Extended Photoperiod Exposure Affects Imidacloprid Toxicity on Juvenile Crayfish Procambarus clarkii by Regulating Oxidative Stress and Neuroendocrine Pathways
Abstract
1. Introduction
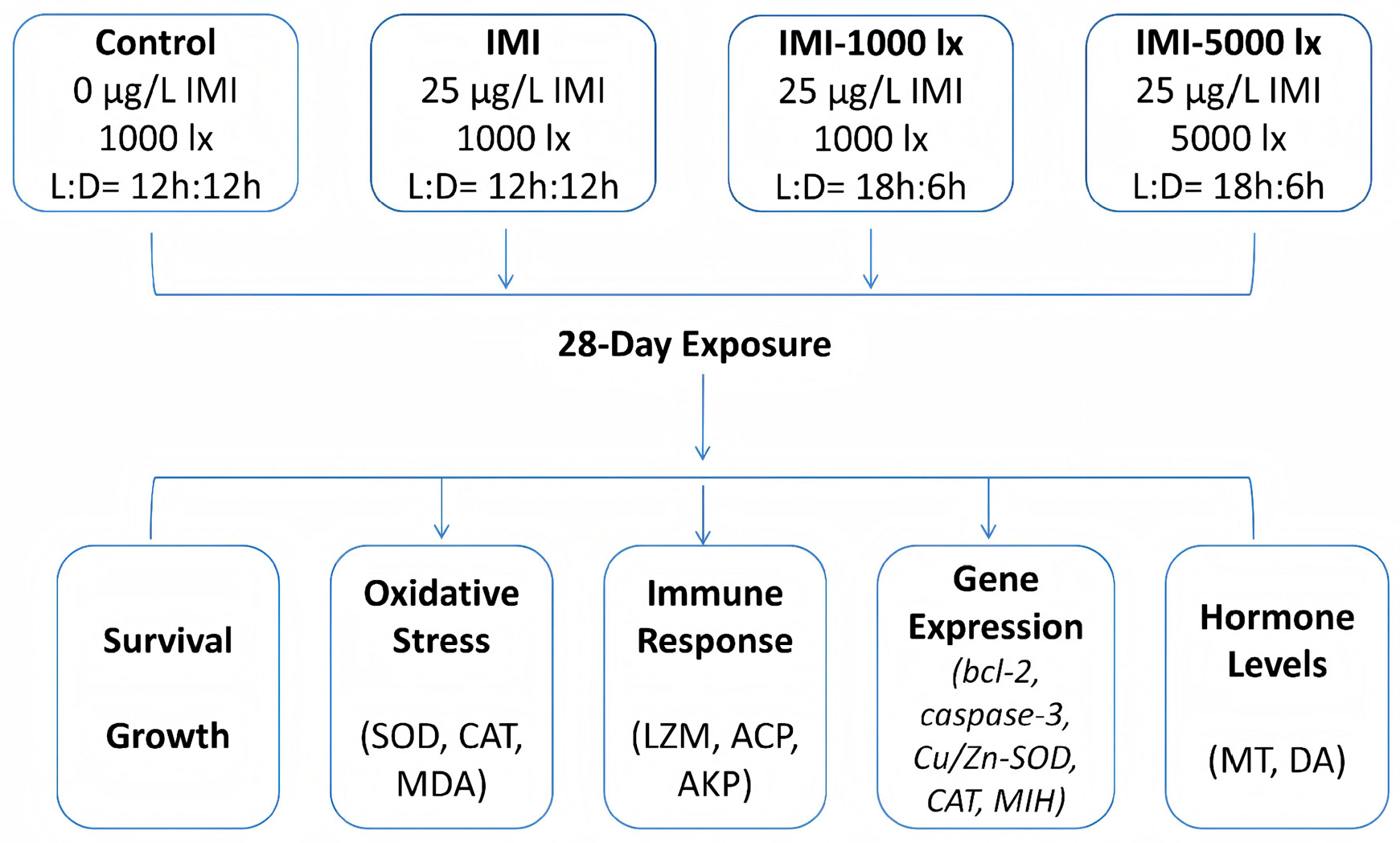
2. Materials and Methods
2.1. Chemicals and Animals
2.2. IMI Exposure and Illumination Control
2.3. Sample Collection
2.4. Biochemical Analysis
2.5. qRT-PCR Analysis
2.6. Detection of MT and DA Levels
2.7. Statistical Analysis
3. Results
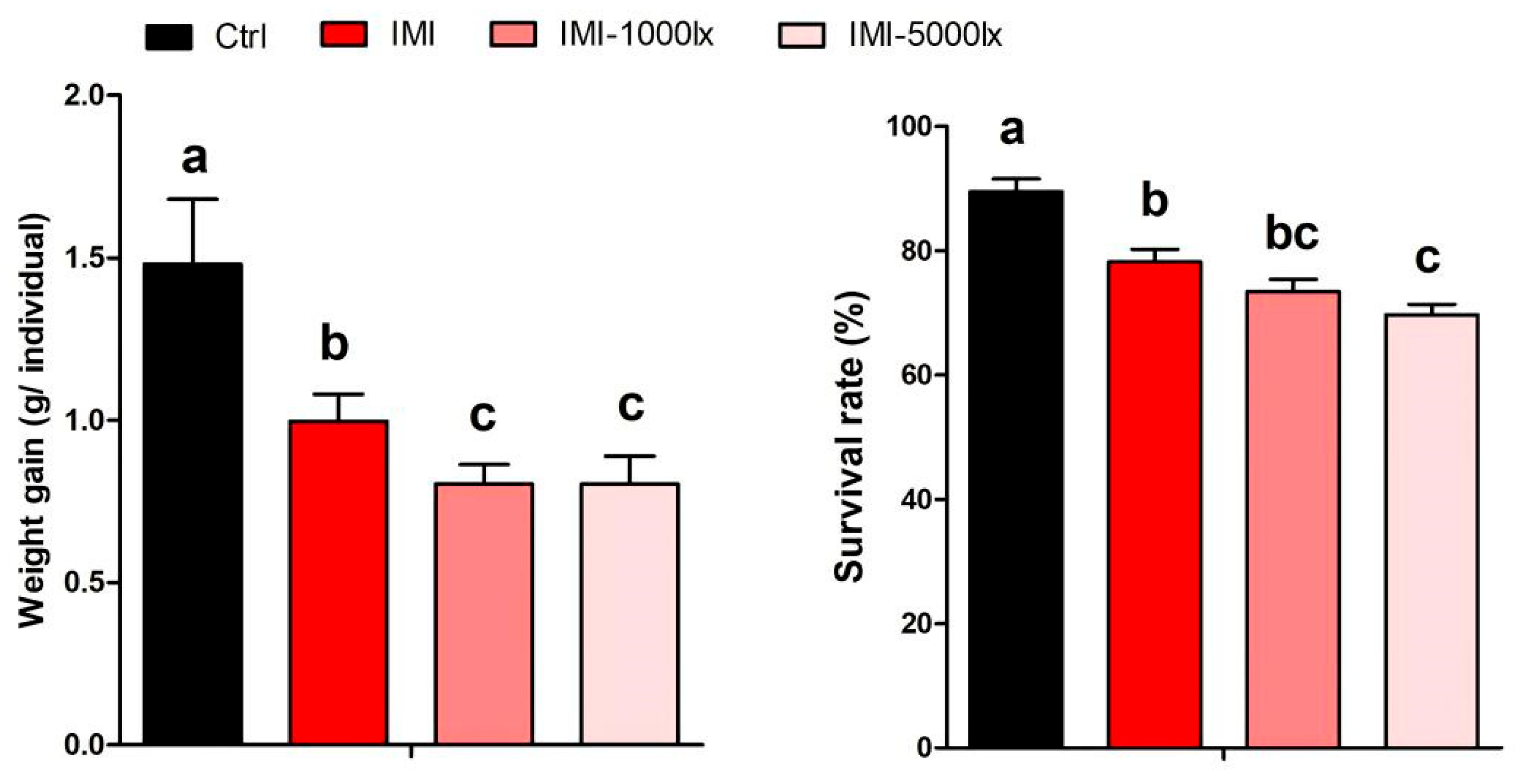
3.1. EPE Affects Growth Performance of P. clarkii Under IMI Exposure
3.2. EPE Affects Oxidative Stress in P. clarkii Under IMI Exposure
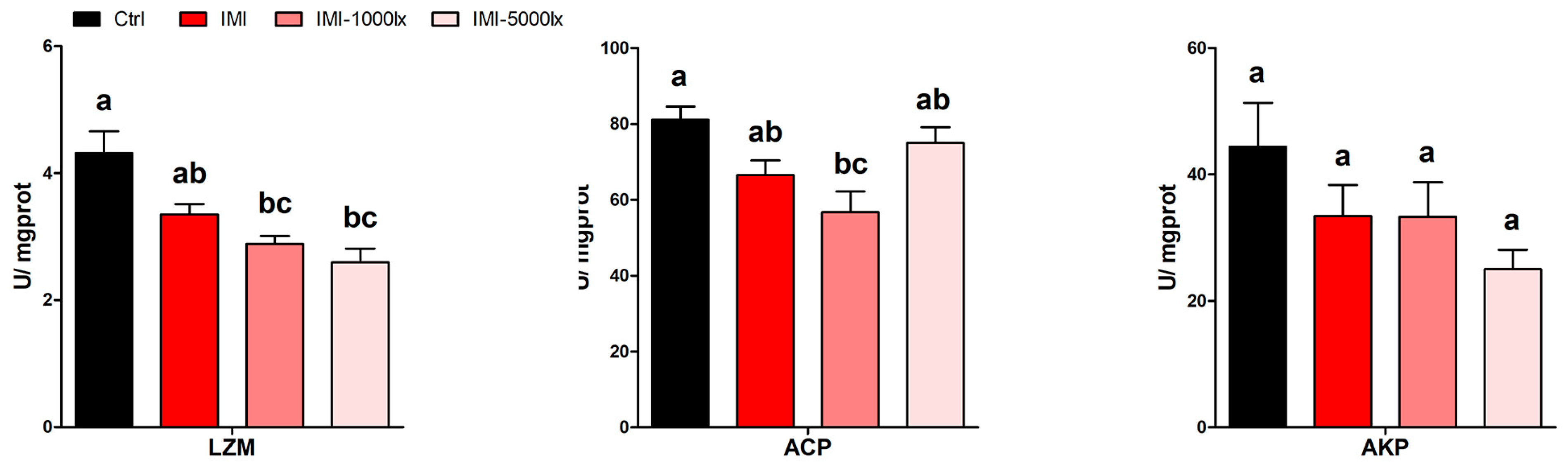
3.3. EPE Affects Immune Response Under IMI Exposure
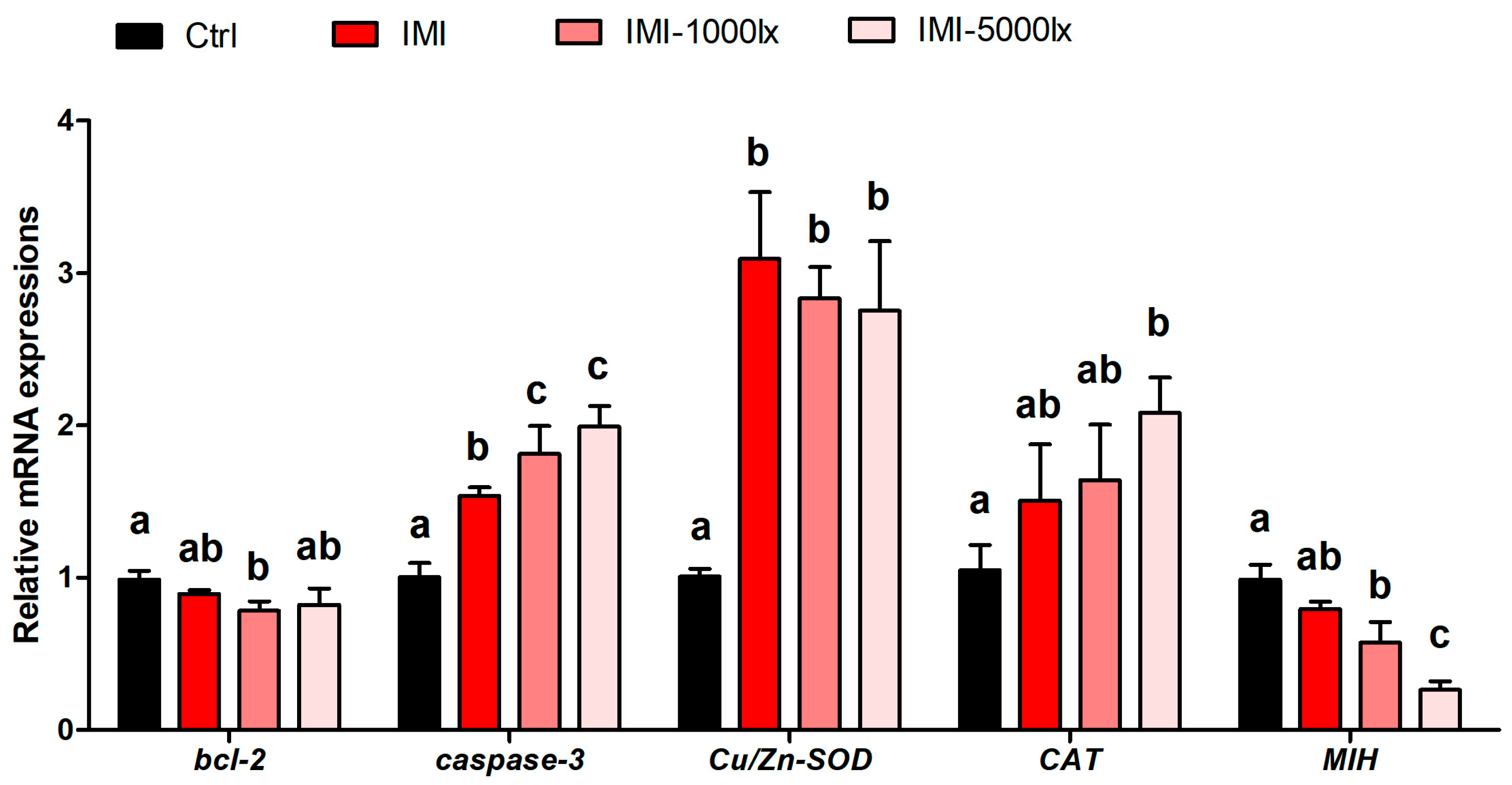
3.4. Gene-Expression Analysis
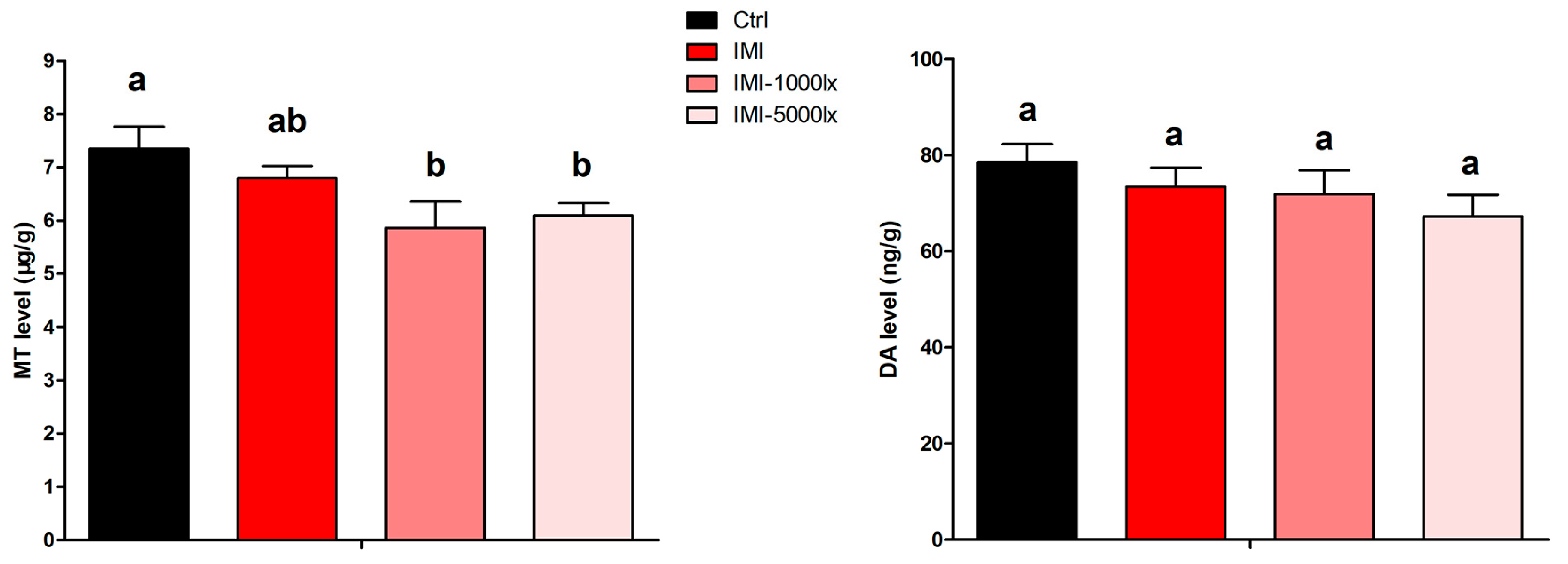
3.5. Effects of IMI and EPE on Neuroendocrine Hormone in Eyestalk of P. clarkii
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buckingham, S.D.; Lapied, B.; Le Corronc, H.; Grolleau, F.; Sattelle, D.B. Imidacloprid actions on insect neuronal acetylcholine receptors. J. Exp. Biol. 1997, 200, 2685–2692. [Google Scholar] [CrossRef]
- Roessink, I.; Merga, L.B.; Zweers, H.J.; Van den Brink, P.J. The neonicotinoid imidacloprid shows high chronic toxicity to mayfly nymphs. Environ. Toxicol. Chem. 2013, 32, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.C.; Dubetz, C.; Palace, V.P. Neonicotinoids in the Canadian aquatic environment: A literature review on current use products with a focus on fate, exposure, and biological effects. Sci. Total Environ. 2015, 505, 409–422. [Google Scholar] [CrossRef]
- Nowell, L.H.; Moran, P.W.; Schmidt, T.S.; Norman, J.E.; Nakagaki, N.; Shoda, M.E.; Mahler, B.J.; Van Metre, P.C.; Stone, W.W.; Sandstrom, M.W.; et al. Complex mixtures of dissolved pesticides show potential aquatic toxicity in a synoptic study of Midwestern U.S. streams. Sci. Total Environ. 2018, 613–614, 1469–1488. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, C.A.; Mineau, P.; Devries, J.H.; Sanchez-Bayo, F.; Liess, M.; Cavallaro, M.C.; Liber, K. Neonicotinoid contamination of global surface waters and associated risk to aquatic invertebrates: A review. Environ. Int. 2015, 74, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Thunnissen, N.W.; Lautz, L.S.; van Schaik, T.W.G.; Hendriks, A.J. Ecological risks of imidacloprid to aquatic species in the Netherlands: Measured and estimated concentrations compared to species sensitivity distributions. Chemosphere 2020, 254, 126604. [Google Scholar] [CrossRef]
