Integration of Gill and Intestinal Osmoregulatory Functions to Assess the Smoltification Window in Atlantic Salmon
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
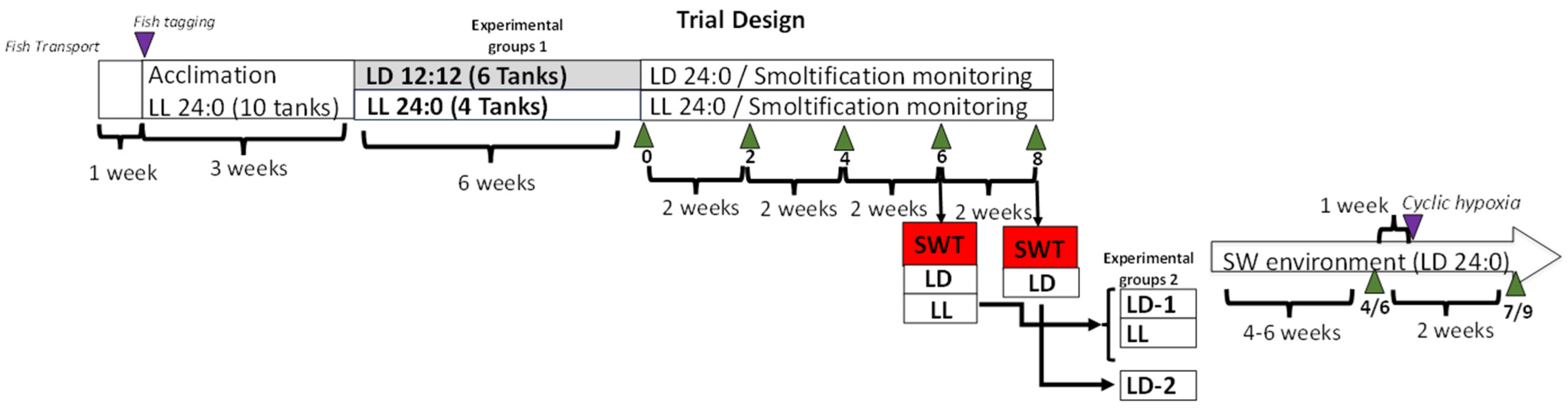
2.2. Experimental Setup
2.3. Sampling
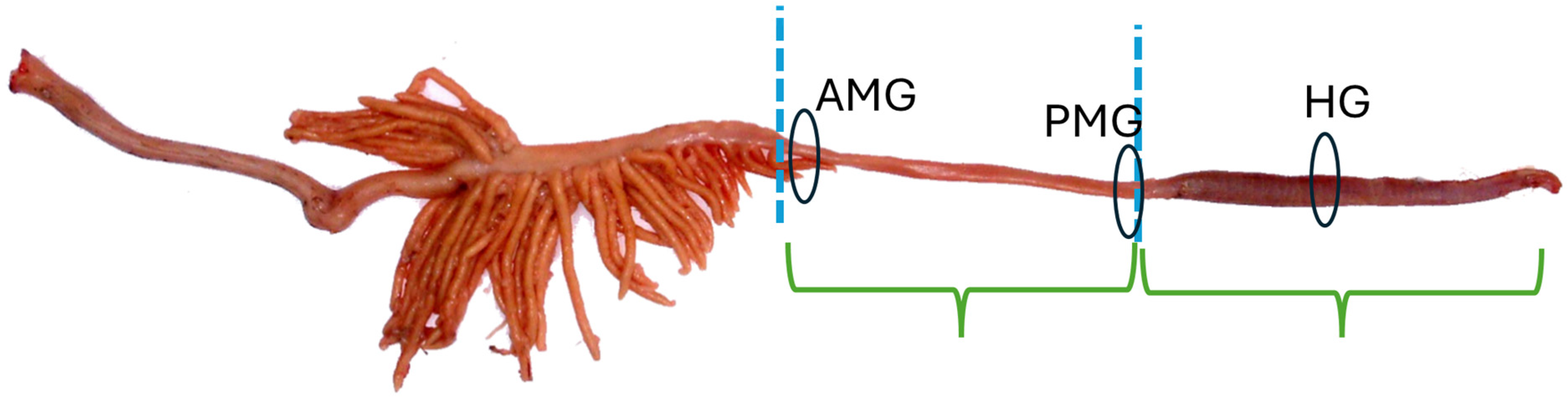
2.4. Morphometrics
2.5. Plasma Analysis
2.6. Gene Expression Analysis
2.7. Na+, K+- ATPase Activity in Gills and Intestine
2.8. Statistical Analysis
3. Results
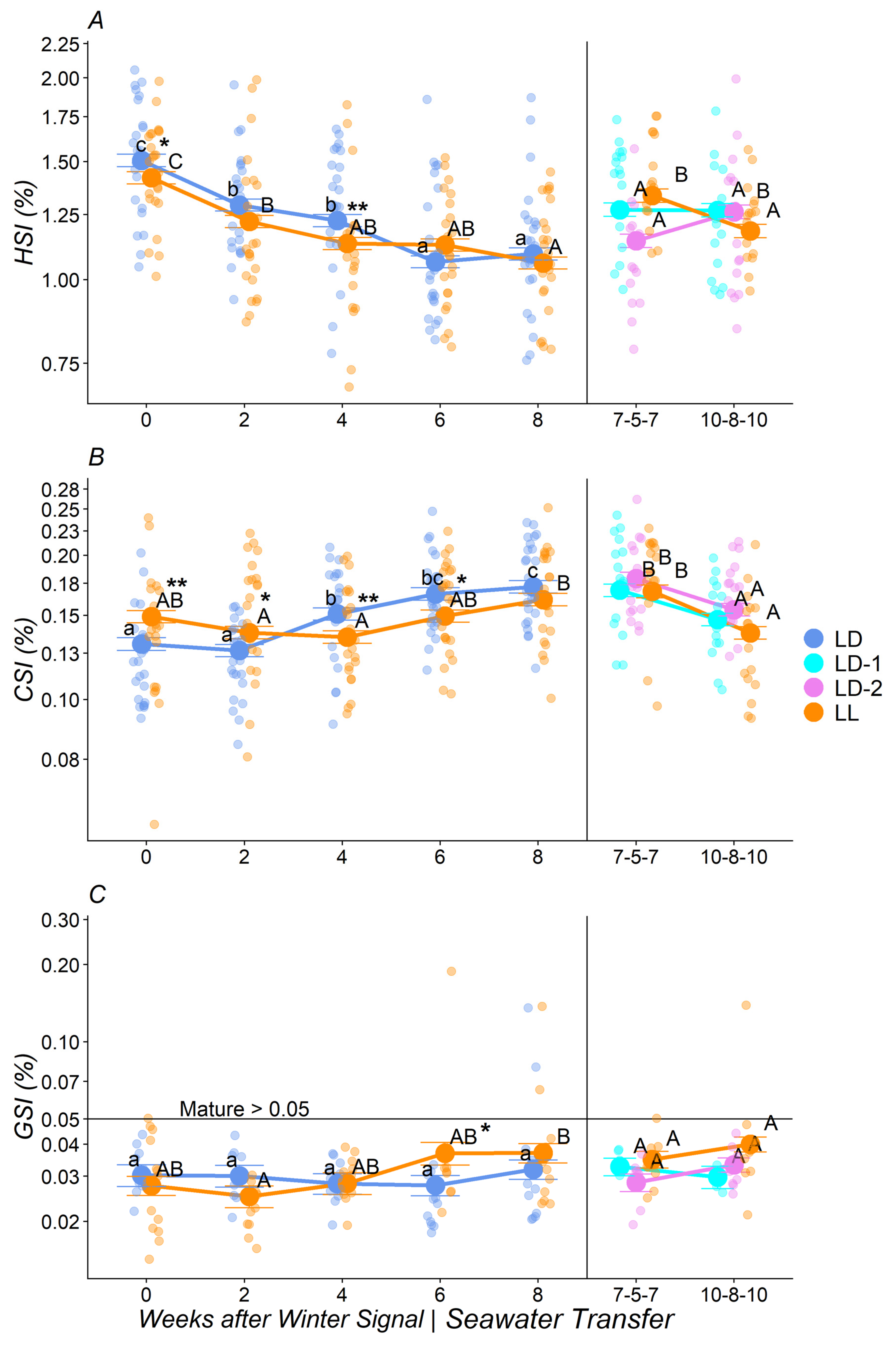
3.1. Morphometrics and Somatic Indexes
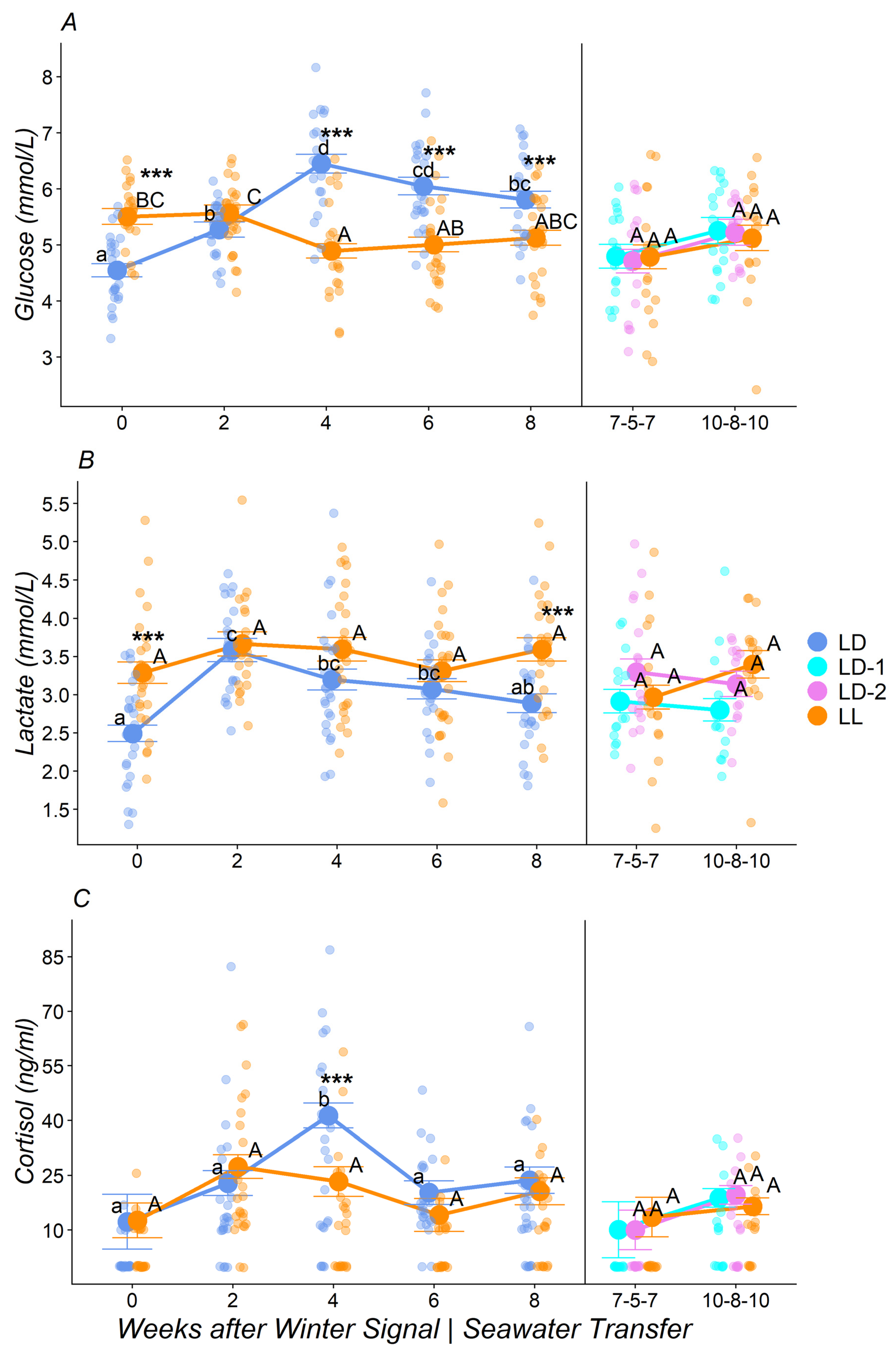
3.2. Plasma Metabolites
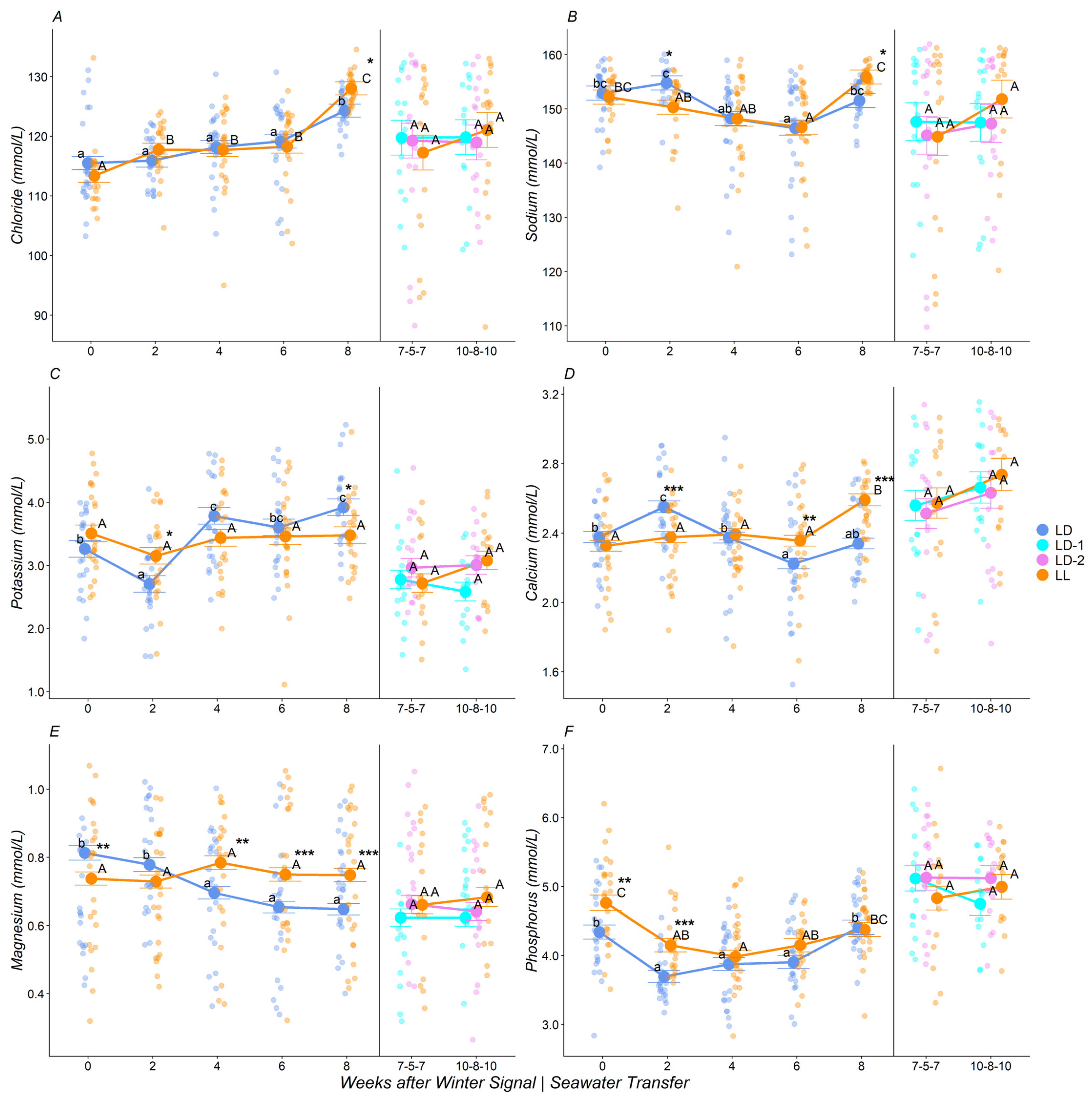
3.3. Plasma Ions
3.4. NKA Activity
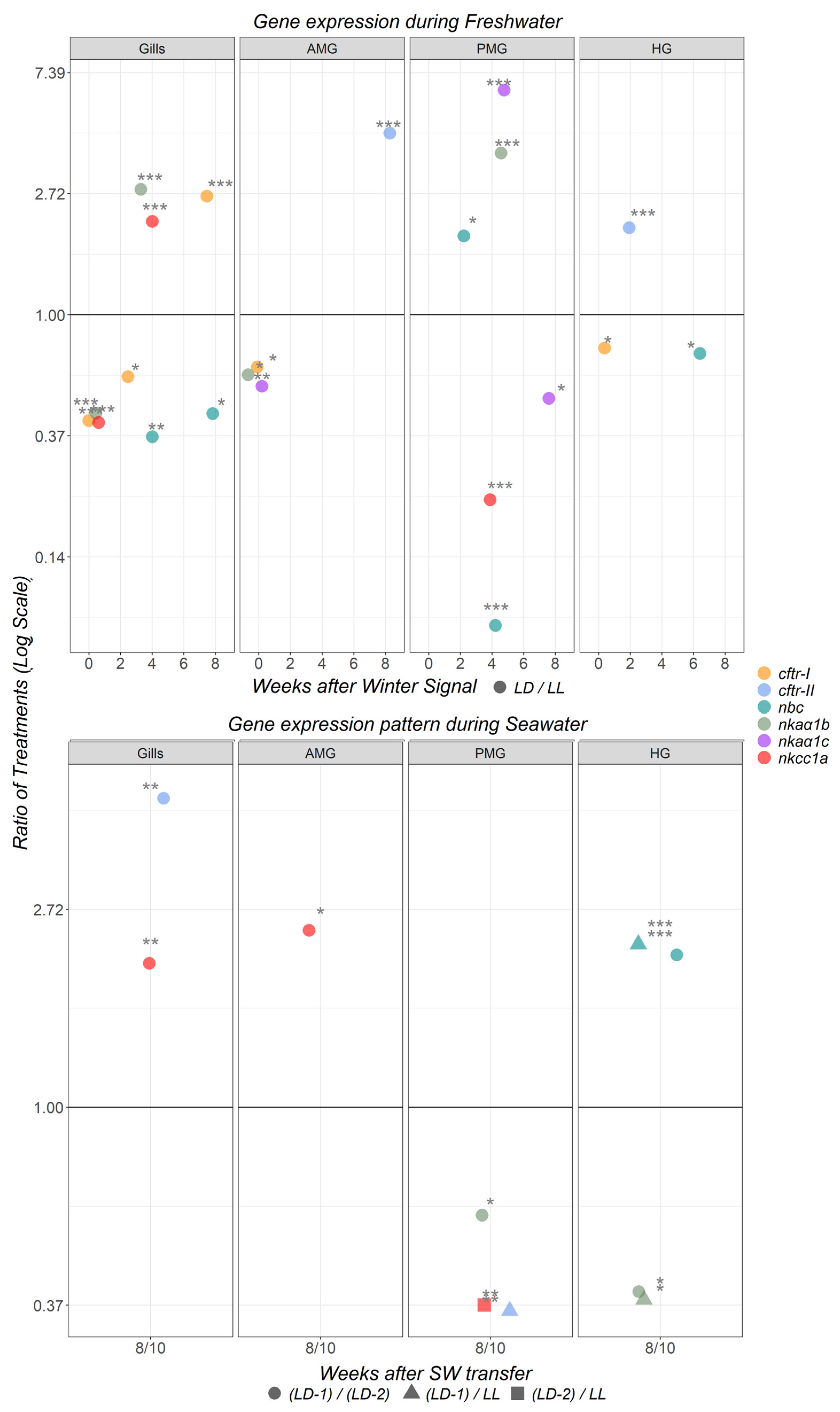
3.5. Gene Expression
4. Discussion
4.1. Atlantic Salmon Growth and Performance
4.2. Osmoregulatory Physiology: Plasma Ions and NKA Activity
4.3. Molecular Markers of Osmoregulation in Gills and Intestine
4.4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCormick, S.D.; Hansen, L.P.; Quinn, T.P.; Saunders, R.L. Movement, Migration, and Smolting of Atlantic Salmon (Salmo salar). Can. J. Fish. Aquat. Sci. 1998, 55, 77–92. [Google Scholar] [CrossRef]
- Björnsson, B.T.; Stefansson, S.O.; McCormick, S.D. Environmental Endocrinology of Salmon Smoltification. Gen. Comp. Endocrinol. 2011, 170, 290–298. [Google Scholar] [CrossRef]
- Hoar, W.S. 4 The Physiology of Smolting Salmonids. Fish Physiol. 1988, 11, 275–343. [Google Scholar] [CrossRef]
- Boeuf, G. Salmonid Smolting: A Pre-Adaptation to the Oceanic Environment. Fish Ecophysiol. 1993, 105–135. [Google Scholar] [CrossRef]
- McCormick, S.D. Smolt Physiology and Endocrinology. In Fish Physiology; Academic Press: Cambridge, MA, USA, 2012; Volume 32, pp. 199–251. [Google Scholar]
- Striberny, A.; Lauritzen, D.E.; Fuentes, J.; Campinho, M.A.; Gaetano, P.; Duarte, V.; Hazlerigg, D.G.; Jørgensen, E.H. More than One Way to Smoltify a Salmon? Effects of Dietary and Light Treatment on Smolt Development and Seawater Growth Performance in Atlantic Salmon. Aquaculture 2021, 532, 736044. [Google Scholar] [CrossRef]
- Ytrestøyl, T.; Hjelle, E.; Kolarevic, J.; Takle, H.; Rebl, A.; Afanasyev, S.; Krasnov, A.; Brunsvik, P.; Terjesen, B.F. Photoperiod in Recirculation Aquaculture Systems and Timing of Seawater Transfer Affect Seawater Growth Performance of Atlantic Salmon (Salmo salar). J. World Aquac. Soc. 2023, 54, 73–95. [Google Scholar] [CrossRef]
- Singer, T.D.; Finstad, B.; McCormick, S.D.; Wiseman, S.B.; Schulte, P.M.; McKinley, R.S. Interactive Effects of Cortisol Treatment and Ambient Seawater Challenge on Gill Na+,K+-ATPase and CFTR Expression in Two Strains of Atlantic Salmon Smolts. Aquaculture 2003, 222, 15–28. [Google Scholar] [CrossRef]
- Kiilerich, P.; Kristiansen, K.; Madsen, S.S. Cortisol Regulation of Ion Transporter MRNA in Atlantic Salmon Gill and the Effect of Salinity on the Signaling Pathway. J. Endocrinol. 2007, 194, 417–427. [Google Scholar] [CrossRef]
- Madsen, S.S. Cortisol Treatment Improves the Development of Hypoosmoregulatory Mechanisms in the Euryhaline Rainbow Trout, Salmo gairdneri. Fish Physiol. Biochem. 1990, 8, 45–52. [Google Scholar] [CrossRef]
