Anesthetic Effects of Clove Basil Essential Oil (Ocimum gratissimum) Microemulsion on Asian Redtail Catfish (Hemibagrus wyckioides) and Its Biochemical Stress Indicators
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparing a Microemulsion Based on Clove Basil Essential Oil
2.2. Assessing the Anesthetic Efficacy of Clove Basil Essential Oil Microemulsion in Asian Redtail Catfish
2.3. Evaluating Various Biochemical Blood Parameters as Stress Indicators in Fish Anesthetized with Clove Basil Essential Oil Microemulsion
3. Results
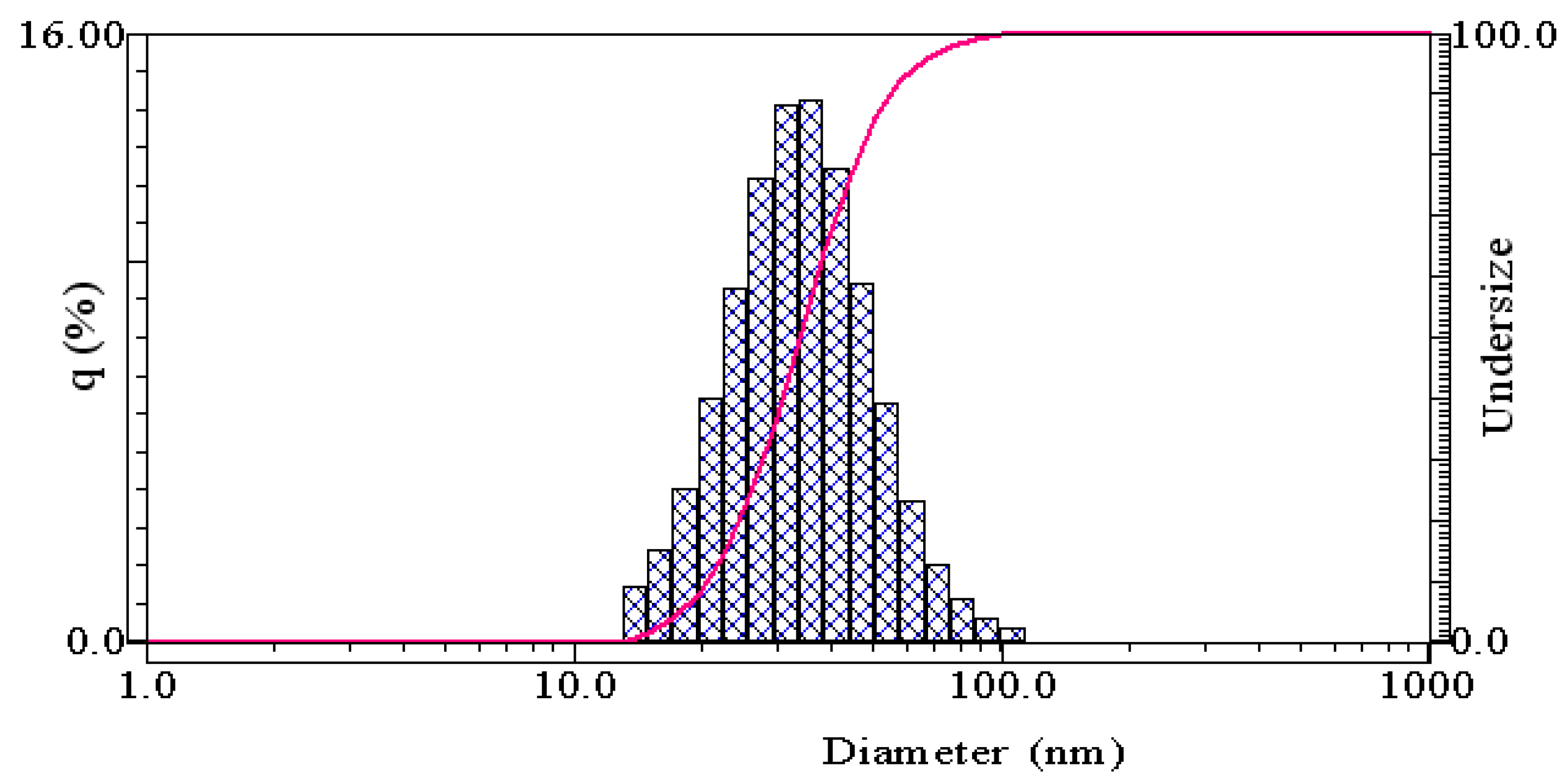
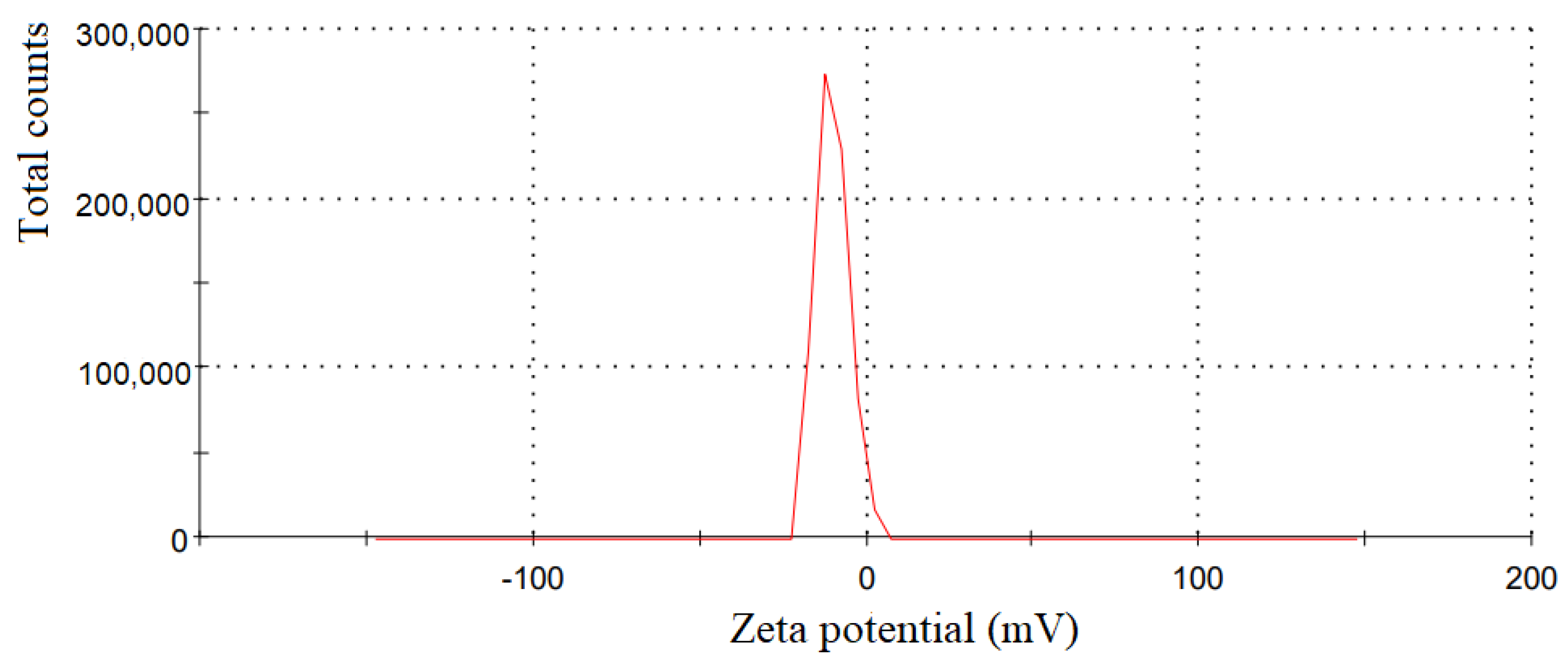
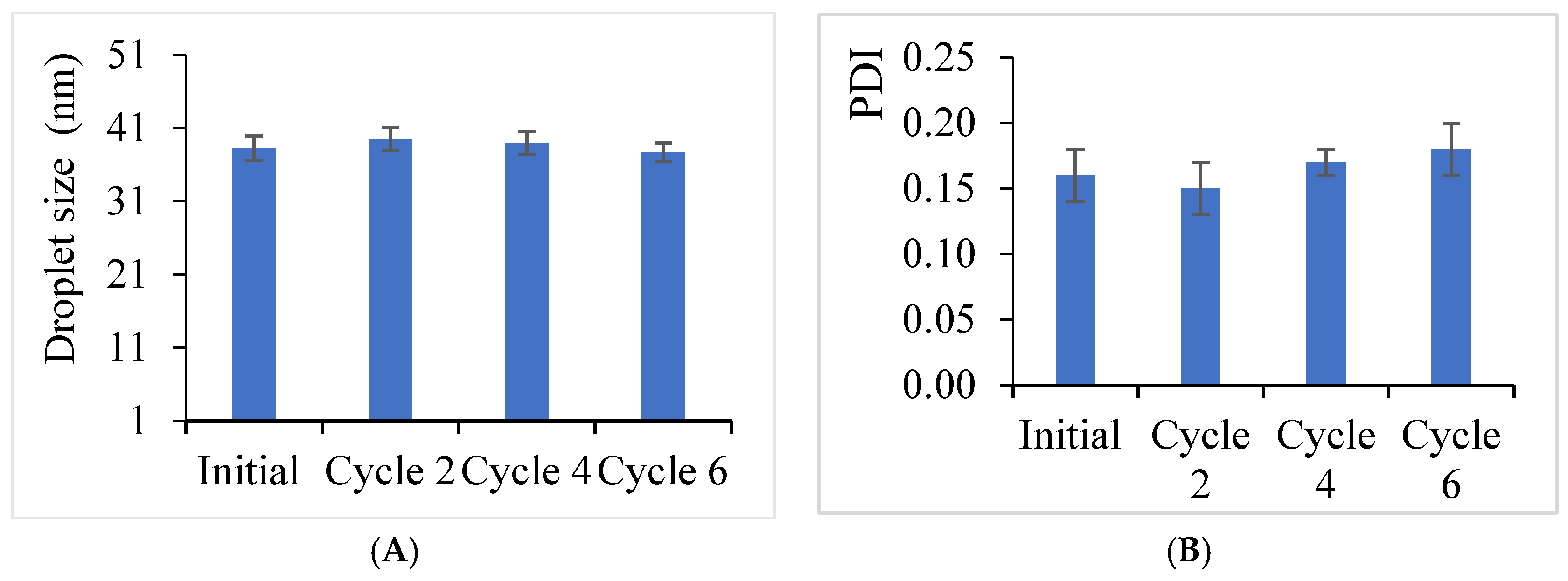
3.1. Results of Synthesizing Clove Basil Essential Oil Microemulsion
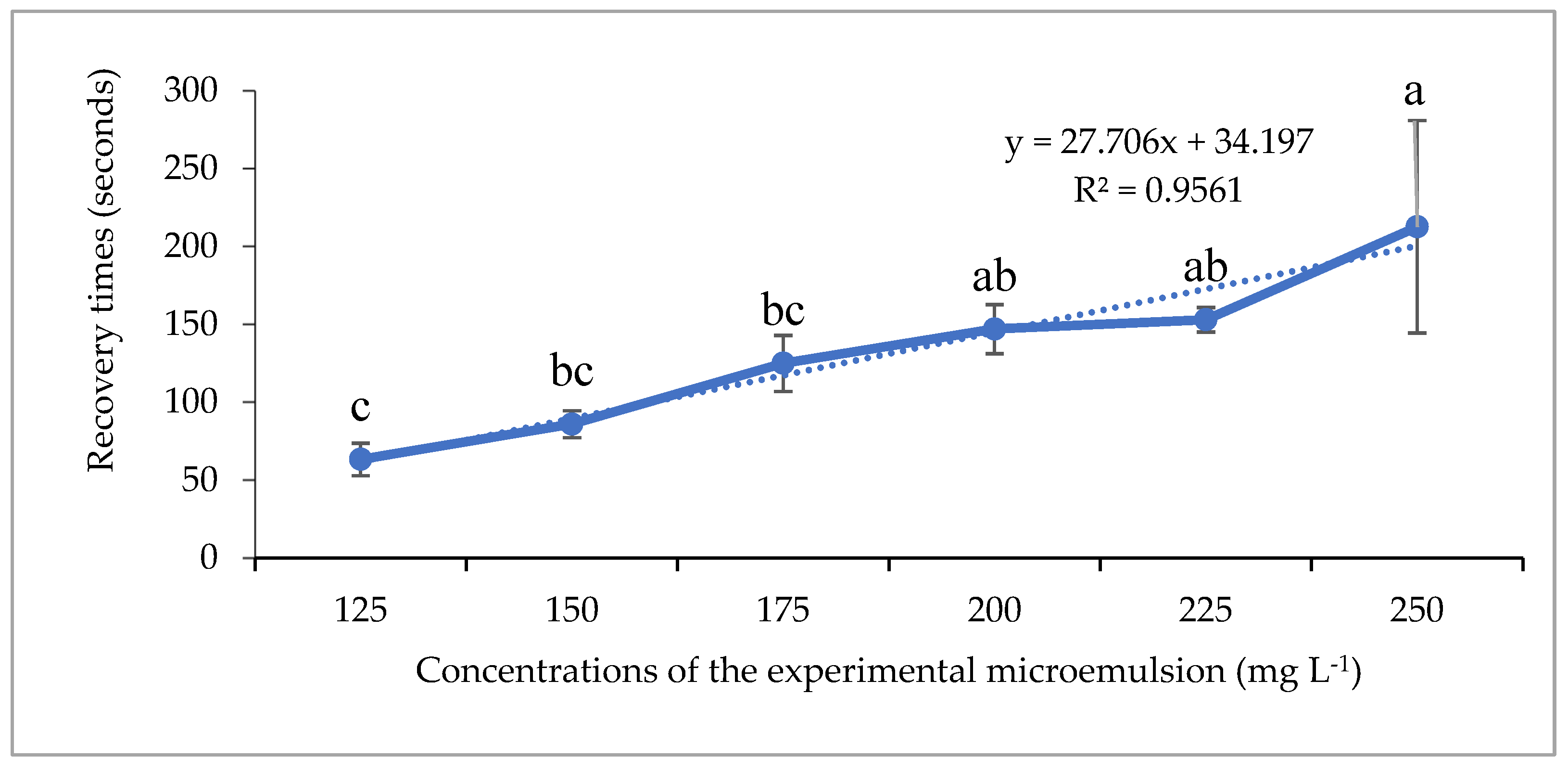
3.2. Results of Evaluating the Anesthetic Effectiveness of Clove Basil Essential Oil Microemulsion in Asian Redtail Catfish
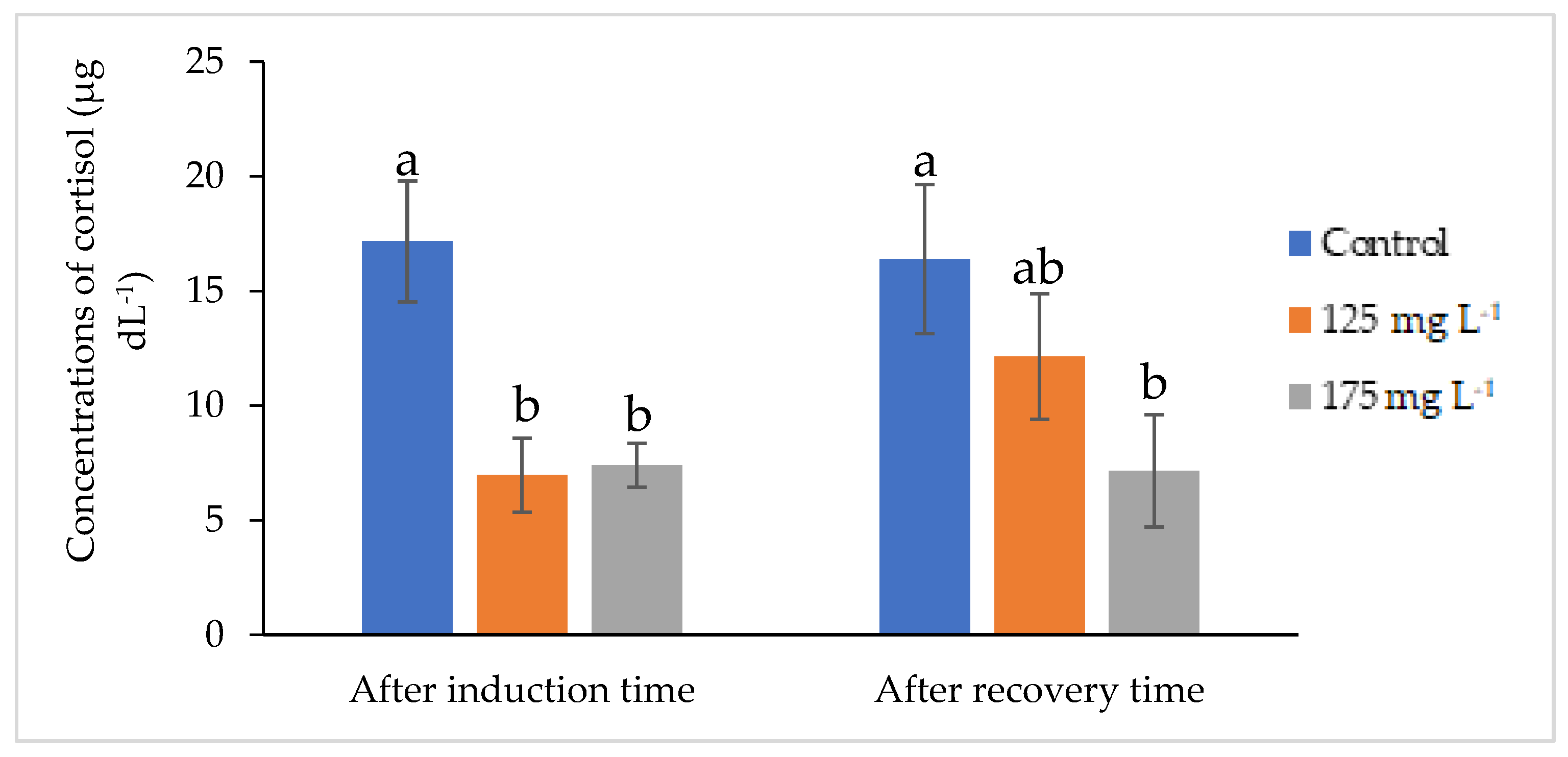
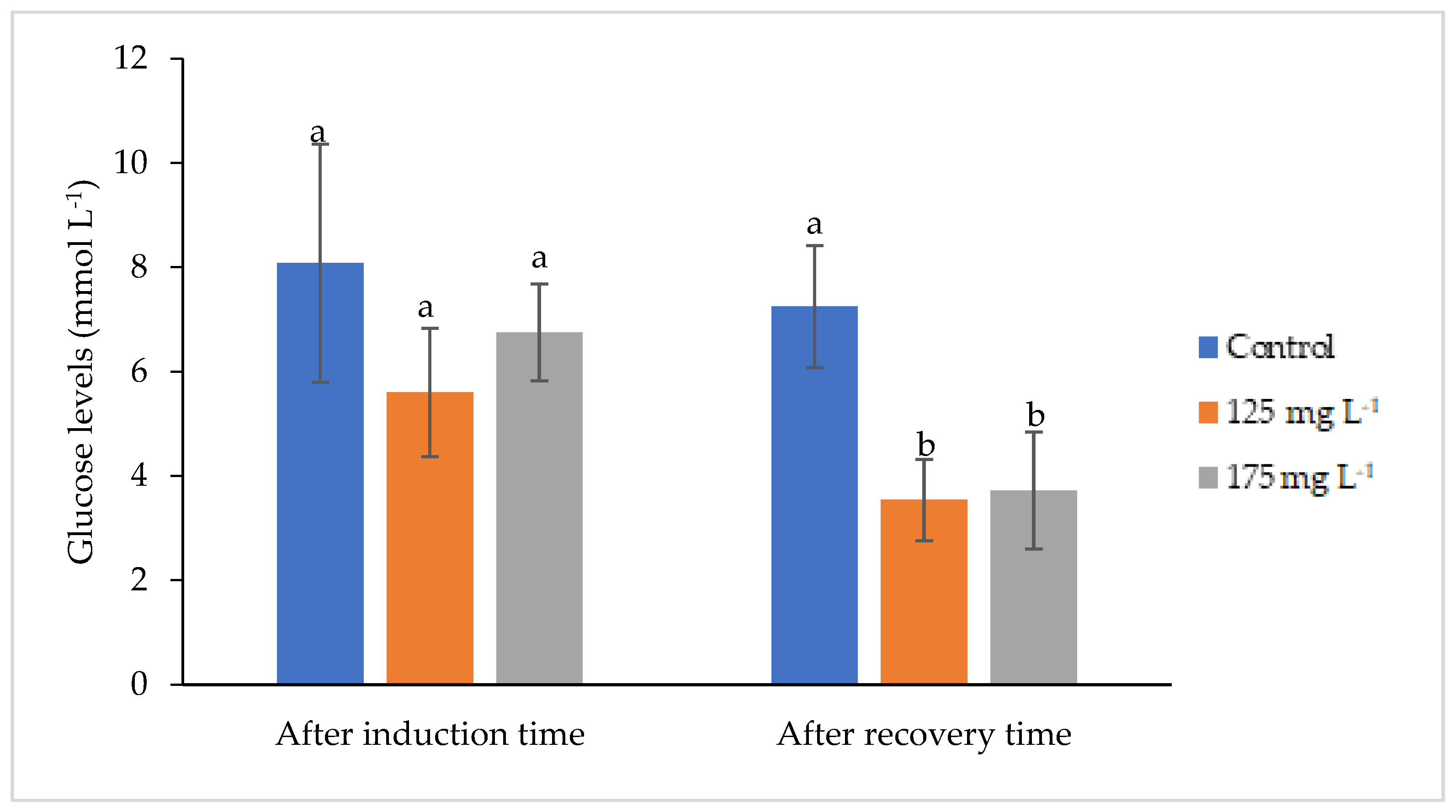
3.3. Results of Glucose, Cortisol, and Lactate Levels of Fish Anesthetized with Clove Basil Essential Oil Microemulsion
4. Discussion
4.1. Characteristics of the Synthesized Clove Basil Essential Oil Microemulsion
4.2. Anesthetic Efficacy of Clove Basil Essential Oil Microemulsion
4.3. Changes of the Blood Cortisol, Lactate, and Glucose Levels in Fish Anesthetized with Clove Basil Essential Oil Microemulsion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dinesh, R.; Prakash, C.; Poojary, N.; Abraham, S.; Kantharajan, G.; Sivagurunathan, U. Role of Sedative and Anaesthetic Drugs in Aquaculture. J. Aquac. Trop. 2017, 32, 305–313. [Google Scholar]
- Purbosari, N.; Warsiki, E.; Syamsu, K.; Santoso, J. Natural versus Synthetic Anesthetic for Transport of Live Fish: A Review. Aquac. Fish. 2019, 4, 129–133. [Google Scholar] [CrossRef]
- Hoseini, S.M.; Taheri Mirghaed, A.; Yousefi, M. Application of Herbal Anaesthetics in Aquaculture. Rev. Aquac. 2019, 11, 550–564. [Google Scholar] [CrossRef]
- Reverter, M.; Tapissier-Bontemps, N.; Sasal, P.; Saulnier, D. Use of Medicinal Plants in Aquaculture. In Diagnosis and Control of Diseases of Fish and Shellfish; Wiley: Hoboken, NJ, USA, 2017; pp. 223–261. ISBN 9781119152125. [Google Scholar]