- Wu, S.; Wu, L.; Xu, H.; Zhao, X.; Wu, C.; Chen, L.; Zhang, H.; Wang, Q. Study on residue of imidacloprid in rice and field environment. Acta Agric. Zhejiangensis 2004, 16, 274–278. [Google Scholar]
- Nugnes, R.; Russo, C.; Orlo, E.; Lavorgna, M.; Isidori, M. Imidacloprid: Comparative toxicity, DNA damage, ROS production and risk assessment for aquatic non-target organisms. Environ. Pollut. 2023, 316, 120682. [Google Scholar] [CrossRef]
- Osterberg, J.S.; Darnell, K.M.; Blickley, T.M.; Romano, J.A.; Rittschof, D. Acute toxicity and sub-lethal effects of common pesticides in post-larval and juvenile blue crabs, Callinectes sapidus. J. Exp. Mar. Biol. Ecol. 2012, 424–425, 5–14. [Google Scholar] [CrossRef]
- Frew, J.A.; Brown, J.T.; Fitzsimmons, P.N.; Hoffman, A.D.; Sadilek, M.; Grue, C.E.; Nichols, J.W. Toxicokinetics of the neonicotinoid insecticide imidacloprid in rainbow trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2018, 205, 34–42. [Google Scholar] [CrossRef]
- Ge, W.; Yan, S.; Wang, J.; Zhu, L.; Chen, A.; Wang, J. Oxidative Stress and DNA Damage Induced by Imidacloprid in Zebrafish (Danio rerio). J. Agric. Food Chem. 2015, 63, 1856–1862. [Google Scholar] [CrossRef]
- Butcherine, P.; Kelaher, B.P.; Taylor, M.D.; Barkla, B.J.; Benkendorff, K. Impact of imidacloprid on the nutritional quality of adult black tiger shrimp (Penaeus monodon). Ecotoxicol. Environ. Saf. 2020, 198, 110682. [Google Scholar] [CrossRef]
- Fu, Z.; Han, F.; Huang, K.; Zhang, J.; Qin, J.G.; Chen, L.; Li, E. Impact of imidacloprid exposure on the biochemical responses, transcriptome, gut microbiota and growth performance of the Pacific white shrimp Litopenaeus vannamei. J. Hazard. Mater. 2022, 424, 127513. [Google Scholar] [CrossRef] [PubMed]
- Souty-Grosset, C.; Anastácio, P.M.; Aquiloni, L.; Banha, F.; Choquer, J.; Chucholl, C.; Tricarico, E. The red swamp crayfish Procambarus clarkii in Europe: Impacts on aquatic ecosystems and human well-being. Limnologica 2016, 58, 78–93. [Google Scholar] [CrossRef]
