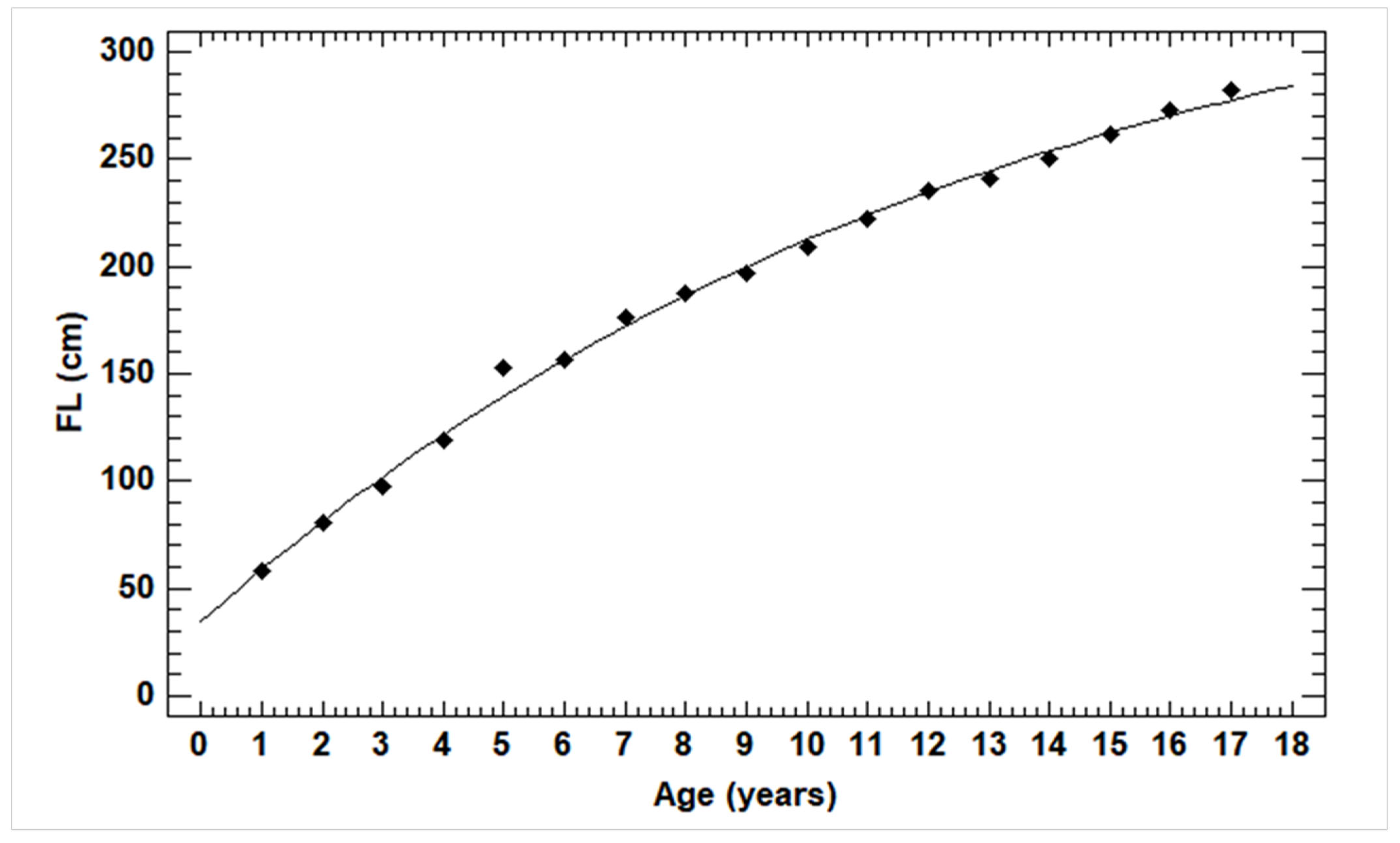
3.1. Size Measurements and Relationships
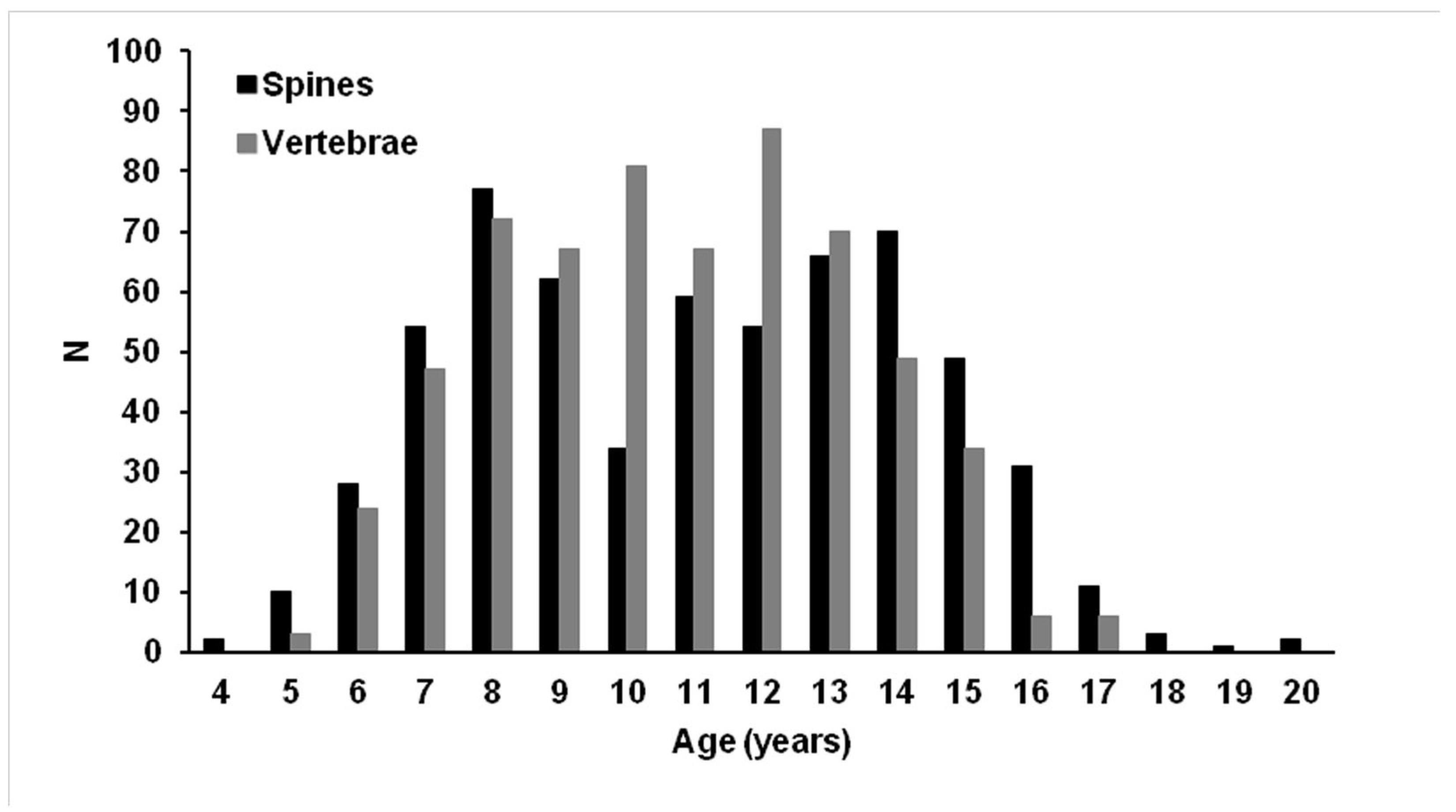
3.2. Age Estimations and Precision
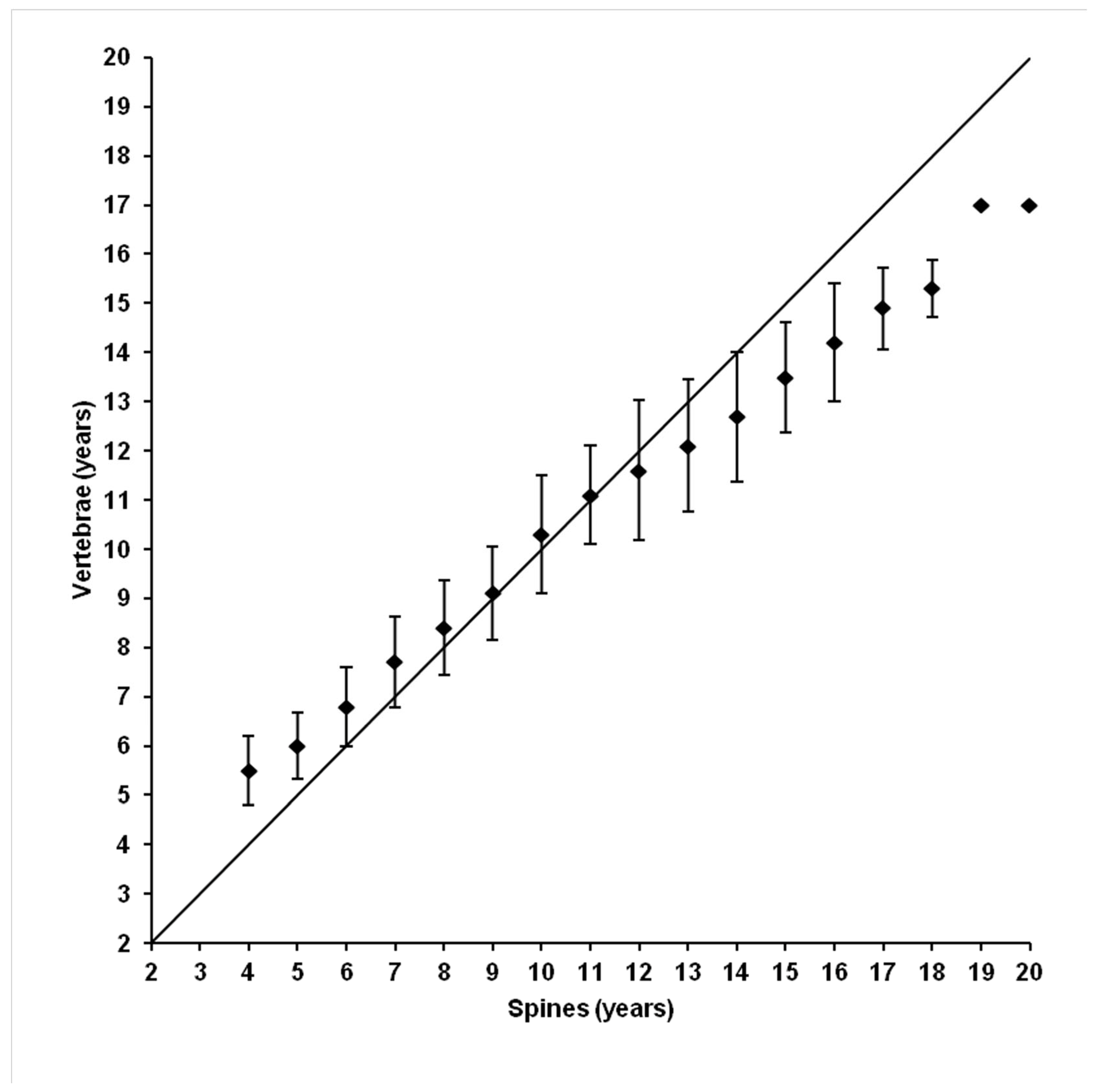
3.3. Ageing Comparison Between the Two Calcified Structures
4. Discussion
4.1. Methodological Limitations, Accuracy of Age Estimation Techniques, and Validation Methods
| Authors | Year | Sampling Area | Stock | Wild/Reared | Calcified Structure | Calcified Structure Comparison | Validation (Yes/No) | Validation Method | CV (%) | APE (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Sella [38] | 1929 | Atlantic Ocean, Mediterranean Sea | eastern | Wild | V | - | No | - | - | - |
| Mather & Schuck [39] | 1960 | Atlantic Ocean | western | Wild | V | - | No | - | - | - |
| Rodríguez-Roda [40] | 1964 | Atlantic Ocean | eastern | Wild | V | - | Yes | Tag-recovery data | - | - |
| Caddy et al. [41] | 1976 | Atlantic Ocean | western | Wild | O | - | No | - | - | - |
| Butler et al. [42] | 1977 | Atlantic Ocean | western | Wild | O | - | No | - | - | - |
| Farrugio [43] | 1980 | Mediterranean Sea | eastern | Wild | V | - | No | - | - | - |
| Lee et al. [9] | 1983 | Atlantic Ocean | western | Wild | O, V | O, V | Yes | Tag-recovery data | - | - |
| Compeán-Jimenez & Bard [34] | 1983 | Atlantic Ocean, Mediterranean Sea | eastern | Wild | S | - | No | - | - | - |
| Hattour [44] | 1984 | Mediterranean Sea | eastern | Wild | O | - | No | - | - | - |
| Cort [15] | 1991 | Atlantic Ocean | eastern | Wild | S | - | No | - | - | - |
| Olafsdóttir & Ingimundardóttir [45] | 2000 | Atlantic Ocean | eastern | Wild | V | - | No | - | - | - |
| Megalofonou & de Metrio [18] | 2000 | Mediterranean Sea | eastern | Wild | S | - | Yes | Marginal Increment Analysis | - | - |
| Farrugia & Rodriguez-Cabello [27] | 2001 | Mediterranean Sea | eastern | Wild | S | - | No | - | - | - |
| El-Kebir et al. [26] | 2002 | Mediterranean Sea | eastern | Wild | S | - | No | - | - | - |
| Megalofonou et al. [46] | 2003 | Mediterranean Sea | eastern | Wild | O | - | No | - | - | - |
| Rodríguez-Marín et al. [47] | 2004 | Atlantic Ocean | eastern | Wild | S | - | No | - | - | - |
| La Mesa et al. [48] | 2005 | Mediterranean Sea | eastern | Wild | O | - | No | - | 0.03 | 0.02 |