- Ugbogu, O.C.; Emmanuel, O.; Agi, G.O.; Ibe, C.; Ekweogu, C.N.; Ude, V.C.; Uche, M.E.; Nnanna, R.O.; Ugbogu, E.A. A Review on the Traditional Uses, Phytochemistry, and Pharmacological Activities of Clove Basil (Ocimum gratissimum L.). Heliyon 2021, 7, e08404. [Google Scholar] [CrossRef]
- Dung, P.N.T.; Dao, T.P.; Le, T.T.; Tran, H.T.; Dinh, T.T.T.; Pham, Q.L.; Tran, Q.T.; Pham, M.Q. Extraction and Analysis of Chemical Composition of Ocimum gratissimum L Essential Oil in the North of Vietnam. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1092, 012092. [Google Scholar] [CrossRef]
- Viegas, R.M.; França, C.L.; Castro, J.S.; Castro, J.J.P.; Santana, T.C.; Costa-Lima, M.P.G.; Neta, R.N.F.C.; Carreiro, C.R.P.; Teixeira, E.G. Eugenol as an Efficient Anesthetic for Neotropical Fish Prochilodus Nigricans (Teleostei, Prochilodontidae). Arq. Bras. Med. Vet. Zootec. 2020, 72, 1813–1820. [Google Scholar] [CrossRef]
- de Souza Valente, C.; dos Santos, G.; Becker, A.G.; Heinzmann, B.M.; Caron, B.O.; Baldisserotto, B.; Ballester, E.L.C. Anaesthetic Effect of Clove Basil (Ocimum gratissimum L.) Essential Oil on the Giant River Prawn (Macrobrachium rosenbergii, De Man 1879) Exposed to Different Water PHs. Aquac. Int. 2024, 32, 1493–1505. [Google Scholar] [CrossRef]
- Silva, L.d.L.; Parodi, T.V.; Reckziegel, P.; Garcia, V.d.O.; Bürger, M.E.; Baldisserotto, B.; Malmann, C.A.; Pereira, A.M.S.; Heinzmann, B.M. Essential Oil of Ocimum gratissimum L.: Anesthetic Effects, Mechanism of Action and Tolerance in Silver Catfish, Rhamdia Quelen. Aquaculture 2012, 350–353, 91–97. [Google Scholar] [CrossRef]
- da Silva, L.A.; Martins, M.A.; Santo, F.E.; Oliveira, F.C.; Chaves, F.C.M.; Chagas, E.C.; Martins, M.L.; de Campos, C.M. Essential Oils of Ocimum gratissimum and Zingiber officinale as Anesthetics for the South American Catfish Pseudoplatystoma reticulatum. Aquaculture 2020, 528, 735595. [Google Scholar] [CrossRef]
