Krüppel Homolog 1 Is Required for the Role of Methyl Farnesoate in Vitellogenesis in the Mud Crab Scylla paramamosain
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Cloning of Sp-Kr-h1 and Sequence Analysis
2.3. Expression Profiles of Sp-Kr-h1 in the Female Mud Crab
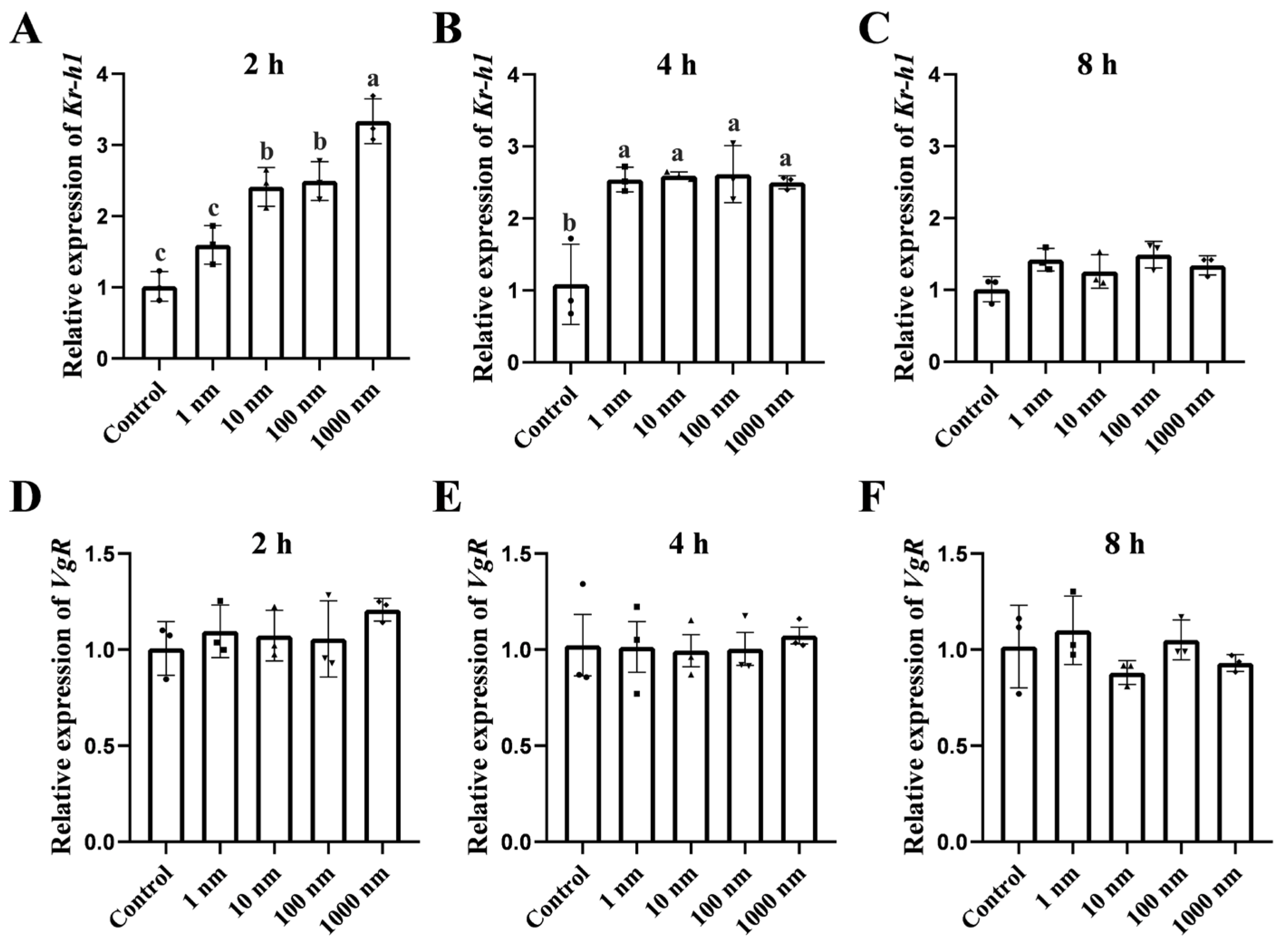
2.4. In Vitro Effect of MF on the Gene Expression in the Hepatopancreas and Ovary
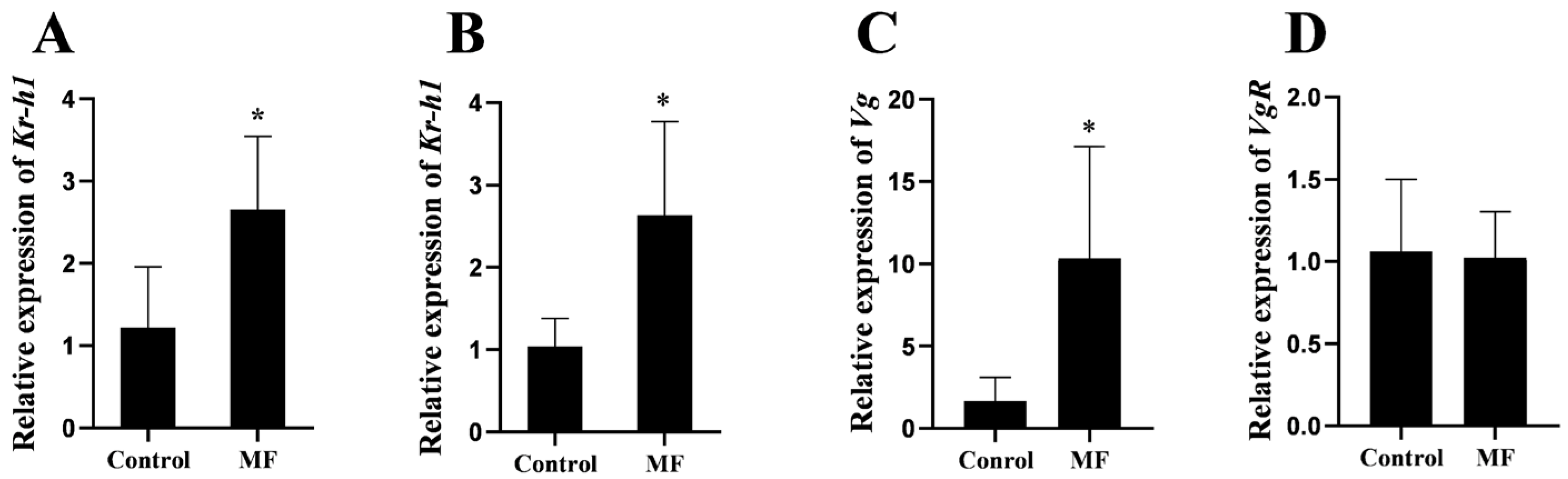
2.5. In Vivo Effects of MF on Ovarian Development of S. paramamosain
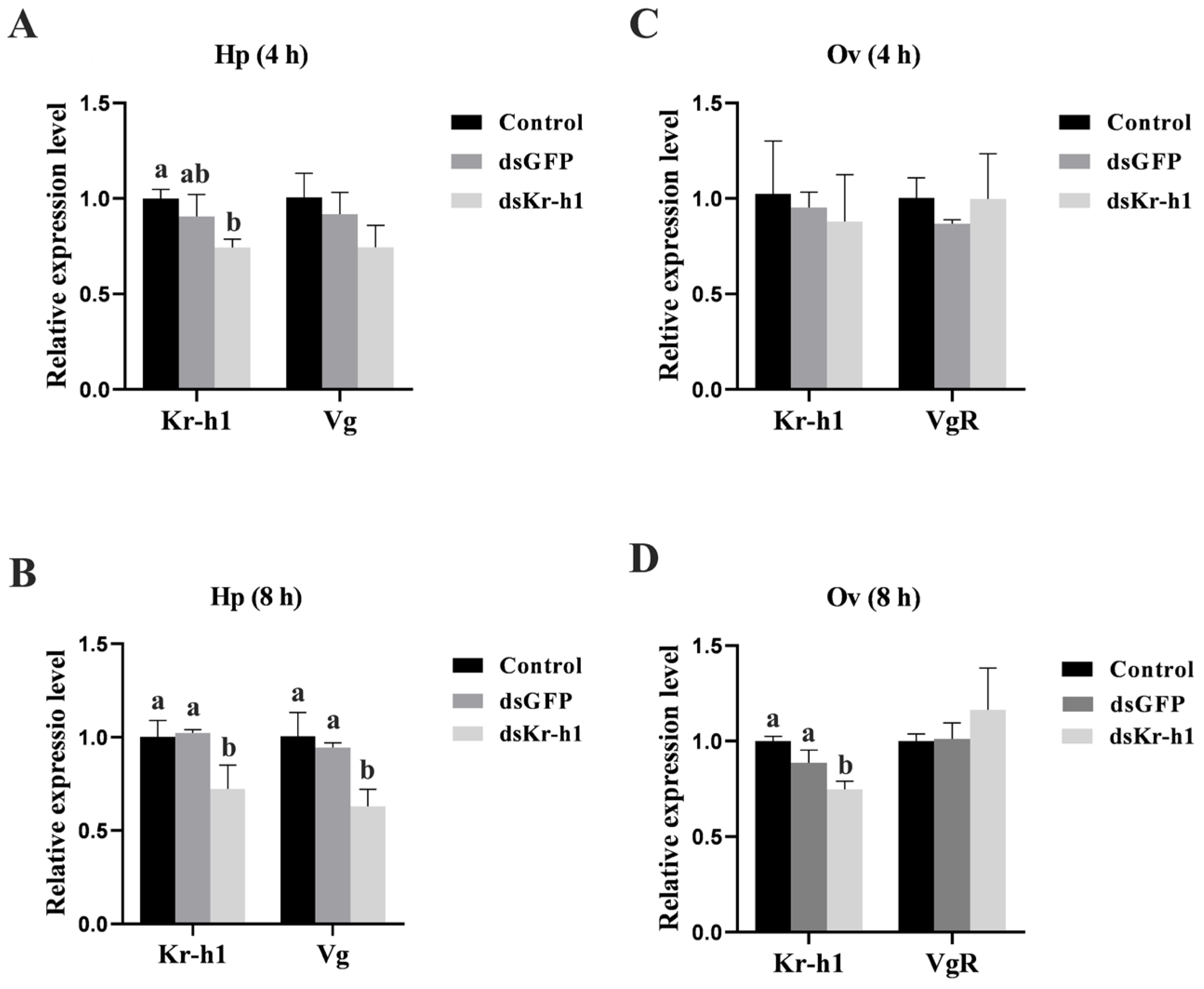
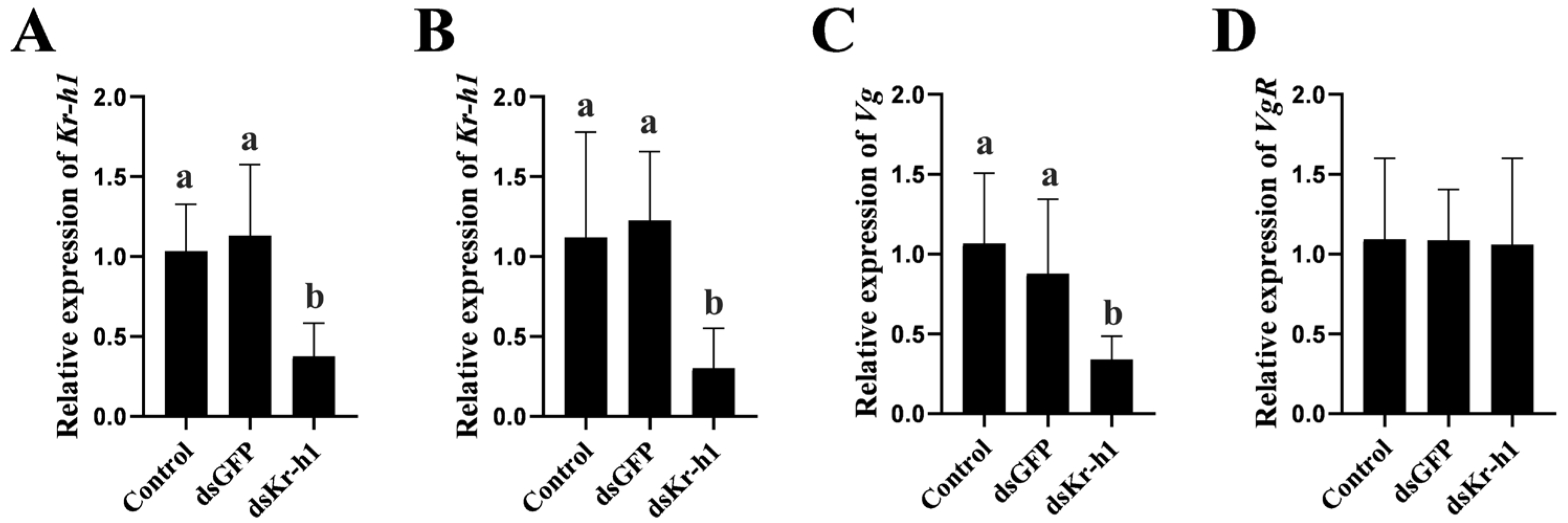
2.6. In Vitro and In Vivo Knockdown of Sp-Kr-h1 on Vitellogenesis
2.7. Histological Observation
2.8. Statistical Analyses
3. Results
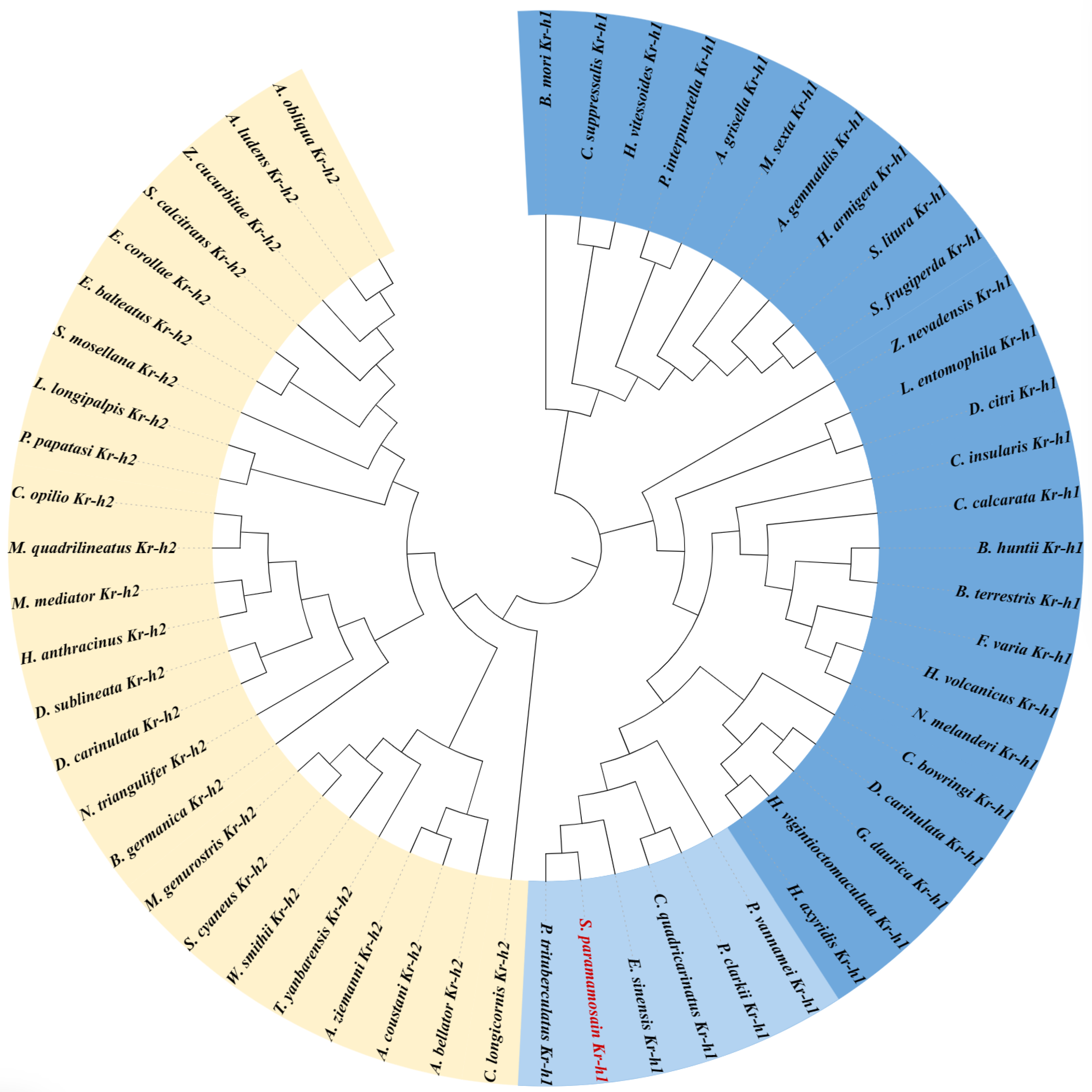
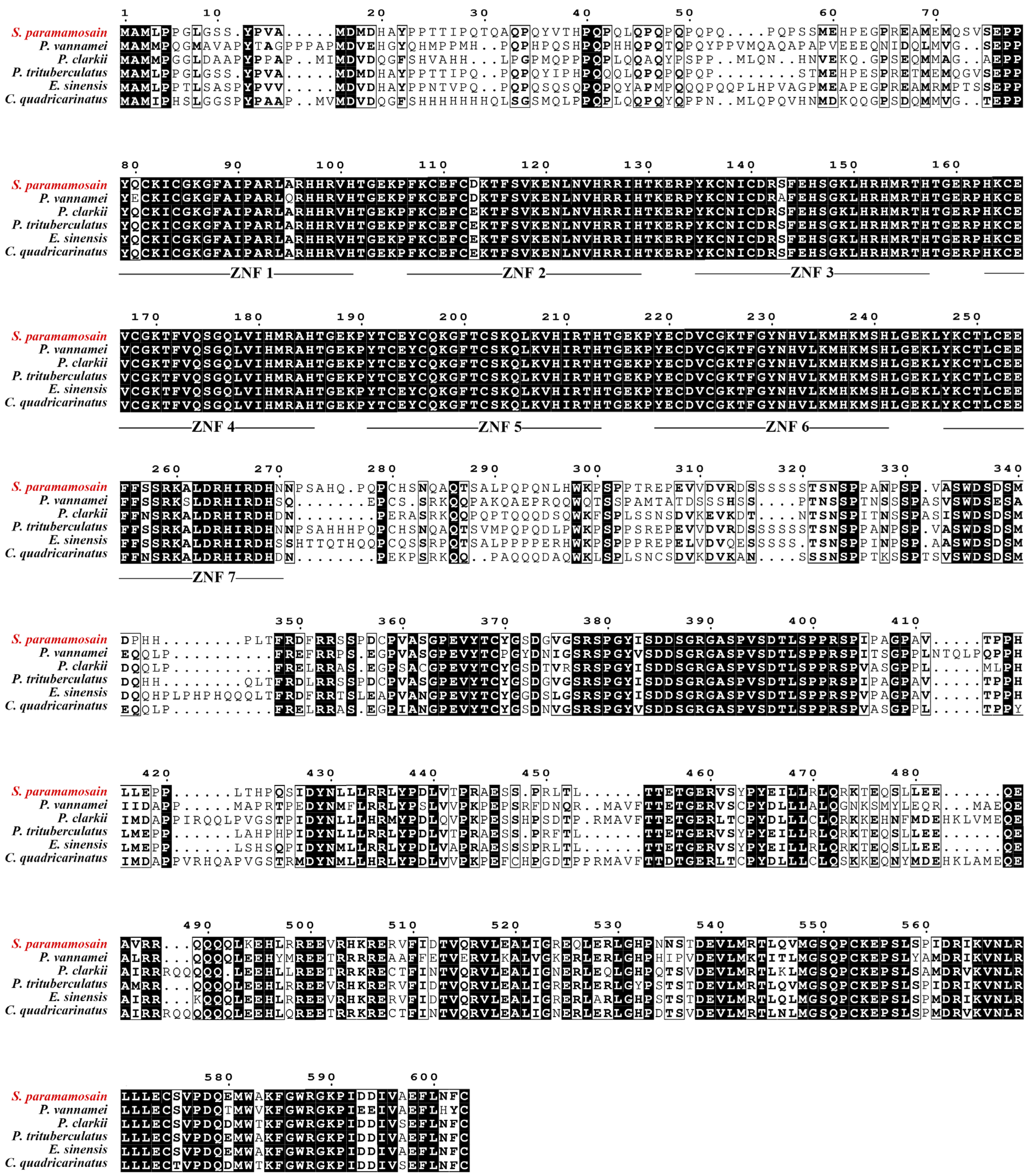
3.1. cDNA Cloning and Sequence Analysis
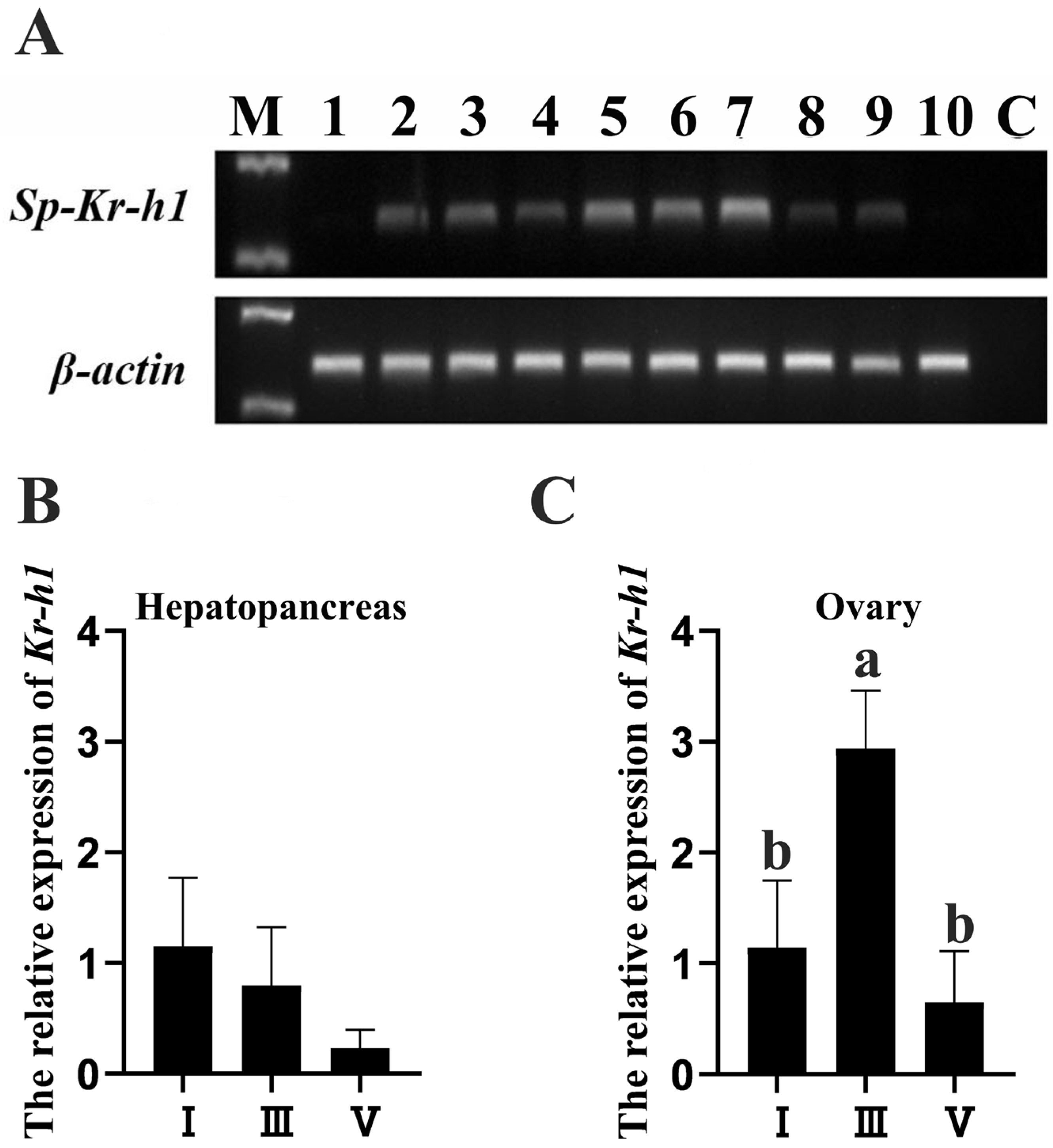
3.2. Expression Profiles of Kr-h1 in the Female Mud Crab
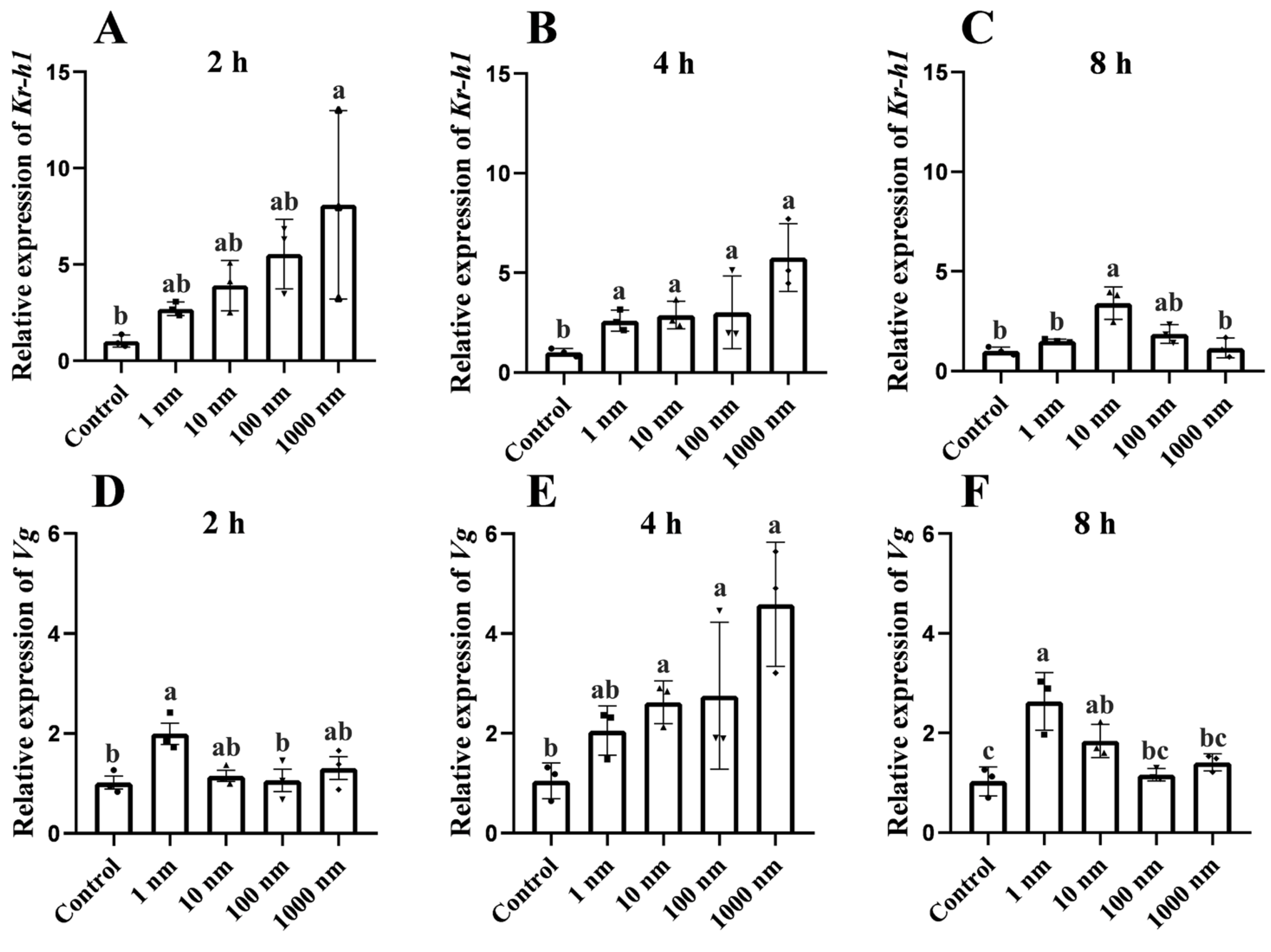
3.3. In Vitro Effect of MF on Kr-h1 Expression and Vitellogenesis of the Mud Crab
3.4. Effect of MF Injection on Kr-h1 Expression and Vitellogenesis of the Mud Crab
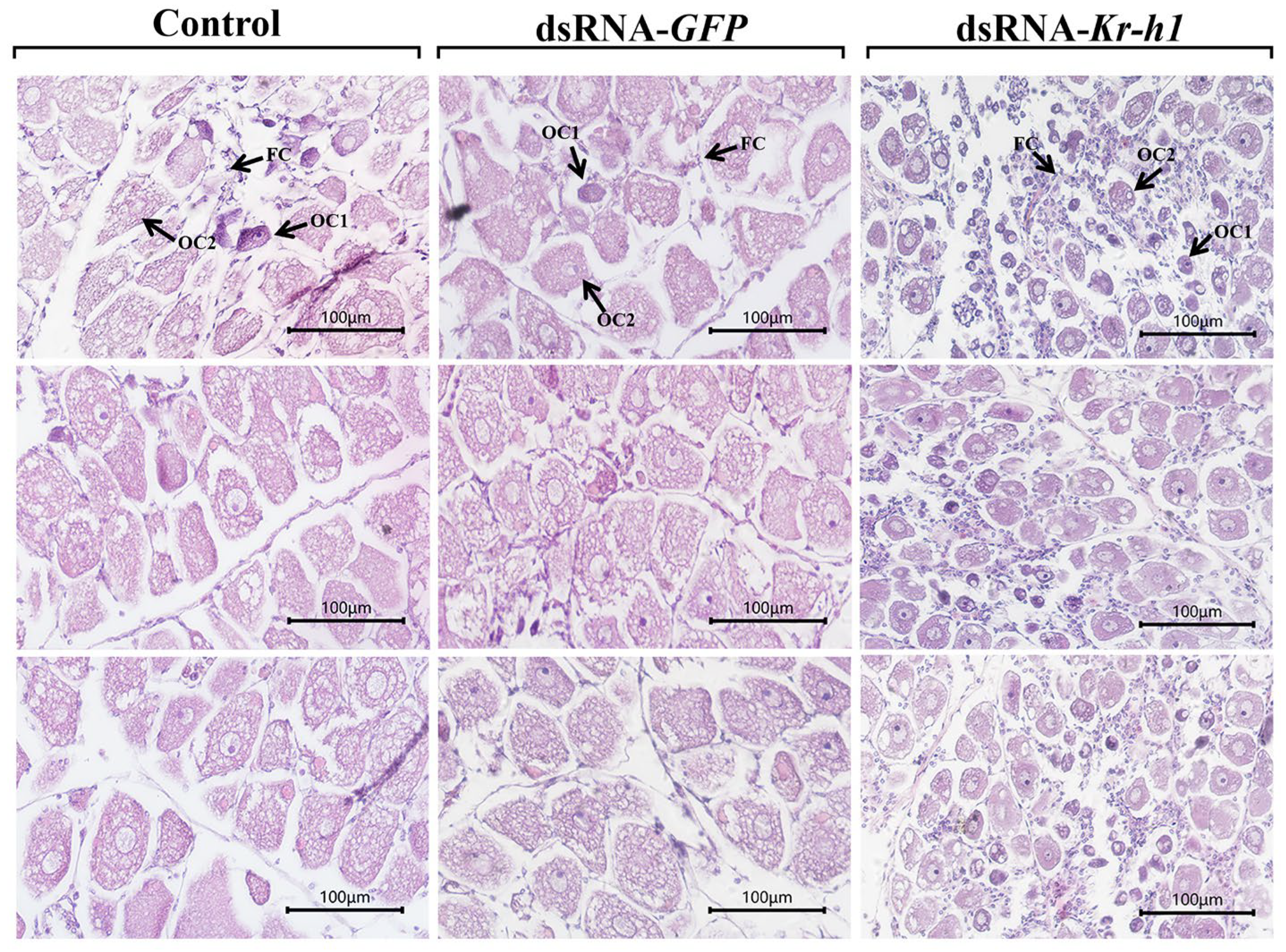
3.5. Effect of Kr-h1 Silencing on Ovarian Development in the Mud Crab
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Kr-h1 | Krüppel homolog 1 |
| JH | juvenile hormone |
| MF | methyl famesoate |
| Met | Methoprene-tolerant |
| ORF | open reading frame |
| RT-PCR | reverse transcription PCR |
| CPS | crustacean physiological saline |
| PFA | paraformaldehyde |
Appendix A
References
- Roy, S.; Saha, T.T.; Zou, Z.; Raikhel, A.S. Regulatory Pathways Controlling Female Insect Reproduction. Annu. Rev. Entomol. 2018, 63, 489–511. [Google Scholar] [CrossRef] [PubMed]