| Rodríguez-Marín et al. [10] | 2006 | Atlantic Ocean | eastern | Wild | S, V | S, V | No | - | 6.2 | - |
| Megalofonou [30] | 2006 | Mediterranean Sea | eastern | Wild | O | - | No | - | 5.26 | - |
| Neilson & Campana [36] | 2008 | Atlantic Ocean | western | Wild | O | - | Yes | Bomb radiocarbon | 0.12 | - |
| Santamaria et al. [33] | 2009 | Mediterranean Sea | eastern | Wild | S | - | No | - | - | - |
| Secor et al. [49] | 2009 | Atlantic Ocean | western | Wild | O | - | No | - | - | - |
| Restrepo et al. [31] | 2010 | Atlantic Ocean | western | Wild | O | - | No | - | - | - |
| Landa et al. [29] | 2011 | Atlantic Ocean, Mediterranean Sea | eastern | Wild | S | - | No | - | - | - |
| Rodríguez-Marín et al. [50] | 2013 | Atlantic Ocean, Mediterranean Sea | eastern | Wild | O, S | O, S | Yes | Bomb radiocarbon | S: 2.4–8.5 O: 4.6–10.4 | - |
| Milatou & Megalofonou [16] | 2014 | Mediterranean Sea | eastern | Reared | V | - | No | - | 2.46 | 1.89 |
| Cort et al. [15] | 2014 | Atlantic Ocean, Mediterranean Sea | eastern, western | Wild, Reared | S | - | Yes | Lmax, Tag-recovery data, first dorsal fin spine analysis, marginal increment analysis | - | - |
| Luque et al. [12] | 2014 | Atlantic Ocean, Mediterranean Sea | eastern | Wild | S | - | Yes | Edge type, marginal increment analysis | 3.1–8.3 | 2.2–5.8 |
| Santamaria et al. [13] | 2015 | Mediterranean Sea | eastern | Wild, Reared | S | - | No | - | - | - |
| Siskey et al. [37] | 2016 | Atlantic Ocean | western | Wild | O | - | Yes | Periodicity of strodium | - | - |
| Rodríguez-Marín et al. [24] | 2022 | Atlantic Ocean, | eastern, western | Wild | O, S | O, S | No | 11 | 8 | |
| Milatou & Megalofonou [14] | 2023 | Mediterranean Sea | eastern | Reared | S | - | No | - | 3.73 | 2.87 |
4.2. Seasonal Formation of Growth Bands in Dorsal Fin Spines and Vertebrae
4.3. Precision of Age Estimates
4.4. Growth Estimation and the Relationship Between Fish Size and Calcified Structures
4.5. Impacts of Including Back-Calculated Juvenile Ages on Growth Parameter Estimation
4.6. Implications for Stock Assessment and Fishery Management
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ailloud, L.E.; Lauretta, M.V.; Hanke, A.R.; Golet, W.J.; Allman, R.J.; Siskey, M.R.; Secor, D.H.; Hoenig, J.M. Improving growth estimates for Western Atlantic bluefin tuna using an integrated modeling approach. Fish. Res. 2017, 191, 17–24. [Google Scholar] [CrossRef]