- Ferreira, A.L.; Favero, G.C.; Boaventura, T.P.; de Freitas Souza, C.; Ferreira, N.S.; Descovi, S.N.; Baldisserotto, B.; Heinzmann, B.M.; Luz, R.K. Essential Oil of Ocimum gratissimum (Linnaeus, 1753): Efficacy for Anesthesia and Transport of Oreochromis niloticus. Fish Physiol. Biochem. 2021, 47, 135–152. [Google Scholar] [CrossRef]
- Boaventura, T.P.; Souza, C.F.; Ferreira, A.L.; Favero, G.C.; Baldissera, M.D.; Heinzmann, B.M.; Baldisserotto, B.; Luz, R.K. Essential Oil of Ocimum Gratissimum (Linnaeus, 1753) as Anesthetic for Lophiosilurus alexandri: Induction, Recovery, Hematology, Biochemistry and Oxidative Stress. Aquaculture 2020, 529, 735676. [Google Scholar] [CrossRef]
- Kalamarz-Kubiak, H. Cortisol in Correlation to Other Indicators of Fish Welfare. In Corticosteroids; InTech: London, UK, 2018. [Google Scholar]
- Pavoni, L.; Perinelli, D.R.; Bonacucina, G.; Cespi, M.; Palmieri, G.F. An Overview of Micro- and Nanoemulsions as Vehicles for Essential Oils: Formulation, Preparation and Stability. Nanomaterials 2020, 10, 135. [Google Scholar] [CrossRef]
- Chaudhari, A.K.; Singh, V.K.; Das, S.; Dubey, N.K. Nanoencapsulation of Essential Oils and Their Bioactive Constituents: A Novel Strategy to Control Mycotoxin Contamination in Food System. Food Chem. Toxicol. 2021, 149, 112019. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, P.; Gupta, V.; Prakash, B. Application of Nanotechnology to Boost the Functional and Preservative Properties of Essential Oils. In Functional and Preservative Properties of Phytochemicals; Elsevier: Amsterdam, The Netherlands, 2020; pp. 241–267. [Google Scholar]
- Swathy, J.S.; Mishra, P.; Thomas, J.; Mukherjee, A.; Chandrasekaran, N. Antimicrobial Potency of High-Energy Emulsified Black Pepper Oil Nanoemulsion against Aquaculture Pathogen. Aquaculture 2018, 491, 210–220. [Google Scholar] [CrossRef]
- Korni, F.M.M.; Mohammed, A.N.; Moawad, U.K. Using Some Natural Essential Oils and Their Nano-Emulsions for Ammonia Management, Anti-Stress and Prevention of Streptococcosis in Nile Tilapia, Oreochromis niloticus. Aquac. Int. 2023, 31, 2179–2198. [Google Scholar] [CrossRef]
