1. Introduction
Fishmeal (FM) has long been a primary feed ingredient in aquaculture due to its high protein content, balanced amino acid composition, and palatability [
1]. However, demand for FM has grown in recent years, while production has remained relatively stable, leading to a sharp rise in FM prices. The Food and Agriculture Organization of the United Nations (FAO) predicts that by 2030, prices will be 28% higher than in 2005 [
2]. In 2020, aquaculture accounted for approximately 86% of global FM consumption, exacerbating overdependence on FM and indicating that future supply may be limited, irrespective of price fluctuations [
2]. Consequently, replacing FM with alternative ingredients in aquafeeds has been widely studied.
Among these, soybean meal (SM) is a widely used substitute due to its high protein content, low cost, and relatively balanced amino acid profile [
3]. However, SM contains various antinutritional factors (ANFs), including phytate, trypsin inhibitors, glycinin, β-conglycinin, and raffinose, which negatively affect growth performance and intestinal health in aquatic animals, particularly carnivorous fish [
4,
5,
6]. Fermentation is an effective method to inactivate ANFs in SM and promote the production of soluble proteins and small peptides [
7,
8]. Fermented soybean meal (FSM) has been successfully used as a key protein source for terrestrial livestock during ANF-sensitive life stages [
9,
10,
11]. Additionally, the use of FSM in aquafeeds has also been extensively studied. He et al. [
12] reported that FSM could replace 30% of FM in the diet of
Micropterus salmoides without negatively affecting its growth performance. Additionally, replacing 10% of FM protein with
Bacillus cereus-fermented soybean meal significantly improved growth performance, antioxidant capacity, and immunity in
Oncorhynchus kisutch [
13]. However, high substitution levels of FSM for FM can significantly affect the growth performance and health of aquatic animals [
14,
15,
16]. This may be due to the reduced feed utilization caused by ANF residues [
17], imbalanced nutrient composition affecting overall intake [
18], potential suppression of immune function, and possible threats to intestinal health [
12], which limits FSM’s use in high proportions. To increase FSM incorporation in feeds, it is crucial to explore supplements that can effectively mitigate the negative effects of high FSM substitution ratios.
Lentinus edodes (
L. edodes) is a widely consumed edible mushroom belonging to the Basidiomycota class [
19]. The mycelium and fruiting bodies of
L. edodes are rich in various nutrients and bioactive compounds, offering numerous health benefits [
19]. As a nutritious food with functional properties,
L. edodes has been shown in several studies to possess a range of pharmacological effects, including anti-inflammatory, hepatoprotective, anti-tumor, hypoglycemic, and antibacterial activities [
20,
21,
22]. Therefore, exploring the use of
L. edodes as a supplement to FSM to mitigate or alleviate the negative effects of substituting FSM for FM represents a valuable research topic.
The Japanese eel (
Anguilla japonica) is an important freshwater economic species in China, valued for its high nutritional and protein content, and is often referred to as the “ginseng of the water” [
23]. Japanese eel farming in China has expanded rapidly over the past decade, with production reaching 291,566 tons in 2023 [
24]. The Japanese eel has high protein requirements, and commercial feeds contain substantial levels of FM, significantly increasing feed costs and investment in aquaculture [
23,
25]. Given the negative effects of replacing FM with a high proportion of FSM, along with the pharmacological effects of
L. edodes, this study aims to investigate the effects of
L. edodes supplementation on Japanese eel consuming high proportions of FSM in their diets, with a focus on growth performance and health issues associated with high FSM substitution.
2. Materials and Methods
2.1. Experimental Diets
This study included four experimental groups: the control group (Con), which was fed a basal diet containing fishmeal and plant proteins (extruded soybean, gluten flour, and α-starch) as the protein source; the fermented soybean meal replacement group (FSM), in which fishmeal was substituted with fermented soybean meal in the basal diet; and two groups where fermented soybean meal was supplemented with 20 g/kg (LEF2) and 30 g/kg (LEF3) of
Lentinus edodes fermentation, respectively. All ingredients were finely crushed, passed through a 100 μm mesh, and then mixed stepwise according to the weight of each feed ingredient, from smallest to largest. The mixture was then sealed, dried, and stored under light-proof, low-temperature conditions. Before each feeding, water was added to the raw materials in a 1:1.2 ratio (feed–water), and the mixture was then formed into a dough-like consistency for feeding. The composition and nutrient levels of the experimental feed are presented in
Table 1.
