Impact of Temperature Manipulations on Growth Performance, Body Composition, and Selected Genes of Koi Carp (Cyprinus carpio koi)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Setup
2.2. Experimental Procedure
2.3. Growth Performance and Survival Rate
2.4. Proximate Analysis of Koi Fish
2.5. Amino Acid Analysis
2.6. Fatty Acid Analysis
2.7. Real-Time PCR Analysis of Selected Genes
2.8. Statistical Analysis
3. Results
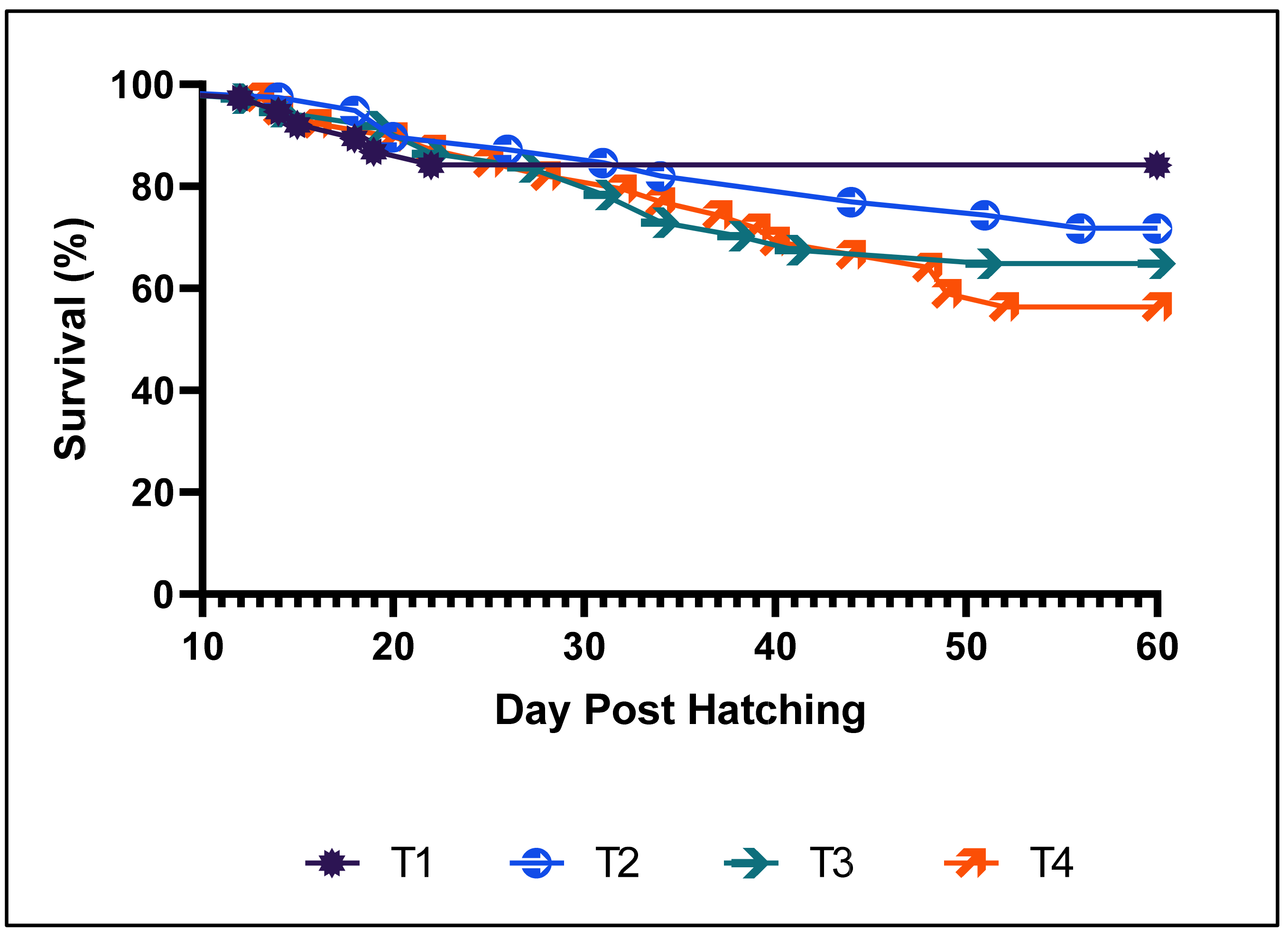
3.1. Growth Performance and Survival Rate in the First Phase
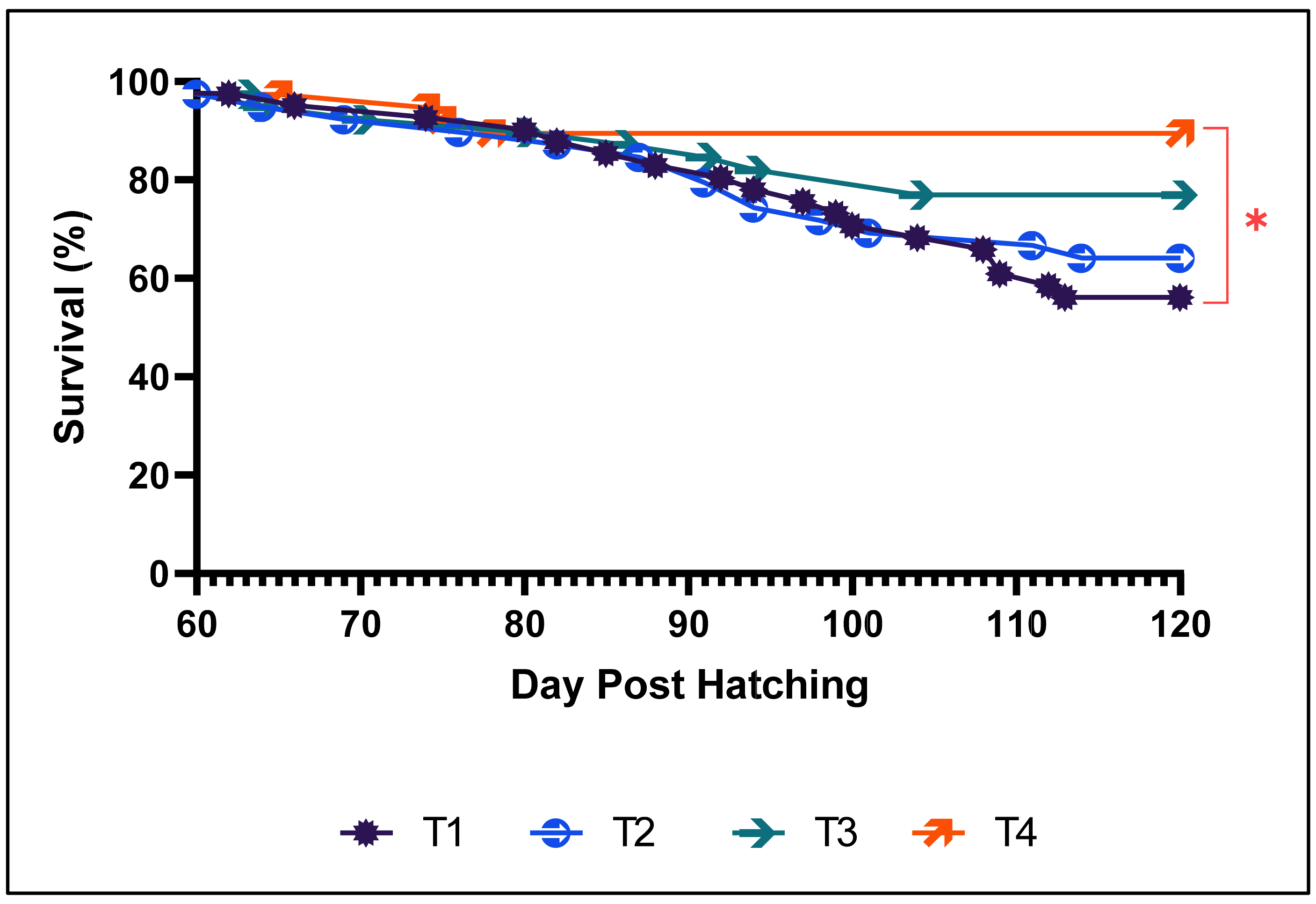
3.2. Growth Performance and Survival Rate in the Second Phase
3.3. Proximate Analysis of Fish in the Second Phase
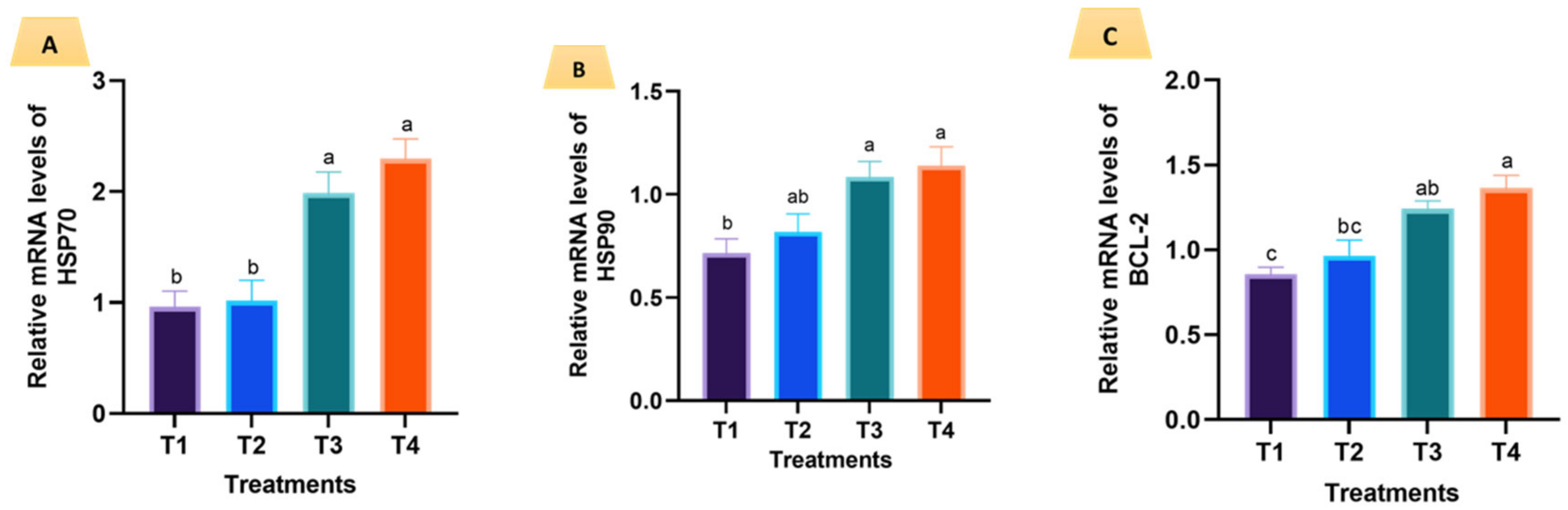
3.4. Expression of Selected Genes in the First Phase
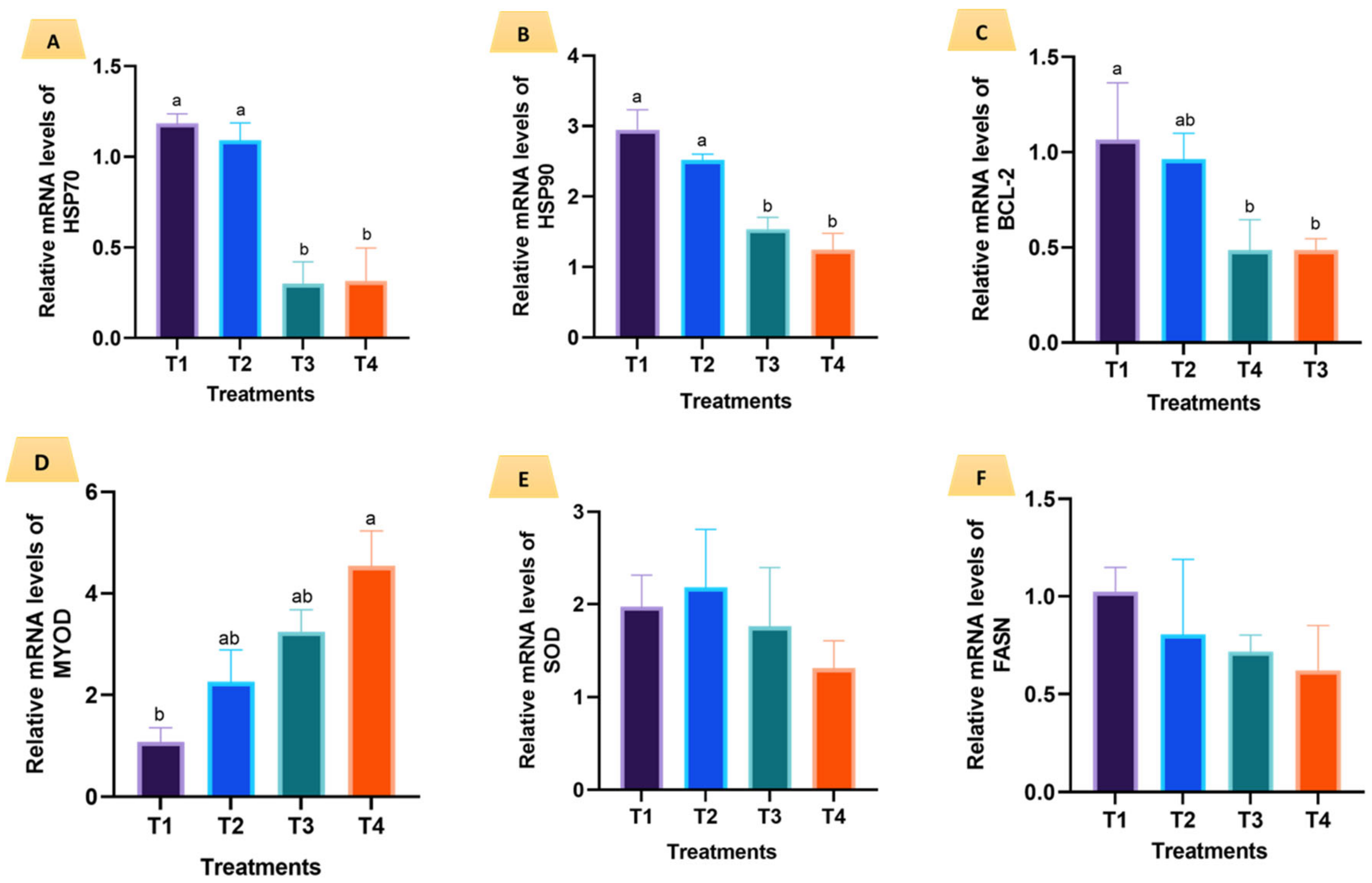
3.5. Expression of Selected Genes in the Second Phase
3.6. Relationships Between Gene Expression and Other Parameters
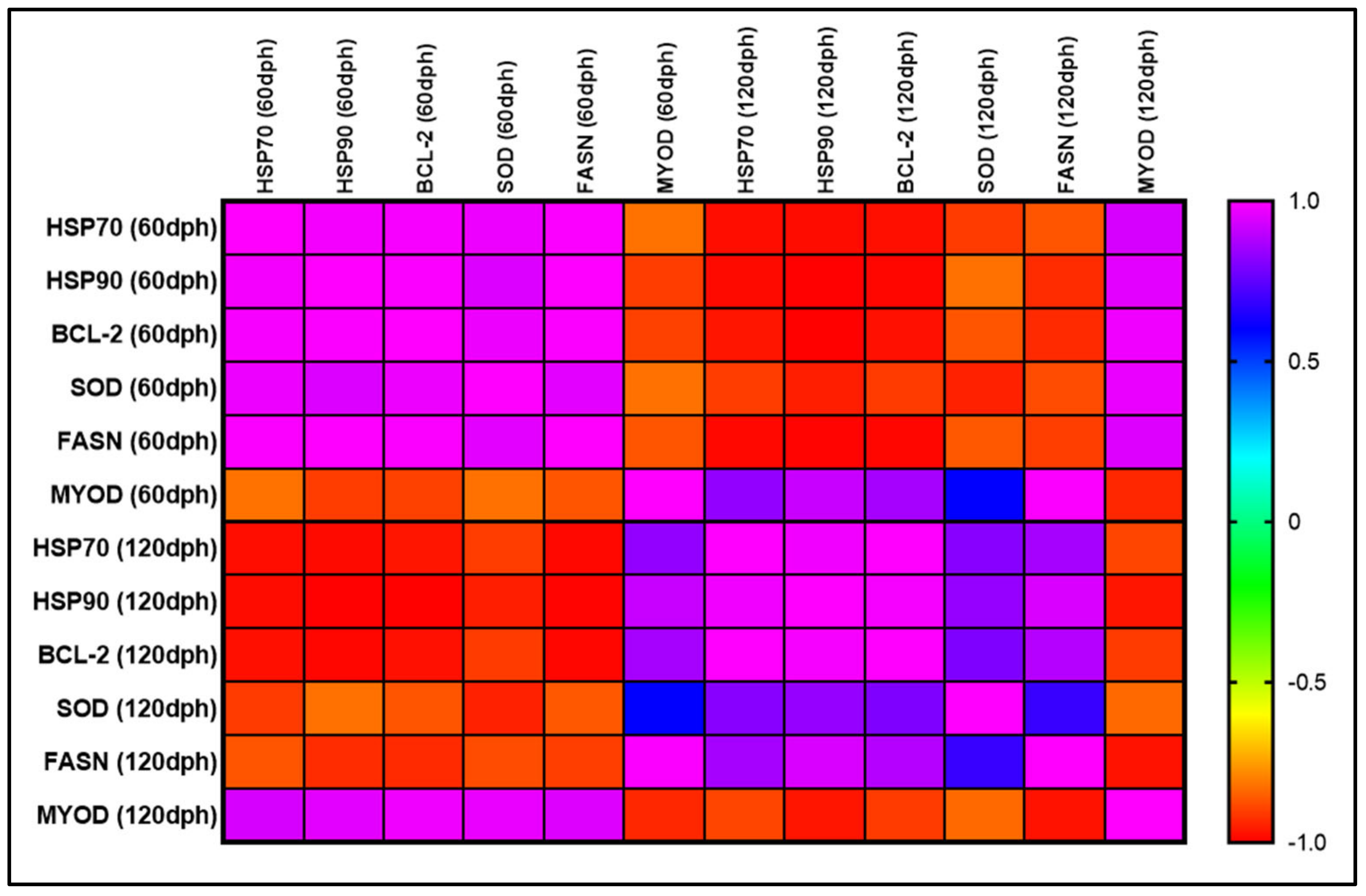
3.6.1. Relationships Among the Expression of Selected Genes Between the First and Second Phase
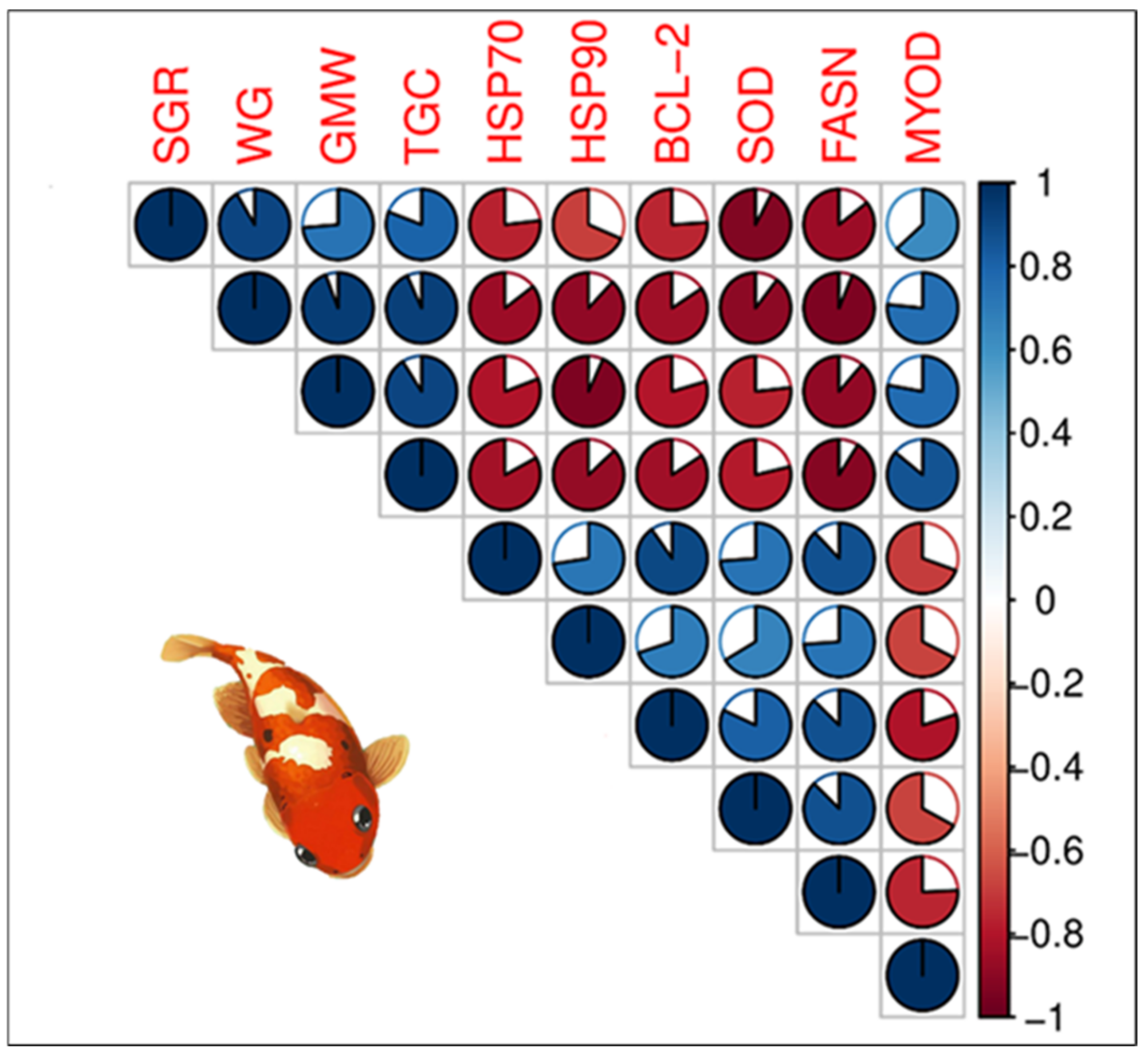
3.6.2. Relationships Between Growth Parameter and Gene Expression in the First Phase
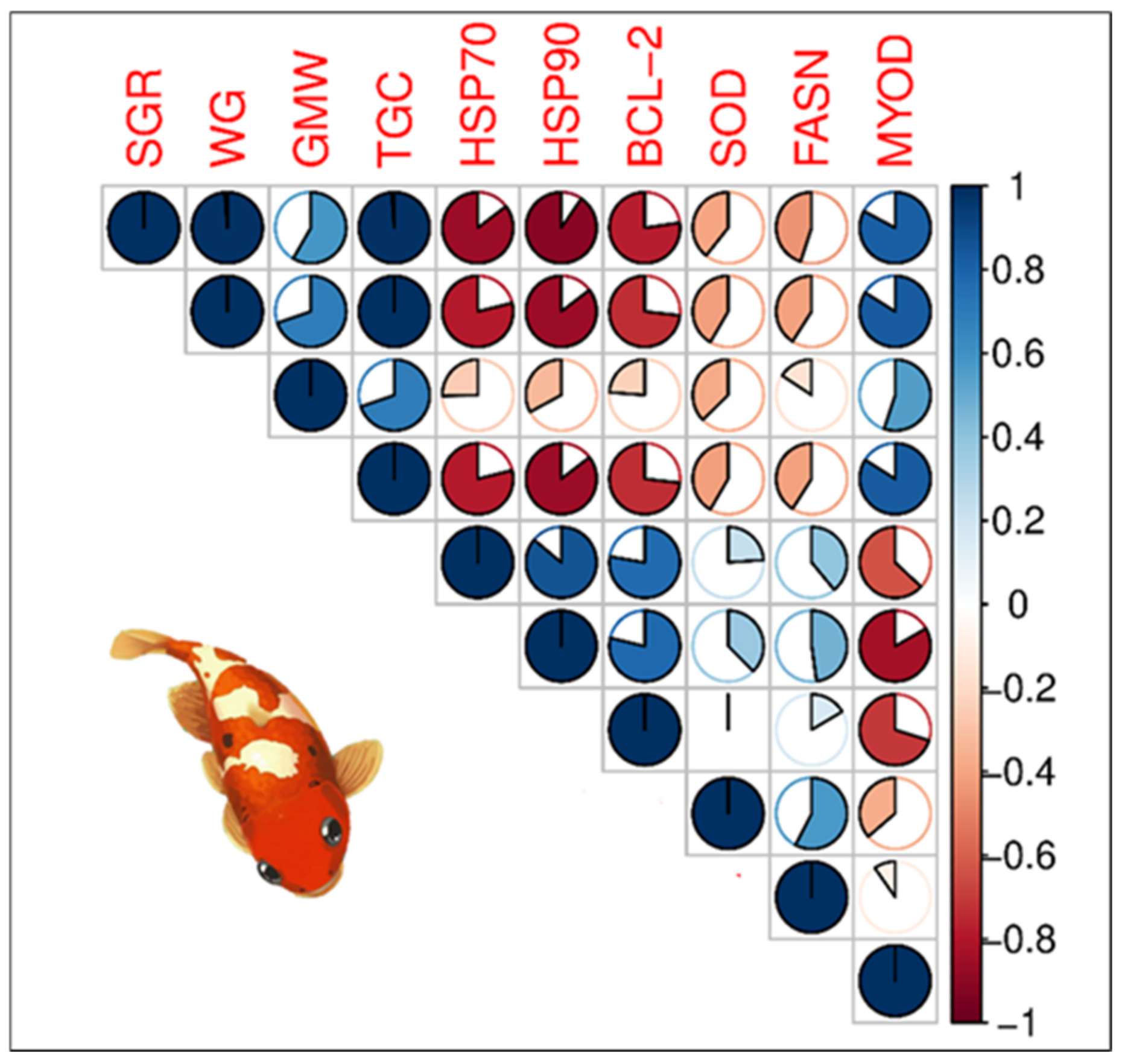
3.6.3. Relationships Between Growth Parameter and Gene Expression in the Second Phase
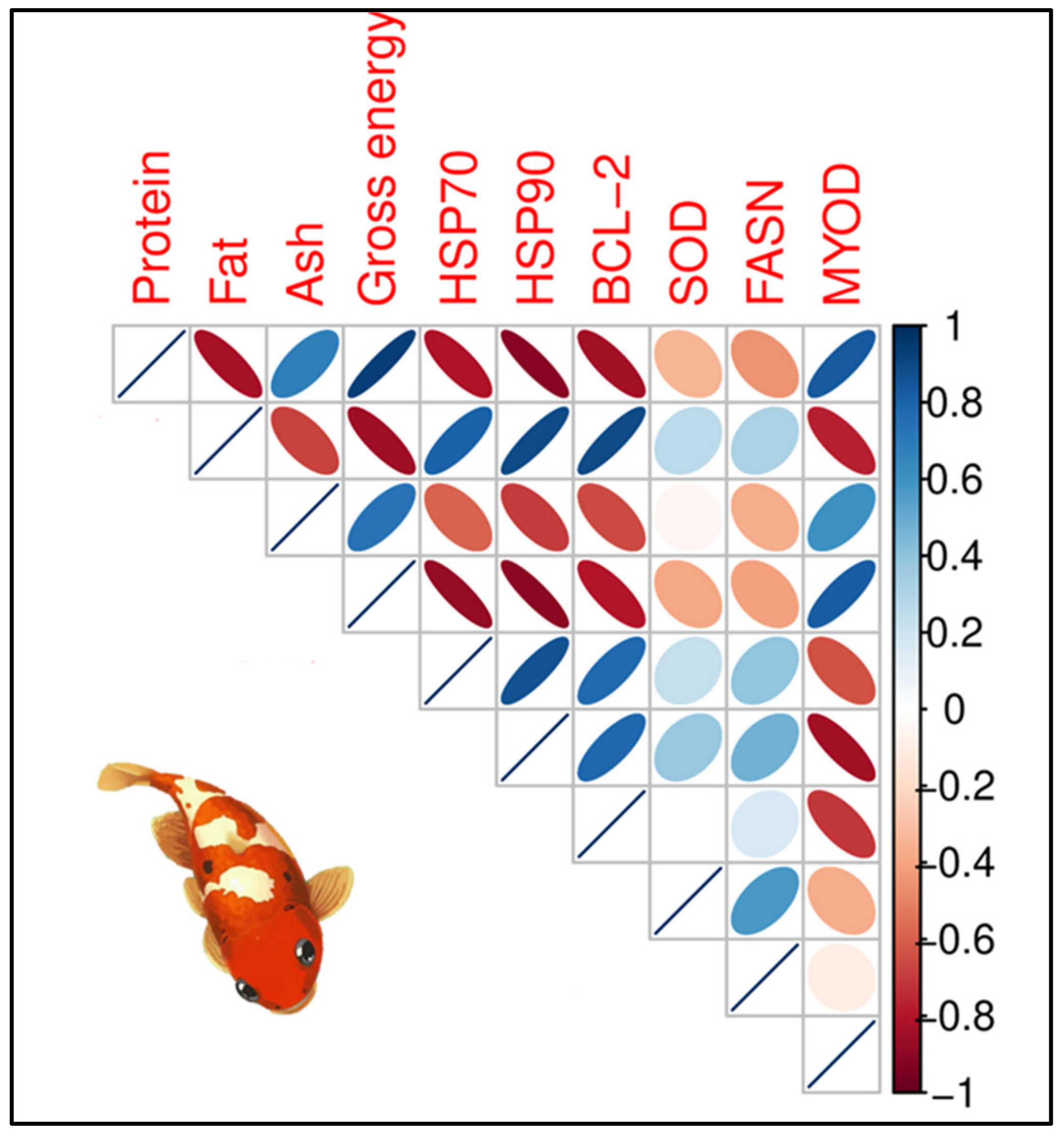
3.6.4. Relationships Between Body Composition and Gene Expression in the Second Phase
3.7. Amino Acid Analysis in the Second Phase
3.8. Fatty Acid Analysis in the Second Phase
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prakash, S. Impact of Climate change on Aquatic Ecosystem and its Biodiversity: An overview. Int. J. Biol. Innov. 2021, 3, 312–317. [Google Scholar] [CrossRef]
- Kroeker, K.J.; Sanford, E. Ecological Leverage Points: Species Interactions Amplify the Physiological Effects of Global Environmental Change in the Ocean. Annu. Rev. Mar. Sci. 2022, 14, 75–103. [Google Scholar] [CrossRef] [PubMed]
- Dudgeon, D.; Strayer, D.L. Bending the curve of global freshwater biodiversity loss: What are the prospects? Biol. Rev. 2024, 100, 205–226. [Google Scholar] [CrossRef] [PubMed]
- Wudu, K.; Abegaz, A.; Ayele, L.; Ybabe, M. The impacts of climate change on biodiversity loss and its remedial measures using nature based conservation approach: A global perspective. Biodivers. Conserv. 2023, 32, 3681–3701. [Google Scholar] [CrossRef]
- Jørgensen, L.B.; Ørsted, M.; Malte, H.; Wang, T.; Overgaard, J. Extreme escalation of heat failure rates in ectotherms with global warming. Nature 2022, 611, 93–98. [Google Scholar] [CrossRef]
- Mugwanya, M.; Dawood, M.A.O.; Kimera, F.; Sewilam, H. Anthropogenic temperature fluctuations and their effect on aquaculture: A comprehensive review. Aquac. Fish. 2022, 7, 223–243. [Google Scholar] [CrossRef]
- Huang, M.; Ding, L.; Wang, J.; Ding, C.; Tao, J. The impacts of climate change on fish growth: A summary of conducted studies and current knowledge. Ecol. Indic. 2021, 121, 106976. [Google Scholar] [CrossRef]
- Bernal, M.A.; Ravasi, T.; Rodgers, G.G.; Munday, P.L.; Donelson, J.M. Plasticity to ocean warming is influenced by transgenerational, reproductive, and developmental exposure in a coral reef fish. Evol. Appl. 2022, 15, 249–261. [Google Scholar] [CrossRef]
- Messina, S.; Costantini, D.; Eens, M. Impacts of rising temperatures and water acidification on the oxidative status and immune system of aquatic ectothermic vertebrates: A meta-analysis. Sci. Total Environ. 2023, 868, 161580. [Google Scholar] [CrossRef]
- Alfonso, S.; Gesto, M.; Sadoul, B. Temperature increase and its effects on fish stress physiology in the context of global warming. J. Fish Biol. 2021, 98, 1496–1508. [Google Scholar] [CrossRef]
- Ern, R.; Andreassen, A.H.; Jutfelt, F. Physiological Mechanisms of Acute Upper Thermal Tolerance in Fish. Physiology 2023, 38, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, J.B.; Fullerton, A.H.; Jordan, C.E.; Ebersole, J.L.; Bellmore, J.R.; Arismendi, I.; Penaluna, B.E.; Reeves, G.H. The importance of warm habitat to the growth regime of cold-water fishes. Nat. Clim. Chang. 2021, 11, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Biela, V.R.; Sergeant, C.J.; Carey, M.P.; Liller, Z.; Russell, C.; Quinn-Davidson, S.; Rand, P.S.; Westley, P.A.H.; Zimmerman, C.E. Premature Mortality Observations among Alaska’s Pacific Salmon During Record Heat and Drought in 2019. Fisheries 2022, 47, 157–168. [Google Scholar] [CrossRef]