- Cailliet, G.M.; Andrews, A.H.; Bourton, E.J.; Watters, D.L.; Kline, D.E.; Ferry, L.A. Age determination and validation studies of marine fishes: Do deep-dwellers live longer? Exp. Gerontol. 2001, 36, 739–764. [Google Scholar] [CrossRef]
- Campana, S.E. Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods. J. Fish Biol. 2001, 59, 197–242. [Google Scholar] [CrossRef]
- Casselman, J.M. Determination of age and growth. In The Biology of Fish Growth; Weatherley, A.H., Gill, H.S., Eds.; Academic Press: London, UK, 1989; pp. 209–242. [Google Scholar]
- Murua, H.; Rodríguez-Marín, E.; Neilson, J.D.; Farley, J.H.; Juan-Jordá, M.J. Fast versus slow growing tuna species: Age, growth, and implications for population dynamics and fisheries management. Rev. Fish Biol. Fish. 2017, 27, 733–773. [Google Scholar] [CrossRef]
- Mather, F.J.; Mason, J.M.; Jones, A.C. Historical Document: Life History and Fisheries of Atlantic Bluefin Tuna; NOAA Tech. Memo.; NMFS-SEFSC-370; U.S. Department of Commerce, National Oceanic and Atmospheric Administration, National Marine Fisheries Service: Silver Spring, MD, USA, 1995; Miami; 165p. [Google Scholar]
- Clay, D. Atlantic bluefin tuna (Thunnus thynnus): A review. Int.-Am. Trop. Tuna Comm. Spec. Rep. 1991, 7, 89–180. [Google Scholar]
- Rodríguez-Marín, E.; Quelle, P.; Ruiz, M.; Busawon, D.; Golet, W.; Dalton, A.; Hanke, A. Updated Comparison of Age Estimates from Paired Calcified Structures from Atlantic Bluefin Tuna. SCRS/2016/134. Available online: https://digital.csic.es/handle/10261/326835 (accessed on 15 May 2025).
- Lee, D.W.; Prince, E.D.; Crow, M.E. Interpretation of growth bands on vertebrae and otoliths of Atlantic bluefin tuna, Thunnus thynnus. NOAA Tech. Rep. NMFS 1983, 8, 61–69. [Google Scholar]
- Rodríguez-Marín, E.; Olafsdottir, D.; Valeiras, J.; Ruiz, M.; Chosson-Pampoulie, V.; Rodríguez Cabello, C. Ageing comparison from vertebrae and spines of bluefin tuna (Thunnus thynnus) coming from the same specimen. Collect. Vol. Sci. Pap. ICCAT 2006, 59, 868–876. [Google Scholar]
