Optimizing Cryopreservation of Pseudoplatystoma magdaleniatum Semen: Evaluation of Two Permeable and Two Non-Permeable Cryoprotectants
Abstract
1. Introduction
2. Materials and Methods
2.1. Location and Biological Material
2.2. Cryopreservation of Semen, Freezing and Thawing
2.3. Semen Quality
2.4. Fertilizing Capacity of Thawed Semen
2.5. Statistical Analysis
3. Results
3.1. Semen Osmolarity and Cryomedia
3.2. Pre-Frozen
3.3. Thawed Semen
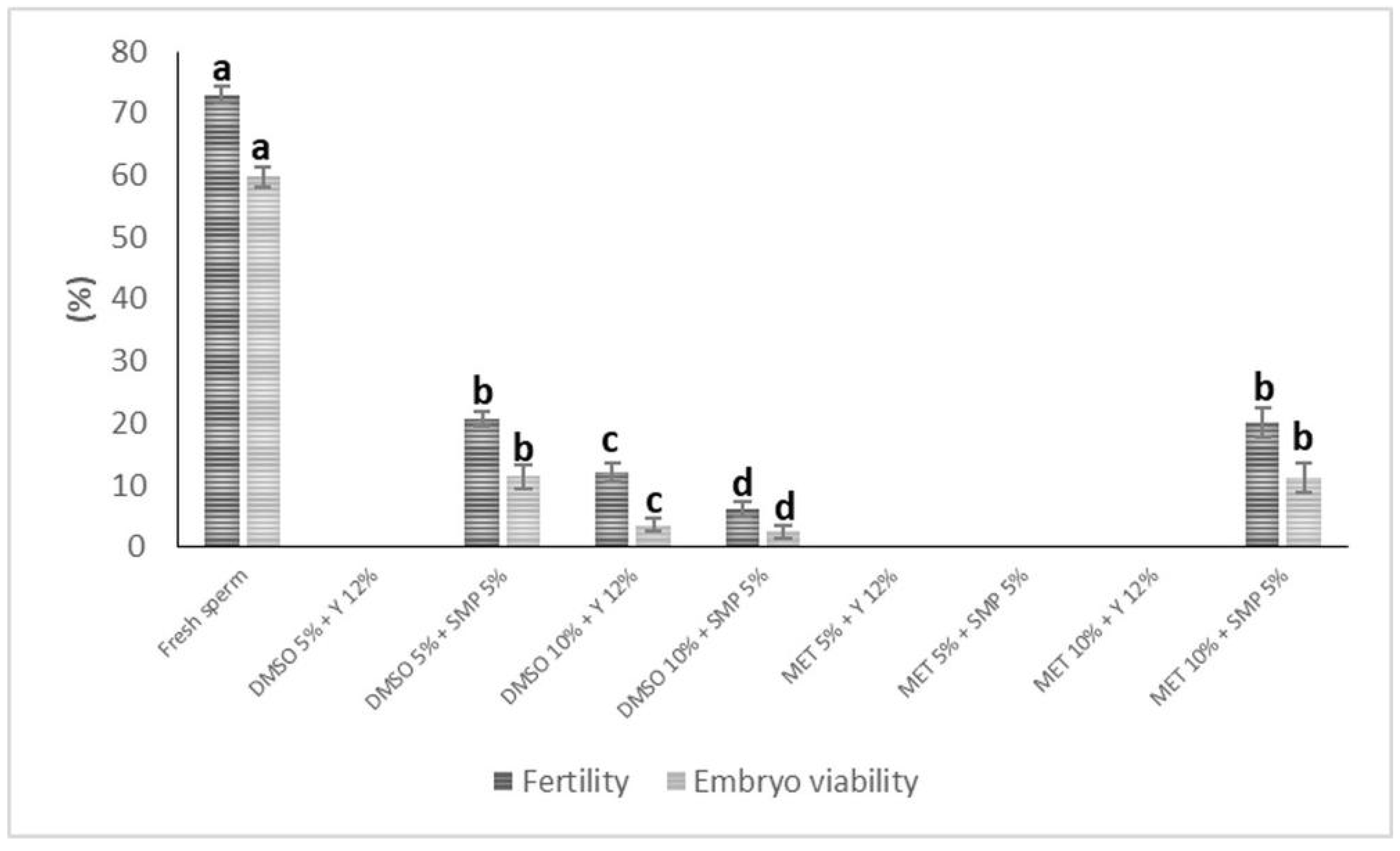
3.4. Fertility and Embryo Viability Rates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- García-Castro, K.; Rangel-Medrano, J.; Landínez-García, R.; Márquez, E. Population genetics of the endangered catfish Pseudoplatystoma magdaleniatum (Siluriformes: Pimelodidae) based on species-specific microsatellite loci. Neotrop. Ichthyol. 2021, 19, e200120. [Google Scholar] [CrossRef]
- Barzotto, E.; Oliveira, M.; Mateus, L. Reproductive biology of Pseudoplatystoma corruscans (Spix and Agassiz, 1829) and Pseudoplatystoma reticulatum (Eigenmann and Eigenmann, 1889), two species of fisheries importance in the Cuiabá River basin, Brazil. J. Appl. Ichthyol. 2017, 33, 29–36. [Google Scholar] [CrossRef]
- Balboni, L.; Vargas, F.; Colautti, D. Age and growth of Pseudoplatystoma corruscans (Siluriformes: Pimelodidae) at the confluence of the Paraná and Paraguay rivers. Neotrop. Ichthyol. 2021, 19, e200101. [Google Scholar] [CrossRef]
- Velarde, J.; Bastos, N.; Carneiro-Leite, L.; Borges, L.; Vieira, E.; Veríssimo-Silveira, R.; Ninhaus-Silveira, A. Dimethyl acetamide and dimethyl sulfoxide associated at glucose and egg yolk for cryopreservation of Pseudoplatystoma corruscans semen. Neotrop. Ichthyol. 2023, 21, e220071. [Google Scholar] [CrossRef]
- Buitrago-Suárez, A.; Burr, B. Taxonomy of the catfish genus Pseudoplatystoma Bleeker (Siluriformes: Pimelodidae) with recognition of eight species. Zootaxa 2007, 1512, 1–38. [Google Scholar] [CrossRef]
