Abstract
Microplastics threaten freshwater fish health, with probiotics potentially alleviating this stress. This study subjected Opsariichthys bidens fry to 10 μg/L polystyrene microplastics (diameter 100 μm) for seven days, then established control (CK, 0 CFU/g), low (LC, 1 × 107 CFU/g), medium (MC, 1 × 108 CFU/g), and high (HC, 1 × 109 CFU/g) Bacillus coagulans supplementation groups for a 56−day rearing experiment. Results indicated that microplastic stress significantly reduced intestinal digestive enzyme activity in O. bidens and induced oxidative stress. Following stress removal, probiotic-treated groups exhibited markedly superior growth performance compared to the control (CK). Notably, the high-concentration probiotic group (HC) demonstrated intestinal trypsin levels approaching pre-stress levels, with no significant differences in liver antioxidant capacity (T−AOC, CAT, SOD) compared to pre-stress levels (p > 0.05). Furthermore, compared to the control group (CK), probiotic-treated fish exhibited upregulated growth- and immune-related genes (igf, ghr, tnf−α, il−1β), alongside optimized gut microbiota composition, characterized by increased abundance of Bacillus and decreased abundance of Pseudomonas and Aeromonas. This study demonstrates that B. coagulans alleviates microplastic-induced stress in O. bidens, offering insights for aquatic ecosystem conservation and biological remediation of microplastic pollution.
Keywords:
Chinese hooksnout carp; microplastic pollution; Bacillus coagulans; intestinal microbial community Key Contribution:
This study confirms that Bacillus coagulans alleviates microplastic stress on juvenile Opsariichthys bidens via enhancing growth, antioxidant function, gene regulation, and gut microbiota optimization. It offers a reference for using probiotics in aquatic microplastic mitigation and ecological protection.
1. Introduction
Global annual plastic consumption exceeds 348 million tons. These non-degradable materials decompose into microplastics (diameter < 5 mm) through physical, chemical, and biological processes in the natural environment [1]. Microplastics can enter various aquatic environments through the water cycle. They not only cause physical abrasion to aquatic organisms, impairing their feeding capacity, reducing their range of movement, and diminishing their energy reserves, but also accumulate within their bodies. This leads to a series of physiological damages, including endocrine disruption, decreased enzyme activity, and gut microbiota imbalance [2,3,4,5].
Probiotics, as beneficial active microorganisms capable of colonizing the host and improving its microbial community composition, demonstrate significant application potential in aquaculture [6]. Research indicates that probiotics not only maintain host gut microbiota balance, regulate mucosal vitality, and enhance systemic immune function [7], but also improve aquaculture water quality and mitigate the negative effects of environmental stress on aquatic organisms [8]. Among these, Bacillus coagulans, a common probiotic strain, has been demonstrated in studies involving Oncorhynchus mykiss and Cyprinus carpio to enhance growth performance, boost antioxidant capacity, and optimize intestinal microecology [9,10]. However, its role and underlying mechanisms in alleviating microplastic−induced functional damage in O. bidens remain unclear.
Chinese hooksnout carp (Opsariichthys bidens Günther, 1873), a typical stream fish belonging to the order Cypriniformes, family Cyprinidae, and genus Opsariichthys, is characterized by rapid growth, strong reproductive capacity, and high nutritional value. Widely distributed throughout the main and tributary rivers of China, it constitutes a vital component of freshwater ecosystems [11]. However, its small size renders it more susceptible to the toxic effects of microplastics, making it a vulnerable victim of microplastic pollution. Current research on the impact of microplastics on the physiological functions and gut microbiota of O. bidens remains limited, necessitating targeted investigations.
This study investigated the restorative effects of probiotics on the physiological functions of O. bidens subjected to microplastic stress by incorporating B. coagulans at varying concentrations into their feed. The research aimed to elucidate the underlying mechanisms of action. It provides scientific evidence and novel approaches for employing biological methods, such as probiotics, to mitigate microplastic pollution, protect aquatic ecosystems, and safeguard biological health.
2. Materials and Methods
2.1. Experimental Materials
A total of 120 juvenile O. bidens (body length: 42.08 ± 1.4 mm; body weight: 1.17 ± 0.19 g) were purchased from Zhejiang Yulaoda Agricultural Technology Co., Ltd. (Quzhou, China) and temporarily housed in the recirculating aquaculture system of the School of Life Sciences at Zhejiang Normal University (comprising multiple glass tanks; dimensions: 40 cm × 45 cm × 50 cm; daily water change percentage: 500%) for seven days to acclimatize to the environment. During the acclimation and experimental periods, water quality parameters were measured using a water quality analyzer (Zhanjiang, China): water temperature, 28 ± 1 °C; dissolved oxygen, 7.5 ± 0.06 mg/L; ammonia nitrogen, 0.37 ± 0.09 mg/L; and nitrite, 0.0064 ± 0.0004 mg/L. The photoperiod was controlled as 12 h light and 12 h dark throughout the experiment. The probiotic used in the experiment was B. coagulans powder (Shandong Zhongke Jiayi Biotechnology Co., Ltd., Shandong, China, 1 × 1010 CFU/g). Microplastics were polystyrene (PS) microspheres with a diameter of 100 μm (Hangzhou Shengli Chemical Co., Ltd., Hangzhou, China) [12]. The basal feed was a commercial diet specifically formulated for O. bidens (Zhejiang Yixiang Biotechnology Co., Ltd., Huzhou, China) with a crude protein content of 42.40% (see Table 1 for detailed formulation and proximate composition).
