Size-Dependent Salinity Tolerance and Osmotic Regulation in Juvenile Lateolabrax japonicus
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Acute Toxicity Experiments
2.3. Low Salinity Stress Experiments
2.4. Measurement of Plasma Osmolality and Blood Ion Concentration
2.5. Tissue Enzyme Activity Assay
2.6. Data Analysis
3. Results
3.1. Survival Results
3.2. Osmotic Pressure
3.3. Blood Ion
3.4. Tissue Enzyme Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saoud, I.P.; Kreydiyyeh, S.; Chalfoun, A.; Fakih, M. Influence of salinity on survival, growth, plasma osmolality and gill Na+–K+–ATPase activity in the rabbitfish Siganus rivulatus. J. Exp. Mar. Biol. Ecol. 2007, 348, 183–190. [Google Scholar] [CrossRef]
- Tsuzuki, M.Y.; Sugai, J.K.; Maciel, J.C.; Francisco, C.J.; Cerqueira, V.R. Survival, growth and digestive enzyme activity of juveniles of the fat snook (Centropomus parallelus) reared at different salinities. Aquaculture 2007, 271, 319–325. [Google Scholar] [CrossRef]
- Kombat, E.O.; Abakari, G.; Alhassan, E.H.; Zhao, J.-L. Effect of acute and chronic salinity exposure on the amino acid composition in muscle, intestine and gill tissues of Nile tilapia (Oreochromis niloticus). Aquaculture 2023, 570, 739406. [Google Scholar] [CrossRef]
- Laiz-Carrión, R.; Sangiao-Alvarellos, S.; Guzmán, J.M.; Martín del Río, M.P.; Soengas, J.L.; Mancera, J.M. Growth performance of gilthead sea bream Sparus aurata in different osmotic conditions: Implications for osmoregulation and energy metabolism. Aquaculture 2005, 250, 849–861. [Google Scholar] [CrossRef]
- Mylonas, C.C.; Pavlidis, M.; Papandroulakis, N.; Zaiss, M.M.; Tsafarakis, D.; Papadakis, I.E.; Varsamos, S. Growth performance and osmoregulation in the shi drum(Umbrina cirrosa)adapted to different environmental salinities. Aquaculture 2009, 287, 203–210. [Google Scholar] [CrossRef]
