Inspection of Gonadal Maturation in Mediterranean Sea Cucumber (Holothuria tubulosa Gmelin 1791) Under a Constant Temperature Regime ‡
Abstract
1. Introduction
2. Materials and Methods
2.1. Wild Broodstock Collection, Acclimation, and Gonad Regression
2.2. Experimental Design
2.3. Gonadal Maturation and Growth Performance
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IMTA | Integrated multi-trophic aquaculture |
| GI | Gonadosomatic index |
| 12L:12D | 12 h light: 12 h dark |
| Wi | Initial wet weight |
| Wf | Final wet weight |
| GW | Gonad weight |
| GBW | Gutted body weight |
| SR | Survival rate |
| SGR | Specific growth rate |
| EAT | Effective accumulated temperature |
References
- FAO. Global Trade Volume of Sea Cucumbers. Available online: https://www.fao.org/fishery/statistics/software/fishstatj/en (accessed on 5 September 2025).
- Engin, S.; Tolon, M.T.; Günay, D.; Emiroğlu, D. Reproductive cycle of the temperate sea cucumber Holothuria tubulosa in the northeastern Aegean Sea. Mar. Coast. Fish. 2024, 16, 10307. [Google Scholar] [CrossRef]
- Hu, M.; Li, Q.; Li, L. Effect of salinity and temperature on salinity tolerance of the sea cucumber Apostichopus japonicus. Fish. Sci. 2010, 76, 267–273. [Google Scholar] [CrossRef]
- Huo, Y.; Drawbridge, M. Spawning, early life stages and optimizing temperature and microalgal diets in larval rearing of the warty sea cucumber Apostichopus parvimensis. Aquaculture 2025, 604, 742454. [Google Scholar] [CrossRef]
- Laguerre, H.; Raymond, G.; Plan, P.; Améziane, N.; Bailly, X.; Le Chevalier, P. First description of embryonic and larval development, juvenile growth of the black sea-cucumber Holothuria forskali (Echinodermata: Holothuroidea), a new species for aquaculture in the north-eastern Atlantic. Aquaculture 2020, 521, 734961. [Google Scholar] [CrossRef]
- Purcell, S.W.; Kirby, D.S. Restocking the sea cucumber Holothuria scabra: Sizing no-take zones through individual-based movement modelling. Fish. Res. 2006, 80, 53–61. [Google Scholar] [CrossRef]
- Purwati, P. Reproductive patterns of Holothuria scabra (Echinodermata: Holothuroidea) in Indonesian waters. Mar. Res. Indones. 2006, 30, 47–55. [Google Scholar] [CrossRef]
- Junus, S.; Kwong, P.; Khoo, G. A review on the recent advances in the biology and aquaculture technology of Holothuria scabra. J. Surv. Fish. Sci. 2018, 4, 5–25. [Google Scholar] [CrossRef]
- Muthiga, N.; Kawaka, J. The breeding pattern and variations in timing and reproductive output of the commercial sea cucumber Holothuria fuscogilva in Kenya. West. Indian Ocean J. Mar. Sci. 2009, 8, 183–192. [Google Scholar] [CrossRef]
- Bahida, A.; Abrehouche, A.; Nhhala, H.; Chadli, H.; Nhhala, I.; Erraioui, H. Reproductive activity of three sea cucumber species Holothuria forskali, Holothuria sanctori and Holothuria tubulosa in WSW Alboran Sea, M’diq Bay, Morocco. Egypt. J. Aquat. Biol. Fish. 2022, 26, 177. [Google Scholar] [CrossRef]