- Shaalan, W.M.; Elbaghdady, H.A.M.; Sayed, A.E.-D.H. Synergistic effects of thermal stress and 4-nonylphenol on oxidative stress and immune responses in juvenile tilapia (Oreochromis niloticus). Environ. Sci. Pollut. Res. 2024, 31, 64024–64032. [Google Scholar] [CrossRef]
- Verberk, W.C.E.P.; Atkinson, D.; Hoefnagel, K.N.; Hirst, A.G.; Horne, C.R.; Siepel, H. Shrinking body sizes in response to warming: Explanations for the temperature–size rule with special emphasis on the role of oxygen. Biol. Rev. 2021, 96, 247–268. [Google Scholar] [CrossRef]
- Friesen, C.R.; Wapstra, E.; Olsson, M. Of telomeres and temperature: Measuring thermal effects on telomeres in ectothermic animals. Mol. Ecol. 2022, 31, 6069–6086. [Google Scholar] [CrossRef]
- Agarwal, D.; Shanmugam, S.A.; Kathirvelpandian, A.; Eswaran, S.; Rather, M.A.; Rakkannan, G. Unraveling the Impact of Climate Change on Fish Physiology: A Focus on Temperature and Salinity Dynamics. J. Appl. Ichthyol. 2024, 2024, 5782274. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation; Food and Agriculture Organization of the United Nations: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Maulu, S.; Hasimuna, O.J.; Haambiya, L.H.; Monde, C.; Musuka, C.G.; Makorwa, T.H.; Munganga, B.P.; Phiri, K.J.; Nsekanabo, J.D. Climate Change Effects on Aquaculture Production: Sustainability Implications, Mitigation, and Adaptations. Front. Sustain. Food Syst. 2021, 5, 609097. [Google Scholar] [CrossRef]
- Vargas-Chacoff, L.; Arjona, F.J.; Polakof, S.; del Río, M.P.M.; Soengas, J.L.; Mancera, J.M. Interactive effects of environmental salinity and temperature on metabolic responses of gilthead sea bream Sparus aurata. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2009, 154, 417–424. [Google Scholar] [CrossRef]
- Vargas-Chacoff, L.; Arjona, F.J.; Ruiz-Jarabo, I.; García-Lopez, A.; Flik, G.; Mancera, J.M. Water temperature affects osmoregulatory responses in gilthead sea bream (Sparus aurata L.). J. Therm. Biol. 2020, 88, 102526. [Google Scholar] [CrossRef]
- Vargas-Chacoff, L.; Regish, A.M.; Weinstock, A.; McCormick, S.D. Effects of elevated temperature on osmoregulation and stress responses in Atlantic salmon Salmo salar smolts in fresh water and seawater. J. Fish Biol. 2018, 93, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Nuwansi, K.; Verma, A.; Chandrakant, M.; Prabhath, G.; Peter, R. Optimization of stocking density of koi carp (Cyprinus carpio var. koi) with gotukola (Centella asiatica) in an aquaponic system using phytoremediated aquaculture wastewater. Aquaculture 2021, 532, 735993. [Google Scholar] [CrossRef]
- Dasgupta, P.; Anandhi, D.U. Adaptation of Koi Carp (Cyprinus carpio) Exposed to Different Temperature Variants. Int. J. Innov. Sci. Res. Technol. (IJISRT) 2024, 9, 824–826. [Google Scholar] [CrossRef]
- Ginanjar, P.; Opipah, S.; Rusmana, D.; Muhlas; Effendi, M.; Hamidi, E. Prototype Smart Fish Farm in Koi Fish Farming. In Proceedings of the 2021 7th International Conference on Wireless and Telematics (ICWT), Bandung, Indonesia, 19–20 August 2021; pp. 1–6. [Google Scholar] [CrossRef]
- Grynevych, N.; Khomiak, O.; Sliusarenko, A.; Trofymchuk, A.; Zharchynska, V.; Osadcha, Y.; Tkachenko, O. Adaptive response of koi carp (Cyprinus carpio koi) to low and high temperatures in experimental conditions. Sci. Messenger LNU Vet. Med. Biotechnol. 2022, 24, 137–145. [Google Scholar] [CrossRef]
- Șerban, D.A.; Barbacariu, C.A.; Burducea, M.; Ivancia, M.; Creangă, Ș. Comparative Analysis of Growth Performance, Morphological Development, and Physiological Condition in Three Romanian Cyprinus carpio Varieties and Koi: Implications for Aquaculture. Life 2024, 14, 1471. [Google Scholar] [CrossRef]