2.2. Experimental Design and Feed Preparation
All experimental fish were first subjected to 10 μg/L PS microplastic stress for seven days, then randomly assigned to four treatment groups (three replicates per group, totaling 12 rearing units, each containing 30 fish). They were fed diets containing different concentrations of B. coagulans: control group (CK, diet without B. coagulans), low-concentration group (LC, diet with 1×107 CFU/g B. coagulans), medium-concentration group (MC, diet with 1 × 108 CFU/g B. coagulans), and high-concentration group (HC, diet with 1 × 109 CFU/g B. coagulans). The B. coagulans addition levels referenced the experimental results from Wang et al. [13]. Feed preparation followed the method of Feng et al. [14]: B. coagulans was sprayed onto the surface of the basal feed at corresponding concentrations (1 × 107, 1 × 108, 1 × 109 CFU/g). After air-drying at room temperature, the feed was portioned into light-protected, sealed bags and stored at 4 °C (the base feed composition is detailed in Table 1). Weekly monitoring confirmed that probiotic activity in the supplemented feed remained stable (LC group: 0.97–0.99 × 107 CFU/g; MC group: 0.97–0.99 × 108 CFU/g; HC group: 0.97–0.99 × 109 CFU/g). During the rearing period, feeding occurred twice daily at 8:00 and 18:00, with a daily feed intake amounting to 5% of the total fish body weight. Uneaten feed was removed after one hour. The experimental cycle lasted 56 days.

Table 1.
Formulation and proximate composition of the experimental diets for O. bidens (unit: %).
Table 1.
Formulation and proximate composition of the experimental diets for O. bidens (unit: %).
| Ingredient (%) | Composition |
|---|---|
| Peruvian fish meal | 25.00 |
| Antarctic krill meal | 5.00 |
| Soybean | 28.00 |
| Enzymatic hydrolysis of soybeans | 5.00 |
| American chicken powder | 5.00 |
| High-gluten flour | 21.00 |
| Fish oil | 2.00 |
| Soybean oil | 2.00 |
| Yeast powder | 2.00 |
| 1 vitamin and mineral premixes | 2.00 |
| Calcium dihydrogen phosphate | 2.00 |
| Total | 100.00 |
| Proximate composition (%) | |
| Crude protein | 42.40 |
| Crude fat | 8.28 |
| Crude ash | 16.00 |
| Moisture | 10.00 |
1 vitamin and mineral premixes provide the following ingredients (g/kg diet): vitamin A 2.4 g; vitamin D 0.45 g; vitamin E 30 g; vitamin K3 1.56 g; vitamin B1 1.03 g; vitamin B2 1.88 g; vitamin B6 2.35 g; vitamin B12 203 g; vitamin C 42.86 g; niacin 10 g; pantothenic acid 6.06 g; folic acid 0.51 g; biotin 3.75 g; inositol 20.71 g; antioxidant 1 g; rice husk 300 g; Cu 4 g; Fe 36.67 g; Mn 3.14 g; Zn 14.49 g; Mg 61.54 g; I 9.71 g; Se 2.73 g; Co 25 g; zeolite powder 600 g; choline chloride 100 g; triglyceride butyrate 50 g; high-efficiency palatogen 50 g; and bentonite 416 g.
2.3. Sample Collection
Samples were collected at the following three time points: before microplastic stress (NP, non−stressed control group), after microplastic stress (HP, end of the stress period), and after the 56−day feeding experiment. At each sampling time, six fish were randomly selected from each group, anesthetized with MS−222 (100 mg/L), measured for body length with a vernier caliper (accuracy 0.01 mm), and measured for body weight with an electronic balance (accuracy 0.01 g), with basic growth data recorded. After being placed on an ice tray, the liver and intestines were quickly dissected and separated (removing surface fat from the intestines and rinsing the contents). The tissue samples were frozen in liquid nitrogen and stored at −80 °C for later use.
2.4. Determination of Physiological and Biochemical Parameters
Liver tissues were used to determine total antioxidant capacity (T−AOC), superoxide dismutase (SOD) activity, catalase (CAT) activity, and malondialdehyde (MDA) content. Intestinal tissues (with contents removed) were used to determine the content of α−amylase (AMY), trypsin (TPS), and lipase (LPS). All assays were performed using commercial kits (Nanjing Jiancheng Bioengineering Research Institute, Nanjing, China) in accordance with the manufacturer’s instructions.
2.5. Gut Microbiota Composition and Diversity Analysis
After the 56-day feeding experiment, an additional six fish were randomly selected from each of the four groups. After starvation for 24 h, the fish were dissected under sterile conditions, and the middle and posterior intestines (excluding the contents) were taken. The intestinal samples were stored at −80 °C and sent to Guangzhou Jidiao Biotechnology Co., Ltd. for high-throughput sequencing of the 16S rRNA gene amplicon. The analysis included operational taxonomic unit (OTU) composition, alpha diversity (Chao1 index, ACE index), and species relative abundance (at the phylum and genus levels).