- Maldonado-Ocampo, J.; Vari, R.; Usma, J. Checklist of the freshwater fishes of Colombia. Biota Colomb. 2008, 9, 143–237. [Google Scholar]
- García-Alzate, C.; DoNascimiento, C.; Villa-Navarro, F.; García-Melo, J.; Herrera, R.G. Diversidad de peces de la cuenca del río Magdalena, Colombia. In Peces de la Cuenca del río Magdalena, Colombia: Diversidad, Conservación y Uso Sostenible; Jiménez Segura, L., Lasso, C.A., Eds.; Instituto de Investigación de Recursos Biológicos Alexander von Humboldt: Bogotá, Colombia, 2020; pp. 85–113. Available online: http://hdl.handle.net/20.500.11761/35752 (accessed on 3 March 2025).
- Duarte, L.; García, E.; Tejeda, K.; Cuello, F.; Gil-Manrique, B.; De León, G.; Curiel, J.; Cuervo, C.; Vargas, O.; Isaza, E.; et al. Estadísticas de Desembarco y Esfuerzo de las Pesquerías Artesanales de Colombia–Enero a Octubre de 2022; Autoridad Nacional de Acuicultura y Pesca (AUNAP); Universidad del Magdalena: Santa Marta, Colombia, 2022; p. 136. Available online: http://sepec.aunap.gov.co/Estadisticas (accessed on 3 March 2025).
- Marrugo-Negrete, J.; Pinedo-Hernández, J.; Díez, S. Assessment of heavy metal pollution, spatial distribution and origin in agricultural soils along the Sinú River Basin, Colombia. Environ. Res. 2017, 154, 380–388. [Google Scholar] [CrossRef]
- Gutiérrez-Moreno, F.; De la Parra-Guerra, A. Contaminación en la cuenca del río Magdalena (Colombia) y su relación con los peces. In Peces de la Cuenca del río Magdalena, Colombia: Diversidad, Conservación y uso Sostenible; Jiménez Segura, L., Lasso, C.A., Eds.; Instituto de Investigación de Recursos Biológicos Alexander von Humboldt: Bogotá, Colombia, 2020; pp. 239–263. Available online: http://hdl.handle.net/20.500.11761/35752 (accessed on 3 March 2025).