- Wang, Q.; Ding, H.; Tao, Z.; Ma, D. Crayfish (Procambarus clarkii) Cultivation in China: A Decade of Unprecedented Development. In Aquaculture in China; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018; pp. 363–377. [Google Scholar]
- Hernández, L.; Maeda-Martínez, A.M.; Ruiz-Campos, G.; Rodríguez-Almaraz, G.; Alonzo-Rojo, F.; Sainz, J.C. Geographic expansion of the invasive red crayfish Procambarus clarkii (Girard, 1852) (Crustacea: Decapoda) in Mexico. Biol. Invasions 2008, 10, 977–984. [Google Scholar] [CrossRef]
- Gherardi, F. Crayfish invading Europe: The case study of Procambarus clarkii. Mar. Freshw. Behav. Physiol. 2006, 39, 175–191. [Google Scholar] [CrossRef]
- Li, Y.; Guo, X.; Cao, X.; Deng, W.; Luo, W.; Wang, W. Population Genetic Structure and Post-Establishment Dispersal Patterns of the Red Swamp Crayfish Procambarus clarkii in China. PLoS ONE 2012, 7, e40652. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Cisnal, R.; García-Sevillano, M.A.; García-Barrera, T.; Gómez-Ariza, J.L.; Abril, N. Metabolomic alterations and oxidative stress are associated with environmental pollution in Procambarus clarkii. Aquat. Toxicol. 2018, 205, 76–88. [Google Scholar] [CrossRef]
- Suárez-Serrano, A.; Alcaraz, C.; Ibáñez, C.; Trobajo, R.; Barata, C. Procambarus clarkii as a bioindicator of heavy metal pollution sources in the lower Ebro River and Delta. Ecotoxicol. Environ. Saf. 2010, 73, 280–286. [Google Scholar] [CrossRef]
- Pastorino, P.; Anselmi, S.; Zanoli, A.; Esposito, G.; Bondavalli, F.; Dondo, A.; Pucci, A.; Pizzul, E.; Faggio, C.; Barceló, D.; et al. The invasive red swamp crayfish (Procambarus clarkii) as a bioindicator of microplastic pollution: Insights from Lake Candia (northwestern Italy). Ecol. Indic. 2023, 150, 110200. [Google Scholar] [CrossRef]
- Vioque-Fernández, A.; de Almeida, E.A.; Ballesteros, J.; García-Barrera, T.; Gómez-Ariza, J.L.; López-Barea, J. Doñana National Park survey using crayfish (Procambarus clarkii) as bioindicator: Esterase inhibition and pollutant levels. Toxicol. Lett. 2007, 168, 260–268. [Google Scholar] [CrossRef]
- Gaston, K.J.; Sánchez de Miguel, A. Environmental Impacts of Artificial Light at Night. Annu. Rev. Environ. Resour. 2022, 47, 373–398. [Google Scholar] [CrossRef]