2.2. Experimental Procedure and Sample Collection
The Japanese eels used in the experiment were provided by Yinhua Biotechnology Co., Ltd. (Dongguan, Guangdong, China). The culture experiment was conducted in ponds at the Yinmei Aquaculture Eel Farm (Zhuhai, Guangdong, China), where experimental cages were installed. Before the formal experiment, the Japanese eels were temporarily housed in 4 m × 4 m × 3 m cages for feed adaptation. After the Japanese eels exhibited normal feeding behavior, 1200 uniformly sized eels (average body weight 62.50 ± 2.14 g) with healthy growth patterns were randomly assigned to four groups, each consisting of three replicate cages (2 m × 2 m × 1.5 m), with 100 fish per cage. Each group was fed one of four different experimental diets over the course of the 12-week culture experiment. The daily feeding rate was 3–4% of the total fish biomass, administered twice daily at 07:00 and 18:00, with each feeding comprising 50% of the total daily ration. Uneaten feed was promptly removed, weighed, and recorded, and feeding rates were adjusted accordingly based on these observations. Dead fish were promptly removed, weighed, and recorded. Water quality parameters, including pH, temperature, ammonia nitrogen concentration, nitrite concentration, and dissolved oxygen, were regularly monitored throughout the experiment. Water conditions were maintained within the following ranges: ammonia nitrogen < 0.06 mg/L, pH 7.6–8.5, temperature 25–30 °C, nitrite < 0.02 mg/L, and dissolved oxygen > 8 mg/L.
After 12 weeks of feeding, the fish were fasted for 24 h and then weighed according to the experimental group. Nine fish from each replicate were randomly selected, anesthetized in MS-222 solution, and individually measured for body weight, visceral weight, and liver weight. The liver, muscle, spleen, and portions of the intestines were removed and stored at −80 °C for subsequent analysis. Blood samples were collected from the caudal vein, centrifuged at 3500 rpm for 10 min at 4 °C, and the separated serum was stored at −80 °C.
The following calculations were performed:
2.3. Analysis Method of Nutritional Components
Nutrient composition analysis of experimental diets and muscles was performed with reference to Zhao et al. [
26]. Samples were dried at 105 °C to a constant weight to determine moisture content. The crude fat and protein content in muscle and feed were determined using the Soxhlet system and the Kjeldahl method (Xianjian Instrument Company, Shanghai, China), respectively. Crude ash content was determined by incineration at a constant weight in a muffle furnace at 550 °C.
To analyze the amino acid composition of the muscle, the samples were dried by a freeze dryer (ALPHA1–2 LDplus, Christco, Ltd., Ottobeuren, Germany) and digested in hydrochloric acid solution (6 M) at 110 °C for 22 h. The samples were then analyzed by an automated amino acid analyzer (L-8900, Hitachi, Tokyo, Japan) with a sodium ion exchange column. To examine the fatty acid composition of muscle, lipids from muscle were extracted after homogenization in a mixture of chloroform and methanol (2:1 v:v). For extraction of fatty acids, 1 mL of 50% KOH was added to 15 mL of 100% ethanol and saponified at 80 °C for 60 min. The fatty acids were then esterified with 7% boron trifluoride in methanol and heated at 80 °C for 20 min. The fatty acid methyl ester formulation was prepared in hexane (20 mg/mL) and analyzed by gas chromatography (HP 6890, Santa Clara, CA, USA).