- Jindra, M.; Palli, S.R.; Riddiford, L.M. The juvenile hormone signaling pathway in insect development. Annu. Rev. Entomol. 2013, 58, 181–204. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Bendena, W.G.; Tobe, S.S.; Hui, J.H.L. Juvenile hormone and sesquiterpenoids in arthropods: Biosynthesis, signaling, and role of MicroRNA. J. Steroid. Biochem. Mol. Biol. 2018, 184, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Hatem, N.E.; Wang, Z.; Nave, K.B.; Koyama, T.; Suzuki, Y. The role of juvenile hormone and insulin/TOR signaling in the growth of Manduca sexta. BMC Biol. 2015, 13, 44. [Google Scholar] [CrossRef]
- Ferreira, H.M.; Da Silva, R.C.; Do Nascimento, F.S.; Wenseleers, T.; Oi, C.A. Reproduction and fertility signalling under joint juvenile hormone control in primitively eusocial Mischocyttarus wasps. Chemoecology 2022, 32, 105–116. [Google Scholar] [CrossRef]
- Shianiou, G.; Teloni, S.; Apidianakis, Y. Intestinal immune deficiency and juvenile hormone signaling mediate a metabolic trade-off in adult Drosophila females. Metabolites 2023, 13, 340. [Google Scholar] [CrossRef]
- Laufer, H.; Borst, D.; Baker, F.C.; Reuter, C.C.; Tsai, L.W.; Schooley, D.A.; Carrasco, C.; Sinkus, M. Identification of a juvenile hormone-like compound in a crustacean. Science 1987, 235, 202–205. [Google Scholar] [CrossRef]
- Jo, Q.T.; Laufer, H.; Biggers, W.J.; Kang, H.S. Methyl farnesoate induced ovarian maturation in the spider crab, Libinia emarginata. Invertebr. Reprod. Dev. 1999, 36, 79–85. [Google Scholar] [CrossRef]
- Borst, D.W.; Laufer, H.; Landau, M.; Chang, E.S.; Hertz, W.A.; Baker, F.C.; Schooley, D.A. Methyl farnesoate and its role in crustacean reproduction and development. Insect Biochem. 1987, 17, 1123–1127. [Google Scholar] [CrossRef]