- Raval, N.; Maheshwari, R.; Kalyane, D.; Youngren-Ortiz, S.R.; Chougule, M.B.; Tekade, R.K. Importance of Physicochemical Characterization of Nanoparticles in Pharmaceutical Product Development. In Basic Fundamentals of Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 369–400. [Google Scholar]
- Clayton, K.N.; Salameh, J.W.; Wereley, S.T.; Kinzer-Ursem, T.L. Physical Characterization of Nanoparticle Size and Surface Modification Using Particle Scattering Diffusometry. Biomicrofluidics 2016, 10, 054107. [Google Scholar] [CrossRef]
- Swastika Braja, I.W.R.; Mayun Permana, I.D.G.; Suhendra, L. Formulation and Stability of Clove Leaf (Syzygium aromaticum L.) Essential Oil Microemulsion. Int. J. Curr. Microbiol. Appl. Sci. 2022, 11, 197–211. [Google Scholar] [CrossRef]
- Liang, Y.; Zou, J.; Zhang, X.; Shi, Y.; Tai, J.; Wang, Y.; Guo, D.; Yang, M. Preparation and Quality Evaluation of a Volatile Oil Microemulsion from Flos Magnoliae and Centipeda Minima. Mol. Med. Rep. 2020, 22, 4531–4540. [Google Scholar] [CrossRef]
- Woody, C. Clove Oil as an Anaesthetic for Adult Sockeye Salmon: Field Trials. J. Fish. Biol. 2002, 60, 340–347. [Google Scholar] [CrossRef]
- Duran, U.; Çenesiz, S.; Şahin, B. Blood Sampling Techniques and Preparing for Analysis in Rainbow Trout (Oncorhynchus mykiss). Black Sea J. Agric. 2023, 6, 68–73. [Google Scholar] [CrossRef]
- Berka, R. The Transport of Live Fish: A Review; Food and Agriculture Organization of the United Nations: Roma, Italy, 1986; ISBN 9251023808. [Google Scholar]
- Cookey, A.I. Efficacy of Some Plant Extracts as Anaesthetics Agent in Tranportation of Nile Tilapia (Oreochromis niloticus). Acad. J. Hydrol. Water Res. 2023, 1, 57–63. [Google Scholar]
- Ackerman, P.A.; Morgan, J.D.; Iwama, G.K. Anesthetics. In CCCA (Canadian Council on Animal Care) Guidelines on the Care and Use of Fish in Research, Teaching and Testing; University of British Columbia: Vancouver, BC, Canada, 2005. [Google Scholar]
- Paliwal, H.; Solanki, R.S.; Chauhan, C.S. Development of Microemulsion Formulation for Oral Delivery of Rosuvastatin Calcium. Int. J. Pharm. Sci. Drug Res. 2020, 12, 35–39. [Google Scholar] [CrossRef]