2.6. Determination of Expression Levels of Growth- and Immunity-Related Genes
Total RNA was extracted from liver samples using the RNAiso Plus kit (TaKaRa, Kusatsu, Japan). The quality and concentration of total RNA were analyzed by agarose gel electrophoresis and spectrophotometry, respectively. Qualified RNA was reverse-transcribed into cDNA using the PrimeScript™ RT Reagent Kit (TaKaRa, Kusatsu, Japan). Primers were designed with Primer Premier 6 software according to the transcriptome data from laboratory. O. bidens; the specific primers for growth- and immunity-related genes are listed in Table 2, and primer synthesis was performed by Tsingke Biotechnology Co., Ltd. (Beijing, China). Quantitative real-time polymerase chain reaction (qRT−PCR) was conducted using TB Green® Premix Ex Taq™ II (Tli RNaseH Plus) (TaKaRa, Kusatsu, Japan). The reaction system (total volume) consisted of 10 μL of TB Green Premix Ex Taq™ II (Tli RNaseH Plus) (2×), 0.8 μL of forward primer, 0.8 μL of reverse primer, 2 μL of cDNA template, and 6.4 μL of sterile water. The reaction conditions were set as follows: denaturation at 95 °C for 15 s, annealing at 60 °C for 30 s, with 40 cycles in total. The relative expression levels of target genes were quantified on a real-time fluorescence quantitative PCR instrument using the 2−ΔΔCt method.

Table 2.
Information of qPCR primers for growth- and immunity-related genes in O. bidens.
2.7. Calculation of Growth Performance Indicators
The formulas for calculating relevant indicators are as follows:
where W1 = initial body weight of each fish before the experiment (g); W2 = final body weight of each fish at the end of the experiment (g); L = final body length of each fish at the end of the experiment (cm); W3 = liver weight of each fish at the end of the experiment (g); and W4 = visceral weight of each fish at the end of the experiment (g).
Weight growth rate (WGR) = [(W2 − W1)/W1] × 100%;
Condition factor (CF) = (W2/L3) × 100%;
Hepatosomatic index (HSI) = W3/W1 × 100%;
Viscerosomatic index (VSI) = W4/W1 × 100%;
2.8. Data Analysis
Data are expressed as mean ± standard deviation (S.D.). The Shapiro–Wilk test and Levene’s test were employed to assess normality of distribution and homogeneity of variance, respectively. Differences between multiple groups were evaluated using one-way analysis of variance (ANOVA), followed by Tukey’s post hoc test. Statistical significance was set at p < 0.05, with significant differences defined as p < 0.01. All statistical analyses were performed using IBM SPSS Statistics 26 software (Chicago, IL, USA).
3. Results
3.1. Growth Performance
Experimental results indicated that at the initial stage of the feeding trial, there were no significant differences in the initial body length and weight among the various groups of O. bidens (p > 0.05). Upon conclusion of the feeding trial, the body length, body weight, and weight growth rate of the LC, MC, and HC groups were significantly higher than those of the CK group (p < 0.05). Among these, the HC group exhibited the highest body length (58.80 ± 6.60 mm), body weight (2.36 ± 0.73 g), and weight gain rate (99.92 ± 4.42%). The LC and MC groups ranked second, with no significant difference between them (p > 0.05). No significant differences were observed among groups in CF, HIS, or VSI (p > 0.05) (Table 3).

Table 3.
Effects of dietary B. coagulans supplementation on growth performance indicators of O. bidens after microplastic stress.
3.2. Digestive Enzyme Activity
Microplastic stress significantly reduced the contents of digestive enzymes such as lipase, trypsin, and α−amylase in the intestines of O. bidens (p < 0.05). After the microplastic stress was alleviated, the activities of digestive enzymes such as lipase, trypsin, and α−amylase in the intestines of O. bidens in the probiotic-supplemented groups increased, but there was no significant difference compared with the NP group and the CK group (p > 0.05), except for the α−amylase content in the CK, LC, and HC groups and the trypsin content in the HC group, which were significantly higher than those in the HP group (p < 0.05). There was no significant difference in the contents of digestive enzymes between the other groups and the HP group (p > 0.05) (Figure 1).
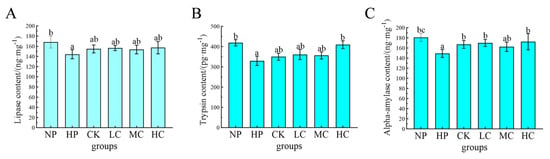
Figure 1.
Effects of dietary B. coagulans supplementation on intestinal digestive enzyme contents of O. bidens after microplastic stress. (A) Lipase; (B) trypsin; (C) α−amylase. NP: non-microplastic stress control; HP: end of the microplastic stress period; CK: control group (without B. coagulans); LC: low−concentration group (1 × 107 CFU/g B. coagulans); MC: medium−concentration group (1 × 108 CFU/g B. coagulans); and HC: high−concentration group (1 × 109 CFU/g B. coagulans). Different letters indicate statistically significant differences between the two groups (p < 0.05).
3.3. Antioxidant Capacity
Microplastic stress significantly increased the activities of T−AOC, CAT, and SOD in the liver of O. bidens (p < 0.05), and the content of MDA also significantly increased (p < 0.05), indicating that the organism was in an oxidative stress state and the antioxidant defense mechanism was activated. After the microplastic stress was relieved, the antioxidant indicators such as T−AOC, SOD, and MDA in the liver of O. bidens in the probiotic-supplemented groups were significantly lower than those in the HP group (p < 0.05), while there was no significant difference in the oxidative indicators between the CK group and the HP group. Moreover, the contents of T−AOC, CAT, and MDA in the MC group and the HC group were not significantly different from those in the NP group (Figure 2).