- Abdu, U.; Takac, P.; Laufer, H.; Sagi, A. Effect of methyl farnesoate on late larval development and metamorphosis in the prawn Macrobrachium rosenbergii (Decapoda, Palaemonidae): A juvenoid-like effect? Biol. Bull. 1998, 195, 112–119. [Google Scholar] [CrossRef]
- Rodríguez, E.M.; Greco, L.S.L.; Medesani, D.A.; Laufer, H.; Fingerman, M. Effect of methyl farnesoate, alone and in combination with other hormones, on ovarian growth of the red swamp crayfish, Procambarus clarkii, during vitellogenesis. Gen. Comp. Endocrinol. 2002, 125, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.R.; Nagaraju, G.P.C.; Reddy, P.S. Involvement of methyl farnesoate in the regulation of molting and reproduction in the freshwater crab Oziotelphusa senex senex. J. Crustac. Biol. 2004, 24, 511–515. [Google Scholar] [CrossRef]
- Arath Raghavan, S.D.; Ayanath, A. Effect of methyl farnesoate administration on ovarian growth and maturation in the freshwater crab Travancoriana schirnerae. Egypt. J. Aquat. Biol. Fish. 2019, 22, 257–271. [Google Scholar] [CrossRef]
- Olmstead, A.W.; Leblanc, G.A. Juvenoid hormone methyl farnesoate is a sex determinant in the crustacean Daphnia magna. J. Exp. Zool. 2002, 293, 736–739. [Google Scholar] [CrossRef]
- Mu, X.; Leblanc, G.A. Cross communication between signaling pathways: Juvenoid hormones modulate ecdysteroid activity in a crustacean. J. Exp. Zool. Part A Comp. Exp. Biol. 2004, 301, 793–801. [Google Scholar] [CrossRef]
- Minakuchi, C.; Zhou, X.; Riddiford, L.M. Krüppel homolog 1 (Kr-h1) mediates juvenile hormone action during metamorphosis of Drosophila melanogaster. Mech. Dev. 2008, 125, 91–105. [Google Scholar] [CrossRef]
- Minakuchi, C.; Namiki, T.; Shinoda, T. Krüppel homolog 1, an early juvenile hormone-response gene downstream of Methoprene-tolerant, mediates its anti-metamorphic action in the red flour beetle Tribolium castaneum. Dev. Biol. 2009, 325, 341–350. [Google Scholar] [CrossRef]