- Madsen, S.S.; Kiilerich, P.; Tipsmark, C.K. Multiplicity of Expression of Na+,K+-ATPase α-Subunit Isoforms in the Gill of Atlantic Salmon (Salmo salar): Cellular Localisation and Absolute Quantification in Response to Salinity Change. J. Exp. Biol. 2009, 212, 78–88. [Google Scholar] [CrossRef]
- McCormick, S.D.; Dickhoff, W.W.; Duston, J.; Nishioka, R.S.; Bern, H.A. Developmental Differences in the Responsiveness of Gill Na+, K+-ATPase to Cortisol in Salmonids. Gen. Comp. Endocrinol. 1991, 84, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, T.O.; Ebbesson, L.O.E.; Kiilerich, P.; Björnsson, B.T.; Madsen, S.S.; McCormick, S.D.; Stefansson, S.O. Endocrine Systems in Juvenile Anadromous and Landlocked Atlantic Salmon (Salmo salar): Seasonal Development and Seawater Acclimation. Gen. Comp. Endocrinol. 2008, 155, 762–772. [Google Scholar] [CrossRef]
- Nilsen, T.O.; Ebbesson, L.O.E.; Madsen, S.S.; McCormick, S.D.; Andersson, E.; Björnsson, B.T.; Prunet, P.; Stefansson, S.O. Differential Expression of Gill Na+,K+-ATPase α- and β-Subunits, Na+,K+,2Cl− Cotransporter and CFTR Anion Channel in Juvenile Anadromous and Landlocked Atlantic Salmon Salmo salar. J. Exp. Biol. 2007, 210, 2885–2896. [Google Scholar] [CrossRef]
- Sundh, H.; Nilsen, T.O.; Lindström, J.; Hasselberg-Frank, L.; Stefansson, S.O.; Mccormick, S.D.; Sundell, K. Development of Intestinal Ion-Transporting Mechanisms during Smoltification and Seawater Acclimation in Atlantic Salmon Salmo salar. J. Fish Biol. 2014, 85, 1227–1252. [Google Scholar] [CrossRef]
- Esbaugh, A.J.; Kristensen, T.; Takle, H.; Grosell, M. The Effects of Sustained Aerobic Swimming on Osmoregulatory Pathways in Atlantic Salmon Salmo salar Smolts. J. Fish Biol. 2014, 85, 1355–1368. [Google Scholar] [CrossRef] [PubMed]
- Pino Martinez, E.; Imsland, A.K.D.; Hosfeld, A.C.D.; Handeland, S.O. Effect of Photoperiod and Transfer Time on Atlantic Salmon Smolt Quality and Growth in Freshwater and Seawater Aquaculture Systems. Fishes 2023, 8, 212. [Google Scholar] [CrossRef]
- Björnsson, B.T.; Bradley, T.M. Epilogue: Past Successes, Present Misconceptions and Future Milestones in Salmon Smoltification Research. Aquaculture 2007, 273, 384–391. [Google Scholar] [CrossRef]
- McCormick, S.D.; Shrimpton, J.M.; Moriyama, S.; Björnsson, B.T. Effects of an Advanced Temperature Cycle on Smolt Development and Endocrinology Indicate That Temperature Is Not a Zeitgeber for Smolting in Atlantic Salmon. J. Exp. Biol. 2002, 205, 3553–3560. [Google Scholar] [CrossRef] [PubMed]
- Zydlewski, G.B.; Zydlewski, J. Gill Na+,K+-ATPase of Atlantic Salmon Smolts in Freshwater Is Not a Predictor of Long-Term Growth in Seawater. Aquaculture 2012, 362–363, 121–126. [Google Scholar] [CrossRef]
- Oliveira, V.H.S.; Dean, K.R.; Qviller, L.; Kirkeby, C.; Bang Jensen, B. Factors Associated with Baseline Mortality in Norwegian Atlantic Salmon Farming. Sci. Rep. 2021, 11, 14702. [Google Scholar] [CrossRef]
- Lai, F.; Rønnestad, I.; Budaev, S.; Balseiro, P.; Gelebart, V.; Pedrosa, C.; Stevnebø, A.; Haugarvoll, E.; Korsøen, Ø.J.; Tangen, K.L.; et al. Freshwater History Influences Farmed Atlantic Salmon (Salmo salar) Performance in Seawater. Aquaculture 2024, 586, 740750. [Google Scholar] [CrossRef]