- Andrian, K.N.; Wihadmadyatami, H.; Wijayanti, N.; Karnati, S.; Haryanto, A. A comprehensive review of current practices, challenges, and future perspectives in Koi fish (Cyprinus carpio var. koi) cultivation. Vet World 2024, 17, 1846–1854. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of Official Analytical Chemists International, 16th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1995. [Google Scholar]
- Wen, B.; Chen, Z.; Qu, H.; Gao, J. Growth and fatty acid composition of discus fish Symphysodon haraldi given varying feed ratios of beef heart, duck heart, and shrimp meat. Aquac. Fish. 2018, 3, 84–89. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, C.; Xing, W.; Li, T.; Xu, G.; Ma, Z.; Jiang, N.; Luo, L. Comparative study on the non-specific immune response and hsp70 gene expression among three strains of koi (Cyprinus carpio) under acute heat stress. Aquac. Rep. 2020, 18, 100461. [Google Scholar] [CrossRef]
- Jiao, X.; Hou, X.-w.; Guo, Z.-y.; Li, Y.-h.; Zhou, J.-x. Influence of heat shock protein 90 on the replication of spring viremia of carp virus. Aquaculture 2024, 588, 740955. [Google Scholar] [CrossRef]
- Li, M.; Xu, C.-J.; Li, D.; Wu, G.-F.; Wu, G.-Q.; Yang, C.-Y.; Pan, Y.-F.; Pan, Z.-Q.; Tan, G.-L.; Liu, Y.-Y. Transcriptome analysis of the spleen provides insight into the immunoregulation of Cyprinus carpio koi under Aeromonas veronii infection. Aquaculture 2021, 540, 736650. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, N.; Liang, J.; Wang, Q.; Zu, X.; Wang, H.; Yuan, H.; Zhang, R.; Guo, S.; Liu, Y.; et al. Emodin resists to Cyprinid herpesvirus 3 replication via the pathways of Nrf2/Keap1-ARE and NF-κB in the ornamental koi carp (Cyprinus carpio haematopterus). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 246, 109023. [Google Scholar] [CrossRef]
- Yao, J.; Hu, P.; Zhu, Y.; Xu, Y.; Tan, Q.; Liang, X. Lipid-Lowering Effects of Lotus Leaf Alcoholic Extract on Serum, Hepatopancreas, and Muscle of Juvenile Grass Carp via Gene Expression. Front. Physiol. 2020, 11, 584782. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, G.; Xie, S.; Pan, L.; Tan, Q. Growth and Meat Quality of Grass Carp (Ctenopharyngodon idellus) Responded to Dietary Protein (Soybean Meal) Level Through the Muscle Metabolism and Gene Expression of Myosin Heavy Chains. Front. Nutr. 2022, 9, 833924. [Google Scholar] [CrossRef] [PubMed]
- Ghinter, L.; Lambert, Y.; Audet, C. Juvenile Greenland halibut (Reinhardtius hippoglossoides) growth in the context of rising temperature in the Estuary and Gulf of St. Lawrence. Fish. Res. 2021, 233, 105766. [Google Scholar] [CrossRef]
- Pacheco-Carlón, N.; Salgado-García, R.L.; Guerrero-Tortolero, D.A.; Kraffe, E.; Campos-Ramos, R.; Racotta, I.S. Biochemical composition and adenylate energy charge shifts in longfin yellowtail (Seriola rivoliana) embryos during development under different temperatures. J. Therm. Biol. 2023, 112, 103470. [Google Scholar] [CrossRef]
- Jutfelt, F. Metabolic adaptation to warm water in fish. Funct. Ecol. 2020, 34, 1138–1141. [Google Scholar] [CrossRef]
- Eme, J.; Mueller, C.A.; Lee, A.H.; Melendez, C.; Manzon, R.G.; Somers, C.M.; Boreham, D.R.; Wilson, J.Y. Daily, repeating fluctuations in embryonic incubation temperature alter metabolism and growth of Lake whitefish (Coregonus clupeaformis). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2018, 226, 49–56. [Google Scholar] [CrossRef]
- Morgan, R.; Andreassen, A.H.; Åsheim, E.R.; Finnøen, M.H.; Dresler, G.; Brembu, T.; Loh, A.; Miest, J.J.; Jutfelt, F. Reduced physiological plasticity in a fish adapted to stable temperatures. Proc. Natl. Acad. Sci. USA 2022, 119, e2201919119. [Google Scholar] [CrossRef]
- Tahir, I.; Fatima, M.; Shah, S.Z.H.; Khan, N.; Ali, W. Interactive effects of temperature and protein levels on the growth performance, proximate composition, digestive enzyme activity and serum biochemistry of Labeo rohita. J. Anim. Physiol. Anim. Nutr. 2024, 108, 403–413. [Google Scholar] [CrossRef]
- Blair, S.D.; Glover, C.N. Acute exposure of larval rainbow trout (Oncorhynchus mykiss) to elevated temperature limits hsp70b expression and influences future thermotolerance. Hydrobiologia 2019, 836, 155–167. [Google Scholar] [CrossRef]