- Brüning, A.; Hölker, F.; Franke, S.; Kleiner, W.; Kloas, W. Impact of different colours of artificial light at night on melatonin rhythm and gene expression of gonadotropins in European perch. Sci. Total Environ. 2016, 543, 214–222. [Google Scholar] [CrossRef]
- Cho, Y.; Ryu, S.-H.; Lee, B.R.; Kim, K.H.; Lee, E.; Choi, J. Effects of artificial light at night on human health: A literature review of observational and experimental studies applied to exposure assessment. Chronobiol. Int. 2015, 32, 1294–1310. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, X.; Luo, Q.; Lin, S.; Lyu, M.; Luo, X.; Ke, C.; You, W. Risk assessment of persistent exposure to artificial light at night revealed altered behavior and metabolic patterns of marine nocturnal shellfish. Ecol. Indic. 2024, 160, 111807. [Google Scholar] [CrossRef]
- Manríquez, P.H.; Jara, M.E.; González, C.P.; Seguel, M.; Quijón, P.A.; Widdicombe, S.; Pulgar, J.M.; Quintanilla-Ahumada, D.; Anguita, C.; Duarte, C. Effects of artificial light at night and predator cues on foraging and predator avoidance in the keystone inshore mollusc Concholepas concholepas. Environ. Pollut. 2021, 280, 116895. [Google Scholar] [CrossRef]
- Jackson, K.M.; Moore, P.A. The intensity and spectrum of artificial light at night alters crayfish interactions. Mar. Freshw. Behav. Physiol. 2019, 52, 131–150. [Google Scholar] [CrossRef]
- Nie, X.; Huang, C.; Wei, J.; Wang, Y.; Hong, K.; Mu, X.; Liu, C.; Chu, Z.; Zhu, X.; Yu, L. Effects of Photoperiod on Survival, Growth, Physiological, and Biochemical Indices of Redclaw Crayfish (Cherax quadricarinatus) Juveniles. Animals 2024, 14, 411. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.R.; James, J.; Newman, R.C.; Riley, W.D.; Griffiths, S.W.; Cable, J. The impact of streetlights on an aquatic invasive species: Artificial light at night alters signal crayfish behaviour. Appl. Anim. Behav. Sci. 2016, 176, 143–149. [Google Scholar] [CrossRef]
- Liu, Z.; Lv, Y.; Ding, R.; Chen, X.; Pu, G. Light Pollution Changes the Toxicological Effects of Cadmium on Microbial Community Structure and Function Associated with Leaf Litter Decomposition. Int. J. Mol. Sci. 2020, 21, 422. [Google Scholar] [CrossRef]