- Loretz, C.A. Electrophysiology of Ion Transport in Teleost Intestinal Cells. Fish Physiol. 1995, 14, 25–56. [Google Scholar] [CrossRef]
- Veillette, P.A.; Sundell, K.; Specker, J.L. Cortisol Mediates the Increase in Intestinal Fluid Absorption in Atlantic Salmon during Parr-Smolt Transformation. Gen. Comp. Endocrinol. 1995, 97, 250–258. [Google Scholar] [CrossRef]
- Sundell, K.; Jutfelt, F.; Ágústsson, T.; Olsen, R.E.; Sandblom, E.; Hansen, T.; Björnsson, B.T. Intestinal Transport Mechanisms and Plasma Cortisol Levels during Normal and Out-of-Season Parr–Smolt Transformation of Atlantic Salmon, Salmo salar. Aquaculture 2003, 222, 265–285. [Google Scholar] [CrossRef]
- Sundell, K.S.; Sundh, H. Intestinal Fluid Absorption in Anadromous Salmonids: Importance of Tight Junctions and Aquaporins. Front. Physiol. 2012, 3, 388. [Google Scholar] [CrossRef]
- Bjørgen, H.; Li, Y.; Kortner, T.M.; Krogdahl, Å.; Koppang, E.O. Anatomy, Immunology, Digestive Physiology and Microbiota of the Salmonid Intestine: Knowns and Unknowns under the Impact of an Expanding Industrialized Production. Fish Shellfish Immunol. 2020, 107, 172–186. [Google Scholar] [CrossRef] [PubMed]
- Collie, N.L.; Ferraris, R.P. Nutrient Fluxes and Regulation in Fish Intestine. Biochem. Mol. Biol. Fishes 1995, 4, 221–239. [Google Scholar] [CrossRef]
- Takei, Y.; Hiroi, J.; Takahashi, H.; Sakamoto, T. Diverse Mechanisms for Body Fluid Regulation in Teleost Fishes. Am. J. Physiol.—Regul. Integr. Comp. Physiol. 2014, 307, R778–R792. [Google Scholar] [CrossRef]
- Fuentes, J.; Eddy, F.B. Drinking in Marine, Euryhaline and Freshwater Teleost Fish. In Ionic Regulation in Animals: A Tribute to Professor W.T.W.Potts; Springer: Berlin/Heidelberg, Germany, 1997; pp. 135–149. ISBN 978-3-642-60415-7. [Google Scholar]
- Takei, Y. The Digestive Tract as an Essential Organ for Water Acquisition in Marine Teleosts: Lessons from Euryhaline Eels. Zool. Lett. 2021, 7, 10. [Google Scholar] [CrossRef]
- Grosell, M. Intestinal Transport. In The Physiology of Fishes; Evans, D.H., Claiborne, J.B., Currie, S., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 175–203. [Google Scholar]
- Takvam, M.; Sundell, K.; Sundh, H.; Gharbi, N.; Kryvi, H.; Nilsen, T.O. New Wine in Old Bottles: Modification of the Na+/K+-ATPase Enzyme Activity Assay and Its Application in Salmonid Aquaculture. Rev. Aquac. 2024, 16, 1087–1098. [Google Scholar] [CrossRef]
- Gharbi, K.; Ferguson, M.M.; Danzmann, R.G. Characterization of Na, K-ATPase Genes in Atlantic Salmon (Salmo salar) and Comparative Genomic Organization with Rainbow Trout (Oncorhynchus mykiss). Mol. Genet. Genomics 2005, 273, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Pino Martinez, E.; Balseiro, P.; Pedrosa, C.; Haugen, T.S.; Fleming, M.S.; Handeland, S.O. The Effect of Photoperiod Manipulation on Atlantic Salmon Growth, Smoltification and Sexual Maturation: A Case Study of a Commercial RAS. Aquac. Res. 2021, 52, 2593–2608. [Google Scholar] [CrossRef]
- Tipsmark, C.K.; Sørensen, K.J.; Hulgard, K.; Madsen, S.S. Claudin-15 and -25b Expression in the Intestinal Tract of Atlantic Salmon in Response to Seawater Acclimation, Smoltification and Hormone Treatment. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2010, 155, 361–370. [Google Scholar] [CrossRef]
