Substituting Fishmeal with Bacillus licheniformis-Fermented Fish By-Products Protein Hydrolysates in Nile Tilapia Diet (Oreochromis niloticus): Impacts on Growth Performance, Humoral Immunity, Oxidative Defense, and Digestive Enzymes
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Microorganisms and Investigation of Protease Properties
2.1.1. Submerged Fermentation of Fish By-Product
2.1.2. Bacterial Growth During Fermentation
2.1.3. Soluble Protein Assay
2.2. Conditions of Experimental Treatments
2.2.1. Feeding Trial
2.2.2. Biological Indices
2.2.3. Blood Sampling
2.2.4. Measuring the Enzyme Activities of Hepatopancreas Tissue
2.2.5. Measuring Digestive Enzyme Activities
2.2.6. Proximate Chemical Composition
2.2.7. Statistical Analysis
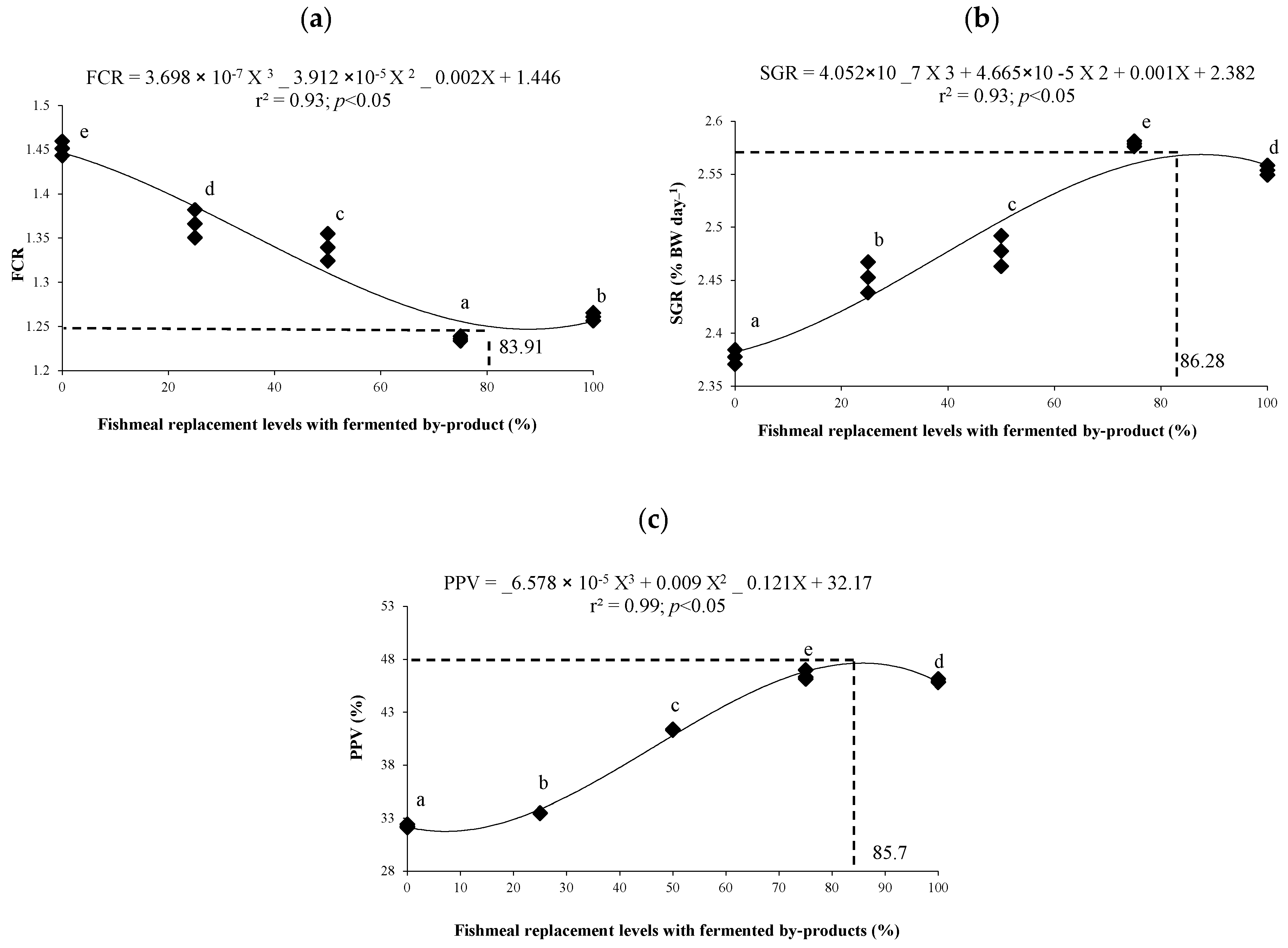
3. Results
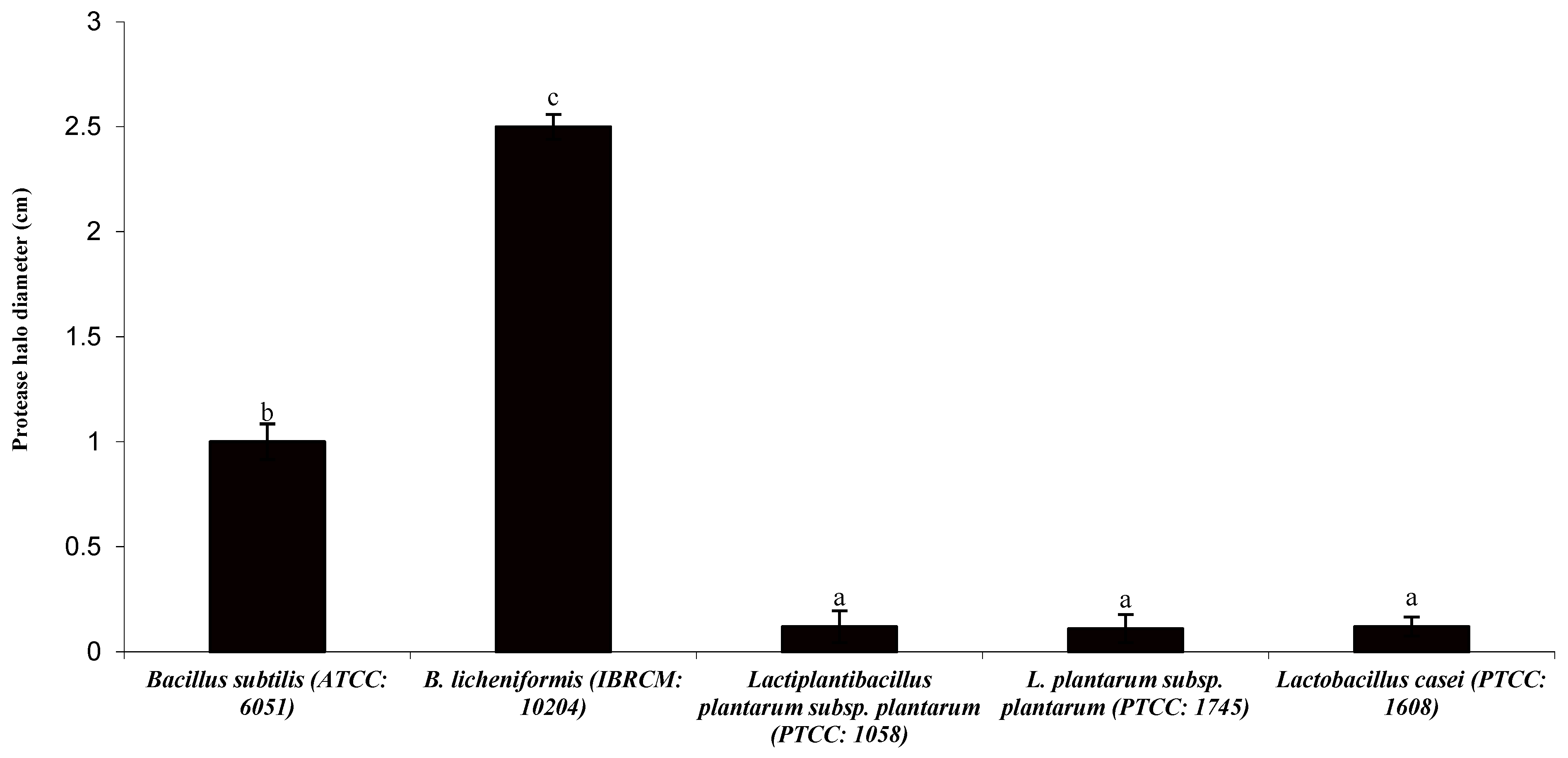
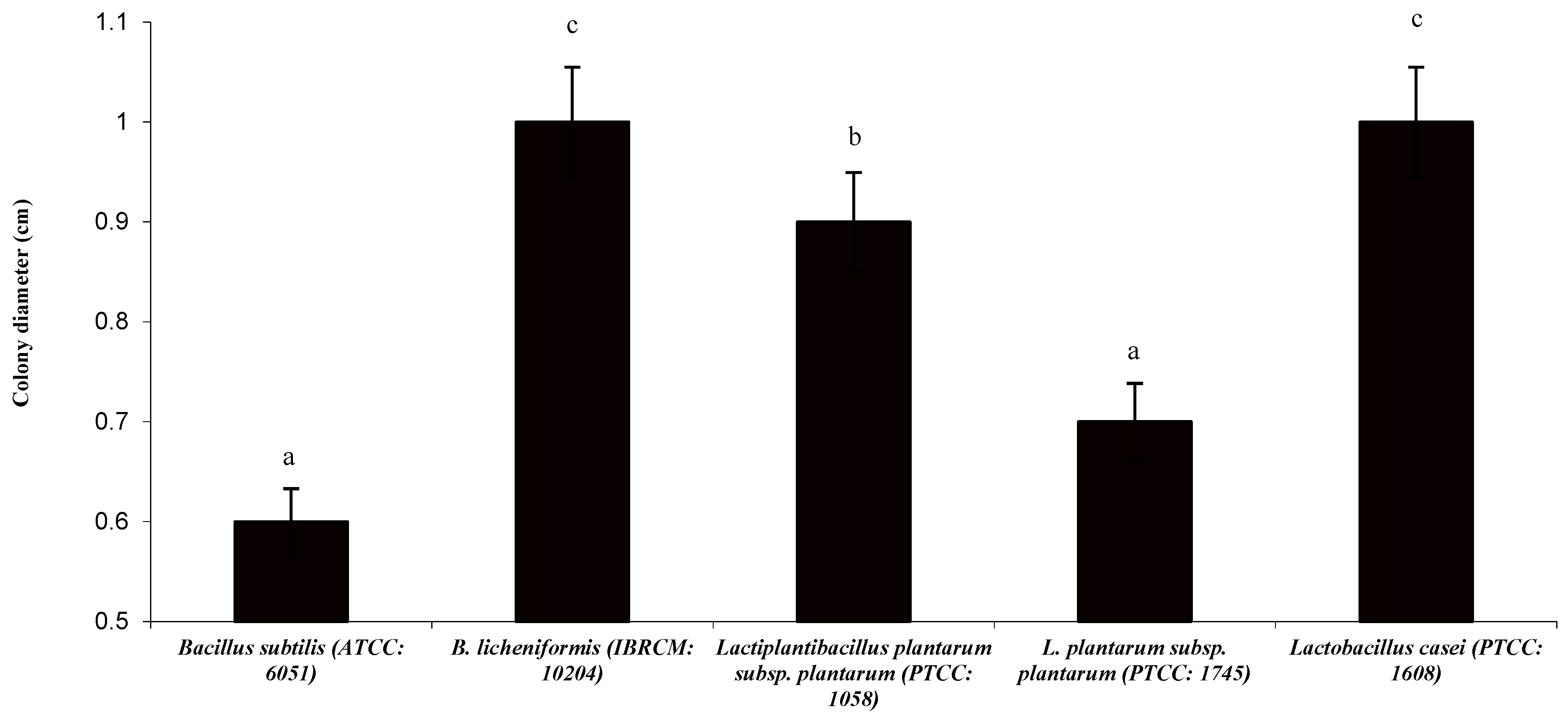
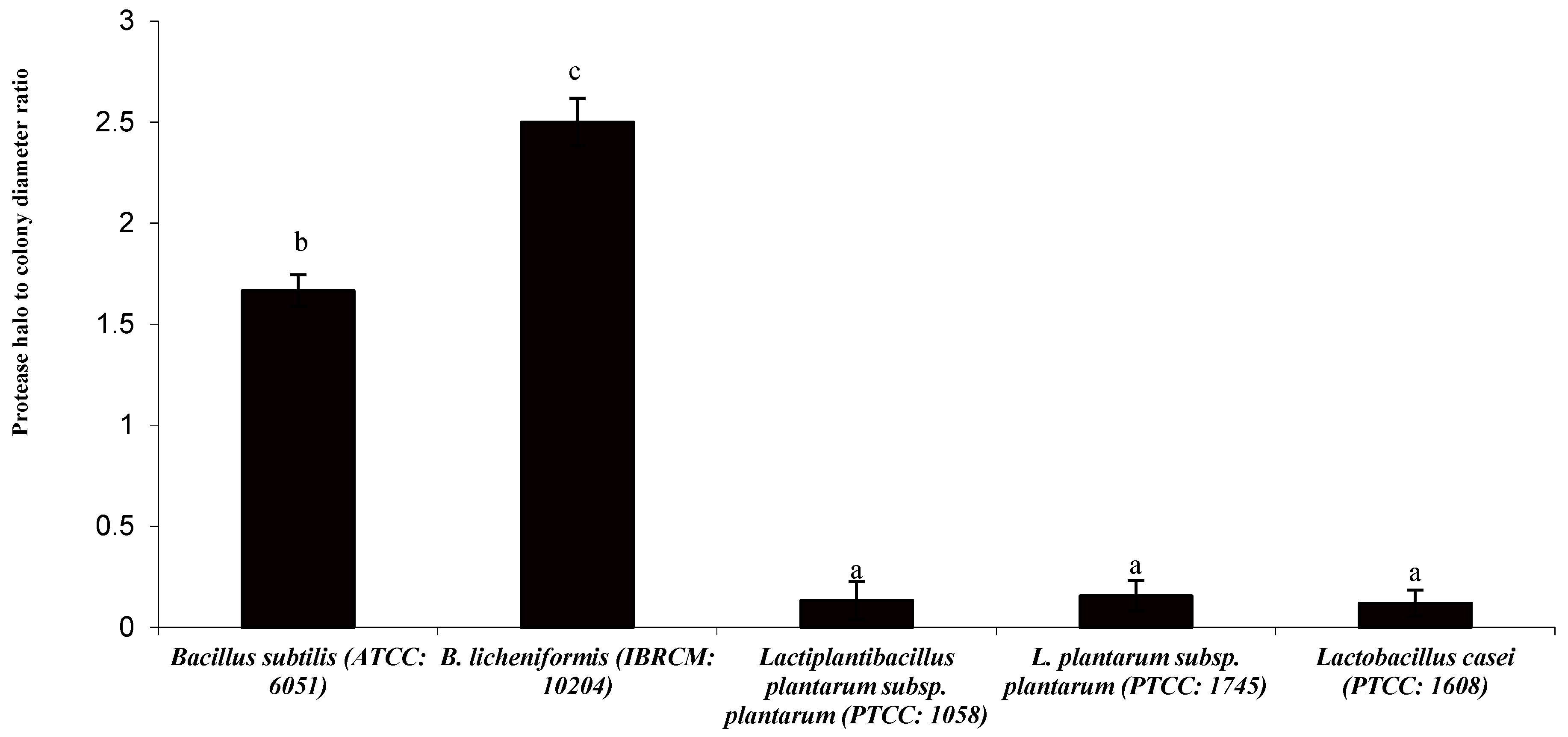
3.1. Protease Halo and Colony Diameter
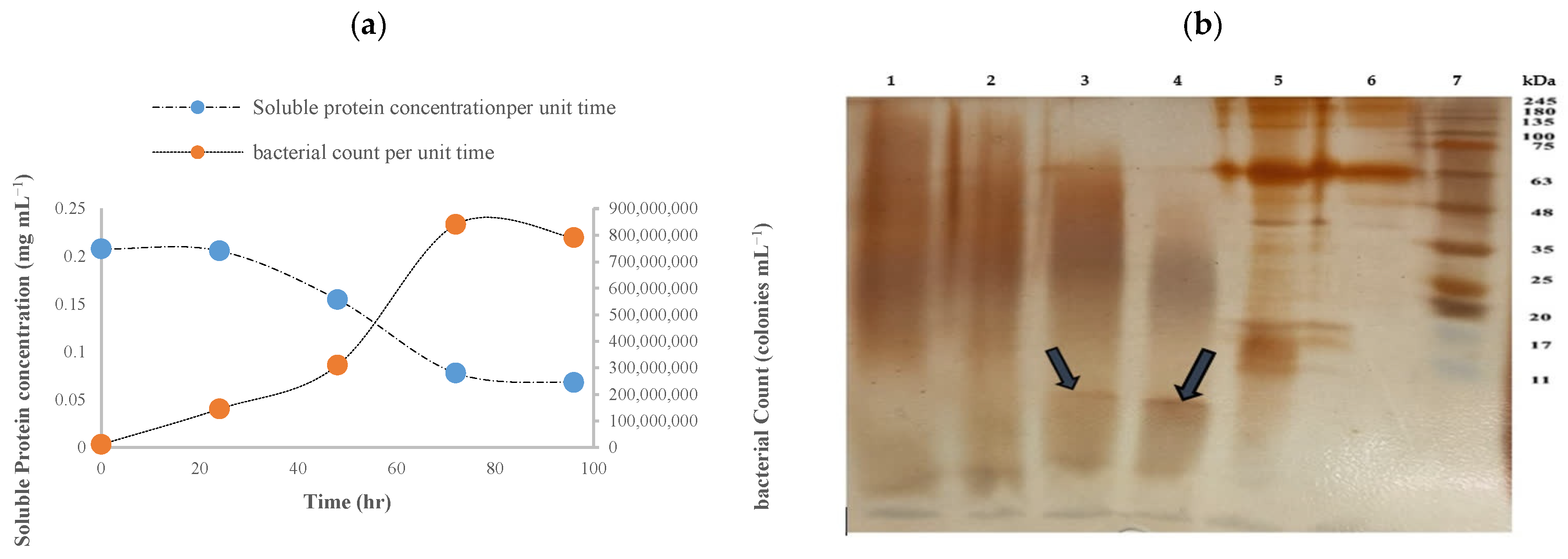
3.2. Soluble Protein Concentration and Bacterial Growth
3.3. Polyacrylamide Gel Electrophoresis of Soluble Protein
3.4. Growth Performance and Carcass Chemical Composition
3.5. Thyroid Hormone Concentrations
3.6. Immune Responses
3.7. Hepatopancreas Enzymes and Antioxidant Indices
3.8. Digestive Enzyme Activities
4. Discussion
4.1. Bacteria Growth and Selection
4.2. Growth Performance and Nutritional Efficiency Indices
4.3. Blood Biochemical Indices
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 2022. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture; FAO Fisheries and Aquaculture Department: Rome, Italy, 2020. [Google Scholar]