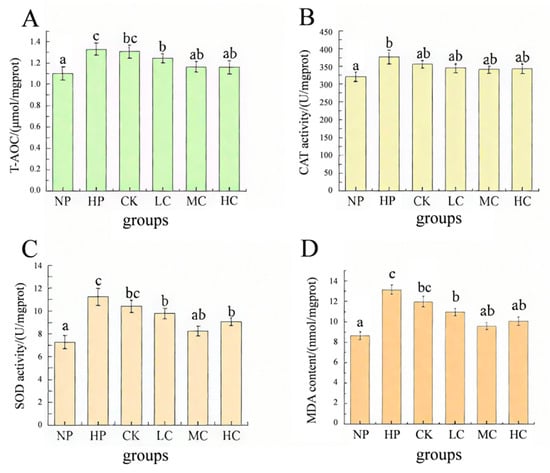
Figure 2.
Effects of dietary B. coagulans supplementation on liver antioxidant indicators of O. bidens after microplastic stress. (A) T−AOC; (B) CAT; (C) SOD; (D) MDA. NP: non-microplastic stress control; HP: end of the microplastic stress period; CK: control group (without B. coagulans); LC: low−concentration group (1 × 107 CFU/g B. coagulans); MC: medium−concentration group (1 × 108 CFU/g B. coagulans); and HC: high−concentration group (1 × 109 CFU/g B. coagulans). Different letters indicate statistically significant differences between the two groups (p < 0.05).
3.4. Expression of Growth- and Immunity-Related Genes
Except for the ghr gene in the LC and MC groups, which showed no significant difference from that in the control group, the expression levels of growth- and immunity-related genes (igf, ghr, tnf−α, il−1β) in the probiotic-treated groups of O. bidens were significantly higher than those in the CK group (p < 0.05) (Figure 3).
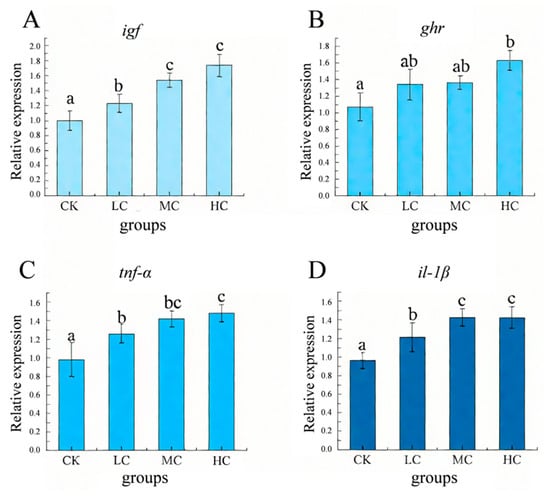
Figure 3.
Effects of dietary B. coagulans supplementation on relative expression levels of growth- and immunity-related genes (igf, ghr, tnf−α, il−1β) in O. bidens after microplastic stress. (A) igf: insulin-like growth factor, (B) ghr: growth hormone receptor, (C) tnf−α: tumor necrosis factor α, and (D) il−1β: interleukin1β. CK: control group (without B. coagulans); LC: low-concentration group (1 × 107 CFU/g B. coagulans); MC: medium-concentration group (1 × 108 CFU/g B. coagulans); and HC: high-concentration group (1 × 109 CFU/g B. coagulans). Different letters indicate statistically significant differences between the two groups (p < 0.05).
3.5. Gut Microbiota Composition and Diversity
OTU Venn analysis of gut microbiota showed that the CK group had 116 unique OTUs, the LC group had 26, the MC group had 28, and the HC group had 15 (Figure 4A). The number of shared OTUs between the LC and CK groups was eleven, between the MC and CK groups was six, and between the HC and CK groups was three. The Chao1 and ACE indices (indicators of microbial abundance) in the probiotic-treated groups were significantly lower than those in the CK group (p < 0.05), with the lowest abundance observed in the HC group (Figure 4B,C).
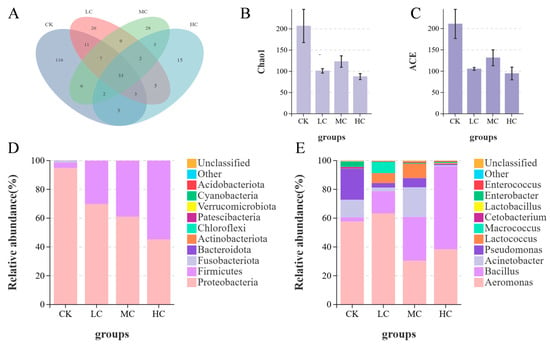
Figure 4.
Effects of dietary B. coagulans supplementation on intestinal microbiota composition and diversity of O. bidens after microplastic stress. (A) VENN diagram of horizontal species; (B) Chao1 index analysis chart; (C) ACE index analysis chart; (D) Horizontal composition analysis of microbiota phylum; (E) Analysis of horizontal composition of microbial communities. CK: control group (without B. coagulans); LC: low-concentration group (1 × 107 CFU/g B. coagulans); MC: medium-concentration group (1 × 108 CFU/g B. coagulans); and HC: high-concentration group (1 × 109 CFU/g B. coagulans).
At the phylum level, the relative abundance of Proteobacteria decreased, the relative abundance of Firmicutes increased, and Fusobacteria almost disappeared with increasing probiotic concentration (Figure 4D). At the genus level, the relative abundance of Bacillus in the probiotic-treated groups was significantly higher than that in the CK group (p < 0.05), with the highest abundance in the HC group (Figure 4E). Additionally, the LC and MC groups had a higher relative abundance of Lactococcus, while the relative abundance of Pseudomonas and Aeromonas in all probiotic-treated groups was significantly lower than that in the CK group (p < 0.05).