- Rodríguez-Marín, E.; Clear, N.; Cort, J.L.; Megalofonou, P.; Neilson, J.D.; Neves Dos Santos, M.; Olafsdottir, D.; Rodriguez-Cabello, C.; Ruiz, M.; Valeiras, J. Report of the 2006 ICCAT workshop for bluefin tuna direct ageing. Collect. Vol. Sci. Pap. ICCAT 2007, 60, 1349–1392. [Google Scholar]
- Luque, P.L.; Rodríguez-Marín, E.; Landa, J.; Ruiz, M.; Quelle, P.; Marcias, D.; Ortiz de Urbina, J.M. Direct ageing of Thunnus thynnus from the east Atlantic Ocean and western Mediterranean Sea using dorsal fin spines. J. Fish Biol. 2014, 84, 1876–1903. [Google Scholar] [CrossRef]
- Santamaria, N.; Bello, G.; Pousis, C.; Vassallo-Agius, R.; de la Gándara, F.; Corriero, A. Fin Spine Bone Resorption in Atlantic Bluefin Tuna, Thunnus thynnus, and Comparison between Wild and Captive-Reared Specimens. PLoS ONE 2015, 10, e0121924. [Google Scholar] [CrossRef]
- Milatou, N.; Megalofonou, P. Direct Ageing and Growth using Dorsal Fin Spines of Bluefin Tuna Thunnus thynnus (Scombridae) Reared in Sea Cages and a Comparison with Wild Populations. J. Ichthyol. 2023, 63, 479–492. [Google Scholar] [CrossRef]
- Cort, J.L. Age and growth of the bluefin tuna (Thunnus thynnus) of the NorthEast Atlantic. Collect. Vol. Sci. Pap. ICCAT 1991, 35, 213–230. [Google Scholar]
- Milatou, N.; Megalofonou, P. Age structure and growth of bluefin tuna (Thunnus thynnus, L.) in the capture-based aquaculture in the Mediterranean Sea. Aquaculture 2014, 424–425, 35–44. [Google Scholar] [CrossRef]
- Milatou, N.; Dassenakis, M.; Megalofonou, P. Mercury concentrations in reared Atlantic bluefin tuna and risk assessment for the consumers: To eat or not to eat? Food Chem. 2020, 331, 127267. [Google Scholar] [CrossRef] [PubMed]
- Megalofonou, P.; De Metrio, G. Age estimation and annulus-formation in dorsal spines of juvenile bluefin tuna, Thunnus thynnus, from the Mediterranean Sea. J. Mar. Biol. Assoc. UK 2000, 80, 753–754. [Google Scholar] [CrossRef]
- Megalofonou, P. Age and growth of Mediterranean albacore. J. Fish Biol. 2000, 57, 700–715. [Google Scholar] [CrossRef]
- Berry, F.H.; Lee, D.W.; Bertolino, A.R. Age estimates in Atlantic bluefin tuna—An objective examination and an intuitive analyses of rhythmic markings in vertebrae and in otoliths. Collect. Vol. Sci. Pap. ICCAT 1977, 6, 305–317. [Google Scholar]
- Beamish, R.J.; Fournier, D.A. A method of comparing the precision of a set of age determinations. Can. J. Fish. Aquat. Sci. 1981, 38, 982–983. [Google Scholar] [CrossRef]