- Tipsmark, C.K.; Madsen, S.S.; Seidelin, M.; Christensen, A.S.; Cutler, C.P.; Cramb, G. Dynamics of Na+,K+,2Cl− Cotransporter and Na+,K+-ATPase Expression in the Branchial Epithelium of Brown Trout (Salmo Trutta) and Atlantic Salmon (Salmo salar). J. Exp. Zool. 2002, 293, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Hiroi, J.; McCormick, S.D. New Insights into Gill Ionocyte and Ion Transporter Function in Euryhaline and Diadromous Fish. Respir. Physiol. Neurobiol. 2012, 184, 257–268. [Google Scholar] [CrossRef]
- Grosell, M. The Role of the Gastrointestinal Tract in Salt and Water Balance; Academic Press: Cambridge, MA, USA, 2010; Volume 30. [Google Scholar]
- Breves, J.P.; McKay, I.S.; Koltenyuk, V.; Nelson, N.N.; Lema, S.C.; McCormick, S.D. Na+/HCO3− Cotransporter 1 (Nbce1) Isoform Gene Expression during Smoltification and Seawater Acclimation of Atlantic Salmon. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2022, 192, 577–592. [Google Scholar] [CrossRef]
- Perry, S.F.; Furimsky, M.; Bayaa, M.; Georgalis, T.; Shahsavarani, A.; Nickerson, J.G.; Moon, T.W. Integrated Responses of Na+/HCO3− Cotransporters and V-Type H+-ATPases in the Fish Gill and Kidney during Respiratory Acidosis. Biochim. Biophys. Acta—Biomembr. 2003, 1618, 175–184. [Google Scholar] [CrossRef]
- Bernard, B.; Leguen, I.; Mandiki, S.N.M.; Cornet, V.; Redivo, B.; Kestemont, P. Impact of Temperature Shift on Gill Physiology during Smoltification of Atlantic Salmon Smolts (Salmo salar L.). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2020, 244, 110685. [Google Scholar] [CrossRef]
- McCormick, S.D.; Regish, A.M.; Christensen, A.K.; Björnsson, B.T. Differential Regulation of Sodium-Potassium Pump Isoforms during Smolt Development and Seawater Exposure of Atlantic Salmon. J. Exp. Biol. 2013, 216, 1142–1151. [Google Scholar] [CrossRef]
- Stefansson, S.O.; Björnsson, B.T.; Ebbesson, L.O.; McCormick, S.D. Smoltification. In Fish Larval Physiology; Finn, R.N., Kapoo, B.G., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 639–681. ISBN 9780429061608. [Google Scholar]
- McCormick, S.D.; Cunjak, R.A.; Dempson, B.; O’Dea, M.F.; Carey, J.B. Temperature-Related Loss of Smolt Characteristics in Atlantic Salmon (Salmo salar) in the Wild. Can. J. Fish. Aquat. Sci. 1999, 56, 1649–1667. [Google Scholar] [CrossRef]
- Handeland, S.O.; Wilkinsson, E.; Stefansson, S.O. Smolting and De-Smolting at Different Temperatures in Two Strains of Atlantic Salmon (Salmo salar L.). Inst. Fisk. Mar. Rapp. Bergen 2001, 10, 1–20. [Google Scholar]
- Zydlewski, G.B.; Haro, A.; McCormick, S.D. Evidence for Cumulative Temperature as an Initiating and Terminating Factor in Downstream Migratory Behavior of Atlantic Salmon (Salmo salar) Smolts. Can. J. Fish. Aquat. Sci. 2005, 62, 68–78. [Google Scholar] [CrossRef]
- Melo, M.C.; Andersson, E.; Fjelldal, P.G.; Bogerd, J.; França, L.R.; Taranger, G.L.; Schulz, R.W. Salinity and Photoperiod Modulate Pubertal Development in Atlantic Salmon (Salmo salar). J. Endocrinol. 2014, 220, 319–332. [Google Scholar] [CrossRef]