2.4. Biochemical Indexes Analysis
Liver samples were homogenized on ice using PBS (phosphate buffered saline) solution at a 1:9 weight ratio. The sample suspensions were further homogenized with a homogenizer in an ice bath and centrifuged at 4 °C (800× g, 10 min). The supernatant was collected for further analysis. The biochemical parameters in serum and liver supernatant, including TC (total cholesterol), LDL-C (low-density lipoprotein cholesterol), HDL-C (high-density lipoprotein cholesterol), AKP (alkaline phosphatase), AST (aspartate aminotransferase), ALT (alanine aminotransferase), MDA (malondialdehyde), T-SOD (total superoxide dismutase), T-AOC (total antioxidant capacity), and CAT (catalase), were measured using commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
2.5. Morphology of Liver and Intestine
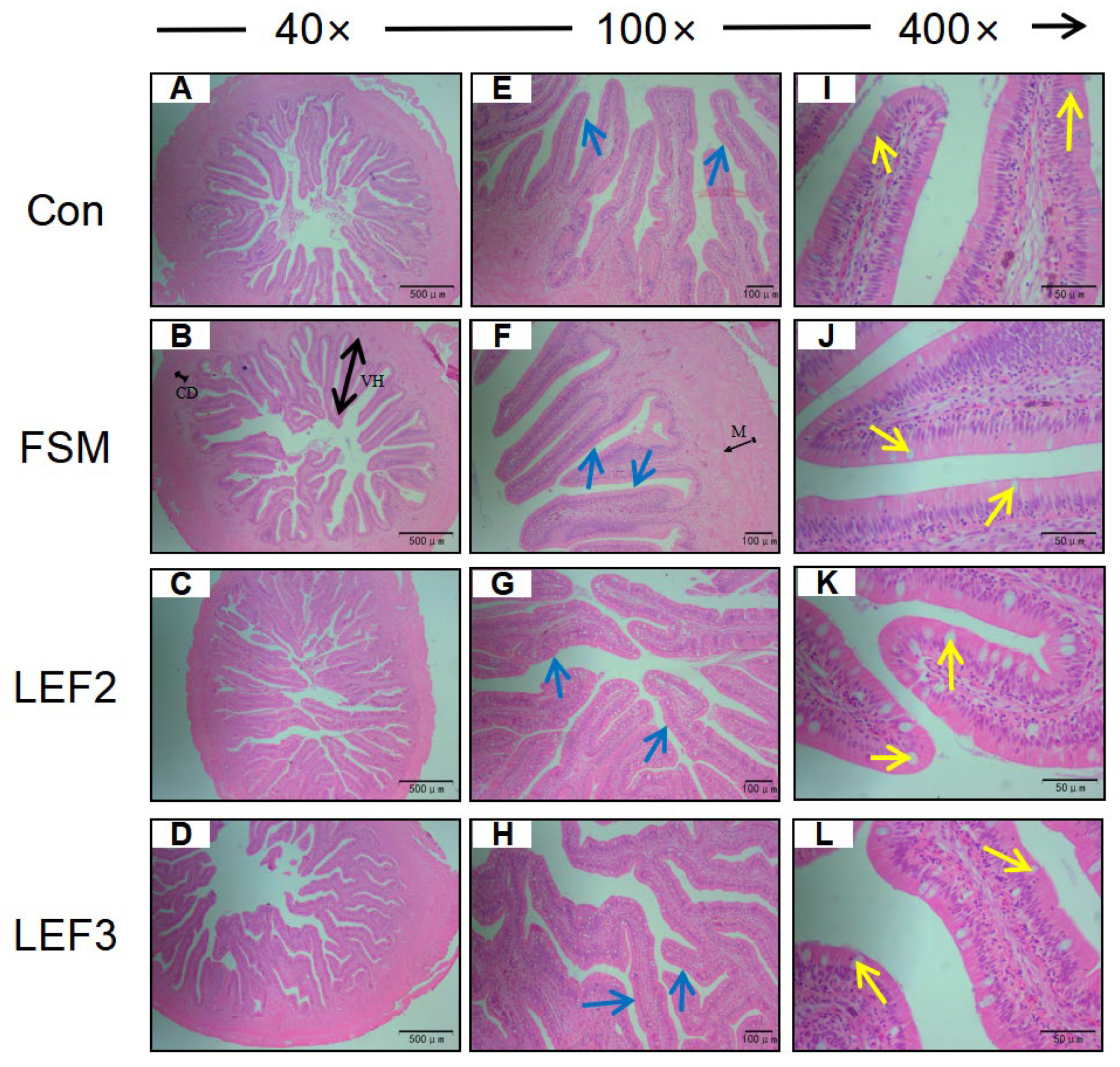
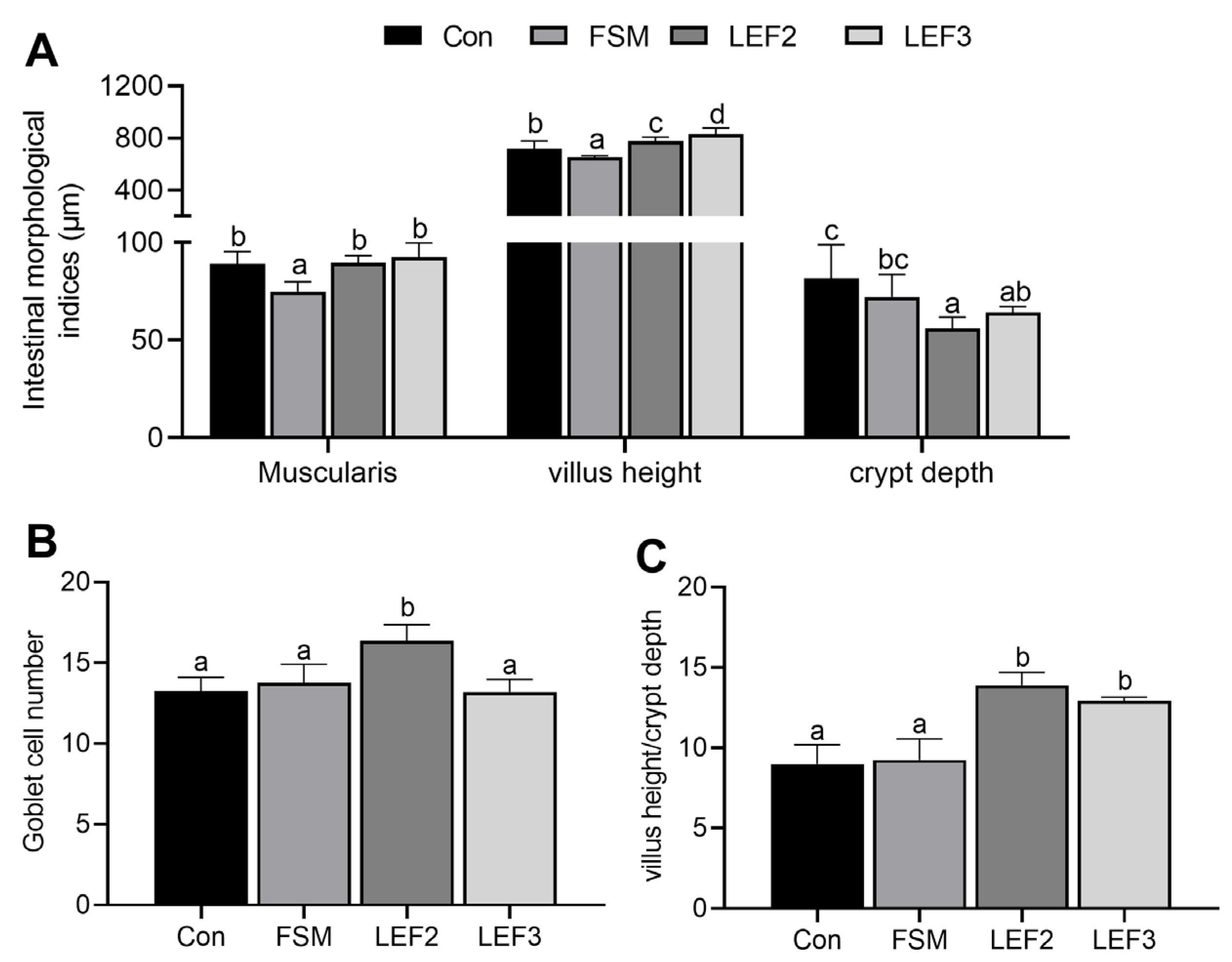
Liver and intestinal tissue samples from each group of five fish were dissected, pre-cooled, and rinsed in PBS, fixed in 4% paraformaldehyde solution for 24–48 h, then dehydrated in gradients of 50%, 75%, 80%, 95%, 100%, and 100% ethanol for 30 min in each gradient, washed in xylene for 1 h, embedded in paraffin wax, sectioned by microtome (5 μm), stained with hematoxylin and eosin, and mounted on neutral resin-mounted slices. Subsequently, the liver and intestinal tissues were observed under a microscope and photographed. The thickness of the intestinal muscularis propria, height of villi, depth of crypts, and number of thrush cells were measured using ImageJ software version V1.8.0.112.
2.6. Quantitative Real-Time Polymerase Chain Reaction
Total RNA was extracted from spleen samples of Japanese eel using Trizol reagent (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA) and electrophoresed on a 1.2% denaturing agarose gel to assess integrity. RNA was treated with RNA-free DNase (Toroivd Technology Co., Ltd., Shanghai, China) to remove DNA contaminants and then reverse-transcribed into cDNA using the PrimeScript™ RT reagent kit (Toroivd Technology Co., Ltd., Shanghai, China) following the manufacturer’s instructions. Real-time RT-PCR was performed using a quantitative thermal cycler (Mastercycler ep realplex, Eppendorf, Germany).
β-actin was used as the housekeeping gene, and the 2
−ΔΔCT algorithm calculated the relative mRNA expression of each target gene. The primer sequences for Japanese eel are provided in
Table 2.