- Chen, J.; Lv, Z.; Guo, M. Research advancement of Apostichopus japonicus from 2000 to 2021. Front. Mar. Sci. 2022, 9, 931903. [Google Scholar] [CrossRef]
- Hamel, J.-F.; Mercier, A. Early development, settlement, growth, and spatial distribution of the sea cucumber Cucumaria frondosa (Echinodermata: Holothuroidea). Can. J. Fish. Aquat. Sci. 1996, 53, 253–271. [Google Scholar] [CrossRef]
- Kazanidis, G.; Lolas, A.; Vafidis, D. Reproductive cycle of the traditionally exploited sea cucumber Holothuria tubulosa (Holothuroidea: Aspidochirotida) in Pagasitikos Gulf, western Aegean Sea, Greece. Turk. J. Zool. 2014, 38, 306–315. [Google Scholar] [CrossRef]
- Pasquini, V.; Porcu, C.; Marongiu, M.F.; Follesa, M.C.; Giglioli, A.A.; Addis, P. New insights upon the reproductive biology of the sea cucumber Holothuria tubulosa (Echinodermata, Holothuroidea) in the Mediterranean: Implications for management and domestication. Front. Mar. Sci. 2022, 9, 1029147. [Google Scholar] [CrossRef]
- Spirina, I.; Dolmatov, I.Y. Morphology of the respiratory trees in the holothurians Apostichopus japonicus and Cucumaria japonica. Russ. J. Mar. Biol. 2001, 27, 367–375. [Google Scholar] [CrossRef]
- Tolon, M.T.; Engin, S. Gonadal development of the holothurian Holothuria polii (Delle Chiaje, 1823) in spawning period at the Aegean Sea (Mediterranean Sea). Ege J. Fish. Aquat. Sci. 2019, 36, 379–385. [Google Scholar] [CrossRef]
- Ramofafia, C.; Byrne, M.; Battaglene, C. Reproduction of the commercial sea cucumber Holothuria scabra (Echinodermata: Holothuroidea) in the Solomon Islands. Mar. Biol. 2003, 142, 281–288. [Google Scholar] [CrossRef]
- Rakaj, A.; Fianchini, A.; Boncagni, P.; Lovatelli, A.; Scardi, M.; Cataudella, S. Spawning and rearing of Holothuria tubulosa: A new candidate for aquaculture in the Mediterranean region. Aquac. Res. 2018, 49, 557–568. [Google Scholar] [CrossRef]
- Sadoul, B.; Caprioli, J.-P.; Barrier-Loiseau, C.; Cimiterra, N.; Laugier, T.; Lagarde, F.; Chary, K.; Callier, M.D.; Guillermard, M.-O.; d’Orbcastel, E.R. Is Holothuria tubulosa the golden goose of ecological aquaculture in the Mediterranean Sea? Aquaculture 2022, 554, 738149. [Google Scholar] [CrossRef]
- Neofitou, N.; Lolas, A.; Ballios, I.; Skordas, K.; Tziantziou, L.; Vafidis, D. Contribution of sea cucumber Holothuria tubulosa on organic load reduction from fish farming operation. Aquaculture 2019, 501, 97–103. [Google Scholar] [CrossRef]
- Tolon, T.; Emiroglu, D.; Günay, D.; Hanci, B. Effect of stocking density on growth performance of juvenile sea cucumber (Gmelin, 1788). Aquac. Res. 2017, 48, 4124–4131. [Google Scholar] [CrossRef]
- Lee, S.; Ford, A.K.; Mangubhai, S.; Wild, C.; Ferse, S.C. Length-weight relationship, movement rates, and in situ spawning observations of Holothuria scabra (sandfish) in Fiji. Beche-de-Mer Inf Bull 2018, 38, 11–14. Available online: https://repository.usp.ac.fj/id/eprint/12619/ (accessed on 30 August 2025).