- Mazumder, S.K.; Debi, S.; Das, S.K.; Salam, M.A.; Alam, M.S.; Rahman, M.L.; Mamun, M.A.; Ibrahim Khalil, S.M.; Pandit, D. Effects of Extreme-Ambient Temperatures in Silver Barb (Barbonymus gonionotus): Metabolic, Hemato-Biochemical Responses, Enzymatic Activity and Gill Histomorphology. Water 2024, 16, 292. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, C.; Zhang, Y.; Zhao, X. Effects of Heat Stress on the Muscle Meat Quality of Rainbow Trout. Fishes 2024, 9, 459. [Google Scholar] [CrossRef]
- Hardison, E.A.; Eliason, E.J. Diet effects on ectotherm thermal performance. Biol. Rev. 2024, 99, 1537–1555. [Google Scholar] [CrossRef]
- Illing, B.; Downie, A.T.; Beghin, M.; Rummer, J.L. Critical thermal maxima of early life stages of three tropical fishes: Effects of rearing temperature and experimental heating rate. J. Therm. Biol. 2020, 90, 102582. [Google Scholar] [CrossRef]
- Garvin, M.R.; Thorgaard, G.H.; Narum, S.R. Differential Expression of Genes that Control Respiration Contribute to Thermal Adaptation in Redband Trout (Oncorhynchus mykiss gairdneri). Genome Biol. Evol. 2015, 7, 1404–1414. [Google Scholar] [CrossRef]
- Lutze, P.; Brenmoehl, J.; Tesenvitz, S.; Ohde, D.; Wanka, H.; Meyer, Z.; Grunow, B. Effects of Temperature Adaptation on the Metabolism and Physiological Properties of Sturgeon Fish Larvae Cell Line. Cells 2024, 13, 269. [Google Scholar] [CrossRef]
- Sun, Y.; Wen, H.; Tian, Y.; Mao, X.; Li, X.; Li, J.; Hu, Y.; Liu, Y.; Li, J.; Li, Y. HSP90 and HSP70 Families in Lateolabrax maculatus: Genome-Wide Identification, Molecular Characterization, and Expression Profiles in Response to Various Environmental Stressors. Front. Physiol. 2021, 12, 784803. [Google Scholar] [CrossRef]
- Chen, Y.; Chang, Q.; Fang, Q.; Zhang, Z.; Wu, D.; Bian, L.; Chen, S. Genome-Wide Identification, Molecular Characterization, and Expression Analysis of the HSP70 and HSP90 Gene Families in Thamnaconus septentrionalis. Int. J. Mol. Sci. 2024, 25, 5706. [Google Scholar] [CrossRef]
- Elabd, H.; Kumar, V.; Eissa, N.; Shen, Z.-G.; Yao, H.; Shaheen, A.; Abbass, A.; Wang, H.-P. Stress, immune and growth responses of bluegill sunfish (Lepomis macrochirus) to different environmental temperatures as referred by related gene expression. Glob. J. Fish. Aquac 2015, 3, 247–256. [Google Scholar]
- Koagouw, W.; Hazell, R.J.; Ciocan, C. Induction of apoptosis in the gonads of Mytilus edulis by metformin and increased temperature, via regulation of HSP70, CASP8, BCL2 and FAS. Mar. Pollut. Bull. 2021, 173, 113011. [Google Scholar] [CrossRef] [PubMed]
- Somero, G.N. The cellular stress response and temperature: Function, regulation, and evolution. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2020, 333, 379–397. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.L.; Do, D.T. The Role of Heat Shock Proteins in Response to Extracellular Stress in Aquatic Organisms. In Heat Shock Proteins in Veterinary Medicine and Sciences; Asea, A.A.A., Kaur, P., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 247–274. [Google Scholar] [CrossRef]
- Chowdhury, S.; Saikia, S. Oxidative Stress in Fish: A Review. J. Sci. Res. 2020, 12, 145–160. [Google Scholar] [CrossRef]
- Liang, Y.-S.; Wu, R.-X.; Niu, S.-F.; Miao, B.-B.; Liang, Z.-B.; Zhai, Y. Liver transcriptome analysis reveals changes in energy metabolism, oxidative stress, and apoptosis in pearl gentian grouper exposed to acute hypoxia. Aquaculture 2022, 561, 738635. [Google Scholar] [CrossRef]
- Elbialy, Z.I.; Atef, E.; Al-Hawary, I.I.; Salah, A.S.; Aboshosha, A.A.; Abualreesh, M.H.; Assar, D.H. Myostatin-mediated regulation of skeletal muscle damage post-acute Aeromonas hydrophila infection in Nile tilapia (Oreochromis niloticus L.). Fish Physiol. Biochem. 2023, 49, 1–17. [Google Scholar] [CrossRef]
- Javid, P.; Farrokhi, N.; Behzadi, S.; Bakhtiarizadeh, M.; Alavi, S.; Ranjbar, M. Genetic Variation in Response to Global Warming in a Coral Reef Species, Porites lobata. Avicenna J. Environ. Health Eng. 2020, 7, 28–33. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, X.; Zhang, X.; Zhu, H.; Zhong, X.; Song, W.; Yuan, J.; Sha, Z.; Li, F. Gene structure, expression and function analysis of the MyoD gene in the Pacific white shrimp Litopenaeus vannamei. Gene 2024, 921, 148523. [Google Scholar] [CrossRef]