- Babikian, J.; Nasser, N.; Saoud, I.P. Effects of salinity on standard metabolic rate of juvenile marbled spinefoot (Siganus rivulatus). Aquac. Res. 2017, 48, 2561–2566. [Google Scholar] [CrossRef]
- Moorman, B.P.; Inokuchi, M.; Yamaguchi, Y.; Lerner, D.T.; Grau, E.G.; Seale, A.P. The osmoregulatory effects of rearing Mozambique tilapia in a tidally changing salinity. Gen. Comp. Endocrinol. 2014, 207, 94–102. [Google Scholar] [CrossRef]
- Trehern, R.H.; Garg, A.; Bigelow, W.B.; Hauptman, H.; Brooks, A.; Hawkes, L.A.; Van Leeuwen, T.E. Low salinity negatively affects metabolic rate, food consumption, digestion and growth in invasive lionfish Pterois spp. Mar. Ecol. Prog. Ser. 2020, 644, 157–171. [Google Scholar] [CrossRef]
- Shi, H.J.; Li, J.F.; Li, X.Y.; Ru, X.Y.; Huang, Y.; Zhu, C.H.; Li, G.L. Survival pressure and tolerance of juvenile greater amberjack (Seriola dumerili) under acute hypo- and hyper-salinity stress. Aquac. Rep. 2024, 36, 102150. [Google Scholar] [CrossRef]
- Resley, M.J.; Webb, K.A.; Holt, G.J. Growth and survival of juvenile cobia, Rachycentron canadum, at different salinities in a recirculating aquaculture system. Aquaculture 2006, 253, 398–407. [Google Scholar] [CrossRef]
- Garcia, L.M.B.; Garcia, C.M.H.; Pineda, A.F.S.; Gammad, E.A.; Canta, J.; Simon, S.P.D.; Hilomen-Garcia, G.V.; Gonzal, A.C.; Santiago, C.B. Survival and growth of bighead carp fry exposed to Low salinities. Aquac. Int. 1999, 7, 241–250. [Google Scholar] [CrossRef]
- Shui, C.; Zhang, H.M.; Shi, Y.H.; Xie, Y.D.; Liu, Y.S.; Lu, G.H.; Xu, J.B. Effects of salinity on growth, osmophysiology and body composition of juvenile soiuy Liza haematocheila. J. Dalian Ocean. Univ. 2015, 30, 634–640. [Google Scholar] [CrossRef]