- Chang, W.Y.B. A statistical method for evaluating the reproducibility of age determination. Can. J. Fish. Aquat. Sci. 1982, 39, 1208–1210. [Google Scholar] [CrossRef]
- Sparre, P. Computer Programs for Fish Stock Assessment: Length-Based Fish Stock Assessment (LFSA); Food and Agriculture Organization of the United Nations: Charlottesville, VA, USA, 1987; Volume 101. [Google Scholar]
- Rodríguez-Marín, E.; Busawon, D.; Luque, P.L.; Castillo, I.; Stewart, N.; Krusic-Golub, K.; Parejo, A.; Hanke, A. Timing of Increment Formation in Atlantic Bluefin Tuna (Thunnus thynnus) Otoliths. Fishes 2022, 7, 227. [Google Scholar] [CrossRef]
- Cort, J.L.; Arregui, I.; Estruch, V.D.; Deguara, S. Validation of the growth equation applicable to the eastern Atlantic bluefin tuna Thunnus thynnus (L.), using Lmax, tag-recapture, and first dorsal spine analysis. Rev. Fish. Sci. Aquac. 2014, 22, 239–255. [Google Scholar] [CrossRef]
- El-Kebir, N.K.; Rodríguez-Cabello, C.; Tawil, Y. Age estimation of bluefin tuna (Thunnus thynnus, L.) caught in traps in Libyan waters based in dorsal spine reading. Collect. Vol. Sci. Pap. ICCAT 2002, 54, 641–648. [Google Scholar]
- Farrugia, A.; Rodríguez-Cabello, C. Preliminary study on the age estimation of bluefin tuna (Thunnus thynnus, L.) around the Maltese Islands. Collect. Vol. Sci. Pap. ICCAT 2001, 52, 771–775. [Google Scholar]
- Itoh, T.; Shiina, Y.; Tsuji, S.; Endo, F.; Tezuka, N. Otolith daily increment formation in laboratory reared larval and juvenile bluefin tuna Thunnus thynnus. J. Fish. Sci. 2000, 66, 834–839. [Google Scholar] [CrossRef]
- Landa, J.; Rodríguez-Marín, E.; Lastra, P.; Ruiz, M.; Quelle, P. Growth of bluefin tuna (Thunnus thynnus) in the North-eastern Atlantic and Mediterranean based on back-calculation of dorsal spine annuli. Fish. Res. 2011, 170, 190–198. [Google Scholar] [CrossRef]
- Megalofonou, P. Comparison of otolith growth and morphology with somatic growth and age in young-of-the-year bluefin tuna. J. Fish Biol. 2006, 68, 1867–1878. [Google Scholar] [CrossRef]
- Restrepo, V.R.; Diaz, G.A.; Walter, J.F.; Neilson, J.D.; Campana, S.E.; Secor, D.; Wingate, R.L. Updated estimate of the growth curve of Western Atlantic bluefin tuna. Aquat. Living Resour. 2010, 23, 335–342. [Google Scholar] [CrossRef]