- Mercier, A.; Hamel, J.-F. Advances in Marine Biology: Endogenous and Exogenous Control of Gametogenesis and Spawning in Echinoderms; Elsevier: London, UK, 2009. [Google Scholar]
- Mohsen, M.; Yang, H. Sea Cucumbers research in the Mediterranean and the Red Seas. In Sea Cucumbers: Aquaculture, Biology and Ecology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 61–101. [Google Scholar]
- Battaglene, S.C.; Seymour, J.E.; Ramofafia, C.; Lane, I. Spawning induction of three tropical sea cucumbers, Holothuria scabra, H. fuscogilva and Actinopyga mauritiana. Aquaculture 2002, 207, 29–47. [Google Scholar] [CrossRef]
- Morgan, A.D. Spawning of the temperate sea cucumber, Australostichopus mollis (Levin). J. World Aquac. Soc. 2009, 40, 363–373. [Google Scholar] [CrossRef]
- Ramofafia, C.; Byrne, M.; Battaglene, S. Reproductive biology of the intertidal sea cucumber Actinopyga mauritiana in the Solomon Islands. J. Mar. Biol. Assoc. United Kingd. 2001, 81, 523–531. [Google Scholar] [CrossRef]
- Naylor, R.L.; Hardy, R.W.; Bureau, D.P.; Chiu, A.; Elliott, M.; Farrell, A.P.; Forster, I.; Gatlin, D.M.; Goldburg, R.J.; Hua, K. Feeding aquaculture in an era of finite resources. Proc. Natl. Acad. Sci. USA 2009, 106, 15103–15110. [Google Scholar] [CrossRef]
- Shiell, G.R.; Uthicke, S. Reproduction of the commercial sea cucumber Holothuria whitmaei [Holothuroidea: Aspidochirotida] in the Indian and Pacific Ocean regions of Australia. Mar. Biol. 2006, 148, 973–986. [Google Scholar] [CrossRef]