- Belles, X.; Santos, C.G. The MEKRE93 (Methoprene tolerant-Krüppel homolog 1-E93) pathway in the regulation of insect metamorphosis, and the homology of the pupal stage. Insect Biochem. Mol. Biol. 2014, 52, 60–68. [Google Scholar] [CrossRef]
- Miyakawa, H.; Watanabe, M.; Araki, M.; Ogino, Y.; Miyagawa, S.; Iguchi, T. Juvenile hormone-independent function of Krüppel homolog 1 in early development of water flea Daphnia pulex. Insect Biochem. Mol. Biol. 2018, 93, 12–18. [Google Scholar] [CrossRef]
- Song, J.; Wu, Z.; Wang, Z.; Deng, S.; Zhou, S. Krüppel-homolog 1 mediates juvenile hormone action to promote vitellogenesis and oocyte maturation in the migratory locust. Insect Biochem. Mol. Biol. 2014, 52, 94–101. [Google Scholar] [CrossRef]
- Lozano, J.; Belles, X. Conserved repressive function of Krüppel homolog 1 on insect metamorphosis in hemimetabolous and holometabolous species. Sci. Rep. 2011, 1, 163. [Google Scholar] [CrossRef] [PubMed]
- Saha, T.T.; Roy, S.; Pei, G.; Dou, W.; Zou, Z.; Raikhel, A.S. Synergistic action of the transcription factors Krüppel homolog 1 and Hairy in juvenile hormone/Methoprene-tolerant-mediated gene-repression in the mosquito Aedes aegypti. PLoS Genet. 2019, 15, e1008443. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Yao, Y.; Wang, B. Methoprene-tolerant (Met) and Krüpple-homologue 1 (Kr-h1) are required for ovariole development and egg maturation in the brown plant hopper. Sci. Rep. 2015, 5, 18064. [Google Scholar] [CrossRef]
- Kayukawa, T.; Jouraku, A.; Ito, Y.; Shinoda, T. Molecular mechanism underlying juvenile hormone-mediated repression of precocious larval–adult metamorphosis. Proc. Natl. Acad. Sci. USA 2017, 114, 1057–1062. [Google Scholar] [CrossRef]