- Churova, M.V.; Shulgina, N.; Kuritsyn, A.; Krupnova, M.Y.; Nemova, N.N. Muscle-specific gene expression and metabolic enzyme activities in Atlantic salmon Salmo salar L. fry reared under different photoperiod regimes. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2020, 239, 110330. [Google Scholar] [CrossRef]
- Fishman, B.; Tauber, E. Epigenetics and seasonal timing in animals: A concise review. J. Comp. Physiol. A 2024, 210, 565–574. [Google Scholar] [CrossRef]
- Jiang, C.; Storey, K.B.; Yang, H.; Sun, L. Aestivation in Nature: Physiological Strategies and Evolutionary Adaptations in Hypometabolic States. Int. J. Mol. Sci. 2023, 24, 14093. [Google Scholar] [CrossRef]
- Mutamiswa, R.; Mbande, A.; Nyamukondiwa, C.; Chidawanyika, F. Thermal adaptation in Lepidoptera under shifting environments: Mechanisms, patterns, and consequences. Phytoparasitica 2023, 51, 929–955. [Google Scholar] [CrossRef]
- Dabrowski, K.; Panicz, R.; Fisher, K.J.; Gomelsky, B.; Eljasik, P. Inherited anoxia tolerance and growth performance can result in enhanced invasiveness in hybrid fish. Biol. Open 2024, 13, bio060342. [Google Scholar] [CrossRef] [PubMed]
- Reid, C.H.; Patrick, P.H.; Rytwinski, T.; Taylor, J.J.; Willmore, W.G.; Reesor, B.; Cooke, S.J. An updated review of cold shock and cold stress in fish. J. Fish Biol. 2022, 100, 1102–1137. [Google Scholar] [CrossRef] [PubMed]
- Rezvi, H.U.A.; Tahjib-Ul-Arif, M.; Azim, M.A.; Tumpa, T.A.; Tipu, M.M.H.; Najnine, F.; Dawood, M.F.A.; Skalicky, M.; Brestič, M. Rice and food security: Climate change implications and the future prospects for nutritional security. Food Energy Secur. 2023, 12, e430. [Google Scholar] [CrossRef]
- Armobin, K.; Ahmadifar, E.; Adineh, H.; Samani, M.N.; Kalhor, N.; Yilmaz, S.; Hoseinifar, S.H.; Van Doan, H. Quercetin Application for Common Carp (Cyprinus carpio): I. Effects on Growth Performance, Humoral Immunity, Antioxidant Status, Immune-Related Genes, and Resistance against Heat Stress. Aquac. Nutr. 2023, 2023, 1168262. [Google Scholar] [CrossRef]
- Shahjahan, M.; Zahangir, M.; Islam, S.; Ashaf-Ud-Doulah, M.; Ando, H. Higher acclimation temperature affects growth of rohu (Labeorohita) through suppression of GH and IGFs genes expression actuating stress response. J. Therm. Biol. 2021, 100, 103032. [Google Scholar] [CrossRef]
- Jiang, X.; Dong, S.; Liu, R.; Huang, M.; Dong, K.; Ge, J.; Gao, Q.; Zhou, Y. Effects of temperature, dissolved oxygen, and their interaction on the growth performance and condition of rainbow trout (Oncorhynchus mykiss). J. Therm. Biol. 2021, 98, 102928. [Google Scholar] [CrossRef]
- Abdollahpour, H.; Falahatkar, B.; Jafari, N.; Lawrence, C. Effect of stress severity on zebrafish (Danio rerio) growth, gonadal development and reproductive performance: Do females and males respond differently? Aquaculture 2020, 522, 735099. [Google Scholar] [CrossRef]
- Jeyachandran, S.; Chellapandian, H.; Park, K.; Kwak, I.-S. A Review on the Involvement of Heat Shock Proteins (Extrinsic Chaperones) in Response to Stress Conditions in Aquatic Organisms. Antioxidants 2023, 12, 1444. [Google Scholar] [CrossRef]
- Jing, Z.; Chen, Q.; Yan, C.; Zhang, C.; Xu, Z.; Huang, X.; Wu, J.; Li, Y.; Yang, S. Effects of Chronic Heat Stress on Kidney Damage, Apoptosis, Inflammation, and Heat Shock Proteins of Siberian Sturgeon (Acipenser baerii). Animals 2023, 13, 3733. [Google Scholar] [CrossRef]
- Sammad, A.; Wang, Y.J.; Umer, S.; Lirong, H.; Khan, I.; Khan, A.; Ahmad, B.; Wang, Y. Nutritional Physiology and Biochemistry of Dairy Cattle under the Influence of Heat Stress: Consequences and Opportunities. Animals 2020, 10, 793. [Google Scholar] [CrossRef] [PubMed]
- Volkoff, H.; Rønnestad, I. Effects of temperature on feeding and digestive processes in fish. Temperature 2020, 7, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, M.; Spriet, L.L. Skeletal muscle energy metabolism during exercise. Nat. Metab. 2020, 2, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Guo, H.; Chen, Z.; Jiang, Y.; Shen, J.; Pang, X.; Li, Y. Effects of acclimation temperature regime on the thermal tolerance, growth performance and gene expression of a cold-water fish, Schizothorax prenanti. J. Therm. Biol. 2021, 98, 102918. [Google Scholar] [CrossRef]