- El-Sayed, A.M.; Fitzsimmons, K. From Africa to the world-the journey of Nile tilapia. Rev. Aquac. 2022, 15, 6–21. [Google Scholar] [CrossRef]
- Ozogul, F.; Cagalj, M.; Šimat, V.; Ozogul, Y.; Tkaczewska, J.; Hassoun, A.; Kaddour, A.A.; Kuley, E.; Rathod, N.B.; Phadke, G.G. Recent developments in valorisation of bioactive ingredients in discard/seafood processing by-products. Trends Food Sci. Technol. 2021, 116, 559–582. [Google Scholar] [CrossRef]
- Rabiei, S.; Rezaei, M.; Asgharzade, S.; Nikoo, M.; Rafieia-Kopai, M. Antioxidant and cytotoxic properties of protein hydrolysates obtained from enzymatic hydrolysis of Klunzinger’s mullet (Liza klunzingeri) muscle. Braz. J. Pharm. Sci. 2021, 55, e18304. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Omega-3 polyunsaturated fatty acids and their health benefts. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef]
- Safari, R.; Reyhani Poul, S.; Yaghoubzadeh, Z.; Bankehsaz, Z.; Taghavi, M.J.; Safari, E. Investigation of some chemical and microbial properties of biosilage produced from rainbow trout (Oncorhynchus mykiss) wastes. J. Fish. 2021, 74, 575–586. [Google Scholar] [CrossRef]
- Ahmed, N.; Thompson, S.; Glaser, M. Global aquaculture productivity, environmental sustainability, and climate change adaptability. Environ. Manag. 2019, 63, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Longo, S.B.; Clark, B.; York, R.; Jorgenson, A.K. Aquaculture and the displacement of fisheries captures. Conserv. Biol. 2019, 33, 832–841. [Google Scholar] [CrossRef]