- Hung, W.-H.; Chen, P.-K.; Fang, C.-W.; Lin, Y.-C.; Wu, P.-C. Preparation and Evaluation of Azelaic Acid Topical Microemulsion Formulation: In Vitro and In Vivo Study. Pharmaceutics 2021, 13, 410. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, A.A.; Güven, U.M. Cefaclor Monohydrate Loaded Microemulsion Formulation for Topical Application: Characterization with New Developed UPLC Method and Stability Study. Marmara Pharm. J. 2019, 23, 426–440. [Google Scholar] [CrossRef]
- Yotsawimonwat, S.; Sirithunyalug, J.; Sirithunyalug, B.; Yotsawimonwat, S.; Okonoki, S.; Krauel, K.; Sirithunyalug, J.; Sirithunyalug, B.; Rades, T. Characterisation of Microemulsions Containing Orange Oil with Water and Propylene Glycol as Hydrophilic Components. Die Pharm.-Int. J. Pharm. Sci. 2006, 61, 920–926. [Google Scholar]
- Sha, R.; Wu, Y.; Cai, C.; Mao, J.; Liu, S.; Wang, W. Preparation of a Nanoscale Garlic Oil Microemulsion and Its Antioxidant Activity. Nanosci. Nanotechnol. Lett. 2017, 9, 123–133. [Google Scholar] [CrossRef]
- Chatzidaki, M.D.; Demisli, S.; Zingkou, E.; Liggri, P.G.V.; Papachristos, D.P.; Balatsos, G.; Karras, V.; Nallet, F.; Michaelakis, A.; Sotiropoulou, G. Essential Oil-in-Water Microemulsions for Topical Application: Structural Study, Cytotoxic Effect and Insect Repelling Activity. Colloids Surf. A Physicochem. Eng. Asp. 2022, 654, 130159. [Google Scholar] [CrossRef]
- Callender, S.P.; Mathews, J.A.; Kobernyk, K.; Wettig, S.D. Microemulsion Utility in Pharmaceuticals: Implications for Multi-Drug Delivery. Int. J. Pharm. 2017, 526, 425–442. [Google Scholar] [CrossRef]
- Leng, K.; Guan, B.; Liu, W.; Jiang, C.; Cong, S.; Peng, B.; Tao, Y. Advance of Microemulsion and Application for Enhanced Oil Recovery. Nanomaterials 2024, 14, 1004. [Google Scholar] [CrossRef]
- Vlaia, L.; Coneac, G.; Muţ, A.M.; Olariu, I.; Vlaia, V.; Anghel, D.F.; Maxim, M.E.; Dobrescu, A.; Hîrjău, M.; Lupuleasa, D. Topical Biocompatible Fluconazole-Loaded Microemulsions Based on Essential Oils and Sucrose Esters: Formulation Design Based on Pseudo-Ternary Phase Diagrams and Physicochemical Characterization. Processes 2021, 9, 144. [Google Scholar] [CrossRef]