- Beyenbach, K.W.; Freire, C.A.; Kinne, R.K.; Kinne-Saffran, E. Epithelial transport of magnesium in the kidney of fish. Miner. Electrolyte Metab. 1993, 19, 241–249. [Google Scholar]
- Piermarini, P.M.; Evans, D.H. Effects of Environmental Salinity on Na+/K+-ATPase in the Gills and Rectal Gland of a Euryhaline Elasmobranch (Dasyatis Sabina). J. Exp. Biol. 2000, 203, 2957–2966. [Google Scholar] [CrossRef]
- Gaumet, F.; Boeuf, G.; Severe, A.; Le Roux, A.; Mayer-Gostan, N. Effects of salinity on the ionic balance and growth of juvenile turbot. J. Fish Biol. 1995, 47, 865–876. [Google Scholar] [CrossRef]
- Nordlie, F.G. Environmental influences on regulation of blood plasma/serum components in teleost fishes: A review. Rev. Fish Biol. Fish. 2009, 19, 481–564. [Google Scholar] [CrossRef]
- Herrera, M.; Vargas-Chacoff, L.; Hachero, I.; Ruíz-Jarabo, I.; Rodiles, A.; Navas, J.I.; Mancera, J.M. Osmoregulatory changes in wedge sole (Dicologoglossa cuneata Moreau, 1881) after acclimation to different environmental salinities. Aquac. Res. 2009, 40, 762–771. [Google Scholar] [CrossRef]
- Al-Jandal, N.J.; Wilson, R.W. A comparison of osmoregulatory responses in plasma and tissues of rainbow trout (Oncorhynchus mykiss) following acute salinity challenges. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2011, 159, 175–181. [Google Scholar] [CrossRef]
- Tian, X.; Wang, G.; Dong, S.; Fang, J.; Liu, Y. Effects of salinity on plasma osmolality and gill Na+/K+-ATPase activity of the tongue Sole (Cynoglossus semilaevis). Mar. Sci. 2011, 35, 27–31. [Google Scholar]
- Liu, X.; Shi, B.; Liu, Y.; Zhang, Y.; Gao, Q.; Xu, Y.; Wang, B.; Jiang, Y.; Song, X. Effects of sharp changes in salinity on osmotic regulation function in juvenile yellowtail kingfish Seriolaaureovittata. J. Dalian Fish. Univ. 2019, 34, 767–775. [Google Scholar] [CrossRef]