- Mojica, J.; Castellanos, C.; Usma, J.; Álvarez, R.; Lasso, C. Libro rojo de Peces Dulceacuícolas de Colombia; Serie Libros Rojos de Especies Amenazadas de Colombia; Universidad Nacional de Colombia; WWF: Manizales, Colombia, 2012; 319p. [Google Scholar]
- Cabrita, E.; Martínez-Páramo, S.; Gavaia, P.; Riesco, M.; Valcarce, D.; Sarasquete, C.; Herráez, M.; Robles, V. Factors enhancing fish sperm quality and emerging tools for sperm analysis. Aquaculture 2014, 432, 389–401. [Google Scholar] [CrossRef]
- Medina-Robles, V.; Duarte-Trujillo, A.; Cruz-Casallas, P. Crioconservación seminal en peces de agua dulce: Aspectos biotecnológicos, celulares y bioquímicos. Orinoquia 2020, 24, 51–78. [Google Scholar] [CrossRef]
- Bernáth, G.; Csorbai, B.; Nagy, B.; Csókás, E.; Molnár, J.; Bartucz, T.; Láng, Z.; Gyurcsák, M.; Hegyi, Á.; Kobolák, J.; et al. The investigation of post-thaw chilled storage and the applicability of large-scale cryopreservation in chub (Squalius cephalus) sperm. Cryobiology 2023, 113, 1–9. [Google Scholar] [CrossRef]
- Cruz-Casallas, P.; Medina-Robles, V.; Velasco-Santamaría, Y. Protocolo para la crioconservación de semen de yamú (Brycon amazonicus Spix & Agassiz 1829). Rev. Colomb. Cienc. Pecu. 2006, 19, 146–151. [Google Scholar] [CrossRef]
- Ramirez-Merlano, J.; Medina-Robles, V.; Cruz-Casallas, P. Variación estacional de las características seminales del bagre rayado Pseudoplatystoma metaense (Telostei, Pimelodidae). Rev. MVZ Córdoba 2011, 16, 2336–2348. [Google Scholar] [CrossRef][Green Version]
- França, T.; Motta, N.; Egger, R.; Oliveira, A.; Murgas, L. Impact of activation solutions on fresh and frozen-thawed sperm motility and fertilization success for two species of migratory freshwater fishes. Theriogenology 2020, 149, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Merlano, J.; Medina-Robles, V.; Cruz-Casallas, P. Crioconservación seminal de bagre rayado Pseudoplatystoma metaense (Teleostei, Pimelodidae), bajo diferentes protocolos de congelación. Arch. Med. Vet. 2011, 43, 135–144. [Google Scholar] [CrossRef]
- Herrera-Cruz, E.; Aristizabal-Regino, J.; Yepes-Blandón, J.; Estrada-Posada, A.; Espinosa-Araujo, J.; Atencio-García, V. Criopreservación de semen de bagre rayado Pseudoplatystoma magdaleniatum con tres diferentes crioprotectoras. Rev. Colomb. Biotecnol. 2019, 21, 55–62. [Google Scholar] [CrossRef]
- Atencio-García, V.; Padilla-Izquierdo, D.; Robles-González, J.; Prieto-Guevara, M.; Pardo-Carrasco, S.; Espinosa-Araujo, J. Damage to Sorubim cuspicaudus sperm cryopreserved with ethylene glycol. Animals 2023, 13, 235. [Google Scholar] [CrossRef]
- Montes-Petro, C.; Yepes-Escobar, J.; Tapia Pacheco, C.; Madariaga-Mendoza, D.; Espinosa-Araujo, J.; Atencio-García, V. Madurez testicular y calidad seminal de Ichthyoelephas longirostris (Prochilodontidae) del Medio río Cauca. Acta Biol. Colomb. 2024, 29, 179–188. [Google Scholar] [CrossRef]
- Vinatea-Arana, L. Principios Químicos de Calidad del Agua en Acuicultura: Una Revisión Para Peces y Camarones; UAM: Unidad Xochimilco, México, 2002; 154p, Available online: https://www.academia.edu/50857947/ (accessed on 3 March 2025).