4. Discussion
4.1. Effects of Probiotics on Growth and Digestive Enzymes of O. bidens
Under microplastic stress, supplementing probiotics in the feed exerted a restorative effect on the growth of O. bidens. Specifically, the body length and weight of O. bidens in all probiotic-supplemented groups (at different concentrations) were significantly higher than those in the control group (p < 0.05), which is consistent with the findings of Fan Ying et al. [9] in their research on O. mykiss. Microplastic stress significantly reduced the digestive enzyme content in the intestinal tract of O. bidens (p < 0.05). After the alleviation of microplastic stress, the contents of intestinal digestive enzymes (including lipase, trypsin, and α−amylase) in the probiotic-supplemented groups increased; however, no significant differences were observed when compared with the NP group and CK group (p > 0.05). This result indicates that probiotic supplementation has a limited effect on restoring the intestinal digestive enzyme content of O. bidens following microplastic stress. A potential explanation for this phenomenon is that microplastics can cause intestinal obstruction and induce a sense of satiety, thereby interfering with the secretion of digestive enzymes [15]. In contrast, probiotics primarily function by indirectly regulating the intestinal microenvironment [16] and the expression of growth- and immunity-related genes [17,18]. While probiotics cannot fully counteract the direct damage caused by microplastics, they can mitigate its effects to some extent.
Gene expression analysis further supported this conclusion: the expression levels of igf and ghr (key regulators of growth) in the HC group were significantly upregulated (p < 0.05), indicating that B. coagulans may promote growth by activating the growth regulatory pathway. It is hypothesized that metabolites of B. coagulans (e.g., short-chain fatty acids, SCFAs) can stimulate the activity of growth hormone receptors through endocrine signaling, thereby enhancing protein synthesis and cell proliferation [19]. Additionally, the high relative abundance of B. coagulans and Lactococcus in the gut of probiotic-treated O. bidens may further contribute to growth: these bacteria secrete digestive enzymes and metabolites that regulate intestinal pH, improve nutrient absorption efficiency, and ultimately synergistically promote growth recovery [20].
4.2. Effects of Probiotics on the Antioxidant Capacity of O. bidens
Microplastic stress induced a significant increase in T−AOC, CAT, and SOD activities, as well as MDA content in O. bidens (p < 0.05), indicating that microplastics trigger oxidative stress in the fish. This finding aligns with the study by Duan et al. [21], who demonstrated that microplastics disrupt the homeostasis of the antioxidant system by promoting free radical accumulation, leading to abnormal elevation in antioxidant enzyme activity and potential impairment of immune function [22].After supplementation with B. coagulans, the HC group showed the most significant recovery in antioxidant capacity: T−AOC, CAT, and SOD showed no significant difference compared to pre-stress levels (p < 0.05), confirming that B. coagulans can mitigate oxidative damage under microplastic stress.
The underlying mechanism may involve two aspects: first, food-borne B. coagulans produces organic acids during fermentation, which exhibit antioxidant activity by scavenging excess free radicals and inhibiting lipid peroxidation chain reactions [23,24]; second, probiotics may enhance the endogenous antioxidant defense system by regulating the expression of antioxidant enzyme genes. Liu et al. [25] reported that Bacillus subtilis upregulates the mRNA expression of tight junction proteins (Zo−1, claudin, and occludin) in the jejunal mucosa to improve intestinal barrier function, which may indirectly reduce the entry of oxidative stress-inducing substances into the bloodstream. Notably, SOD activity in the HC group was slightly higher than that in the MC group (p < 0.05). This may be due to the colonization of B. coagulans in the gut: its secreted metabolites (e.g., butyric acid and antioxidant peptides) can bind to Keap1 protein, activate the Nrf2−Keap1 signaling pathway, and further enhance antioxidant capacity [26].
4.3. Effects of Probiotics on Gut Microbiota of O. bidens
The gut is a critical organ for nutrient absorption, and the composition and balance of gut microbiota are essential for host health and growth [27]. After microplastic stress, the gut microbiota of O. bidens was dominated by Proteobacteria, Firmicutes, and Fusobacteria. Among these, Proteobacteria contained a high abundance of pathogenic genera (e.g., Aeromonas, which causes bacterial septicemia in fish, and Pseudomonas, which induces visceral white spot disease [28,29,30]), while Firmicutes (which includes beneficial genera such as Lactococcus and Bacillus that enhance growth and inhibit pathogens [31]) had a low relative abundance. This suggests that microplastics disrupt the stability of the gut microbiota in O. bidens. Venn analysis and alpha diversity indices (Chao1 and ACE) showed that high-concentration probiotics significantly reduced gut microbiota diversity (p < 0.05) Specifically, the LC and MC groups had a higher relative abundance of Lactococcus, while the MC and HC groups had significantly lower relative abundances of Aeromonas and Pseudomonas compared with the CK and LC groups (p < 0.05). The relative abundance of Bacillus in the LC, MC, and HC groups was 15.37%, 30.51%, and 57.7%, respectively—far higher than the 3.05% in the CK group—confirming the successful colonization of B. coagulans in the gut of O. bidens and significantly increased the relative abundance of beneficial bacteria. This result is consistent with the study by Sharifuzzaman et al. [32]
The antibacterial mechanism of B. coagulans may involve two pathways: (1) secretion of bacteriocins, which disrupt the integrity of pathogenic bacterial cell membranes and inhibit their proliferation; and (2) production of SCFAs (e.g., butyric acid), which lower intestinal pH, inhibit the growth of acid-sensitive pathogens (e.g., Pseudomonas), and enhance the intestinal epithelial barrier to reduce pathogen translocation [33,34]. Although probiotic intervention reduced microbial diversity (decreased Chao1 index, p < 0.05), the colonization of Bacillus may compensate for this loss through metabolic complementarity (e.g., vitamin synthesis) [35]. Chandrasekaran et al. [36] also reported that high-concentration probiotics maintain gut homeostasis through a “core functional microbiota” even when diversity is reduced.