- Liu, S.; Sun, J.; Ru, X.; Hamel, J.-F.; Mercier, A. Broodstock conditioning and spawning. In Developments in Aquaculture and Fisheries Science; Elsevier: Amsterdam, The Netherlands, 2015; Volume 39, pp. 101–110. [Google Scholar]
- Despalatovic, M.; Grubelic, I.; Simunovic, A.; Antolic, B.; Zuljevic, A. Reproductive biology of the holothurian Holothuria tubulosa (Echinodermata) in the Adriatic Sea. J. Mar. Biol. Assoc. U. K. 2004, 84, 409. [Google Scholar] [CrossRef]
- Andriyono, S.; Masruroh, N.; Masithah, E.; Triastuti, J.; Winarni, D. Effect of thermal stimulation on gonad maturation in sea cucumber Phyllophorus sp.(Grube, 1840). J. Bio-Sci. 2015, 23, 39–46. [Google Scholar] [CrossRef]
- Ru, X.; Zhang, L.; Liu, S.; Sun, J.; Yang, H. Energy budget adjustment of sea cucumber Apostichopus japonicus during breeding period. Aquac. Res. 2018, 49, 1657–1663. [Google Scholar] [CrossRef]
- Setiawati, K.M.; Widiastuti, Z.; Sembiring, S.B.M.; Giri, N. Growth and reproduction performance of sea cucumber (Stichopus sp.) fed with different feed regime. In International Seminar on Fish and Fisheries Sciences (ISFFS 2021), Proceedings of the ISFFS 2021, Indonesia, 13–14 July 2021; E3S Web of Conferences: Les Ulis, France, 2021; p. Art. 02009. [Google Scholar] [CrossRef]
- Mezali, K.; Soualili, D.L.; Neghli, L.; Conand, C. Reproductive cycle of the sea cucumber Holothuria (Platyperona) sanctori (Holothuroidea: Echinodermata) in the southwestern Mediterranean Sea: Interpopulation variability. Invertebr. Reprod. Dev. 2014, 58, 179–189. [Google Scholar] [CrossRef]