- Rodríguez-Marín, E.; Di Natale, A.; Quelle, P.; Ruiz, M.; Allman, R.; Bellodi, A.; Busawon, D.; Farley, J.; Garibaldi, F.; Ishihara, T.; et al. Report of the age calibration exchange within the Atlantic Wide Research Programme for bluefin tuna (GBYP) Collect. Vol. Sci. Pap. ICCAT 2015, 71, 1174–1214. [Google Scholar]
- Santamaria, N.; Bello, G.; Corriero, A.; Deflorio, M.; Vassalo-Agius, R.; Bök, T.; De Metrio, G. Age and growth of Atlantic bluefin tuna, Thunnus thynnus, (Osteichthyes: Thunnidae), in the Mediterranean Sea. J. Appl. Ichthyol. 2009, 25, 38–45. [Google Scholar] [CrossRef]
- Compeán-Jiménez, G.; Bard, F.X. Growth increments on dorsal spines of eastern Atlantic bluefin tuna (Thunnus thynnus, L.) and their possible relation to migrations patterns. NOAA Tech. Rep. NMFS 1983, 8, 77–86. [Google Scholar]
- Tuset, V.M.; González, M.S.; García, A. Age estimation of Atlantic bluefin tuna from the Mediterranean Sea using calcified structures. Fish. Sci. 2008, 74, 573–585. [Google Scholar]
- Neilson, J.D.; Campana, S.E. A validated description of age and growth of western Atlantic bluefin tuna (Thunnus thynus). Can. J. Fish. Aquat. Sci. 2008, 65, 1523–1527. [Google Scholar] [CrossRef]
- Siskey, M.R.; Wilberg, M.J.; Allman, R.J.; Barnett, B.K.; Secor, D.H. Forty years of fishing: Changes in age structure and stock mixing in northwestern Atlantic bluefin tuna (Thunnus thynnus) associated with size-selective and long-term exploitation. ICES J. Mar. Sci. 2016, 73, 2518–2528. [Google Scholar] [CrossRef]
- Sella, M. Migrations and Habitat of the Tuna (Thunnus thynnus), Studied by Method of the Hooks, with Observations on Growth, on the Operation of the Fisheries, Etc.; Van Campen, W.G., Translator; U.S. Department of the Interior Fish and Wildlife Service: Washington, DC, USA, 1929; SSRF No. 76; 20p. [Google Scholar]
- Mather, F.J.; Schuck, H.A. Growth of bluefin tuna of the western North Atlantic. Fish. Bull. 1960, 61, 39–52. [Google Scholar]
- Rodríguez-Roda, J. Movimientos Migratorios del Atún, Thunnus thynnus (L.), Deducidos por Nuestraspropias “Marcaciones” en Aguas Españolas; Publicaciones Técnicas de la Junta de Estudios de Pesca, Dirección General de Pesca Marítima: Madrid, Spain, 1964; Volume 3, pp. 279–304. [Google Scholar]