- Huang, Y.; Hong, Y.; Yin, H.; Yan, G.; Huang, Q.; Li, Z.; Huang, Z. Imidacloprid induces locomotion impairment of the freshwater crayfish, Procambarus clarkii via neurotoxicity and oxidative stress in digestive system. Aquat. Toxicol. 2021, 238, 105913. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M.A. A Rapid and Sensitive Method for Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 25, 248–256. [Google Scholar] [CrossRef]
- Han, Z.; Li, X.; Li, X.; Xu, W.; Li, Y. Circadian rhythms of melatonin in haemolymph and optic lobes of Chinese mitten crab (Eriocheir sinensis) and Chinese grass shrimp (Palaemonetes sinensis). Biol. Rhythm Res. 2019, 50, 400–407. [Google Scholar] [CrossRef]
- Tinikul, Y.; Poljaroen, J.; Kornthong, N.; Chotwiwatthanakun, C.; Anuracpreeda, P.; Poomtong, T.; Hanna, P.J.; Sobhon, P. Distribution and changes of serotonin and dopamine levels in the central nervous system and ovary of the Pacific white shrimp, Litopenaeus vannamei, during ovarian maturation cycle. Cell Tissue Res. 2011, 345, 103–124. [Google Scholar] [CrossRef]
- Eberhardt, L.; Binde Doria, H.; Bulut, B.; Feldmeyer, B.; Pfenninger, M. Transcriptomics predicts Artificial Light at Night’s (ALAN) impact on fitness: Nightly illumination alters gene expression pattern and negatively affects fitness components in the midge Chironomus riparius (Diptera: Chironomidae). bioRxiv 2024. [Google Scholar] [CrossRef]
- Huang, Y.; Hong, Y.; Wu, S.; Yang, X.; Huang, Q.; Dong, Y.; Xu, D.; Huang, Z. Prolonged darkness attenuates imidacloprid toxicity through the brain-gut-microbiome axis in zebrafish, Danio rerio. Sci. Total Environ. 2023, 881, 163481. [Google Scholar] [CrossRef]
- Vignet, C.; Cappello, T.; Fu, Q.; Lajoie, K.; De Marco, G.; Clérandeau, C.; Mottaz, H.; Maisano, M.; Hollender, J.; Schirmer, K.; et al. Imidacloprid induces adverse effects on fish early life stages that are more severe in Japanese medaka (Oryzias latipes) than in zebrafish (Danio rerio). Chemosphere 2019, 225, 470–478. [Google Scholar] [CrossRef]