- Domínguez-Godino, J.A.; González-Wangüemert, M. Breeding and larval development of Holothuria mammata, a new target species for aquaculture. Aquac. Res. 2018, 49, 1430–1440. [Google Scholar] [CrossRef]
- Dong, Y.W.; Dong, S.L.; Ji, T.T. Effect of different thermal regimes on growth and physiological performance of the sea cucumber Apostichopus japonicus Selenka. Aquaculture 2008, 275, 329–334. [Google Scholar] [CrossRef]
- Ennas, C.; Pasquini, V.; Abyaba, H.; Addis, P.; Sarà, G.; Pusceddu, A. Sea cucumbers bioturbation potential outcomes on marine benthic trophic status under different temperature regimes. Sci. Rep. 2023, 13, 11558. [Google Scholar] [CrossRef] [PubMed]
- Al-Harbi, M. Effects of diet quantity on growth performance of juvenile sea cucumbers. Oceanol. Hydrobiol. Stud. 2022, 51, 158–166. [Google Scholar] [CrossRef]
- Okorie, O.E.; Ko, S.H.; Go, S.; Bae, J.Y.; Yoo, G.Y.; Lee, J.H.; Kim, T.I.; Bai, S.C. Preliminary Study of the Optimum Dietary Riboflavin Level in Sea Cucumber, (Selenka). J. World Aquac. Soc. 2011, 42, 657–666. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Q.; Yuan, L.; Liu, X. Effects of dietary taurine on the growth, digestive enzymes, and antioxidant capacity in juvenile sea cucumber, Apostichopus japonicus. J. World Aquac. Soc. 2017, 48, 478–487. [Google Scholar] [CrossRef]
- Escudier, R.; Clementi, E.; Omar, M.; Cipollone, A.; Pistoia, J.; Aydogdu, A.; Drudi, M.; Grandi, A.; Lyubartsev, V.; Lecci, R.; et al. Mediterranean Sea Physical Reanalysis (CMEMS MED-Currents). Copernic. Monit. Environ. Mar. Serv. 2020. [Google Scholar] [CrossRef]