- Trejo-Albarrán, R.; Flores-Ibarra, K.; Trujillo-Jiménez, P.; Granados-Ramírez, J.; Gómez-Márquez, J.; Delgado Sánchez, L. Water quality in fish farming ponds using some physical and chemical parameters. Braz. J. Anim. Environ. Res. 2021, 4, 5490–5509. [Google Scholar] [CrossRef]
- Martínez, J.; Atencio-García, V.; Pardo-Carrasco, S. DNA fragmentation and membrane damage of bocachico Prochilodus magdalenae (Ostariophysi, Prochilodontidae) sperm following cryopreservation with dimethylsulfoxide and glucose. Neotrop. Ichthyol. 2012, 10, 577–586. [Google Scholar] [CrossRef]
- Figueroa, E.; Merino, O.; Risopatrón, J.; Isachenko, V.; Sánchez, R.; Effer, B.; Isachenko, E.; Farias, J.; Valdevenito, I. Effect of seminal plasma on Atlantic salmon (Salmo salar) sperm vitrification. Theriogenology 2015, 83, 238–245. [Google Scholar] [CrossRef]
- Figueroa, E.; Valdebenito, I.; Zepeda, A.; Figueroa, C.; Dumorné, K.; Castillo, R.; Farias, J. Effects of cryopreservation on mitochondria of fish spermatozoa. Rev. Aquac. 2017, 9, 76–87. [Google Scholar] [CrossRef]
- Neyrão, I.; Santos, F.; Rodrigues, R.; Streit, D.; Godoy, L. Use of powdered milk in semen cryopreservation protocols for fish: A systematic review. Biopreserv. Biobank 2024, 22, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Riesco, M.F.; Oliveira, C.; Soares, F.; Gavaia, P.J.; Dinis, M.T.; Cabrita, E. Solea senegalensis sperm cryopreservation: New insights on sperm quality. PLoS ONE 2017, 12, e0186542. [Google Scholar] [CrossRef] [PubMed]
- Xin, M.; Niksirat, H.; Shaliutina-Kolešová, A.; Siddique, M.A.M.; Sterba, J.; Boryshpolets, S.; Linhart, O. Molecular and subcellular cryoinjury of fish spermatozoa and approaches to improve cryopreservation. Rev. Aquac. 2019, 12, 909–924. [Google Scholar] [CrossRef]
- Handayani, L.; Maulida, S.; Rahayu, S.; Razi, N.M.; Kocabas, M.; Kocabas, F.K.; Wilkes, M.; Siti-Azizah, M.N.; Eriani, K.; Fadli, N.; et al. Effect of cryoprotectant and concentration on the sperm quality of walking catfish, Clarias batrachus, post-cryopreservation. CryoLetters 2024, 45, 320–328. [Google Scholar] [CrossRef]
- Pereira, J.R.; Pereira, F.A.; Perry, C.T.; Pires, D.M.; Muelbert, J.R.E.; Garcia, J.R.E.; Corcini, C.D.; Varela-Junior, A.S. Dimethylsulfoxide, methanol and methylglycol in the seminal cryopreservation of Suruvi, Steindachneridion scriptum. Anim. Reprod. Sci. 2019, 200, 7–13. [Google Scholar] [CrossRef]
- Pardo-Carrasco, S.; Salas-Villalva, J.; Reza-Gaviria, L.; Espinosa-Araújo, J.; Atencio-García, V. Criopreservación de semen de bagre blanco (Sorubim cuspicaudus) con dimetilacetamida como crioprotector. Rev. CES Med. Zootec. 2015, 10, 122–131. [Google Scholar]