- Sampaio, L.s.A.; Bianchini, A. Salinity effects on osmoregulation and growth of the euryhaline flounder Paralichthys orbignyanus. J. Exp. Mar. Biol. Ecol. 2002, 269, 187–196. [Google Scholar] [CrossRef]
- Stewart, H.A.; Noakes, D.L.G.; Cogliati, K.M.; Peterson, J.T.; Iversen, M.H.; Schreck, C.B. Salinity effects on plasma ion levels, cortisol, and osmolality in Chinook salmon following lethal sampling. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2016, 192, 38–43. [Google Scholar] [CrossRef]
- Zhang, C.J.; Shi, Z.H.; Wang, J.G.; Gao, Q.X. On salinity-related effects on osmoregulation mechanism in marine teleost. Mar. Fish. 2013, 35, 108–116. [Google Scholar] [CrossRef]
- Tian, L.; Tan, P.; Yang, L.; Zhu, W.L.; Xu, D.D. Effects of salinity on the growth, plasma ion concentrations, osmoregulation, non-specific immunity, and intestinal microbiota of the yellow drum (Nibea albiflora). Aquaculture 2020, 528, 735470. [Google Scholar] [CrossRef]
- Xu, L.W.; Liu, G.F.; Wang, R.X.; Su, Y.L.; Guo, Z.X.; Feng, J. Effects of abrupt salinity stress on osmoregulation of juvenile Rachycentron canadum. Chin. J. Appl. Ecol. 2007, 18, 1596–1600. [Google Scholar]
- Becker, A.; Gonçalves, J.; Toledo, J.; Burns, M.; Garcia, L.; Vieira, J.; Baldisserotto, B. Plasma ion levels of freshwater and marine/estuarine teleosts from Southern Brazil. Neotrop. Ichthyol. 2010, 9, 895–900. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Saad, M.F.; Shukry, M.; El-Keredy, A.M.S.; Nasif, O.; Van Doan, H.; Dawood, M.A.O. Physiological and ion changes of Nile tilapia (Oreochromis niloticus) under the effect of salinity stress. Aquac. Rep. 2021, 19, 100567. [Google Scholar] [CrossRef]