- Siddik, M.A.B.; Howieson, J.; Ilham, I.; Fotedar, R. Growth, biochemical response and liver health of juvenile barramundi (Lates calcarifer) fed fermented and nonfermented tuna hydrolysate as fishmeal protein replacement ingredients. Aquat. Biol. PeerJ 2018, 6, e4870. [Google Scholar]
- Chai, K.F.; Voo, A.Y.H.; Chen, W.N. Bioactive peptides from food fermentation: A comprehensive review of their sources, bioactivities, applications, and future development. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3825–3885. [Google Scholar] [CrossRef]
- Barzkar, N.; Khan, Z.; Tamadoni Jahromi, S.; Pourmozaffar, S.; Gozari, M.; Nahavandi, R. A critical review on marine serine protease and its inhibitors: A new wave of drugs? Int. J. Biol. Macromol. 2021, 170, 674–687. [Google Scholar] [CrossRef]
- Jemil, I.; Jridi, M.; Nasri, R.; Ktari, N.; Salem, R.B.S.-B.; Mehiri, M.; Hajji, M.; Nasri, M. Functional, antioxidant and antibacterial properties of protein hydrolysates prepared from fish meat fermented by Bacillus subtilis A26. Process. Biochem. 2014, 49, 963–972. [Google Scholar] [CrossRef]
- Mao, X.; Liu, P.; He, S.; Xie, J.; Kan, F.; Yu, C.; Li, Z.; Xue, C.; Lin, H. Antioxidant properties of bioactive substances from shrimp head fermented by Bacillus licheniformis OPL-007. Appl. Biochem. Biotechnol. 2013, 171, 1240–1252. [Google Scholar] [CrossRef]
- Soltan, M.A.; El- Laithy, S.M. Evaluation of fermented silage mae from fish, tomato and potato by-products as a feed ingredient for Nil Tilapia, Oreochromis niloticus. Egypt. J. Aquat. Biol. Fish. 2008, 12, 25–41. [Google Scholar] [CrossRef]