4.4. Potential Role of Probiotics in Alleviating Microplastic-Induced Digestive Impairment
Microplastic stress significantly suppressed the digestive capacity of O. bidens, with markedly reduced intestinal digestive enzyme activity—especially trypsin (p < 0.05). This suggests that microplastics may disrupt intestinal epithelial cells through physical damage or chemical toxicity, thereby inhibiting enzyme secretion. Concurrently, the activities of antioxidant indicators T−AOC, SOD, and CAT increased significantly (p < 0.05), whilst MDA content rose extremely significantly (p < 0.01). This indicates that microplastics induce free radical accumulation, triggering oxidative stress that leads to lipid peroxidation and cell membrane damage.
Following exposure to microplastics, the gut microbiota exhibited an increased proportion of the Proteobacteria phylum, alongside elevated abundances of pathogenic bacteria such as Pseudomonas and Aeromonas, thereby heightening disease susceptibility. Following B. coagulans intervention, both the MC and HC groups demonstrated significant effects in increasing body length and weight while alleviating oxidative stress. Furthermore, gut microbiota composition was optimized, suggesting that intestinal probiotics contribute to a degree of microplastic degradation within O. bidens. This hypothesis is supported by previous studies: Park et al. [37] cultured Bacillus subtilis in a medium with polyethylene (PE) as the sole carbon source for 60 days and observed a 14.7% reduction in PE dry weight; Bozkurt et al. [38] confirmed that Bifidobacterium can use polypropylene (PP) as an energy and carbon source. Additionally, certain bacterial strains secrete degrading enzymes (e.g., laccase and lipase) that induce surface modification and degradation of microplastics [39]. Furthermore, Teng et al. [40] found that probiotics upregulate the expression of tight junction proteins, which prevent microplastics from penetrating the intestinal barrier, reduce the level of pro-inflammatory factors, and inhibit inflammatory responses. This indicates that B. coagulans alleviates microplastic-induced intestinal damage through two complementary mechanisms: enhancing the intestinal barrier and potentially promoting microplastic degradation.
5. Conclusions
Microplastic stress significantly inhibits the growth of O. bidens, reduces its digestive enzyme activity, and triggers inflammation. After alleviating microplastic stress, supplementing B. coagulans in feed can significantly increase the fish’s body length and weight, alleviate oxidative stress, enhance immunity, and optimize intestinal microbiota composition. Though it has little effect on reversing changes in the fish’s intestinal digestive enzyme activity, it still mitigates the negative impacts of microplastics to some extent. Additionally, while the B. coagulans-supplemented group showed significantly improved physiological indicators, this was accompanied by reduced intestinal microbiota diversity. Long-term application may pose ecological safety risks, thus necessitating further optimization of B. coagulans dosage and in-depth analysis of its mechanisms in restoring the physiological functions of O. bidens.
Author Contributions
M.H.: Conceptualization; Writing—Original Draft; Methodology; Software; Data Curation; Investigation; and Formal Analysis. Y.L.: Methodology; Investigation; Software; Data Curation; Validation; and Writing—Review and Editing. L.P.: Validation; Formal Analysis; Supervision; Software; and Writing—Review and Editing. M.W.: Validation; Formal Analysis; Supervision; and Writing—Review and Editing. S.Z.: Funding Acquisition; Writing—Review and Editing; Visualization; Project Administration; Resources; and Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support for this work was provided by Zhejiang Province’s “San nong Jiu fang ” Project (2025SNJF044) (2025SNJF023).
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee of Zhejiang Normal University (Approval No. 20250925331, approval date 20 september 2024). This study adheres to ethical standards, including ethics committee approval and consent procedure, and follows standard biosafety and institutional safety protocols.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lu, Z. The Immunopathological Effects of Microplasticsfrom Zhanjiang Offshore Sediments on Zebrafish. Master’s Thesis, Guangdong Ocean University, Zhanjiang, China, 2021. [Google Scholar]
- Yin, L.Y.; Chen, B.J.; Xia, B.; Shi, X.T.; Qu, K.M. Polystyrene Microplastics Alter the Behavior, Energy Reserve and Nutritional Composition of Marine Jacopever (Sebastes schlegelii). J. Hazard. Mater. 2018, 360, 97–105. [Google Scholar] [CrossRef]