- He, Q.; Zhang, Y. Kr-h1, a cornerstone gene in insect life history. Front. Physiol. 2022, 13, 905441. [Google Scholar] [CrossRef]
- Kayukawa, T.; Tateishi, K.; Shinoda, T. Establishment of a versatile cell line for juvenile hormone signaling analysis in Tribolium castaneum. Sci. Rep. 2013, 3, 1570. [Google Scholar] [CrossRef]
- Xie, X.; Liu, M.; Jiang, Q.; Zheng, H.; Zheng, L.; Zhu, D. Role of Kruppel homolog 1 (Kr-h1) in methyl farnesoate-mediated vitellogenesis in the swimming crab Portunus trituberculatus. Gene 2018, 679, 260–265. [Google Scholar] [CrossRef]
- Li, X.; Chen, T.; Jiang, H.; Huang, J.; Huang, M.; Xu, R.; Xie, Q.; Zhu, H.; Su, S. Effects of methyl farnesoate on Krüppel homolog 1 (Kr-h1) during vitellogenesis in the Chinese mitten crab (Eriocheir sinensis). Anim. Reprod. Sci. 2021, 224, 106653. [Google Scholar] [CrossRef]
- Huang, X.; Ye, H.; Huang, H.; Yang, Y.; Gong, J. An insulin-like androgenic gland hormone gene in the mud crab, Scylla paramamosain, extensively expressed and involved in the processes of growth and female reproduction. Gen. Comp. Endocrinol. 2014, 204, 229–238. [Google Scholar] [CrossRef]
- Shinoda, T. Methyl farnesoate. In Handbook of Hormones, 2nd ed.; Hironori, A., Ed.; Academic Press: New York, NY, USA, 2021; pp. 991–992. [Google Scholar]
- Kayukawa, T.; Murata, M.; Kobayashi, I.; Muramatsu, D.; Okada, C.; Uchino, K.; Sezutsu, H.; Kiiuchi, M.; Tamura, T.; Hiruma, K.; et al. Hormonal regulation and developmental role of Krüppel homolog 1, a repressor of metamorphosis, in the silkworm Bombyx mori. Dev. Biol. 2014, 388, 48–56. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, S.; Lin, Z.; Aweya, J.J.; Zheng, Z.; Zhao, Y.; Chen, X.; Li, S.; Zhang, Y. Krüppel homolog 1 modulates ROS production and antimicrobial peptides expression in shrimp hemocytes during infection by the Vibrio parahaemolyticus strain that causes AHPND. Front. Immunol. 2023, 14, 1246181. [Google Scholar] [CrossRef] [PubMed]