- Abd Rashid, N.Y.; Abdul Manan, M.; Faizal Pa’ee, K.; Saari, N.; Faizal Wong, F.W. Evaluation of antioxidant and antibacterial activities of fish protein hydrolysate produced from Malaysian fish sausage (Keropok Lekor) by-products by indigenous Lactobacillus casei fermentation. J. Clean. Prod. 2022, 347, 131303. [Google Scholar] [CrossRef]
- Rajendran, S.R.C.K.; Mohan, A.; Khiari, Z.; Udenigwe, C.C.; Mason, B. Yield, physicochemical, and antioxidant properties of Atlantic salmon visceral hydrolysate: Comparison of lactic acid bacterial fermentation with flavourzyme proteolysis and formic acid treatment. J. Food Process. Preserv. 2018, 42, e13620. [Google Scholar] [CrossRef]
- Choksawangkarn, W.; Phiphattananukoon, S.; Jaresitthikunchai, J.; Roytrakul, S.; Resources, N. Antioxidative peptides from fish sauce by-product: Isolation and characterization. Agric. Nat. Resour. 2018, 5, 460–466. [Google Scholar] [CrossRef]
- Ruthu, N.; Murthy, P.S.; Rai, A.K.; Bhaskar, N. Fermentative recovery of lipids and proteins from freshwater fish head waste with reference to antimicrobial and antioxidant properties of protein hydrolysate. J. Food Sci. Technol. 2014, 51, 1884–1892. [Google Scholar] [CrossRef]
- El-Dakar, A.; Elgamal, A.; Amer, M.A.; Mohammed, A.S.; Abdel-Aziz, M.F. Evaluation of fermented soybean meal by Bacillus subtilis as an alternative to fishmeal on the growth, and physiological status of Nile tilapia Oreochromis niloticus fingerlings. Heliyon 2023, 9, e19602. [Google Scholar] [CrossRef]
- Hassaan, M.S.; Soltan, M.A.; Abdel-Moez, A.M. Nutritive value of soybean meal after solid state fermentation with Saccharomyces cerevisiae for Nile tilapia, Oreochromis niloticus. Anim. Feed. Sci. Technol. 2015, 201, 89–98. [Google Scholar] [CrossRef]