- Dietrich, M.; Arnold, J.; Fröhlich, T.; Otte, K.; Dietrich, G.; Ciereszko, A. Proteomic analysis of extracellular medium of cryopreserved carp (Cyprinus carpio L.) semen. Comp. Biochem. Physiol. (CBPD) 2015, 15, 49–57. [Google Scholar] [CrossRef]
- Martínez-Páramo, S.; Horváth, Á.; Labbé, C.; Zhang, T.; Robles, V.; Herráez, P.; Suquet, M.; Adams, S.; Viveiros, A.; Tiersch, T.; et al. Cryobanking of aquatic species. Aquaculture 2017, 472, 156–177. [Google Scholar] [CrossRef]
- Nynca, J.; Arnold, G.; Fröhlich, T.; Ciereszko, A. Cryopreservation induced alterations in protein composition of rainbow trout semen. Proteomics 2015, 15, 2643–2654. [Google Scholar] [CrossRef]
- Dumorné, K.; Figueroa, E.; Cosson, J.; Lee-Estevez, M.; Ulloa-Rodríguez, P.; Valdebenito, I.; Farías, J. Protein phosphorylation and ions effects on salmonid sperm motility activation. Aquaculture 2017, 10, 727–737. [Google Scholar] [CrossRef]
- Galo, M.; Streit-Junior, D.; Oliveira, C.; Povh, J.; Fornari, C.; Digmayer, M.; Ribero, P. Quality of fresh and cryopreserved semen and their influence on the rates of fertilization, hatching and quality of the larvae of Piaractus mesopotamicus. Braz. J. Biol. 2019, 79, 438–445. [Google Scholar] [CrossRef]
- El Kamouh, M.; Brionne, A.; Sayyari, A.; Laurent, A.; Labbé, C. Cryopreservation effect on DNA methylation profile in rainbow trout spermatozoa. Sci. Rep. 2023, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, B.; Lassen, P.; Streit, P. Cryopreservation-induced morphological changes in freshwater fish sperm: A systematic review. Biopreserv. Biobank. 2024, 22, 416–427. [Google Scholar] [CrossRef]
- Martínez, J.; Pardo, S. Crioconservación de semen en peces: Efectos sobre la movilidad espermática y la fertilidad. Acta Biol. Colomb. 2010, 15, 3–24. [Google Scholar]
- Müller, T.; Szabó, T.; Kollár, T.; Csorbai, B.; Marinović, Z.; Horváth, L.; Kucska, B.; Bodnár, Á.; Urbányi, B.; Horváth, Á. Artificial insemination of African catfish (Clarias gariepinus) using cryopreserved sperm. Theriogenology 2019, 123, 145–150. [Google Scholar] [CrossRef]
- Mazur, P.; Leibo, S.; Chu, E. A two-factor hypothesis of freezing injury: Evidence from Chinese hamster tissue-culture cells. Exp. Cell Res. 1972, 71, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Yeste, M. Sperm cryopreservation update: Cryodamage, markers and factors affecting the sperm freezability in pigs. Theriogenology 2016, 85, 47–64. [Google Scholar] [CrossRef]
- Sieme, H.; Oldenhof, H.; Wolkers, W. Sperm membrane behaviour during cooling and cryopreservation. Reprod. Domest Anim. 2015, 50 (Suppl. 3), 20–26. [Google Scholar] [CrossRef]
- Roca, J.; Parrilla, I.; Gil, M.; Cuello, C.; Martinez, E.; Rodriguez-Martinez, H. Non-viable sperm in the ejaculate: Lethal escorts for contemporary viable sperm. Anim. Reprod. Sci. 2016, 169, 24–31. [Google Scholar] [CrossRef]