- Das Mandal, S.; Patel, V. Development of Microemulsion Formulation for the Solubility Enhancement of Flunarizine. Pharm. Lett. 2010, 2, 227–236. [Google Scholar]
- Kiromah, N.Z.W.; Sugihartini, N.; Nurani, L.H. Development and Characterization of Clove Oil Microemulsion. Pharmacia 2023, 70, 233–241. [Google Scholar] [CrossRef]
- Bandeira Junior, G.; Bianchini, A.E.; de Freitas Souza, C.; Descovi, S.N.; da Silva Fernandes, L.; de Lima Silva, L.; Cargnelutti, J.F.; Baldisserotto, B. The Use of Cinnamon Essential Oils in Aquaculture: Antibacterial, Anesthetic, Growth-Promoting, and Antioxidant Effects. Fishes 2022, 7, 133. [Google Scholar] [CrossRef]
- Nuanmanee, S.; Sriwanayos, P.; Boonyo, K.; Chaisri, W.; Saengsitthisak, B.; Tajai, P.; Pikulkaew, S. Synergistic Effect between Eugenol and 1,8-Cineole on Anesthesia in Guppy Fish (Poecilia reticulata). Vet. Sci. 2024, 11, 165. [Google Scholar] [CrossRef]
- Ribeiro, P.A.P.; Miranda-Filho, K.C.; Melo, D.C.d.; Luz, R.K. Efficiency of Eugenol as Anesthetic for the Early Life Stages of Nile Tilapia (Oreochromis niloticus). Anais da Academia Brasileira de Ciências 2015, 87, 529–535. [Google Scholar] [CrossRef]
- Diemer, O.; Neu, D.H.; Bittencourt, F.; Signor, A.; Boscolo, W.R.; Feiden, A. Eugenol Como Anestésico Para Jundiá (Rhamdia voulezi) Em Diferentes Pesos. Semina Ciências Agrárias 2012, 33, 1495–1500. [Google Scholar] [CrossRef]
- Parodi, T.V.; Gressler, L.T.; Silva, L.d.L.; Becker, A.G.; Schmidt, D.; Caron, B.O.; Heinzmann, B.M.; Baldisserotto, B. Chemical Composition of the Essential Oil of Aloysia triphylla under Seasonal Influence and Its Anaesthetic Activity in Fish. Aquac. Res. 2020, 51, 2515–2524. [Google Scholar] [CrossRef]
- Kurniawan, K.; Atmadi Prakoso, V.; Gustiano, R.; Subagja, J.; Heru Prihadi, T.; Iriana Kusmini, I.; Zenal Arifin, O.; Radona, D.; Hari Kristanto, A. Tolerance Level of Domesticated Asian Red-Tail Catfish Hemibagrus Nemurus to Salinity, Acidity, and Temperature Variability. Journal of Hunan University (Natural Sciences) 2022, 49, 75–84. [Google Scholar] [CrossRef]