- Touitou, Y.; Reinberg, A.; Touitou, D. Association between light at night, melatonin secretion, sleep deprivation, and the internal clock: Health impacts and mechanisms of circadian disruption. Life Sci. 2017, 173, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Fu, R.; Zhang, Y.; Mao, L.; Zhu, L.; Zhang, L.; Liu, X.; Jiang, H. Integrate transcriptomic and metabolomic analysis reveals the underlying mechanisms of behavioral disorders in zebrafish (Danio rerio) induced by imidacloprid. Sci. Total Environ. 2023, 870, 161541. [Google Scholar] [CrossRef] [PubMed]
- Rubí, B.; Maechler, P. Minireview: New Roles for Peripheral Dopamine on Metabolic Control and Tumor Growth: Let’s Seek the Balance. Endocrinology 2010, 151, 5570–5581. [Google Scholar] [CrossRef] [PubMed]
- Janner, D.E.; Gomes, N.S.; Poetini, M.R.; Poleto, K.H.; Musachio, E.A.S.; de Almeida, F.P.; de Matos Amador, E.C.; Reginaldo, J.C.; Ramborger, B.P.; Roehrs, R.; et al. Oxidative stress and decreased dopamine levels induced by imidacloprid exposure cause behavioral changes in a neurodevelopmental disorder model in Drosophila melanogaster. NeuroToxicology 2021, 85, 79–89. [Google Scholar] [CrossRef]
- Romeo, S.; Viaggi, C.; Di Camillo, D.; Willis, A.W.; Lozzi, L.; Rocchi, C.; Capannolo, M.; Aloisi, G.; Vaglini, F.; Maccarone, R.; et al. Bright light exposure reduces TH-positive dopamine neurons: Implications of light pollution in Parkinson’s disease epidemiology. Sci. Rep. 2013, 3, 1395. [Google Scholar] [CrossRef]
- Vieira, C.E.D.; Pérez, M.R.; Acayaba, R.D.A.; Raimundo, C.C.M.; dos Reis Martinez, C.B. DNA damage and oxidative stress induced by imidacloprid exposure in different tissues of the Neotropical fish Prochilodus lineatus. Chemosphere 2018, 195, 125–134. [Google Scholar] [CrossRef]
- Rios, F.M.; Wilcoxen, T.E.; Zimmerman, L.M. Effects of imidacloprid on Rana catesbeiana immune and nervous system. Chemosphere 2017, 188, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Raap, T.; Casasole, G.; Costantini, D.; AbdElgawad, H.; Asard, H.; Pinxten, R.; Eens, M. Artificial light at night affects body mass but not oxidative status in free-living nestling songbirds: An experimental study. Sci. Rep. 2016, 6, 35626. [Google Scholar] [CrossRef] [PubMed]
- Czarnecka, M.; Jermacz, Ł.; Glazińska, P.; Kulasek, M.; Kobak, J. Artificial light at night (ALAN) affects behaviour, but does not change oxidative status in freshwater shredders. Environ. Pollut. 2022, 306, 119476. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, P.; Cai, M.; Wu, D.; Zhang, M.; Chen, M.; Zhao, Y. Effects of microplastics on the innate immunity and intestinal microflora of juvenile Eriocheir sinensis. Sci. Total Environ. 2019, 685, 836–846. [Google Scholar] [CrossRef]