- Qiang, L.Y.; Cheng, J.P. Exposure to Microplastics Decreases Swimming Competence in Larval Zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2019, 176, 226–233. [Google Scholar] [CrossRef]
- Chen, Q.Q.; Lackmann, C.; Wang, W.Y.; Seiler, T.-B.; Hollert, H.; Shi, H.H. Microplastics Lead to Hyperactive Swimming Behaviour in Adult Zebrafish. Aquat. Toxicol. 2020, 224, 105521. [Google Scholar] [CrossRef]
- Qiao, R.X.; Deng, Y.F.; Zhang, S.H.; Wolosker, M.B.; Zhu, Q.D.; Ren, H.Q.; Zhang, Y. Accumulation of Different Shapes of Microplastics Initiates Intestinal Injury and Gut Microbiota Dysbiosis in the Gut of Zebrafish. Chemosphere 2019, 236, 124334. [Google Scholar] [CrossRef]
- Otles, S.; Cagindi, O.; Akcicek, E. Probiotics and Health. Asian Pac. J. Cancer Prev. APJCP 2003, 4, 369–372. [Google Scholar]
- Peng, Y.L. The application effect of compound microbial ecological preparations in aquaculture. Agric. Eng. Technol. 2020, 40, 82–83. [Google Scholar]
- Saengrung, J.; Bunnoy, A.; Du, X.M.; Huang, L.L.; An, R.; Liang, X.G.; Srisapoome, P. Effects of Ribonucleotide Supplementation in Modulating the Growth of Probiotic Bacillus subtilis and the Synergistic Benefits for Improving the Health Performance of Asian Seabass (Lates calcarifer). Fish Shellfish Immunol. 2023, 140, 108983. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Ye, H.B.; Wang, X.L.; Li, L.; Wang, Y.Q.; Diao, J. Effects of Clostridium butyricum and Bacillus coagulans on growth performance, hepatic function and intestinal microbiotain rainbow trout Oncorhynchus mykiss. J. Dalian Ocean Univ. 2019, 34, 198–203. [Google Scholar]
- Chang, X.L.; Chen, Y.Y.; Kang, M.R.; Feng, J.C.; Zhang, J.X. Effects of Bacillus coagulans on the cadmium content, antioxidant capacity andinflammatory response in the liver of Cyprinus carpio under cadmium exposure. J. Fish. China 2023, 47, 152–160. [Google Scholar]
- Du, G.H. Biological characteristics and artificial breeding technology of Opsariichthys bidens. North. Chin. Fish. 2021, 40, 50–52. [Google Scholar]
- Huang, Y.; Li, W.; Dong, K.; Li, X.; Li, W.; Wang, D. Effect of Polystyrene Microplastic Exposure on Individual, Tissue, and Gene Expression in Juvenile Crucian Carp (Carassius auratus). Fishes 2024, 9, 385. [Google Scholar] [CrossRef]
- Wang, N.; Gao, C.; Zhang, P.; Guan, L.; Wang, Y.; Qin, Y.; Li, Y. Effect of Bacillus cereus against Cadmium Induced Hematological Disturbances and Immunosuppression in Carassius auratus gibelio. Fish Shellfish Immunol. 2019, 89, 141–148. [Google Scholar] [CrossRef]
- Feng, J.; Chang, X.; Zhang, Y.; Yan, X.; Zhang, J.; Nie, G. Effects of Lactococcus lactis from Cyprinus carpio L. as Probiotics on Growth Performance, Innate Immune Response and Disease Resistance against Aeromonas hydrophila. Fish Shellfish Immunol. 2019, 93, 73–81. [Google Scholar] [CrossRef]
- Huang, J.N.; Wen, B.; Zhu, J.G.; Zhang, Y.S.; Gao, J.Z.; Chen, Z.Z. Exposure to Microplastics Impairs Digestive Performance, Stimulates Immune Response and Induces Microbiota Dysbiosis in the Gut of Juvenile Guppy (Poecilia reticulata). Sci. Total Environ. 2020, 733, 138929. [Google Scholar] [CrossRef] [PubMed]
- Assan, D.; Kuebutornye, F.K.A.; Hlordzi, V.; Chen, H.P.; Mraz, J.; Mustapha, U.F.; Abarike, E.D. Effects of Probiotics on Digestive Enzymes of Fish (Finfish and Shellfish); Status and Prospects: A Mini Review. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2022, 257, 110653. [Google Scholar] [CrossRef] [PubMed]
- Shadrack, R.S.; Manabu, I.; Koshio, S.; Yokoyama, S.; Zhang, Y.; Mzengereza, K.; El Basuini, M.F.; Dawood, M.A.O. Effects of Single and Mixture Probiotic Supplements on Growth, Digestive Activity, Antioxidative Status, Immune and Growth-Related Genes, and Stress Response of Juvenile Red Sea Bream (Pagrus major). Aquac. Nutr. 2022, 2022, 8968494. [Google Scholar] [CrossRef]
- Munir, M.B.; Hashim, R.; Chai, Y.H.; Marsh, T.L.; Nor, S.A.M. Dietary Prebiotics and Probiotics Influence Growth Performance, Nutrient Digestibility and the Expression of Immune Regulatory Genes in Snakehead (Channa striata) Fingerlings. Aquaculture 2016, 460, 59–68. [Google Scholar] [CrossRef]
- Mann, E.R.; Lam, Y.K.; Uhlig, H.H. Short-Chain Fatty Acids: Linking Diet, the Microbiome and Immunity. Nat. Rev. Immunol. 2024, 24, 577–595. [Google Scholar] [CrossRef]
- Qiu, B.S.; Lin, W.T.; Yan, J.G.; Wu, J.L. Application of Probiotics in Aquaculture. Fish. Sci. 2004, 7, 39–41. [Google Scholar]
- Duan, Y.F.; Xiong, D.L.; Wang, Y.; Zhang, Z.; Li, H.; Dong, H.B.; Zhang, J.S. Toxicological Effects of Microplastics in Litopenaeus vannamei as Indicated by an Integrated Microbiome, Proteomic and Metabolomic Approach. Sci. Total Environ. 2021, 761, 143311. [Google Scholar] [CrossRef]