- Li, S.; Li, G.; Pan, Y.; Zhang, L.; Li, Z.; Lin, Z.; Sun, B.; Li, H.; Jiang, L. Study on the biological zero point and effective accumulated temperature for the gonad development of sea cucumber. J. Anhui Agric. Sci. 2016, 44, 81–82. [Google Scholar]
- Sui, X.; Chen, Y.; Hu, Q.; Shang, B. The advancement and outlook of cultural breeding and aquatics technique of Apostichopus japonicus. Fish. Sci. 2004, 23, 29–31. [Google Scholar]
- Chang, Y.; Ding, J.; Song, J.; Yang, W. Culture and Biological Study of Sea Cucumber and Sea Urchin; Ocean Press: Beijing, China, 2004; pp. 1–118. (In Chinese) [Google Scholar]
- Navarro, P.G.; García-Sanz, S.; Tuya, F. Reproductive biology of the sea cucumber Holothuria sanctori (Echinodermata: Holothuroidea). Sci. Mar. 2012, 76, 741–752. [Google Scholar] [CrossRef]
- Smiley, S.; McEuen, F.; Chaffee, C.; Krishnan, S. Echinodermata: Holothuroidea. Reprod. Mar. Invertebr. 1991, 6, 663–750. [Google Scholar]
- Thongbuakaew, T.; Suwansa-Ard, S.; Chaiyamoon, A.; Cummins, S.F.; Sobhon, P. Sex steroids and steroidogenesis-related genes in the sea cucumber, Holothuria scabra and their potential role in gonad maturation. Sci. Rep. 2021, 11, 2194. [Google Scholar] [CrossRef]
- Venâncio, E.; Félix, P.M.; Brito, A.C.; Sousa, J.; e Silva, F.A.; Simões, T.; Narciso, L.; Amorim, A.; Dâmaso, L.; Pombo, A. Do broodstock diets influence viability and larval development of Holothuria mammata? Aquaculture 2021, 536, 736431. [Google Scholar] [CrossRef]