2.7. Statistical Analysis
Data were analyzed using SPSS software version 27.0. The homogeneity of variance was assessed using Levene’s test. Significant differences were determined using one-way analysis of variance (ANOVA) followed by Duncan’s multiple range test. Statistical significance was defined as p < 0.05, and the results are presented as means ± S.E.M. (standard error of means).
4. Discussion
Fermented soybean meal (FSM) has been demonstrated as a nutritionally superior alternative to fish meal (FM) in aquafeeds, primarily due to its reduced anti-nutritional factors (ANFs) and enhanced nutrient profile. However, studies indicate that FM substitution with FSM may impair nutrient utilization in aquatic species [
27], adversely affecting growth performance, particularly at high replacement levels [
28]. In the present study, a 19% FM replacement with FSM significantly reduced growth performance and increased the feed conversion ratio (FCR) in Japanese eel. These results align with previous findings in black sea bream (
Acanthopagrus schlegeli) [
16] and rainbow trout (
Oncorhynchus mykiss) [
29].
Lentinula edodes exhibits multiple physiological benefits, including growth promotion, nutrient absorption enhancement, and immune modulation. Wang et al. [
30] demonstrated that lentinan supplementation significantly improved growth rate and feed efficiency in largemouth bass (
Micropterus salmoides) after 8 weeks. Conversely, Song et al. [
31] reported no significant effects on growth parameters in sea cucumber (
Apostichopus japonicus) with pleurotus ostreatus polysaccharide (POPS) supplementation. In our experiment, 3% LEF supplementation effectively counteracted the growth performance decline induced by FSM and enhanced feed efficiency. These findings suggest species-specific responses to fungal supplements, potentially influenced by mushroom species, processing methods, and dosage. Notably, LEF supplementation altered muscle composition, reducing crude lipid, α-linolenic acid (18:3n-3), and eicosapentaenoic acid (EPA) content while significantly increasing docosahexaenoic acid (DHA) levels. As these findings are novel, the mechanisms warrant further investigation. Free amino acids (FAAs), particularly flavor-associated amino acids, significantly influence taste quality. Glutamic acid, phenylalanine, and aspartic acid contribute to umami taste, while alanine and glycine impart sweetness [
32]. In this study, LEF supplementation significantly increased total essential amino acids (TEAAs), non-essential amino acids (NEAAs), total free amino acids (TFAAs), and glutamate in muscle tissue. The microbial fermentation-derived LEF may enhance FAA content and modify muscle FAA composition [
19,
33].
LDL-C is a lipoprotein responsible for transporting cholesterol to peripheral tissues [
34]. In contrast, HDL-C facilitates reverse cholesterol transport from extrahepatic tissues to the liver and steroidogenic organs, where it contributes to the synthesis of vitamin D, bile acids, steroid hormones, and lipoproteins [
35]. TC is a key component of blood lipids, and its concentration serves as an indicator of lipid absorption and metabolism, closely reflecting the nutritional status of fish [
36]. In the present study, FM replacement with FSM significantly elevated LDL-C levels while reducing HDL-C, suggesting impaired lipid transport and metabolism. However, dietary supplementation with LEF significantly lowered TC and LDL-C levels while increasing HDL-C. These findings align with previous studies on barramundi (
Lates calcarifer) [
37] and zebrafish (
Danio rerio) [
38], indicating that LEF may enhance lipid transport and metabolic efficiency.
ALT and AST are well-established biomarkers of hepatic dysfunction. In the present study, elevated serum ALT and AST levels in the FSM-fed group indicated liver injury, corroborated by histological observations of disorganized hepatocyte architecture. Dietary supplementation with LEF ameliorated these effects, suggesting improved lipid metabolism and hepatoprotective activity. The intestinal tract plays a pivotal role in nutrient digestion and absorption in aquatic species, with mucosal integrity being essential for optimal feed efficiency. Our results demonstrated that FM replacement with FSM significantly reduced muscularis thickness and villus height, whereas LEF supplementation restored intestinal morphology by enhancing crypt depth and goblet cell density. Previous studies have reported that
L. edodes derivatives exert anti-inflammatory effects and modulate gut microbiota [
39,
40,
41], potentially explaining the observed intestinal structural improvements in Japanese eel.