- Hassaan, M.S.; Soltan, M.A.; Mohammady, E.Y.; Elashry, M.A.; El-Haroun, E.R.; Davies, S.J. Growth and physiological responses of Nile tilapia, Oreochromis niloticus fed dietary fermented sunflower meal inoculated with Saccharomyces cerevisiae and Bacillus subtilis. Aquaculture 2018, 495, 592–601. [Google Scholar] [CrossRef]
- Dawood, M.A.; Eweedah, N.M.; Khalafalla, M.M.; Khalid, A.; El Asely, A.; Fadl, S.E.; Amin, A.A.; Paray, B.A.; Ahmed, H.A. Saccharomyces cerevisiae increases the acceptability of Nile tilapia (Oreochromis niloticus) to date palm seed meal. Aquac. Rep. 2020, 17, 100314. [Google Scholar] [CrossRef]
- Ismail, T.; Hegazi, E.; Nassef, E.; El-Din, M.T.S.; Dawood, M.A.; Abdo, S.E.; Gewaily, M.S. Gut immune-related gene expression, histomorphometry and hematoimmunological assays in Nile tilapia (Oreochromis niloticus) fed Aspergillus oryzae fermented olive cake. Fish Shellfish Immunol. 2021, 117, 299–310. [Google Scholar] [CrossRef]
- Mohammady, E.Y.; Aboseif, A.M.; Soaudy, M.R.; Ramadan, E.A.; Hassaan, M.S. Appraisal of fermented wheat bran by Saccharomyces cerevisiae on growth, feed utilization, blood indices, intestinal and liver histology of Nile tilapia, Oreochromis niloticus. Aquaculture 2023, 575, 739755. [Google Scholar] [CrossRef]
- Sherif, E.M.; El-Razek, I.M.A.; El-Sharawy, M.E.; Amer, A.A.; Zaineldin, A.I.; Gewaily, M.S.; Ashry, A.M.; Younis, N.A.; Ahmed, H.A.; Dawood, M.A. Growth performance, antioxidative status, and immune response of Nile tilapia (Oreochromis niloticus) fed dietary fermented Spirulina platensis. Aquac. Rep. 2024, 39, 102324. [Google Scholar] [CrossRef]