- Günay, D.; Emiroğlu, D.; Tolon, T.; Özden, O.; Saygi, H. Growth and Survival Rate of Juvenile Sea Cucumbers (Holothuria tubulosa, Gmelin, 1788) at Various Temperatures. Turk. J. Fish. Aquat. Sci. 2015, 15, 539–547. [Google Scholar] [CrossRef]
- Kwon, I.; Jung, J.-H.; Lee, H.-Y.; Moon, S.-J.; Oh, M.-H.; Kim, T. Comparison Between the Survivals and Growth Rate of Rearing Systems for Sea Cucumber (Apostichopus japonicus, Selenka) Grow-out in Indoor Running Water Tank Tests. Fish. Technol. Res. Inst. Rep. 2014, 7, 40–45. [Google Scholar] [CrossRef]
- González-Durán, E.; Hernández-Flores, Á.; Headley, M.D.; Canul, J.D. On the effects of temperature and pH on tropical and temperate holothurians. Conserv. Physiol. 2021, 9, coab092. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, L.; Xia, S.; Liu, S.; Ru, X.; Xu, Q.; Zhang, T.; Yang, H. Effects of dietary protein levels on the growth, energy budget, and physiological and immunological performance of green, white and purple color morphs of sea cucumber, Apostichopus japonicus. Aquaculture 2016, 450, 375–382. [Google Scholar] [CrossRef]
- Wang, T. The technique of Apostichopus japonicus seedlings production at elevated temperature. Sci. Fish Farm. 2009, 3, 22–23. [Google Scholar]
- Dereli, H.; Çulha, S.T.; Çulha, M.; Özalp, H.B.; Tekinay, A.A. Reproduction and population structure of the sea cucumber Holothuria tubulosa in the Dardanelles Strait (Turkey). Mediterr. Mar. Sci. 2016, 17, 47–55. [Google Scholar] [CrossRef]