- Ewere, E.E.; Reichelt-Brushett, A.; Benkendorff, K. The neonicotinoid insecticide imidacloprid, but not salinity, impacts the immune system of Sydney rock oyster, Saccostrea glomerata. Sci. Total Environ. 2020, 742, 140538. [Google Scholar] [CrossRef]
- Giannoulia-Karantana, A.; Vlachou, A.; Polychronopoulou, S.; Papassotiriou, I.; Chrousos, G.P. Melatonin and Immunomodulation: Connections and Potential Clinical Applications. Neuroimmunomodulation 2007, 13, 133–144. [Google Scholar] [CrossRef]
- Medrano-Campillo, P.; Sarmiento-Soto, H.; Álvarez-Sánchez, N.; Álvarez-Ríos, A.I.; Guerrero, J.M.; Rodríguez-Prieto, I.; Castillo-Palma, M.J.; Lardone, P.J.; Carrillo-Vico, A. Evaluation of the immunomodulatory effect of melatonin on the T-cell response in peripheral blood from systemic lupus erythematosus patients. J. Pineal Res. 2015, 58, 219–226. [Google Scholar] [CrossRef]
- Taha, M.A.I.; Badawy, M.E.I.; Abdel-Razik, R.K.; Younis, H.M.; Abo-El-Saad, M.M. Mitochondrial dysfunction and oxidative stress in liver of male albino rats after exposing to sub-chronic intoxication of chlorpyrifos, cypermethrin, and imidacloprid. Pestic. Biochem. Physiol. 2021, 178, 104938. [Google Scholar] [CrossRef]
- Miao, Z.; Miao, Z.; Wang, S.; Wu, H.; Xu, S. Exposure to imidacloprid induce oxidative stress, mitochondrial dysfunction, inflammation, apoptosis and mitophagy via NF-kappaB/JNK pathway in grass carp hepatocytes. Fish Shellfish Immunol. 2022, 120, 674–685. [Google Scholar] [CrossRef]
- Xu, Y.-Q.; Zhang, D.; Jin, T.; Cai, D.-J.; Wu, Q.; Lu, Y.; Liu, J.; Klaassen, C.D. Diurnal Variation of Hepatic Antioxidant Gene Expression in Mice. PLoS ONE 2012, 7, e44237. [Google Scholar] [CrossRef]
- Techa, S.; Chung, J.S. Ecdysteroids Regulate the Levels of Molt-Inhibiting Hormone (MIH) Expression in the Blue Crab, Callinectes sapidus. PLoS ONE 2015, 10, e0117278. [Google Scholar] [CrossRef]