- Dale, J.F.; Tobe, S.S. Biosynthesis and titre of juvenile hormone during the first gonotrophic cycle in isolated and crowded Locusta migratoria females. J. Insect Physiol. 1986, 32, 763–769. [Google Scholar] [CrossRef]
- Cai, S.; Liu, H. Biological characteristics and synthesis of vitellogenin in Decapoda, crustacean. J. Fish Chin 2017, 41, 150–158. [Google Scholar]
- Li, K.; Chen, L.; Zhou, Z.; Li, E.; Zhao, X.; Guo, H. The site of vitellogenin synthesis in Chinese mitten-handed crab Eriocheir sinensis. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2006, 143, 453–458. [Google Scholar] [CrossRef]
- Schneider, W.J. Vitellogenin receptors: Oocyte-specific members of the low-density lipoprotein receptor supergene family. Int. Rev. Cytol. 1996, 166, 103–137. [Google Scholar]
- Sappington, T.W.; Raikhel, A.S. Molecular characteristics of insect vitellogenins and vitellogenin receptors. Insect Biochem. Mol. Biol. 1998, 28, 277–300. [Google Scholar] [CrossRef]
- Willnow, T.E. The low-density lipoprotein receptor gene family: Multiple roles in lipid metabolism. J. Mol. Med. 1999, 77, 306–315. [Google Scholar] [CrossRef]
- Ruan, Y.; Wong, N.K.; Zhang, X.; Luo, P.; Chen, T. Vitellogenin receptor (VgR) mediates oocyte maturation and ovarian development in the Pacific white shrimp (Litopenaeus vannamei). Front. Physiol. 2020, 11, 529982. [Google Scholar] [CrossRef]
- Kuang, S.; Tang, Y.; Gao, Q.; He, H.; Ding, W.; Xue, J.; Li, Y.; Qiu, L. Identification of Potential Target Transcription Factor Genes Regulated by Krüppel Homolog 1 in Chilo suppressalis (Lepidoptera: Crambidae). J. Entomol. Sci. 2023, 58, 318–334. [Google Scholar] [CrossRef]