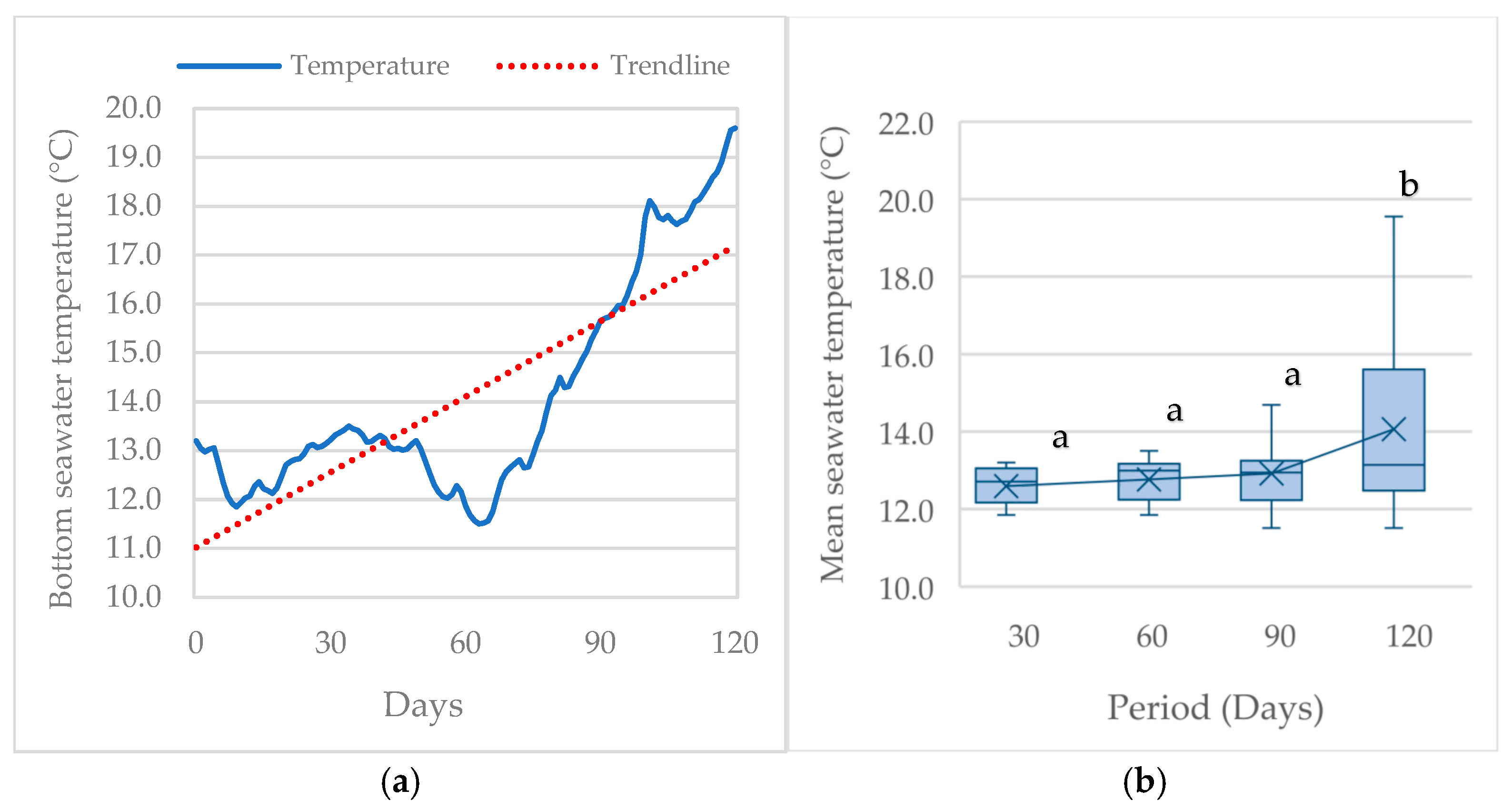
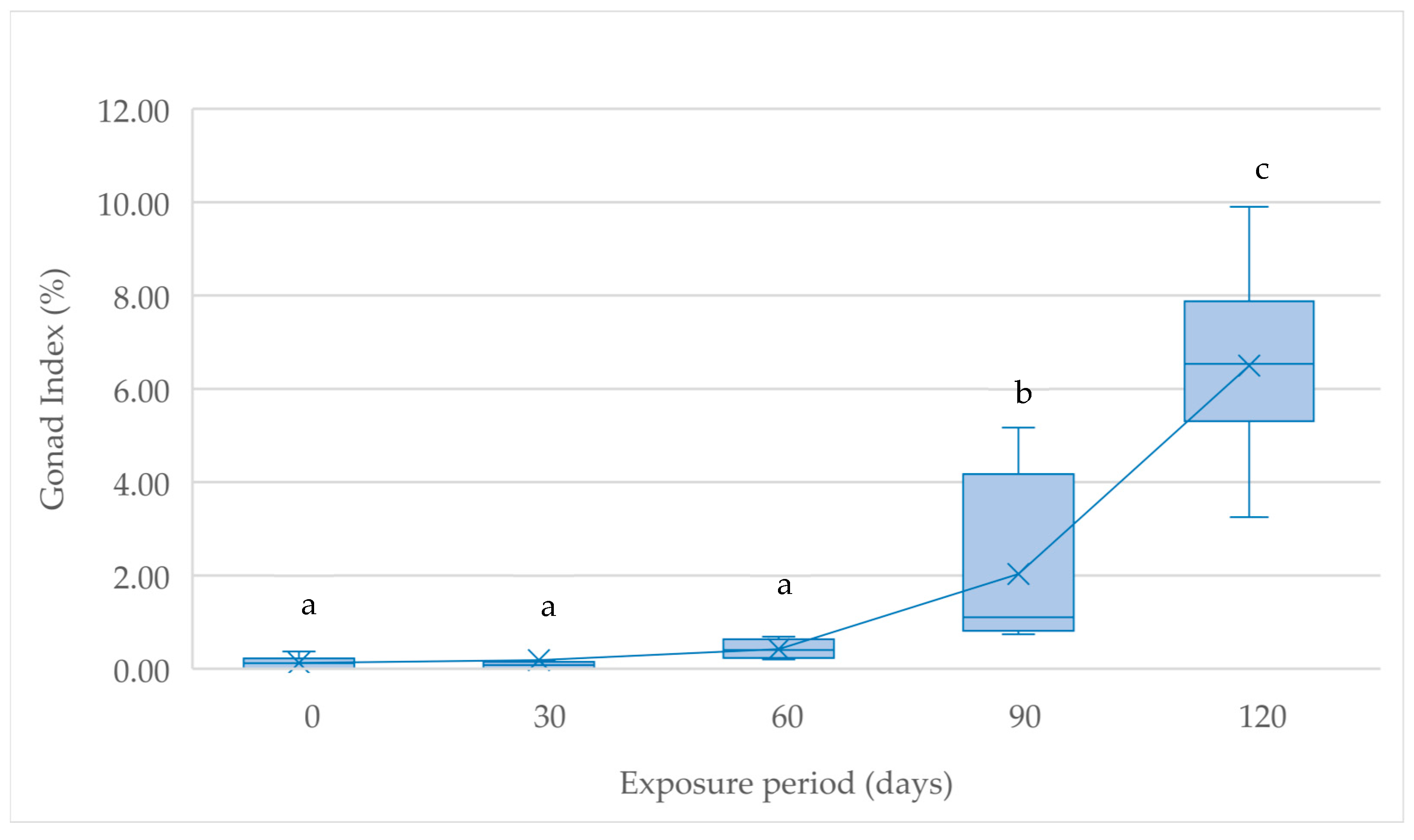
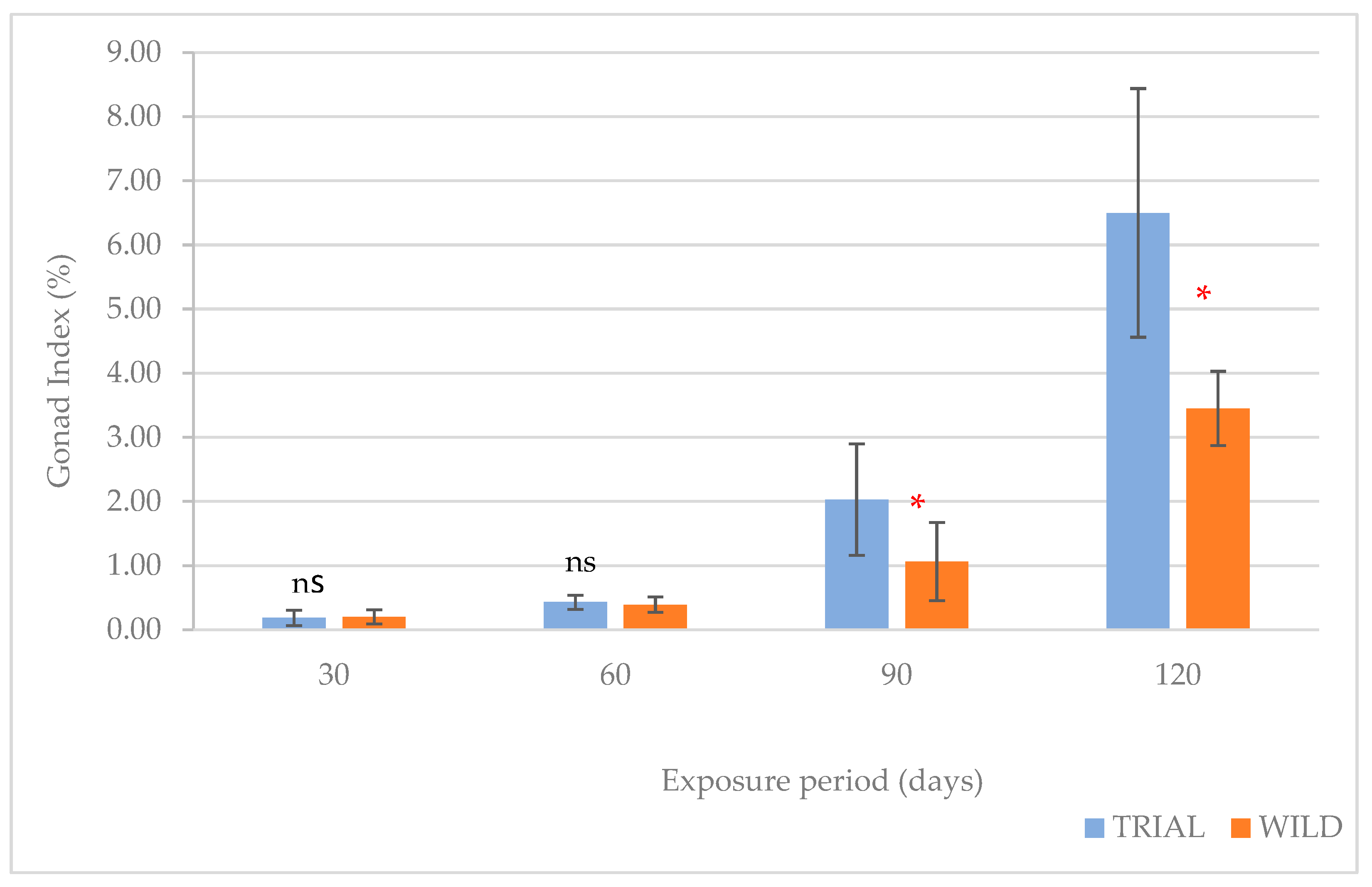
| D30 | D60 | D90 | D120 | ||
|---|---|---|---|---|---|
| Effective accumulative temperature (°C·d) | Trial tanks | 339.9 | 684.6 | 1031.4 | 1370.4 |
| Control | 17.7 | 45.9 | 82.5 | 247.5 |
| D30 | D60 | D90 | D120 | |
|---|---|---|---|---|
| Mean final wet weight (g) | 133.60 ± 24.51 a | 158.16 ± 35.18 b | 168.53 ± 35.99 c | 176.18 ± 33.23 d |
| SGR (% d−1) | 0.03 a | 0.32 b | 0.27 b | 0.24 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerçeklioğlu, B.; Tolon, M.T. Inspection of Gonadal Maturation in Mediterranean Sea Cucumber (Holothuria tubulosa Gmelin 1791) Under a Constant Temperature Regime. Fishes 2025, 10, 560. https://doi.org/10.3390/fishes10110560
Gerçeklioğlu B, Tolon MT. Inspection of Gonadal Maturation in Mediterranean Sea Cucumber (Holothuria tubulosa Gmelin 1791) Under a Constant Temperature Regime. Fishes. 2025; 10(11):560. https://doi.org/10.3390/fishes10110560
Chicago/Turabian StyleGerçeklioğlu, Bengi, and Mustafa Tolga Tolon. 2025. "Inspection of Gonadal Maturation in Mediterranean Sea Cucumber (Holothuria tubulosa Gmelin 1791) Under a Constant Temperature Regime" Fishes 10, no. 11: 560. https://doi.org/10.3390/fishes10110560
APA StyleGerçeklioğlu, B., & Tolon, M. T. (2025). Inspection of Gonadal Maturation in Mediterranean Sea Cucumber (Holothuria tubulosa Gmelin 1791) Under a Constant Temperature Regime. Fishes, 10(11), 560. https://doi.org/10.3390/fishes10110560