- Elshafey, A.E.; Khalafalla, M.M.; Zaid, A.A.A.; Mohamed, R.A.; Abdel-Rahim, M.M. Source diversity of Artemia enrichment boosts goldfish (Carassius auratus) performance, β-carotene content, pigmentation, immune-physiological and transcriptomic responses. Sci. Rep. 2023, 13, 21801. [Google Scholar] [CrossRef]
- Wang, Y.; Han, G.; Pham, C.; Koyanagi, K.; Song, Y.; Sudo, R.; Lauwereyns, J.; Cockrem, J.; Furuse, M.; Chowdhury, V. An acute increase in water temperature can increase free amino acid concentrations in the blood, brain, liver, and muscle in goldfish (Carassius auratus). Fish Physiol. Biochem. 2019, 45, 1343–1354. [Google Scholar] [CrossRef]
- Pelusio, N.; Scicchitano, D.; Parma, L.; Dondi, F.; Brini, E.; D’Amico, F.; Candela, M.; Yúfera, M.; Gilannejad, N.; Moyano, F.; et al. Interaction Between Dietary Lipid Level and Seasonal Temperature Changes in Gilthead Sea Bream Sparus aurata: Effects on Growth, Fat Deposition, Plasma Biochemistry, Digestive Enzyme Activity, and Gut Bacterial Community. Front. Mar. Sci. 2021, 8, 664701. [Google Scholar] [CrossRef]
- Zhao, H.; Ke, H.; Zhang, L.; Zhao, Z.; Lai, J.; Zhou, J.; Huang, Z.; Li, H.; Du, J.; Li, Q. Integrated analysis about the effects of heat stress on physiological responses and energy metabolism in Gymnocypris chilianensis. Sci. Total Environ. 2022, 806, 151252. [Google Scholar] [CrossRef]
- Knapp, B.D.; Huang, K.C. The Effects of Temperature on Cellular Physiology. Annu. Rev. Biophys. 2022, 51, 499–526. [Google Scholar] [CrossRef]
- Ge, J.; Huang, M.; Zhou, Y.; Liu, C.; Han, C.; Gao, Q.; Dong, Y.; Dong, S. Effects of different temperatures on seawater acclimation in rainbow trout Oncorhynchus mykiss: Osmoregulation and branchial phospholipid fatty acid composition. J. Comp. Physiol. B 2021, 191, 669–679. [Google Scholar] [CrossRef]


| Gene | Gene Bank Code | Sequence (5′-3′) | Annealing Temperature (°C) | Efficiency (%) | Product Length | Refs. |
|---|---|---|---|---|---|---|
| HSP70 | XM_042771327.1 | F: GTGTCCATCCTGACCATTGAAGA | 60 | 97.97 | 79 | [32] |
| R: CTGACTGATGTCCTTCTTGTGCTTC | ||||||
| HSP90 | XM_042778180.1 | F: TCCGTGGTGTGGACTCTG | 60 | 97.89 | 71 | [33] |
| R: TCCAGGCACTTCTTGACGATGTTC | ||||||
| BCL-2 | XM_042759195.1 | F: GGCGTAAGGGATAGGTCAACA | 62 | 98.01 | 151 | [34] |
| R: GGTCCCGAGCAGTTCAGAAA | ||||||
| SOD | XM_042761153.1 | F: GATGGCAGCCTTGGAAGTGAC | 60 | 97.91 | 89 | [35] |
| R: TCAGAACAATCAGGAAGGAGGAA | ||||||
| FASN | KY378913.1 | F: TGTATGCCACCGCTTATTATTCC | 60 | 97.9 | 146 | [36] |
| R: TCCTTTGCCCTGAGTGTTGA | ||||||
| MYOD | XM_019068329.2 | F: ATGGAGTTGTCGGATATTCCCTTC | 61 | 97.88 | 100 | [37] |
| R: GCGGTCAGCGTTGGTTGTT | ||||||
| β-actin | XM_019089433.2 | F: CCGTAAGGACCTGTATGCCAAC | 61 | 98.12 | 78 | [32] |
| R: GACAGAGTATTTACGCTCAGGTGG |
| Parameter | Treatment | p-Value | |||
|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | ||
| IBW (g) | 0.3100 ± 0.006 | 0.3033 ± 0.009 | 0.3000 ± 0.000 | 0.3033 ± 0.007 | 0.7227 |
| FBW (g) | 29.61 ± 0.504 a | 28.69 ± 0.192 a | 26.17 ± 0.279 b | 24.60 ± 0.867 b | 0.0005 |
| WG (g) | 29.30 ± 0.506 a | 28.38 ± 0.183 a | 25.87 ± 0.279 b | 24.29 ± 0.867 b | 0.0005 |
| DWG (g) | 0.586 ± 0.010 a | 0.568 ± 0.004 a | 0.517 ± 0.006 b | 0.486 ± 0.017 b | 0.0005 |
| WG (%) | 9461 ± 271.5 a | 9369 ± 213.2 a | 8622 ± 92.90 ab | 8016 ± 325.9 b | 0.0089 |
| SGR (%/day) | 9.119 ± 0.056 a | 9.100 ± 0.045 a | 8.937 ± 0.021 ab | 8.790 ± 0.082 b | 0.0095 |
| GMW (g) | 3.029 ± 0.034 a | 2.949 ± 0.052 ab | 2.802 ± 0.015 bc | 2.730 ± 0.058 c | 0.0046 |
| TGC (g/°C⋅Day) | 1.859 ± 0.015 a | 1.707 ± 0.001 b | 1.533 ± 0.007 c | 1.597 ± 0.025 c | 0.0001 |
| IBL (cm) | 0.110 ± 0.006 | 0.108 ± 0.006 | 0.117 ± 0.033 | 0.110 ± 0.044 | 0.9960 |
| FBL (cm) | 10.95 ± 0.432 a | 9.163 ± 0.120 b | 8.800 ± 0.083 bc | 8.050 ± 0.119 c | 0.0001 |
| LG (cm) | 10.84 ± 0.427 a | 9.056 ± 0.119 b | 8.683 ± 0.104 bc | 7.940 ± 0.090 c | 0.0001 |