- Ciani, E.; von Krogh, K.; Nourizadeh-Lillabadi, R.; Mayer, I.; Fontaine, R.; Weltzien, F.A. Sexual Maturation in Atlantic Salmon Male Parr May Be Triggered Both in Late Summer and Early Spring under Standard Farming Conditions. Aquaculture 2021, 544, 737086. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A New Mathematical Model for Relative Quantification in Real-Time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Olsvik, P.A.; Lie, K.K.; Jordal, A.E.O.; Nilsen, T.O.; Hordvik, I. Evaluation of Potential Reference Genes in Real-Time RT-PCR Studies of Atlantic Salmon. BMC Mol. Biol. 2005, 6, 21. [Google Scholar] [CrossRef]
- Gaetano, P. Intestinal Physiology in Atlantic Salmonpreparatory Changes during Smolting and After Seawater Entry. Doctoral Dissertation, University of Cadiz, Cádiz, Spain, 2024. [Google Scholar]
- McCormick, S.D. Methods for Nonlethal Gill Biopsy and Measurement of Na+,K+-ATPase Activity. Can. J. Fish. Aquat. Sci. 1993, 50, 656–658. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Posit Team. RStudio: Integrated Development Environment for R; Posit Team: Boston, MA, USA, 2023. [Google Scholar]
- Delignette-Muller, M.L.; Dutang, C. Fitdistrplus: An R Package for Fitting Distributions. J. Stat. Softw. 2015, 64, 1–34. [Google Scholar] [CrossRef]
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Mächler, M.; Bolker, B.M. GlmmTMB Balances Speed and Flexibility among Packages for Zero-Inflated Generalized Linear Mixed Modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- Dunn, P.K.; Smyth, G.K. Randomized Quantile Residuals. J. Comput. Graph. Stat. 1996, 5. [Google Scholar] [CrossRef]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models, R Package Version 0.4.6; 2022. Available online: http://florianhartig.github.io/DHARMa (accessed on 6 March 2025).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression: Appendices, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019; ISBN 141297514X. [Google Scholar]
- Lenth, R.V. Emmeans: Estimated Marginal Means, Aka Least-Squares Means, R Package Version 1.8.4-1; 2023. Available online: https://rvlenth.github.io/emmeans/ (accessed on 6 March 2025).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; Volume 35. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed]
- Lock, E.J.; Ornsrud, R.; Aksnes, L.; Spanings, F.A.T.; Waagbø, R.; Flik, G. The Vitamin D Receptor and Its Ligand 1α,25-Dihydroxyvitamin D3 in Atlantic Salmon (Salmo salar). J. Endocrinol. 2007, 193, 459–471. [Google Scholar] [CrossRef] [PubMed]
- van Rijn, C.A.; Jones, P.L.; Evans, B.S.; Afonso, L.O.B. Physiological and Growth Responses of Juvenile Atlantic Salmon (Salmo salar) Transferred to Seawater during Different Stages of Smolt Development. Aquaculture 2021, 538, 736527. [Google Scholar] [CrossRef]
- Handeland, S.O.; Imsland, A.K.; Björnsson, B.T.; Stefansson, S.O. Long-Term Effects of Photoperiod, Temperature and Their Interaction on Growth, Gill Na+, K+-ATPase Activity, Seawater Tolerance and Plasma Growth-Hormone Levels in Atlantic Salmon Salmo salar. J. Fish Biol. 2013, 83, 1197–1209. [Google Scholar] [CrossRef]