- Nkhata, S.G.; Ayua, E.; Kamau, E.H.; Shingiro, J.B. Fermentation and germination improve nutritional value of cereals and legumes through activation of endogenous enzymes. Food Sci. Nutr. 2018, 6, 2446–2458. [Google Scholar] [CrossRef] [PubMed]
- Baron, E.J.; Finegold, S.M. Baily and Scott’s Diagnostic Microbiology, 8th ed.; The C.V. Mosby Company: St. Louis, MO, USA; Baltmore, MD, USA; Philadelphia, PA, USA; Toronto, ON, Canada, 1990; p. 451456. [Google Scholar]
- Sneath, P.H.A.; Sharpe, M.E.; Holt, J.G. Bergey’s Manual of Systematic Bacteriology, 1st ed.; The Williams & Wilkins Company: Baltimore, MD, USA, 1984; Volume 2, pp. 1104–1139. [Google Scholar]
- Downes, F.P.; Lto, K. Compendium of Methods for the Microbiological Analytic Examination of Foods, 4th ed.; APHA: Washington, DC, USA, 2001. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (NRC). Nutrient Requirements of Fish; National Academy Press: Washington, DC, USA, 1996. [Google Scholar]
- El-Sayed, A.-F.M. Environmental Requirements. In Tilapia Culture; CABI Publishing: Oxford, UK, 1950; pp. 34–46. [Google Scholar]
- Khanzadeh, S.; Shahsavani, D.; Safari, O. Synergistic effects of synbiotic and hydrolyzed yeast extract in the diet on growth performance, hemato-immunological responses, and digestive enzyme activities of Nile tilapia (Oreochromis niloticus) fry. Aquac. Int. 2025, 33, 236. [Google Scholar] [CrossRef]
- Refstie, S.; Olli, J.J.; Standal, H. Feed intake, growth, and protein utilisation by post-smolt Atlantic salmon (Salmo salar) in response to graded levels of fish protein hydrolysate in the diet. Aquaculture 2004, 239, 331–349. [Google Scholar] [CrossRef]