- Mahmud, F.; Sarker, D.B.; Jocelyn, J.A.; Sang, Q.-X.A. Molecular and Cellular Effects of Microplastics and Nanoplastics: Focus on Inflammation and Senescence. Cells 2024, 13, 1788. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.P.; Wang, Z.; Wang, J.; Zhang, S.B.; Meng, J.; Ding, C.H. Screening of food—Borne Bacillus coagulans and preliminary evaluation of itshypoglycemic and hypolipidemic functions. J. Henan Univ. Technol. Sci. Ed. 2023, 44, 89–97. [Google Scholar]
- Lastochkina, O.; Yuldashev, R.; Avalbaev, A.; Allagulova, C.; Veselova, S. The Contribution of Hormonal Changes to the Protective Effect of Endophytic Bacterium Bacillus subtilis on Two Wheat Genotypes with Contrasting Drought Sensitivities under Osmotic Stress. Microorganisms 2023, 11, 2955. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Yu, J.; Chen, Z.; Zhang, S.Z.; Zhang, Y.; Zhang, L.; Zhang, Y.Y.; Li, J.D.; Ding, L.G.; Wu, J.Q. Isolation of Bacillus cereus and Its Probiotic Effect on Growth Performance, Antioxidant Capacity, and Intestinal Barrier Protection of Broilers. Poult. Sci. 2025, 104, 104944. [Google Scholar] [CrossRef]
- Chen, X.; Zheng, A.J.; Li, S.Z.; Wang, Z.D.; Chen, Z.M.; Chen, J.; Zou, Z.H.; Liang, H.J.; Liu, G.H. Bacillus amyloliquefaciens Regulates the Keap1/Nrf2 Signaling Pathway to Improve the Intestinal (Caco-2 Cells and Chicken Jejunum) Oxidative Stress Response Induced by Lipopolysaccharide (LPS). Antioxidants 2024, 13, 1550. [Google Scholar] [CrossRef]
- Yuan, P.; Hu, X.G.; Zhou, Q.X. Progress on Cumulative Toxicity and Ecological Hazards of Microplastics in Intestinal Tract. J. Xuzhou Inst. Technol. Sci. Ed. 2019, 34, 54–58. [Google Scholar]
- Liu, Y.L.; Lu, H.Y.; Li, H.D.; Gao, Y. Effect of temperature on the composition of intestinal flora of Paramisgurnus dabryanus. China Fish. 2022, 1, 94–97. [Google Scholar]
- Yan, L. Pathogenicity of Fish Pathogen Pseudomonas Plecoglossicida and Preparation of Its Inactivated Vaccine. Master’s Thesis, Hunan Normal University, Changsha, China, 2021. [Google Scholar]
- Sterniša, M.; Purgatorio, C.; Paparella, A.; Mraz, J.; Smole Možina, S. Combination of Rosemary Extract and Buffered Vinegar Inhibits Pseudomonas and Shewanella Growth in Common Carp (Cyprinus carpio). J. Sci. Food Agric. 2020, 100, 2305–2312. [Google Scholar] [CrossRef]
- Hung, A.T.; Lin, S.-Y.; Yang, T.-Y.; Chou, C.-K.; Liu, H.-C.; Lu, J.-J.; Wang, B.; Chen, S.-Y.; Lien, T.-F. Effects of Bacillus coagulans ATCC 7050 on Growth Performance, Intestinal Morphology, and Microflora Composition in Broiler Chickens. Anim. Prod. Sci. 2012, 52, 874. [Google Scholar] [CrossRef]
- Sharifuzzaman, S.M.; Al-Harbi, A.H.; Austin, B. Characteristics of Growth, Digestive System Functionality, and Stress Factors of Rainbow Trout Fed Probiotics Kocuria SM1 and Rhodococcus SM2. Aquaculture 2014, 418–419, 55–61. [Google Scholar] [CrossRef]
- Wang, Y.F.; Cen, C.N.; Liu, F.Q.; Bao, W.C.; Fu, L.L.; Wang, Y.B. Research Progress on the Probiotic Characteristics and Mechanism of Bacillus coagulans. Sci. Technol. Food Ind. 2023, 44, 458–464. [Google Scholar]
- Hays, K.E.; Pfaffinger, J.M.; Ryznar, R. The Interplay between Gut Microbiota, Short-Chain Fatty Acids, and Implications for Host Health and Disease. Gut Microbes 2024, 16, 2393270. [Google Scholar] [CrossRef]
- Abdel-Baki, R.M.M.; Ahmed, M.N.; Barakat, O.S.; Khalafalla, G.M. Enhanced Vitamin B12 Production by Isolated Bacillus Strains with the Application of Response Surface Methodology. BMC Biotechnol. 2024, 24, 90. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, P.; Weiskirchen, S.; Weiskirchen, R. Effects of Probiotics on Gut Microbiota: An Overview. Int. J. Mol. Sci. 2024, 25, 6022. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, C.G. Biodegradation of Micro-Polyethylene Particles by Bacterial Colonization of a Mixed Microbial Consortium Isolated from a Landfill Site. Chemosphere 2019, 222, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, H.S.; Yörüklü, H.C.; Bozkurt, K.; Denktaş, C.; Bozdoğan, A.; Özdemir, O.; Özkaya, B. Biodegradation of Microplastic by Probiotic Bifidobacterium. Int. J. Glob. Warm. 2022, 26, 429. [Google Scholar] [CrossRef]
- Zhu, Z.Y.; Wu, Y.Q.; Fang, X.L.; Zhong, R.Y.; Gong, H.; Yan, M.T. Bacillus subtilis, a Promising Bacterial Candidate for Trapping Nanoplastics during Water Treatment. J. Hazard. Mater. 2025, 483, 136679. [Google Scholar] [CrossRef] [PubMed]
- Teng, X.; Zhang, T.X.; Rao, C.T. Novel Probiotics Adsorbing and Excreting Microplastics in Vivo Show Potential Gut Health Benefits. Front. Microbiol. 2025, 15, 1522794. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).