- Kumar, A.; Prasad, J.; Srivastava, N.; Ghosh, S.K. Strategies to minimize various stress-related freeze-thaw damages during conventional cryopreservation of mammalian spermatozoa. Biopreserv. Biobank. 2019, 17, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Carolsfeld, J.; Godinho, H.; Zaniboni-Filho, E.; Harvey, B. Cryopreservation of sperm in Brazilian migratory fish conservation. J. Fish Biol. 2003, 63, 472–489. [Google Scholar] [CrossRef]
| Cryoprotectant Solutions | Cryomedia Solutions Osmolarity (mOsmol/Kg) | Osmolarity of Seminal Plasma (mOsmol/Kg) |
|---|---|---|
| DMSO 5% + SMP 5% | 1562.3 ± 10.3 b | |
| DMSO 10% + SMP 5% | 2971.6 ± 37.0 e | |
| DMSO 5% + Y 12% | 1248.3 ± 11.5 a | |
| DMSO 10% + Y 12% | 2906.3 ± 14.4 e | 251.1 ± 3.3 |
| MET 5% + SMP 5% | 2156.3 ± 6.9 d | |
| MET 10% + SMP 5% | 2456.0 ± 0.6 e | |
| MET 5% + Y 12% | 2073.6 ± 7.5 c | |
| MET 10% + Y 12% | 3488.2 ± 1.0 f |
| Treatments | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameters | Fresh Sperm | DMSO 5% + Y 12% | DMSO 5% + SMP 5% | DMSO 10% +Y 12% | DMSO 10% +SMP 5% | MET 5% +Y 12% | MET 5% +SMP 5% | MET 10% +Y 12% | MET 10% +SMP 5% |
| Mt (%) | 94.4 ± 6.0 a | 57.7 ± 6.4 bc | 65.3 ± 5.8 b | 59.3 ± 6.5 bc | 50.6 ± 3.4 de | 55.8 ± 6.2 bcd | 62.5 ± 8.7 b | 50.6 ± 1.9 de | 47.3 ± 6.9 e |
| Pro (%) | 44.0 ± 11.4 a | 6.1 ± 1.2 c | 16.5 ± 1.1 b | 3.2 ± 2.5 d | 4.6 ± 3.1 dd | 6.7 ± 1.9 c | 12.4 ± 2.4 b | 5.1 ± 1.1 d | 5.1 ± 1.9 d |
| Rap (%) | 14.0 ± 12.7 a | 1.0 ± 0.9 b | 1.2 ± 1.4 b | 0.6 ± 0.9 b | 1.2 ± 1.6 b | 2.0 ± 1.3 b | 1.2 ± 1.0 b | 2.3 ± 1.5 b | 0.8 ± 0.5 b |
| Med (%) | 50.6 ± 10.5 a | 9.1 ± 1.6 d | 20.2 ± 1.7 b | 5.0 ± 2.7 e | 4.7 ± 2.8 e | 9.8 ± 2.3 d | 15.4 ± 2.5 c | 7.1 ± 0.8 de | 6.2 ± 1.9 e |
| Slo (%) | 29.8 ± 11.7 a | 47.6 ± 4.6 b | 43.9 ± 5.2 b | 53.6 ± 6.4 c | 44.5 ± 3.8 b | 44.1 ± 3.8 b | 45.8 ± 8.0 b | 42.3 ± 2.0 b | 40.3 ± 6.8 b |
| Imm (%) | 5.6 ± 5.9 a | 42.3 ± 6.4 bc | 34.7 ± 5.8 b | 40.7 ± 6.5 c | 49.4 ± 3.4 cd | 44.2 ± 6.2 cd | 37.5 ± 8.7 b | 49.4 ± 1.8 cd | 52.8 ± 6.9 e |
| VCL (µm/s) | 65.8 ± 15.2 a | 29.2 ± 2.6 cd | 38.5 ± 1.8 b | 25.3 ± 5.1 d | 29.2 ± 6.7 cd | 32.5 ± 4.2 bc | 34.2 ± 3.2 bc | 29.6 ± 0.8 cd | 27.7 ± 2.7 cd |
| VSL (µm/s) | 39.0 ± 7.6 a | 13.6 ± 0.5 c | 22.2 ± 1.8 b | 9.2 ± 2.8 e | 11.3 ± 2.0 d | 13.0 ± 1.4 d | 18.5 ± 1.7 c | 12.8 ± 0.8 d | 11.9 ± 2.8 de |
| Md (s) | 31.5 ± 1.3 | - | - | - | - | - | - | - | - |
| Sc (×106 Spz/mL) | 19,271.5 ± 1444.4 | - | - | - | - | - | - | - | - |
| Treatments | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameters | Fresh Sperm | DMSO 5% +Y 12% | DMSO 5%+SMP 5% | DMSO 10% +Y 12% | DMSO 10% +SMP 5% | MET 5% +Y 12% | MET 5% +SMP 5% | MET 10% +Y 12% | MET 10% +SMP 5% |