- Caddy, J.F.; Dickson, C.A.; Butler, M.J.A. Age and growth of giant bluefin tuna (Thunnus thynnus) taken in Canadian waters in 1975. Fish. Res. Board Can. Manuscr. Rep. Ser. 1976, 1395, 17. [Google Scholar]
- Butler, M.J.A.; Caddy, J.F.; Dickson, C.A.; Hunt, J.J.; Burnett, C.D. Apparent age and growth based on otolith analysis, of giant bluefin tuna, (Thunnus thynnus) in the 1975–1976 Canadian catch. Collect. Vol. Sci. Pap. ICCAT 1977, 6, 318–330. [Google Scholar]
- Farrugio, H. Age et croissance du thon rouge (Thunnus thynnus) dans la pecherie francaise de surface en Mediterranee. Cybium 1980, 3, 45–59. [Google Scholar]
- Hattour, A. Analyse de l’âge, de la croissance et des captures des thons rouges (Thunnus thynnus) et des thonines (Euthynnus alletteratus) pêchés dans les eaux tunisiennes. Bull. Inst. Nat. Scient. Tech. Océanogr. Pêc. Sal. 1984, 11, 5–39. [Google Scholar]
- Ólafsdóttir, D.; Ingimundardóttir, T. Preliminary report on experimental fisheries and biological research on bluefin tuna (Thunnus thynnus) within the Islandic EEZ in 1996-98. ICCAT Sci. Pap. 2000, 51, 827–838. [Google Scholar]
- Megalofonou, P.; Platis, K.; De Metrio, G.; Santamaria, N. Age estimation of juvenile bluefin tuna, Thunnus thynnus, from the Mediterranean Sea. Cah. Options Méditerr. 2003, 60, 123–125. [Google Scholar]
- Rodríguez-Marín, E.; Landa, J.; Ruiz, M.; Godoy, D.; Rodríguez-Cabello, C. Age estimation of adult bluefin tuna (Thunnus thynnus) from dorsal spine reading. Collect. Vol. Sci. Pap. ICCAT 2004, 56, 1168–1174. [Google Scholar]
- La Mesa, M.; Sinopoli, M.; Andaloro, F. Age and growth rate of juvenile bluefin tuna Thunnus Thynnus from the Mediterranean Sea (Sicily, Italy). Sci. Mar. 2005, 69, 241–249. [Google Scholar] [CrossRef]
- Secor, D.H.; Wingate, R.L.; Neilson, J.D.; Rooker, J.R.; Campana, S.E. Growth of Atlantic bluefin tuna: Direct age estimates. Collect. Vol. Sci. Pap. ICCAT 2009, 64, 405–416. [Google Scholar]
- Rodríguez-Marín, E.; Luque, P.L.; Busawon, D.; Campana, S.; Golet, W.; Koob, E.; Neilson, J.; Quelle, P.; Ruiz, M. An Attempt of Validation of Atlantic Bluefin Tuna (Thunnus thynnus) Ageing Using Dorsal Fin Spines. SCRS/2013/081. Available online: https://digital.csic.es/handle/10261/326831 (accessed on 15 May 2025).