- Crosby, T.C.; Hill, J.E.; Watson, C.A.; Yanong, R.P.E.; Strange, R. Effects of Tricaine Methanesulfonate, Hypno, Metomidate, Quinaldine, and Salt on Plasma Cortisol Levels Following Acute Stress in Threespot Gourami Trichogaster trichopterus. J. Aquat. Anim. Health 2006, 18, 58–63. [Google Scholar] [CrossRef]
- Pamukas, N.A.; Tang, U.M. Hematology of Asian Redtail Catfish (Hemibagrus Nemurus) in Different Stocking Densities Using an Aquaponic Recirculation System. AACL Bioflux 2021, 14, 1534–1547. [Google Scholar]
- Fazio, F.; Ferrantelli, V.; Fortino, G.; Arfuso, F.; Giangrosso, G.; Faggio, C. The Influence of Acute Handling Stress on Some Blood Parameters in Cultured Sea Bream (Sparus aurata Linnaeus, 1758). Ital. J. Food Saf. 2015, 4, 4174. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.d.L.; da Silva, D.T.; Garlet, Q.I.; Cunha, M.A.; Mallmann, C.A.; Baldisserotto, B.; Longhi, S.J.; Pereira, A.M.S.; Heinzmann, B.M. Anesthetic Activity of Brazilian Native Plants in Silver Catfish (Rhamdia quelen). Neotrop. Ichthyol. 2013, 11, 443–451. [Google Scholar] [CrossRef]
- Matsche, M.A. Efficacy and Physiological Response to Chemical Anesthesia in Wild Hickory Shad during Spawning Season. Mar. Coast. Fish. 2017, 9, 296–304. [Google Scholar] [CrossRef]
- Hanley, C.S.; Clyde, V.L.; Wallace, R.S.; Paul-Murphy, J.; Patterson, T.A.; Keuler, N.S.; Sladky, K.K. Effects of Anesthesia and Surgery on Serial Blood Gas Values and Lactate Concentrations in Yellow Perch (Perca flavescens), Walleye Pike (Sander vitreus), and Koi (Cyprinus carpio). J. Am. Vet. Med. Assoc. 2010, 236, 1104–1108. [Google Scholar] [CrossRef]

| Name of Samples | Essential Oil (% w/w) | Water (% w/w) | Ratio of Polysorbate 20 and Propylene Glycol |
|---|---|---|---|
| M1 | 8 | 60 | 2:1 |
| M2 | 10 | 50 | 3:1 |
| M3 | 8 | 60 | 3:1 |
| Name of Samples | Particle Size (nm) | PDI | Conductivity (μS/cm) | Viscosity (mPa.s) | Turbidity (%) |
|---|---|---|---|---|---|
| M1 | 44.30 ± 3.44 | 0.49 ± 0.27 | 110.50 ± 0.26 | 66.09 ± 11.18 | 0.07 ± 0.00 |
| M2 | 61.37 ± 8.86 | 1.02 ± 0.24 | 106.53 ± 0.21 | 98.95 ± 1.55 | 0.06 ± 0.01 |
| M3 | 36.33 ± 1.84 | 0.17 ± 0.04 | 124.97 ± 0.42 | 85.01 ± 11.57 | 0.07 ± 0.00 |
| Stages (Seconds) | Concentrations of Microemulsion (mg L−1) | p-Value | |||||