- Stefansson, S.O.; Nilsen, T.O.; Ebbesson, L.O.E.; Wargelius, A.; Madsen, S.S.; Björnsson, B.T.; McCormick, S.D. Molecular Mechanisms of Continuous Light Inhibition of Atlantic Salmon Parr-Smolt Transformation. Aquaculture 2007, 273, 235–245. [Google Scholar] [CrossRef]
- Sheridan, M.A.; Woo, N.Y.S.; Bern, H.A. Changes in the Rates of Glycogenesis, Glycogenolysis, Lipogenesis, and Lipolysis in Selected Tissues of the Coho Salmon (Oncorhynchus kisutch) Associated with Parr-smolt Transformation. J. Exp. Zool. 1985, 236, 35–44. [Google Scholar] [CrossRef]
- Robertson, J.C.; Bradley, T.M. Hepatic Ulstrastructure Changes Associated with the Parr-smolt Transformation of Atlantic Salmon (Salmo salar). J. Exp. Zool. 1991, 260, 135–148. [Google Scholar] [CrossRef]
- Ji, H.; Bradley, T.M.; Tremblay, G.C. Lactate-Dependent Gluconeogenesis and Atractyloside-Sensitive Flux through Pyruvate Carboxylase Are Reduced during Smoltification of Atlantic Salmon (Salmo Solar). J. Exp. Zool. 1996, 276, 375–386. [Google Scholar] [CrossRef]
- Peter, M.C.S. The Role of Thyroid Hormones in Stress Response of Fish. Gen. Comp. Endocrinol. 2011, 172, 198–210. [Google Scholar] [CrossRef]
- Handeland, S.O.; Imsland, A.K.; Stefansson, S.O. The Effect of Temperature and Fish Size on Growth, Feed Intake, Food Conversion Efficiency and Stomach Evacuation Rate of Atlantic Salmon Post-Smolts. Aquaculture 2008, 283, 36–42. [Google Scholar] [CrossRef]
- Brown, M.S.; Jones, P.L.; Tromp, J.J.; van Rijn, C.A.; Collins, R.A.; Afonso, L.O.B. The Physiology of Saltwater Acclimation in Large Juvenile Atlantic Salmon Salmo salar. J. Fish Biol. 2018, 93, 540–549. [Google Scholar] [CrossRef]
- Good, C.; Davidson, J. A Review of Factors Influencing Maturation of Atlantic Salmon, Salmo salar, with Focus on Water Recirculation Aquaculture System Environments. J. World Aquac. Soc. 2016, 47, 605–632. [Google Scholar] [CrossRef]
- Pino Martinez, E.; Balseiro, P.; Stefansson, S.O.; Kaneko, N.; Norberg, B.; Fleming, M.S.; Imsland, A.K.D.; Handeland, S.O. Interaction of Temperature and Feed Ration on Male Postsmolt Maturation of Atlantic Salmon (Salmo salar L.). Aquaculture 2023, 562, 738877. [Google Scholar] [CrossRef]
- Skjold, V.; Rørvik, K.-A.; Sveen, L.; Burgerhout, E.; Mota, V.C.; Weihe, R.; Ytrestøyl, T.; Bou, M.; Jacobsen, H.J.; Allaoui, G.; et al. Gradually Decreasing Daylength after Smoltification Induced by “winter Signal” Reduced Sexual Maturation in Male Atlantic Salmon. Front. Aquac. 2024, 2, 1235584. [Google Scholar] [CrossRef]
- Loncoman, C.A.; Saravia, J.; Gutierrez, L.; Contreras, C.; Oyarzún, R.; Strobel, P.; Enriquez, R.; Isla, A.; Figueroa, J.; Vargas-Chacoff, L.; et al. BK Potassium Channel MRNA Level Changes in Gills of Atlantic Salmon after Brackish Water Transfer. Aquaculture 2018, 491, 184–189. [Google Scholar] [CrossRef]
- Robertson, L.S.; McCormick, S.D. Transcriptional Profiling of the Parr-Smolt Transformation in Atlantic Salmon. Comp. Biochem. Physiol. Part D Genom. Proteom. 2012, 7, 351–360. [Google Scholar] [CrossRef] [PubMed]