- Campana, S.E.; Annand, M.C.; MC Millan, I. Graphical and Statistical Methods for Determining the Consistency of Age Determinations. Trans. Am. Fish. Soc. 1995, 124, 131–138. [Google Scholar] [CrossRef]
- Foreman, T. Estimates of age and growth, and an assessment of ageing techniques, for northern bluefin tuna, Thunnus thynnus, in the Pacific Ocean. IATTC Bull. 1996, 21, 2. [Google Scholar]

| Mean FL (cm) | ||||
|---|---|---|---|---|
| Estimated Age | Spines | Vertebrae | Student Test | p-Value |
| 4 | 141.0 | |||
| 5 | 162.7 | 146.7 | 0.64 | 0.5901 |
| 6 | 165.8 | 158.1 | 1.89 | 0.0717 |
| 7 | 183.1 | 173.3 | 3.60 | 0.0008 * |
| 8 | 190.4 | 187.2 | 1.66 | 0.1486 |
| 9 | 197.9 | 197.7 | 0.08 | 0.9334 |
| 10 | 211.3 | 214.3 | −1.87 | 0.0700 |
| 11 | 222.7 | 226.3 | −2.67 | 0.0099 * |
| 12 | 232.2 | 234.3 | −1.72 | 0.0915 |
| 13 | 236.5 | 242.2 | −2.77 | 0.0073 * |
| 14 | 242.1 | 251.7 | −4.12 | 0.0001 * |
| 15 | 249.5 | 259.4 | −4.49 | 0.0000 * |
| 16 | 255.2 | 262.7 | −1.97 | 0.1057 |
| 17 | 256.7 | 269.5 | −3.63 | 0.0151 * |
| 18 | 250.0 | |||
| 19 | 255.0 | |||
| 20 | 273.5 | |||
| Annual Band Difference (ABD) | 0 ‡ | −1 ‡ | −2 ‡ | −3 ‡ | |
|---|---|---|---|---|---|
| Medium BFT | N | 89 | 74 | 20 | 6 |
| % | 47.60 | 39.57 | 10.69 | 2.14 | |
| Large BFT | N | 126 | 136 | 96 | 68 |
| % | 29.58 | 31.93 | 22.53 | 15.96 | |
| All samples | N | 215 | 210 | 116 | 72 |
| % | 35.07 | 34.26 | 18.92 | 11.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milatou, N.; Megalofonou, P. Ageing Mediterranean Bluefin Tuna: A Comparative Study Between Dorsal Fin Spines and Vertebrae. Fishes 2025, 10, 260. https://doi.org/10.3390/fishes10060260
Milatou N, Megalofonou P. Ageing Mediterranean Bluefin Tuna: A Comparative Study Between Dorsal Fin Spines and Vertebrae. Fishes. 2025; 10(6):260. https://doi.org/10.3390/fishes10060260
Chicago/Turabian StyleMilatou, Niki, and Persefoni Megalofonou. 2025. "Ageing Mediterranean Bluefin Tuna: A Comparative Study Between Dorsal Fin Spines and Vertebrae" Fishes 10, no. 6: 260. https://doi.org/10.3390/fishes10060260
APA StyleMilatou, N., & Megalofonou, P. (2025). Ageing Mediterranean Bluefin Tuna: A Comparative Study Between Dorsal Fin Spines and Vertebrae. Fishes, 10(6), 260. https://doi.org/10.3390/fishes10060260