- Zhang, W.N.; Ma, L.; Liu, C.; Chen, L.; Xiao, H.J.; Liang, G.M. Dissecting the role of Krüppel homolog 1 in the metamorphosis and female reproduction of the cotton bollworm, Helicoverpa armigera. Insect Mol. Biol. 2018, 27, 492–504. [Google Scholar] [CrossRef]
- Zhu, Z.; Tong, C.; Qiu, B.; Yang, H.; Xu, J.; Zheng, S.; Song, Q.; Feng, Q.; Deng, H. 20E-mediated regulation of BmKr-h1 by BmKRP promotes oocyte maturation. BMC Biol. 2021, 19, 39. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.B.; Miao, S.Y.; Lu, Y.J.; Wang, S.S.; Wang, Z.Y.; Zhao, Y.R. Involvement of Methoprene-tolerant and Kruppel homolog 1 in juvenile hormone-mediated vitellogenesis of female Liposcelis entomophila (end.) (Psocoptera: Liposcelididae). Arch. Insect Biochem. Physiol. 2022, 112, e21973. [Google Scholar]
- Song, Z.C.; Gao, Y.Q.; Yan, Z.Q.; Zhao, Y.F.; Zhang, A.Y.; Liu, F.H.; Sun, J.H.; Xu, Y.Y.; Tian, H.G.; Li, S.; et al. Krüppel-homolog 1 is key in development and parthenogenetic reproduction in pea aphids. Entomol. Gen. 2025; early access. [Google Scholar] [CrossRef]
- Han, S.P.; Wang, X.Q.; Han, H.; Wang, D.; He, Y.Z. Hairy and Kruppel homolog 1 Comediate the Action of Juvenile Hormone/Methoprene-Tolerant Signaling Pathway in Vitellogenesis of Spodoptera frugiperda (JE Smith). J. Agric. Food Chem. 2025, 73, 1122–1130. [Google Scholar] [CrossRef]
- Smykal, V.; Bajgar, A.; Provaznik, J.; Fexova, S.; Buricova, M.; Takaki, K.; Hodkova, M.; Dolezel, D. Juvenile hormone signaling during reproduction and development of the linden bug, Pyrrhocoris apterus. Insect Biochem. Mol. Biol. 2014, 45, 69–76. [Google Scholar] [CrossRef]
- Gujar, H.; Palli, S.R. Juvenile hormone regulation of female reproduction in the common bed bug, Cimex lectularius. Sci. Rep. 2016, 6, 35546. [Google Scholar] [CrossRef]