|---|---|---|---|---|---|---|---|
| 125 | 150 | 175 | 200 | 225 | 250 | ||
| 1 | 230.33 ± 53.90 a | 126.33 ± 10.79 b | 115.00 ± 15.59 b | 130.33 ± 13.28 b | 131.00 ± 9.54 b | 121.33 ± 7.51 b | 0.001 |
| 2 | 369.67 ± 53.90 a | 363.67 ± 13.20 a | 144.00 ± 31.18 d b | 189.67 ± 44.02 b | 156.00 ± 22.27 b | 111.00 ± 10.58 b | <0.001 |
| 3a | - | 110.00 ± 3.46 c | 341.00 ± 15.59 a | 280.00 ± 33.42 a b | 241.00 ± 25.24 b | 151.33 ± 41.86 c | <0.001 |
| 3b | - | - | - | - | 72.00 ± 8.72 b | 216.33 ± 44.81 a | 0.005 |
| Recovery | 63.33 ± 10.41 c | 86.00 ± 8.66 b c | 125.00 ± 18.03 b c | 147.00 ± 15.72 a b | 153.00 ± 7.94 a b | 212.67 ± 68.13 a | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lam, P.H.; Vo, H.D.N.; Truong, L.M.T.; Dang, D.M.T.; Dang, C.M.; Doan, T.C.D.; Mollaamin, F.; Monajjemi, M. Anesthetic Effects of Clove Basil Essential Oil (Ocimum gratissimum) Microemulsion on Asian Redtail Catfish (Hemibagrus wyckioides) and Its Biochemical Stress Indicators. Fishes 2025, 10, 104. https://doi.org/10.3390/fishes10030104
Lam PH, Vo HDN, Truong LMT, Dang DMT, Dang CM, Doan TCD, Mollaamin F, Monajjemi M. Anesthetic Effects of Clove Basil Essential Oil (Ocimum gratissimum) Microemulsion on Asian Redtail Catfish (Hemibagrus wyckioides) and Its Biochemical Stress Indicators. Fishes. 2025; 10(3):104. https://doi.org/10.3390/fishes10030104
Chicago/Turabian StyleLam, Phuong Hong, Huyen Da Nguyen Vo, Linh My Thi Truong, Dung My Thi Dang, Chien Mau Dang, Tin Chanh Duc Doan, Fatemeh Mollaamin, and Majid Monajjemi. 2025. "Anesthetic Effects of Clove Basil Essential Oil (Ocimum gratissimum) Microemulsion on Asian Redtail Catfish (Hemibagrus wyckioides) and Its Biochemical Stress Indicators" Fishes 10, no. 3: 104. https://doi.org/10.3390/fishes10030104
APA StyleLam, P. H., Vo, H. D. N., Truong, L. M. T., Dang, D. M. T., Dang, C. M., Doan, T. C. D., Mollaamin, F., & Monajjemi, M. (2025). Anesthetic Effects of Clove Basil Essential Oil (Ocimum gratissimum) Microemulsion on Asian Redtail Catfish (Hemibagrus wyckioides) and Its Biochemical Stress Indicators. Fishes, 10(3), 104. https://doi.org/10.3390/fishes10030104