| Mt (%) | 95.4 ± 2.1 a | 57.2 ± 6.4 b | 45.7 ± 3.8 c | 52.3 ± 4.3 b | 35.3 ± 1.7 d | 41.0 ± 1.5 cd | 45.2 ± 5.1 c | 34.9 ± 1.9 d | 40.3 ± 1.6 cd |
| Pro (%) | 42.0 ± 1.9 a | 10.7 ± 1.8 b | 5.6 ± 1.4 d | 7.7 ± 0.8 c | 1.2 ± 0.3 f | 3.4 ± 1.3 ef | 5.1 ± 1.1 de | 1.2 ± 0.2 f | 1.4 ± 0.1 f |
| Rap (%) | 15.0 ± 2.0 a | 6.0 ± 1.1 b | 4.2 ± 0.7 c | 3.0 ± 0.4 c | 0.4 ± 0.1 d | 4.4 ± 0.5 c | 1.5 ± 0.2 d | 0.7 ± 0.1 d | 0.5 ± 0.1 d |
| Med (%) | 51.5 ± 0.4 a | 15.5 ± 1.7 b | 5.3 ± 0.7 d | 10.7 ± 0.5 c | 2.2 ± 0.7 ef | 4.9 ± 0.8 d | 3.3 ± 1.0 e | 1.4 ± 0.8 f | 1.7 ± 0.7 f |
| Slo (%) | 28.6 ± 0.6 a | 38.3 ± 1.9 cd | 41.1 ± 1.0 e | 41.7 ± 2.2 e | 32.4 ± 1.2 b | 34.1 ± 7.2 c | 40.1 ± 6.1 d | 32.7 ± 1.8 b | 36.6 ± 3.9 cd |
| Imm (%) | 5.1 ± 0.5 a | 39.4 ± 2.8 b | 54.7 ± 3.9 e | 48.1 ± 4.0 c | 64.7 ± 1.7 d | 60.6 ± 4.2 de | 54.2 ± 5.1 d | 65.0 ± 1.9 d | 61.3 ± 4.5 d |
| VCL (µm/s) | 63.7 ± 4.9 a | 45.6 ± 3.1 b | 20.2 ± 0.9 e | 35.2 ± 3.5 c | 23.0 ± 0.9 d | 30.5 ± 6.0 c | 29.8 ± 8.2 cd | 20.5 ± 0.4 e | 21.1 ± 0.8 e |
| VSL (µm/s) | 38.3 ± 1.5 a | 12.8 ± 1.1 c | 6.8 ± 1.4 d | 16.7 ± 2.0 b | 5.5 ± 0.9 d | 6.6 ± 1.5 d | 6.5 ± 1.4 d | 6.5 ± 2.3 d | 4.9 ± 0.5 d |
| Md (s) | 33.2 ± 2.0 | - | - | - | - | - | - | - | - |
| Sc (×106 Spz/mL) | 20,270.1 ± 1245.1 | - | - | - | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montes-Petro, C.; Rodríguez-Peroza, B.; Madariaga-Mendoza, D.; Tapia-Pacheco, C.; Espinosa-Araujo, J.; Atencio-García, V. Optimizing Cryopreservation of Pseudoplatystoma magdaleniatum Semen: Evaluation of Two Permeable and Two Non-Permeable Cryoprotectants. Fishes 2025, 10, 183. https://doi.org/10.3390/fishes10040183
Montes-Petro C, Rodríguez-Peroza B, Madariaga-Mendoza D, Tapia-Pacheco C, Espinosa-Araujo J, Atencio-García V. Optimizing Cryopreservation of Pseudoplatystoma magdaleniatum Semen: Evaluation of Two Permeable and Two Non-Permeable Cryoprotectants. Fishes. 2025; 10(4):183. https://doi.org/10.3390/fishes10040183
Chicago/Turabian StyleMontes-Petro, César, Betty Rodríguez-Peroza, Diana Madariaga-Mendoza, Carlos Tapia-Pacheco, José Espinosa-Araujo, and Víctor Atencio-García. 2025. "Optimizing Cryopreservation of Pseudoplatystoma magdaleniatum Semen: Evaluation of Two Permeable and Two Non-Permeable Cryoprotectants" Fishes 10, no. 4: 183. https://doi.org/10.3390/fishes10040183
APA StyleMontes-Petro, C., Rodríguez-Peroza, B., Madariaga-Mendoza, D., Tapia-Pacheco, C., Espinosa-Araujo, J., & Atencio-García, V. (2025). Optimizing Cryopreservation of Pseudoplatystoma magdaleniatum Semen: Evaluation of Two Permeable and Two Non-Permeable Cryoprotectants. Fishes, 10(4), 183. https://doi.org/10.3390/fishes10040183