- Squires, E.J. Applied Animal Endocrinology; CABI Publishing: Oxford, UK, 2003; p. 234. [Google Scholar]
- Amar, E.C.; Kiron, V.; Satoh, S.; Okamoto, N.; Watanabe, T. Effects of dietary β-carotene on the immune response of rainbow trout Oncorhynchus mykiss. Fish. Sci. 2000, 66, 1068–1075. [Google Scholar] [CrossRef]
- Siwicki, A.K.; Anderson, D.P.; Rumsey, G.L. Dietary intake of immunostimulants by rainbow trout affects non-specific immunity and protection against furunculosis. Vet. Immunol. Immunopathol. 1994, 41, 125–139. [Google Scholar] [CrossRef]
- Goth, L. A simple method for determination of serum catalase activity and revision of reference range. Clin. Chim. Acta 1991, 196, 143–151. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide Dismutase: Improved Assays and an Assay Applicable to Acrylamide Gels. Anal. Biochem. 1971, 44, 276. [Google Scholar] [CrossRef] [PubMed]
- Bolann, B.; Ulvik, R. Improvement of a direct spectrophotometric assay for routine determination of superoxide dismutase activity. Clin. Chem. 1992, 37, 1993–1999. [Google Scholar] [CrossRef]
- Bergmeyer, H.U.; Hodar, M.; Rej, R. Approved recommendation (1985) on IFCC method for the measurement of catalytic concentration of enzymes Part 3. IFCC method for alanine aminotransferase (Lalanine:2- oxoglutarate aminotransferase, EC 2.6.1.2). J. Clin. Chem. Clin. Biochem. 1986, 24, 497–508. [Google Scholar]
- Moss, D.W.; Henderson, A.R. Clinical enzymology. In Tietz Textbook of Clinical Chemistry, 3rd ed.; Burtis, C.A., Ashwood, E.R., Eds.; W.B. Saunders Company: Philadelphia, PA, USA, 1999; pp. 617–721. [Google Scholar]