- Lopina, O.D.; Bukach, O.V.; Sidorenko, S.V.; Klimanova, E.A. Na+, K+-ATPase As a Polyfunctional Protein. Biochem. Suppl. Ser. A Membr. Cell Biol. 2022, 16, 207–216. [Google Scholar] [CrossRef]
- Huang, S.; Dong, W.; Lin, X.; Bian, J. Na+/K+-ATPase: Ion pump, signal transducer, or cytoprotective protein, and novel biological functions. Neural Regen. Res. 2024, 19, 2684–2697. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Wen, H.S.; Qi, X.; Zhang, K.Q.; Liu, Y.; Fan, H.Y.; Yu, P.; Tian, Y.; Li, Y. Na+-K+-ATPase and nka genes in spotted sea bass (Lateolabrax maculatus) and their involvement in salinity adaptation. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2019, 235, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.C.; Chu, K.F.; Yang, W.K.; Lee, T.H. Na+, K+-ATPase β1 subunit associates with α1 subunit modulating a “higher-NKA-in-hyposmotic media” response in gills of euryhaline milkfish, Chanos chanos. J. Comp. Physiol. B 2017, 187, 995–1007. [Google Scholar] [CrossRef]
- Hwang, P.-P.; Lee, T.-H. New insights into fish ion regulation and mitochondrion-rich cells. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2007, 148, 479–497. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.B.; Zhao, F.; Wang, S.K.; Zhang, T.; Yang, G.; Miao, Z.F.; Zuang, P. Habitat traits of Lateolabrax japonicus in different subhabitats of Yangtze River Estuary. South China Fish. Sci. 2021, 17, 1–8. [Google Scholar]
- Song, J.Y.; Zhang, C.X.; Wang, L.; Song, K.; Hu, S.C.; Zhang, L. Effects of dietary calcium levels on growth and tissue mineralization in Japanese seabass, Lateolabrax japonicus. Aquac. Nutr. 2017, 23, 637–648. [Google Scholar] [CrossRef]