| K | 2.289 ± 0.236 c | 3.735 ± 0.129 b | 3.843 ± 0.110 b | 4.713 ± 0.058 a | 0.0001 |
| Parameter | Treatment | p-Value | |||
|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | ||
| IBW (g) | 29.61 ± 0.504 a | 28.69 ± 0.192 a | 26.17 ± 0.279 b | 24.60 ± 0.867 b | 0.0005 |
| FBW(g) | 75.43 ± 2.875 c | 83.46 ± 0.922 bc | 91.80 ± 2.530 b | 103.3 ± 2.053 a | 0.0001 |
| WG(g) | 45.82 ± 2.421 d | 54.78 ± 0.777 c | 65.63 ± 2.253 b | 78.67 ± 1.225 a | 0.0001 |
| DWG (g) | 0.764 ± 0.040 d | 0.913 ± 0.013 c | 1.094 ± 0.038 b | 1.311 ± 0.020 a | 0.0001 |
| WG (%) | 154.6 ± 5.938 d | 190.9 ± 2.038 c | 250.7 ± 6.023 b | 320.3 ± 7.075 a | 0.0001 |
| SGR(%/day) | 1.556 ± 0.038 d | 1.780 ± 0.012 c | 2.091 ± 0.029 b | 2.393 ± 0.028 a | 0.0001 |
| GMW(g) | 47.26 ± 1.278 | 48.93 ± 0.413 | 49.01 ± 0.937 | 50.39 ± 1.381 | 0.3025 |
| TGC (g/°C⋅Day) | 0.628 ± 0.021 c | 0.727 ± 0.006 c | 0.856 ± 0.017 b | 0.991 ± 0.005 a | 0.0005 |
| IBL (cm) | 10.95 ± 0.432 a | 9.163 ± 0.120 b | 8.800 ± 0.083 bc | 8.050 ± 0.119 c | 0.0001 |
| FBL (cm) | 18.40 ± 0.557 b | 20.29 ± 0.300 ab | 20.87 ± 0.384 a | 22.29 ± 0.643 a | 0.0035 |
| LG (cm) | 7.447 ± 0.494 c | 11.12 ± 0.241 b | 12.07 ± 0.367 b | 14.24 ± 0.575 a | 0.0001 |
| K | 1.217 ± 0.076 | 1.003 ± 0.051 | 1.011 ± 0.030 | 0.9403 ± 0.073 | 0.0526 |
| Parameters | Treatments | p-Value | |||
|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | ||
| Moisture% | 73.59 ± 0.377 a | 73.42 ± 0.207 a | 72.36 ± 0.176 ab | 71.14 ± 0.609 b | 0.0067 |
| protein (%DM) | 71.63 ± 1.335 b | 74.27 ± 0.997 b | 78.64 ± 0.678 a | 81.72 ± 0.532 a | 0.0003 |
| Fat (%DM) | 13.53 ± 0.692 a | 13.04 ± 0.243 a | 10.70 ± 0.350 b | 10.47 ± 0.287 b | 0.0017 |
| Ash (%DM) | 6.196 ± 0.084 b | 7.235 ± 0.072 a | 7.493 ± 0.107 a | 7.221 ± 0.106 a | 0.0001 |
| Carbohydrate (%DM) | 7.668 ± 2.530 | 5.558 ± 1.518 | 2.176 ± 0.521 | 1.789 ± 0.254 | 0.0694 |
| Gross energy (kcal−1⋅100 g−1) | 123.4 ± 1.276 d | 130.8 ± 0.546 c | 142.9 ± 1.552 b | 148.6 ± 0.579 a | 0.0001 |
| Amino Acid (g/100 g) | Treatments | p-Value | |||
|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | ||
| Essential | |||||
| Threonine | 3.237 ± 0.013 | 3.322 ± 0.027 | 3.331 ± 0.011 | 3.337 ± 0.045 | 0.1011 |
| Valine | 3.211 ± 0.007 b | 3.260 ± 0.037 b | 3.291 ± 0.026 b | 3.517 ± 0.028 a | 0.0002 |
| Methionine | 2.038 ± 0.010 b | 2.060 ± 0.007 ab | 2.106 ± 0.021 ab | 2.125 ± 0.021 a | 0.0162 |
| Isoleucine | 3.062 ± 0.052 b | 3.071 ± 0.023 b | 3.096 ± 0.041 b | 3.345 ± 0.038 a | 0.0028 |
| Leucine | 5.547 ± 0.050 b | 5.622 ± 0.023 b | 5.628 ± 0.039 b | 5.807 ± 0.031 a | 0.0067 |
| Phenylalanine | 3.404 ± 0.048 b | 3.481 ± 0.067 ab | 3.442 ± 0.020 b | 3.653 ± 0.029 a | 0.0190 |
| Lysine | 6.900 ± 0.030 bc | 6.995 ± 0.018 ac | 6.849 ± 0.021 b | 7.082 ± 0.015 a | 0.0003 |
| Histidine | 2.639 ± 0.041 c | 2.800 ± 0.027 b | 2.792 ± 0.022 b | 2.948 ± 0.015 a | 0.0004 |
| Arginine | 4.955 ± 0.073 b | 5.088 ± 0.030 b | 4.994 ± 0.068 b | 5.496 ± 0.061 a | 0.0008 |
| Total essential amino acid | 34.991 ± 0.209 c | 35.699 ± 0.118 b | 35.528 ± 0.147 bc | 37.311 ± 0.036 a | 0.0001 |
| Non-essential | |||||
| Aspartic acid | 7.539 ± 0.043 c | 7.722 ± 0.030 b | 7.729 ± 0.028 b | 7.989 ± 0.039 a | 0.0002 |
| Serine | 2.942 ± 0.003 ab | 3.032 ± 0.019 ab | 3.032 ± 0.003 ab | 2.914 ± 0.080 a | 0.1659 |
| Glutamic acid | 11.136 ± 0.035 c | 11.453 ± 0.034 b | 11.528 ± 0.016 b | 11.714 ± 0.071 a | 0.0001 |
| Glycine | 3.888 ± 0.013 b | 3. 853 ± 0.031 b | 4.064 ± 0.058 ab | 4.163 ± 0.069 a | 0.0056 |
| Alanine | 4.358 ± 0.012 c | 4.432 ± 0.030 bc | 4.563 ± 0.008 ab | 4.637 ± 0.053 a | 0.0009 |
| Tyrosine | 3.264 ± 0.064 | 3.304 ± 0.041 | 3.237 ± 0.066 | 3.388 ± 0.033 | 0.2808 |
| Proline | 2.382 ± 0.043 b | 2.626 ± 0.048 b | 2.655 ± 0.031 b | 2.658 ± 0.029 a | 0.0025 |
| Cysteine | 0.443 ± 0.012 c | 0.440 ± 0.008 c | 0.398 ± 0.006 b | 0.502 ± 0.007 a | 0.0002 |
| Total non-essential amino acid | 35.953 ± 0.195 c | 36.676 ± 0.253 bc | 37.207 ± 0.065 ab | 37.965 ± 0.270 a | 0.0009 |
| Total amino acid | 70.94 ± 0.390 c | 72.38 ± 0.370 bc | 72.74 ± 0.210 b | 75.28 ± 0.304 a | 0.0001 |
| Fatty Acid (%) | Treatment | p-Value | |||
|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | ||