- McCormick, S.D.; Regish, A.; O’Dea, M.F.; Shrimpton, J.M. Are We Missing a Mineralocorticoid in Teleost Fish? Effects of Cortisol, Deoxycorticosterone and Aldosterone on Osmoregulation, Gill Na+,K+-ATPase Activity and Isoform MRNA Levels in Atlantic Salmon. Gen. Comp. Endocrinol. 2008, 157, 35–40. [Google Scholar] [CrossRef]
- Gaetano, P.; Duarte, V.; Striberny, A.; Hazlerigg, D.; Jørgensen, E.H.; Campinho, M.A.; Fuentes, J. Photoperiod and Dietary Treatment in Freshwater Modulate the Short-Term Intestinal Response to Seawater in Atlantic Salmon (Salmo salar). Aquaculture 2023, 568, 739316. [Google Scholar] [CrossRef]
- Bisbal, G.A.; Specker, J.L. Cortisol Stimulates Hypo-osmoregulatory Ability in Atlantic Salmon, Salmo salar L. J. Fish Biol. 1991, 39, 421–432. [Google Scholar] [CrossRef]
- Kiilerich, P.; Pedersen, S.H.; Kristiansen, K.; Madsen, S.S. Corticosteroid Regulation of Na+,K+-ATPase A1-Isoform Expression in Atlantic Salmon Gill during Smolt Development. Gen. Comp. Endocrinol. 2011, 170, 283–289. [Google Scholar] [CrossRef]
- Breves, J.P.; Runiewicz, E.R.; Richardson, S.G.; Bradley, S.E.; Hall, D.J.; McCormick, S.D. Transcriptional Regulation of Esophageal, Intestinal, and Branchial Solute Transporters by Salinity, Growth Hormone, and Cortisol in Atlantic Salmon. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2024, 341, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Singer, T.D.; Clements, K.M.; Semple, J.W.; Schulte, P.M.; Bystriansky, J.S.; Finstad, B.; Fleming, I.A.; Scott McKinley, R. Seawater Tolerance and Gene Expression in Two Strains of Atlantic Salmon Smolts. Can. J. Fish. Aquat. Sci. 2002, 59, 125–135. [Google Scholar] [CrossRef]
- Morro, B.; Balseiro, P.; Albalat, A.; Pedrosa, C.; Mackenzie, S.; Nakamura, S.; Shimizu, M.; Nilsen, T.O.; Sveier, H.; Ebbesson, L.O.; et al. Effects of Different Photoperiod Regimes on the Smoltification and Seawater Adaptation of Seawater-Farmed Rainbow Trout (Oncorhynchus mykiss): Insights from Na+, K+–ATPase Activity and Transcription of Osmoregulation and Growth Regulation Genes. Aquaculture 2019, 507, 282–292. [Google Scholar] [CrossRef]
- Jutfelt, F.; Olsen, R.E.; Björnsson, B.T.; Sundell, K. Parr-Smolt Transformation and Dietary Vegetable Lipids Affect Intestinal Nutrient Uptake, Barrier Function and Plasma Cortisol Levels in Atlantic Salmon. Aquaculture 2007, 273, 298–311. [Google Scholar] [CrossRef]
- Madsen, S.S.; Olesen, J.H.; Bedal, K.; Engelund, M.B.; Velasco-Santamaría, Y.M.; Tipsmark, C.K. Functional Characterization of Water Transport and Cellular Localization of Three Aquaporin Paralogs in the Salmonid Intestine. Front. Physiol. 2011, 2, 56. [Google Scholar] [CrossRef]
- Grosell, M. Intestinal Anion Exchange in Marine Teleosts Is Involved in Osmoregulation and Contributes to the Oceanic Inorganic Carbon Cycle. Acta Physiol. 2011, 202, 421–434. [Google Scholar] [CrossRef]
- Veillette, P.A.; White, R.J.; Specker, J.L. Changes in Intestinal Fluid Transport in Atlantic Salmon (Salmo salar L.) during Parr-Smolt Transformation. Fish Physiol. Biochem. 1993, 12, 193–202. [Google Scholar] [CrossRef]


| Gene | GenBank Acc. No. | Sequences (5′>3′) | Reference |
|---|---|---|---|
| ef1α | NM_001123629.1 | CCCCTCCAGGACGTTTACAAA | [51] |
| CACACGGCCCACAGGTACA | |||
| b-actin | NM_001123525.1 | AGGGACAACACTGCCTGGAT | [51] |