- Xu, H.; Dong, X.; Zuo, R.; Mai, K.; Ai, Q. Response of juvenile Japanese seabass (Lateolabrax japonicus) to different dietary fatty acid profiles: Growth performance, tissue lipid accumulation, liver histology and flesh texture. Aquaculture 2016, 461, 40–47. [Google Scholar] [CrossRef]
- Bae, S.E.; Kim, J.-K.; Kim, J.H. Evidence of incomplete lineage sorting or restricted secondary contact in Lateolabrax japonicus complex (Actinopterygii: Moronidae) based on morphological and molecular traits. Biochem. Syst. Ecol. 2016, 66, 98–108. [Google Scholar] [CrossRef]
- Li, Y.H.; Zhou, D.L.; Liu, W.; Feng, W.; Chen, C.H.; Guo, J.Y.; Luo, Z.P.; Wu, Q.F. Research on the industrialized aquaculture technology of pondperch in coastal low salinity areas of China. Anim. Breed. Feed. 2024, 23, 32–38. [Google Scholar] [CrossRef]
- Han, Z.Q.; Han, G.; Wang, Z.Y.; Shui, B.N.; Gao, T.X. The genetic divergence and genetic structure of two closely related fish species Lateolabrax maculatus and Lateolabrax japonicus in the Northwestern Pacific inferred from AFLP markers. Genes Genom. 2015, 37, 471–477. [Google Scholar] [CrossRef]
- Jia, P.; Jia, K.T.; Chen, L.M.; Le, Y.; Jin, Y.L.; Zhang, J.; Zhu, L.M.; Zhang, L.; Yi, M.S. Identification and characterization of the melanoma differentiation—Associated gene 5 in sea perch, Lateolabrax japonicus. Dev. Comp. Immunol. 2016, 61, 161–168. [Google Scholar] [CrossRef]
- Carneiro, M.D.D.; Medeiros, R.S.d.; Monserrat, J.M.; Rodrigues, R.V.; Sampaio, L.A. Growth and oxidative stress of clownfish Amphiprion ocellaris reared at different salinities. Fishes 2024, 9, 30. [Google Scholar] [CrossRef]
- Galkanda-Arachchige, H.S.C.; Davis, R.P.; Nazeer, S.; Ibarra-Castro, L.; Davis, D.A. Effect of salinity on growth, survival, and serum osmolality of red snapper, Lutjanus campechanus. Fish Physiol. Biochem. 2021, 47, 1687–1696. [Google Scholar] [CrossRef]