CAT and SOD constitute essential components of the endogenous antioxidant defense system in animals. These enzymes collaboratively catalyze the dismutation of superoxide radicals (-O
2) into hydrogen peroxide (H
2O
2), which is subsequently decomposed into water and molecular oxygen [
42]. This enzymatic cascade provides critical protection against the oxidative damage induced by reactive oxygen species (ROS) and reactive nitrogen species (RNS) [
43,
44]. Previous investigations have demonstrated that lentinan (LNT) upregulates SOD and CAT activities in murine pulmonary tissue [
45] and enhances SOD activity in human keratinocytes [
46]. Similarly, studies in largemouth bass (
Micropterus salmoides) revealed that LEF elevates hepatic CAT activity [
41]. Our findings corroborate these observations, showing that LEF supplementation significantly enhanced both CAT and SOD activities in the liver and serum of Japanese eel. This enhanced antioxidant capacity was further evidenced by increased T-AOC and reduced MDA levels. The observed effects may be attributed to the bioactive polysaccharides present in
L. edodes, including β-D-glucan, eritadenine, xylomannan, lentinan, and heteroglucan [
47], which have been shown to modulate antioxidant enzyme activities and mitigate ROS accumulation [
48,
49,
50]. AKP, a crucial metabolic enzyme, contributes to innate immunity through pathogen catabolism and phagocyte activation [
51]. In agreement with Wang et al.’s findings in sea cucumber (
Apostichopus japonicus) [
52], our study demonstrated that 3% LEF supplementation significantly augmented AKP activity. Collectively, these results indicate that LEF effectively enhances both antioxidant defenses and immune function in Japanese eel.
The spleen, as the largest secondary lymphoid organ, plays a pivotal role in orchestrating diverse immune responses [
53]. In the present study, we investigated the expression patterns of key immune-related genes in this critical immunoregulatory organ. The inflammatory response is tightly regulated through a dynamic balance between proinflammatory and anti-inflammatory mediators [
54]. Notably, tumor necrosis factor-α (TNF-α) and interleukin-1 receptor-associated kinase 4 (IRAK4) serve as crucial proinflammatory factors. TNF-α not only initiates inflammatory cascades but also enhances phagocytic activity and T-cell proliferation to combat pathogenic challenges [
55]. IRAK4, a key component of the IL-1 receptor (IL-1R)-associated kinase family, is essential for toll-like receptor (TLR)-mediated signaling pathways [
56]. Additionally, caspase-1 mediates the proteolytic activation of pro-interleukin (IL)-1β, facilitating its secretion by monocytes and macrophages [
57]. Our findings revealed that FM replacement with FSM significantly upregulated splenic
tnf-α expression. In contrast, LEF supplementation effectively downregulated the expression of
tnf-α,
caspase-1, and
irak4, suggesting its potential to modulate innate immunity through the suppression of proinflammatory cytokine production. Interferons (IFNs), particularly IFN-α and IFN-γ, represent critical antiviral proteins that are secreted in response to various immunological stimuli [
58,
59]. Importantly, LEF supplementation counteracted the FSM-induced suppression of
ifn-α and
ifn-γ expression, aligning with previous observations by Ren et al. [
60]. Given that IFN-γ serves as a key mediator of adaptive immunity, these results imply that LEF may exert immunomodulatory effects across both innate and adaptive immune systems. Further analysis demonstrated that LEF significantly enhanced the expression of interferon regulatory factors (IRFs), particularly
irf3 and
irf11, which are known to govern IFN responses and immune cell differentiation [
61]. The mitochondrial antiviral signaling (MAVS) protein, acting through TNF receptor-associated factor 3 (TRAF3), plays a central role in regulating inflammation-associated apoptotic pathways [
62]. Notably, 2% LEF supplementation markedly elevated mavs and
traf3 expression levels, indicating its potential anti-inflammatory effects via the MAVS/TRAF3 axis, consistent with the LNT-mediated immunomodulation reported in inflammatory disease models [
63,
64,
65]. The suppressor of the cytokine signaling 1 (SOCS1) protein serves as a critical negative regulator of cytokine signaling, maintaining immune homeostasis [
66]. This regulatory function is underscored by studies showing that SOCS1-deficient mice develop severe inflammatory conditions [
67]. Intriguingly, while FSM substitution significantly reduced
socs1 expression, LEF supplementation restored its expression to normal levels. These findings, coupled with the existing evidence that fermented plant products can modulate piscine innate immunity [
68,
69,
70], collectively demonstrate that FSM replacement compromises splenic immunocompetence, whereas LEF exerts comprehensive immunoenhancing effects. To our knowledge, this represents the first comprehensive demonstration that dietary LEF supplementation enhances immune function in Japanese eel through the multifaceted modulation of immune-related genes and signaling pathways.