| Primer | Primer Sequence (5′-3′) | Application |
|---|---|---|
| Sp-Kr-h1-OF | ATGGCCATGCTGCCG | cDNA cloning |
| Sp-Kr-h1-OR | CTAGCAGAAGTTGAGGAACTC | cDNA cloning |
| Sp-Kr-h1-QF | CTGTGCGAGGAGTTCTTCAG | qRT-PCR |
| Sp-Kr-h1-QR | TCACGAAATGTCAGCGGATG | qRT-PCR |
| Sp-Vg-QF | GAGTGATGATGGAGGTGTCCTG | qRT-PCR |
| Sp-Vg-QR | GACCTTGAGCGATTCTGGTGACGA | qRT-PCR |
| Sp-VgR-QF | TTCTATACCAGGCCACTACC | qRT-PCR |
| Sp-VgR-QR | TTTTCACTCCAAGCACACTC | qRT-PCR |
| β-actin-QF | GAGCGAGAAATCGTTCGTGAC | qRT-PCR |
| β-actin-QR | GGAAGGAAGGCTGGAAGAGAG | qRT-PCR |
| dsKr-h1-F | TAATACGACTCACTATAGGGGGAAGGGCTTTGCTATCCC | RNAi |
| dsKr-h1-R | TAATACGACTCACTATAGGAGCACGTGAACCCCTTCTG | RNAi |
| GFP-F | CACAAGTTCAGCGTGTCCG | RNAi |
| GFP-R | AGTTCACCTTGATGCCGTTC | RNAi |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, Y.; Lu, L.; Gong, S.; Liu, F.; Ye, H. Krüppel Homolog 1 Is Required for the Role of Methyl Farnesoate in Vitellogenesis in the Mud Crab Scylla paramamosain. Fishes 2025, 10, 103. https://doi.org/10.3390/fishes10030103
Lai Y, Lu L, Gong S, Liu F, Ye H. Krüppel Homolog 1 Is Required for the Role of Methyl Farnesoate in Vitellogenesis in the Mud Crab Scylla paramamosain. Fishes. 2025; 10(3):103. https://doi.org/10.3390/fishes10030103
Chicago/Turabian StyleLai, Yongqi, Li Lu, Shaoming Gong, Fang Liu, and Haihui Ye. 2025. "Krüppel Homolog 1 Is Required for the Role of Methyl Farnesoate in Vitellogenesis in the Mud Crab Scylla paramamosain" Fishes 10, no. 3: 103. https://doi.org/10.3390/fishes10030103
APA StyleLai, Y., Lu, L., Gong, S., Liu, F., & Ye, H. (2025). Krüppel Homolog 1 Is Required for the Role of Methyl Farnesoate in Vitellogenesis in the Mud Crab Scylla paramamosain. Fishes, 10(3), 103. https://doi.org/10.3390/fishes10030103