- Becker, A.G.; Baldisserotto, B. Chapter 12—Osmotic and ionic regulation. In Biology and Physiology of Freshwater Neotropical Fish; Baldisserotto, B., Urbinati, E.C., Cyrino, J.E.P., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 273–285. [Google Scholar]
- Seale, A.P.; Breves, J.P. Endocrine and osmoregulatory responses to tidally-changing salinities in fishes. Gen. Comp. Endocrinol. 2022, 326, 114071. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, Y.; Zhang, L.Z.; Zhuang, P.; Liu, J.Y. Survival, growth, food conversion efficiency and plasma osmolality of juvenile Siganus guttatus (Bloch, 1787): Experimental analyses of salinity effects. Fish Physiol. Biochem. 2013, 39, 1025–1030. [Google Scholar] [CrossRef]
- Liang, W.S. The osmotic pressure of the internal environment in the human body and the factors influencing it. Biol. Teach. 2020, 45, 72–73. [Google Scholar]
- LeBreton, G.T.O.; Beamish, F.W.H. The influence of salinity on ionic concentrations and osmolarity of blood serum in lake sturgeon, Acipenser fulvescens. Environ. Biol. Fishes 1998, 52, 477–482. [Google Scholar] [CrossRef]
- Huang, M.; Gao, Q.; Yang, X.; Jiang, W.; Hao, L.; Yu, Y.; Tian, Y. Free amino acids in response to salinity changes in fishes: Relationships to osmoregulation. Fish Physiol. Biochem. 2023, 49, 1031–1042. [Google Scholar] [CrossRef] [PubMed]
- Cataldi, E.; Ciccotti, E.; Dimarco, P.; Disanto, O.; Bronzi, P.; Cataudella, S. Acclimation trials of juvenile Italian sturgeon to different salinities: Morpho-physiological descriptors. J. Fish Biol. 1995, 47, 609–618. [Google Scholar] [CrossRef]
- Karnaky, K.J.J. Structure and function of the chloride cell of Fundulus heteroclitus and other teleosts. Am. Zool. 1986, 26, 209–224. [Google Scholar] [CrossRef]