| C14:0 | 0.822 ± 0.011 b | 0.896 ± 0.004 a | 0.839 ± 0.003 b | 0.673 ± 0.003 c | 0.0001 |
| C15:0 | 0.161 ± 0.006 b | 0.164 ± 0.001 b | 0.185 ± 0.001 a | 0.152 ± 0.001 b | 0.0003 |
| C15:1n5 | 0.0157 ± 0.0004 b | 0.0155 ± 0.0003 b | 0.021 ± 0.0001 a | 0.0148 ± 0.0005 b | 0.0001 |
| C16:0 | 14.548 ± 0.607 b | 15.106 ± 0.020 b | 15.933 ± 0.331 ab | 17.129 ± 0.142 a | 0.0043 |
| C16:1n7 | 8.812 ± 0.420 a | 8.037 ± 0.080 a | 6.283 ± 0.288 b | 4.888 ± 0.140 c | 0.0001 |
| C17:0 | 0.325 ± 0.005 ab | 0.303 ± 0.003 b | 0.345 ± 0.012 a | 0.188 ± 0.007 c | 0.0001 |
| C17:1n7 | 0.310 ± 0.005 c | 0. 302 ± 0. 002 c | 0.298 ± 0.013 bc | 0.268 ± 0.002 ab | 0.0171 |
| C18:0 | 5.856 ± 0.008 b | 5.834 ± 0.014 b | 6.694 ± 0.015 a | 6.749 ± 0.019 a | 0.0001 |
| C18:1n9c | 38.042 ± 0.072 a | 37.811 ± 0.100 a | 36.370 ± 0.085 b | 37.781 ± 0.116 a | 0.0001 |
| C18:2n6c | 22.780 ± 0.148 a | 22.561 ± 0.189 a | 22.733 ± 0.083 a | 21.481 ± 0.095 b | 0.0004 |
| C20:0 | 0.255 ± 0.003 ab | 0.223 ± 0.010 b | 0.271 ± 0.012 a | 0.233 ± 0.003 b | 0.0126 |
| C18:3n6 | 0.263 ± 0.004 d | 0.324 ± 0.008 c | 0.612 ± 0.012 a | 0.494 ± 0.014 b | 0.0001 |
| C20:1 | 1.673 ± 0.012 b | 1.658 ± 0.022 b | 1.827 ± 0.023 a | 1.801 ± 0.014 a | 0.0003 |
| C18:3n3 | 0.576 ± 0.022 c | 0.662 ± 0.008 c | 0.836 ± 0.011 b | 0.964 ± 0.045 a | 0.0001 |
| C20:2 | 0.634 ± 0.005 c | 0.642 ± 0.007 c | 0.733 ± 0.006 a | 0.702 ± 0.005 b | 0.0001 |
| C22:0 | 0.206 ± 0.001 c | 0.213 ± 0.002 bc | 0.240 ± 0.003 a | 0.225 ± 0.005 b | 0.0002 |
| C20:3n6 | 1.210 ± 0.006 d | 1.364 ± 0.007 c | 1.465 ± 0.003 b | 1.553 ± 0.017 a | 0.0001 |
| C22:1n9 | 0.070 ± 0.001 b | 0.073 ± 0.001 bc | 0.084 ± 0.001 a | 0.079 ± 0.002 ac | 0.0002 |
| C20:3n3 | 0.126 ± 0.006 b | 0.134 ± 0.002 bc | 0.159 ± 0.002 a | 0.146 ± 0.005 ac | 0.0021 |
| C20:4n6 | 1.032 ± 0.008 c | 1.067 ± 0.006 c | 1.242 ± 0.006 b | 1.355 ± 0.029 a | 0.0001 |
| C23:0 | 0.049 ± 0.002 bc | 0.044 ± 0.00 c | 0.055 ± 0.001 ab | 0.062 ± 0.003 a | 0.0003 |
| C22:2n6 | 0.131 ± 0.004 c | 0.147 ± 0.001 c | 0.179 ± 0.002 b | 0.205 ± 0.005 a | 0.0001 |
| C20:5n3 (EPA) | 0.441 ± 0.001 c | 0.471 ± 0.002 b | 0.489 ± 0.002 a | 0.469 ± 0.006 b | 0.0001 |
| C24:1n9 | 0.140 ± 0.002 b | 0.159 ± 0.004 ab | 0.176 ± 0.001 a | 0.166 ± 0.008 a | 0.0032 |
| C22:6n3 (DHA) | 1.522 ± 0.016 d | 1.790 ± 0.022 c | 1.929 ± 0.021 b | 2.130 ± 0.051 a | 0.0001 |
| ΣSFAs | 22.238 ± 0.616 b | 22.798 ± 0.003 b | 24.583 ± 0.302 a | 25.352 ± 0.072 a | 0.0006 |
| ΣMUFAs | 51.262 ± 2.096 a | 47.738 ± 0.187 ab | 44.741 ± 0.363 b | 44.715 ± 0.206 b | 0.0075 |
| ΣPUFAs | 28.715 ± 0.130 c | 29.161 ± 0.185 bc | 30.379 ± 0.076 a | 29.499 ± 0.108 b | 0.0001 |
| Σn-3 PUFAs | 2.665 ± 0.013 d | 3.057 ± 0.013 c | 3.414 ± 0.012 b | 3.709 ± 0.011 a | 0.0001 |
| Σn-6 PUFAs | 25.416 ± 0. 141 b | 25.462 ± 0.190 b | 26.231 ± 0.088 a | 25.088 ± 0.060 b | 0.0016 |
| n-3/n-6 | 0.105 ± 0.001 d | 0.120 ± 0.001 c | 0.130 ± 0.001 b | 0.148 ± 0.0003 a | 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amuneke, K.E.; Elshafey, A.E.; Liu, Y.; Gao, J.; Amankwah, J.F.; Wen, B.; Chen, Z. Impact of Temperature Manipulations on Growth Performance, Body Composition, and Selected Genes of Koi Carp (Cyprinus carpio koi). Fishes 2025, 10, 95. https://doi.org/10.3390/fishes10030095
Amuneke KE, Elshafey AE, Liu Y, Gao J, Amankwah JF, Wen B, Chen Z. Impact of Temperature Manipulations on Growth Performance, Body Composition, and Selected Genes of Koi Carp (Cyprinus carpio koi). Fishes. 2025; 10(3):95. https://doi.org/10.3390/fishes10030095
Chicago/Turabian StyleAmuneke, Kennedy Emeka, Ahmed E. Elshafey, Yuanhao Liu, Jianzhong Gao, Justice Frimpong Amankwah, Bin Wen, and Zaizhong Chen. 2025. "Impact of Temperature Manipulations on Growth Performance, Body Composition, and Selected Genes of Koi Carp (Cyprinus carpio koi)" Fishes 10, no. 3: 95. https://doi.org/10.3390/fishes10030095
APA StyleAmuneke, K. E., Elshafey, A. E., Liu, Y., Gao, J., Amankwah, J. F., Wen, B., & Chen, Z. (2025). Impact of Temperature Manipulations on Growth Performance, Body Composition, and Selected Genes of Koi Carp (Cyprinus carpio koi). Fishes, 10(3), 95. https://doi.org/10.3390/fishes10030095