| CCAAAGCCAACAGGGAGAAG | |||
| nkaα1a | XM_045722983.1 | CCAGGATCACTCAATGTCACTCT | (Modified from [14]) |
| CAAAGGCAAATGAGTTTAATATC | |||
| nkaα1b | XM_014150738.2 | GCTACATCTCAACCAACAACATTACAC | [14] |
| TGCAGCTGAGTGCACCAT | |||
| nkaα1c | XM_014152158.2 | AGGGAGACGTACTACTAGAAAGCAT | [14] |
| CAGAACTTAAAATTCCGAGCAGCAA | |||
| cftrI | NM_001123533.1 | CCTTCTCCAATATGGTTGAAGAGGCAAG | [14] |
| GAGGCACTTGGATGAGTCAGCAG | |||
| cftrII | NM_001123534.1 | TGCTTAAGGTTAGTGCCTCAGG | [52] |
| AAGGCTACTTCAGGTTAATCAC | |||
| nkcc1a | NM_001123683.1 | GATGATCTGCGGCCATGTTC | [14,16] |
| TCTGGTCATTGGACAGCTCTTTG | |||
| nbc | XM_014140909.2 | TGGACCTGTTCTGGGTAGCAA | [41] |
| AGCACTGGGTCTCCATCTTCAG |
| Variable | Contrast | Weeks After SWT | Ratio | SE | df | LCL | UCL | Null | Stat | p value |
|---|---|---|---|---|---|---|---|---|---|---|
| Final weight | LD-1/LL | 8–10 | 0.841 | 0.057 | 89 | 0.716 | 0.987 | 1 | −2.58 | 0.031 |
| LD-2/LL | 8–10 | 0.814 | 0.055 | 89 | 0.694 | 0.956 | 1 | −3.05 | 0.009 | |
| RGR | LD-2/LL | 8–10 | 0.822 | 0.066 | Inf | 0.682 | 0.991 | 1 | −2.45 | 0.038 |
| K | LD-1/LL | 5–7 | 0.934 | 0.017 | 89 | 0.894 | 0.975 | 1 | −3.74 | <0.001 |
| LD-1/LL | 8–10 | 0.921 | 0.017 | 89 | 0.882 | 0.962 | 1 | −4.48 | <0.001 | |
| LD-2/LL | 5–7 | 0.921 | 0.017 | 89 | 0.882 | 0.962 | 1 | −4.47 | <0.001 | |
| LD-2/LL | 8–10 | 0.924 | 0.017 | 89 | 0.884 | 0.965 | 1 | −4.33 | <0.001 | |
| HSI | LD-1/LD-2 | 5–7 | 1.113 | 0.036 | 89 | 1.029 | 1.203 | 1 | 3.26 | 0.004 |
| LD-2/LL | 5–7 | 0.856 | 0.028 | 89 | 0.792 | 0.926 | 1 | −4.74 | <0.001 | |
| GSI | LD-1/LL | 8–10 | 0.746 | 0.090 | 46 | 0.558 | 0.998 | 1 | −2.44 | 0.048 |
| CSI | LD-2/LL | 8–10 | 1.119 | 0.048 | Inf | 1.012 | 1.237 | 1 | 2.62 | 0.024 |
| Lactate in plasma | LD-1/LL | 8–10 | 0.825 | 0.061 | Inf | 0.693 | 0.982 | 1 | −2.59 | 0.026 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva-Marrero, J.I.; Lai, F.; Handeland, S.O.; Pedrosa, C.; Gelebart, V.; Balseiro, P.; Fuentes, J.; Rønnestad, I.; Gomes, A.S. Integration of Gill and Intestinal Osmoregulatory Functions to Assess the Smoltification Window in Atlantic Salmon. Fishes 2025, 10, 119. https://doi.org/10.3390/fishes10030119
Silva-Marrero JI, Lai F, Handeland SO, Pedrosa C, Gelebart V, Balseiro P, Fuentes J, Rønnestad I, Gomes AS. Integration of Gill and Intestinal Osmoregulatory Functions to Assess the Smoltification Window in Atlantic Salmon. Fishes. 2025; 10(3):119. https://doi.org/10.3390/fishes10030119
Chicago/Turabian StyleSilva-Marrero, Jonás I., Floriana Lai, Sigurd O. Handeland, Cindy Pedrosa, Virginie Gelebart, Pablo Balseiro, Juan Fuentes, Ivar Rønnestad, and Ana S. Gomes. 2025. "Integration of Gill and Intestinal Osmoregulatory Functions to Assess the Smoltification Window in Atlantic Salmon" Fishes 10, no. 3: 119. https://doi.org/10.3390/fishes10030119
APA StyleSilva-Marrero, J. I., Lai, F., Handeland, S. O., Pedrosa, C., Gelebart, V., Balseiro, P., Fuentes, J., Rønnestad, I., & Gomes, A. S. (2025). Integration of Gill and Intestinal Osmoregulatory Functions to Assess the Smoltification Window in Atlantic Salmon. Fishes, 10(3), 119. https://doi.org/10.3390/fishes10030119