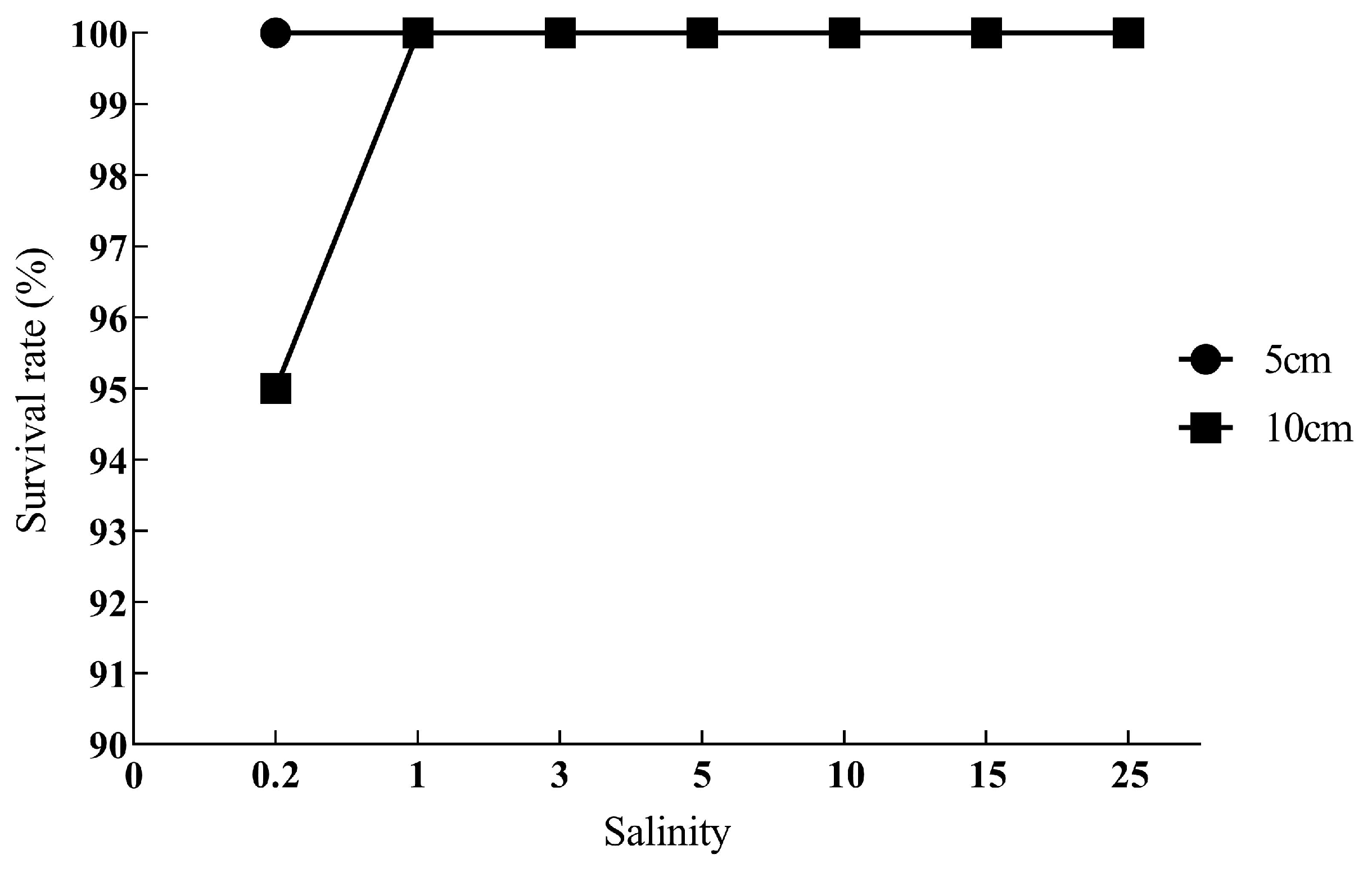
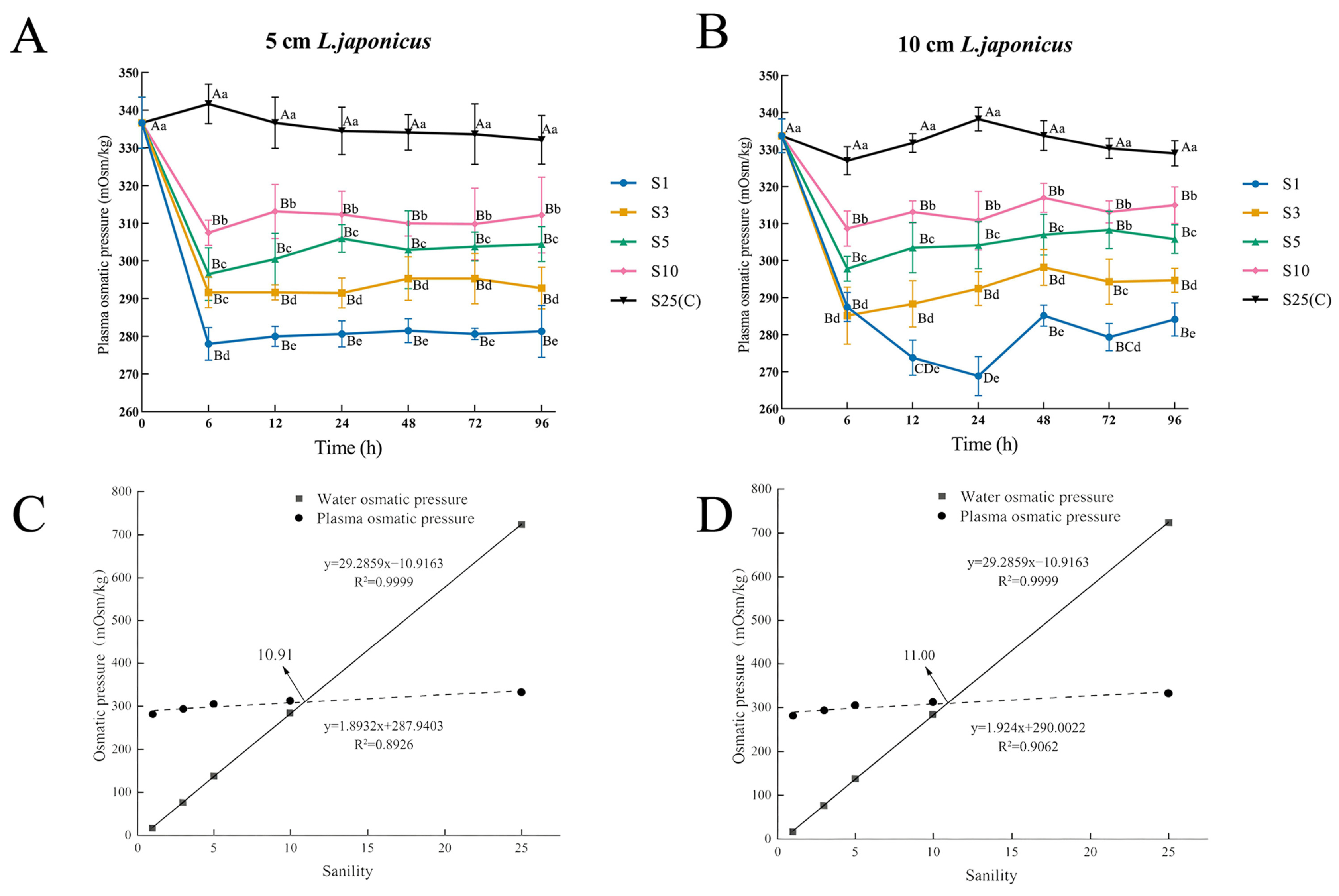
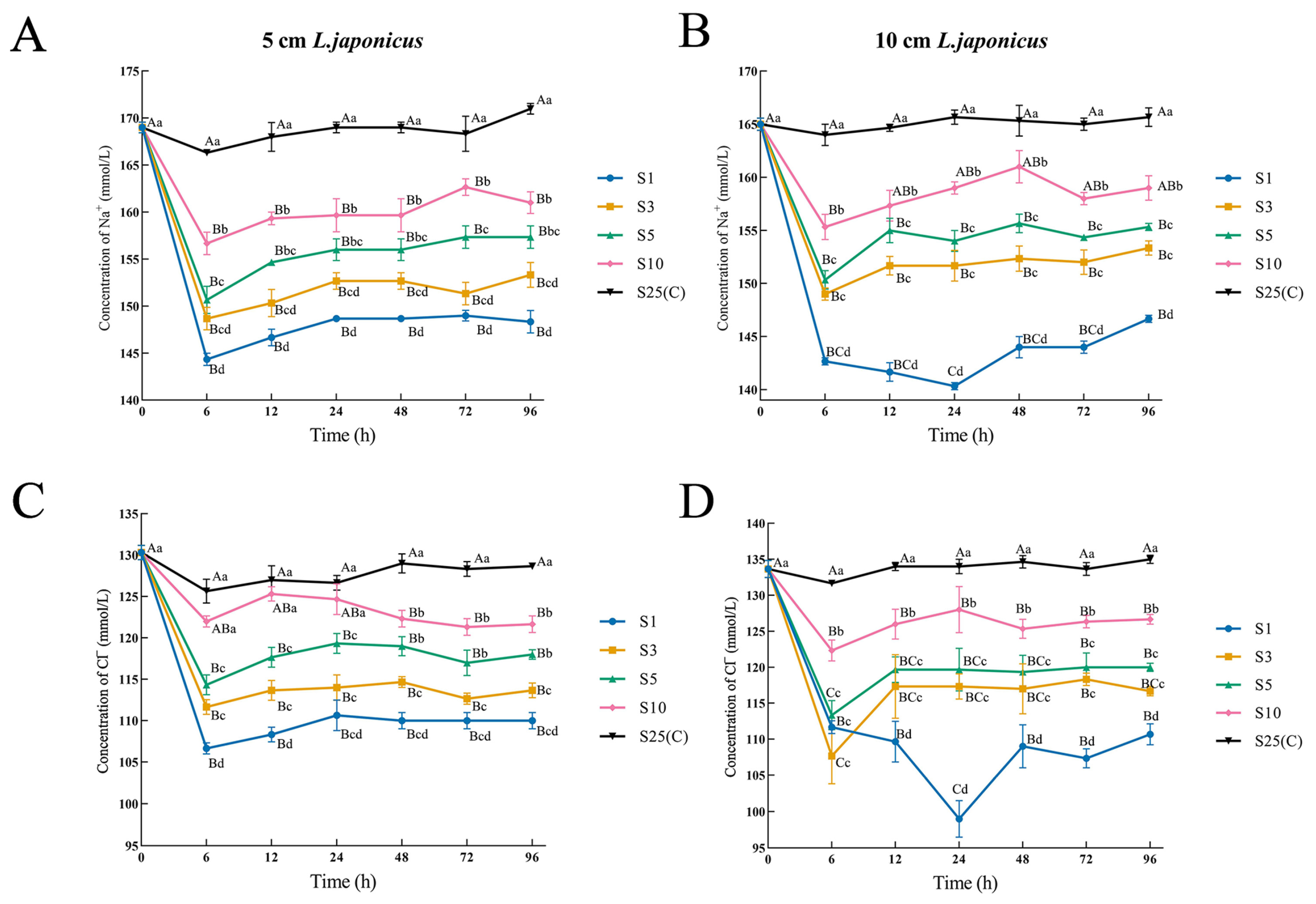
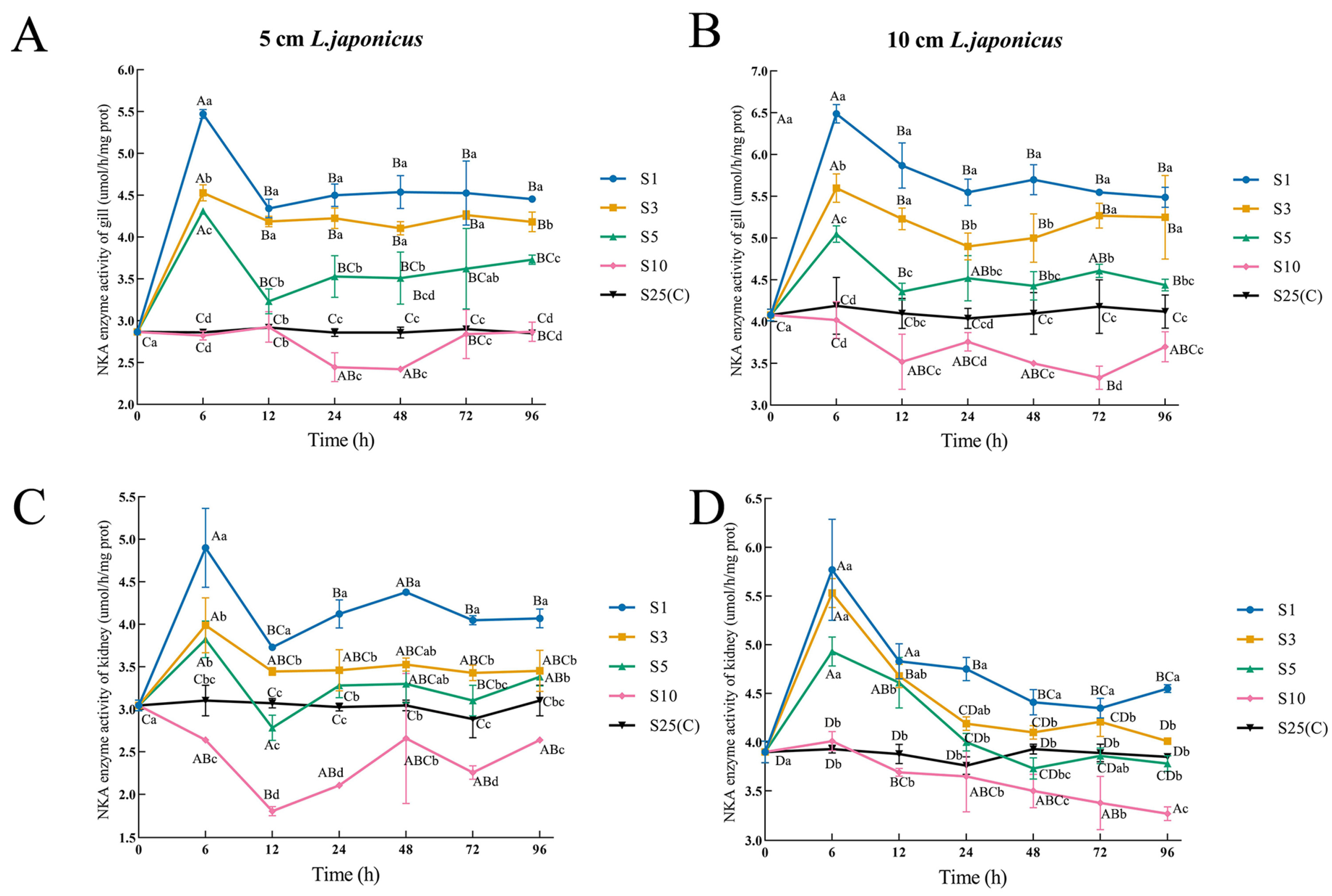
| Salinity | 12 h | 24 h | 48 h | 72 h | 96 h |
|---|---|---|---|---|---|
| Number of Deaths/Individuals | Number of Deaths/Individuals | Number of Deaths/Individuals | Number of Deaths/Individuals | Number of Deaths/Individuals | |
| 0.2 | 0 | 1 | 1 | 0 | 1 |
| 1 | 0 | 0 | 0 | 0 | 0 |
| 3 | 0 | 0 | 0 | 0 | 0 |
| 5 | 0 | 0 | 0 | 0 | 0 |
| 10 | 0 | 0 | 0 | 0 | 0 |
| 15 | 0 | 0 | 0 | 0 | 0 |
| 25 | 0 | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, P.; Yuan, Z.; Ma, Y.; Li, Y.; Yao, Z.; Zhou, K.; Sun, Z.; Wei, Y.; Liu, H.; Yang, F.; et al. Size-Dependent Salinity Tolerance and Osmotic Regulation in Juvenile Lateolabrax japonicus. Fishes 2025, 10, 600. https://doi.org/10.3390/fishes10120600
Gao P, Yuan Z, Ma Y, Li Y, Yao Z, Zhou K, Sun Z, Wei Y, Liu H, Yang F, et al. Size-Dependent Salinity Tolerance and Osmotic Regulation in Juvenile Lateolabrax japonicus. Fishes. 2025; 10(12):600. https://doi.org/10.3390/fishes10120600
Chicago/Turabian StyleGao, Pengcheng, Zhichang Yuan, Yanwu Ma, Yiming Li, Zongli Yao, Kai Zhou, Zhen Sun, Yuxing Wei, Hong Liu, Fan Yang, and et al. 2025. "Size-Dependent Salinity Tolerance and Osmotic Regulation in Juvenile Lateolabrax japonicus" Fishes 10, no. 12: 600. https://doi.org/10.3390/fishes10120600
APA StyleGao, P., Yuan, Z., Ma, Y., Li, Y., Yao, Z., Zhou, K., Sun, Z., Wei, Y., Liu, H., Yang, F., Li, Y., & Lai, Q. (2025). Size-Dependent Salinity Tolerance and Osmotic Regulation in Juvenile Lateolabrax japonicus. Fishes, 10(12), 600. https://doi.org/10.3390/fishes10120600