- Nakatsuji, T.; Lee, C.-Y.; Watson, R.D. Crustacean molt-inhibiting hormone: Structure, function, and cellular mode of action. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2009, 152, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Al-Badran, A.A.; Fujiwara, M.; Mora, M.A. Effects of insecticides, fipronil and imidacloprid, on the growth, survival, and behavior of brown shrimp Farfantepenaeus aztecus. PLoS ONE 2019, 14, e0223641. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, Y.; Qin, Q.; Chen, L.; Dang, X.; Ma, Z.; Zhou, Z. Imidacloprid disrupts larval molting regulation and nutrient energy metabolism, causing developmental delay in honey bee Apis mellifera. eLife 2024, 12, RP88772. [Google Scholar] [CrossRef] [PubMed]

| Primer Name | Sequence (5′ to 3′) |
|---|---|
| bcl-2 Fw | CTGGGAGGTGTGTGAGGTGTT |
| bcl-2 Rv | TCAGAGGTGAGAGTGAGGGAGA |
| caspase-3 Fw | TGAAGAATCGCTGAATCTGCTC |
| caspase-3 Rv | CATATCCTCCTCCAACCTGCT |
| Cn/Zn SOD Fw | AACCAAATCAGTGGCAGGCT |
| Cu/Zn SOD Rv | CCTGGAGTCAGCCCATACAC |
| CAT Fw | AGTTCAAGAAGAGCCAGAC |
| CAT Rv | AGGAATGCGTTCTCTATCAA |
| MIH | AGGTTCTACGAGTTGCTTG |
| MIH | TGCCGTTGTCTGCTGT |
| β-actin Fw | TGCCGCCTCATCCTCTTC |
| β-actin Rv | CCTCTCGTTGCCAATGGTAATG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Qi, D.; Li, X.; Hu, X.; Huang, Q.; Huang, Z. Extended Photoperiod Exposure Affects Imidacloprid Toxicity on Juvenile Crayfish Procambarus clarkii by Regulating Oxidative Stress and Neuroendocrine Pathways. Fishes 2025, 10, 562. https://doi.org/10.3390/fishes10110562
Huang Y, Qi D, Li X, Hu X, Huang Q, Huang Z. Extended Photoperiod Exposure Affects Imidacloprid Toxicity on Juvenile Crayfish Procambarus clarkii by Regulating Oxidative Stress and Neuroendocrine Pathways. Fishes. 2025; 10(11):562. https://doi.org/10.3390/fishes10110562
Chicago/Turabian StyleHuang, Yi, Dongming Qi, Xiaoyan Li, Xiaodan Hu, Qiang Huang, and Zhiqiu Huang. 2025. "Extended Photoperiod Exposure Affects Imidacloprid Toxicity on Juvenile Crayfish Procambarus clarkii by Regulating Oxidative Stress and Neuroendocrine Pathways" Fishes 10, no. 11: 562. https://doi.org/10.3390/fishes10110562
APA StyleHuang, Y., Qi, D., Li, X., Hu, X., Huang, Q., & Huang, Z. (2025). Extended Photoperiod Exposure Affects Imidacloprid Toxicity on Juvenile Crayfish Procambarus clarkii by Regulating Oxidative Stress and Neuroendocrine Pathways. Fishes, 10(11), 562. https://doi.org/10.3390/fishes10110562