- Furné, M.; García-Gallego, M.; Hidalgo, M.C.; Morales, A.E.; Domezain, A.; Domezain, J.; Sanz, A. Effect of starvation and refeeding on digestive enzyme activities in sturgeon (Acipenser naccarii) and trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2008, 149, 420–442. [Google Scholar] [CrossRef] [PubMed]
- Gimenez, A.F.; García-Carreño, F.; del Toro, M.N.; Fenucci, J. Digestive proteinases of red shrimp Pleoticus muelleri (Decapoda, Penaeoidea): Partial characterization and re lationship with molting. Comp. Biochem. Physiol. 2001, 130, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Worthington, C.C. Worthington Enzyme Manual Related Biochemical, 3rd ed.; Worthington Biochemical Corporation: Freehold, NJ, USA, 1991; pp. 38–42. [Google Scholar]
- López-López, S.; Nolasco, H.; Vega-Villasante, F. Characterization of digestive gland esterase–lipase activity of juvenile redclaw crayfish Cherax quadricarinatus. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2003, 135, 337–347. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists Arlington: Washington, DC, USA, 2005. [Google Scholar]
- Lichon, M.J. Sample preparation. In Handbook of Food Analysis; Nollet, L.M.L., Ed.; Marcel Dekker: New York, NY, USA, 1996; Volume 1, pp. 1–19. [Google Scholar]
- Zar, J.H. Biostatistical Analysis; Prentice-Hall, Inc.: Upper Saddle River, NJ, USA, 1999. [Google Scholar]
- De Souza, P.M.; Bittencourt, M.L.A.; Caprara, C.C.; de Freitas, M.; de Almeida, R.P.C. A biotechnology perspective of fungal proteases. Braz. J. Microbiol. 2015, 46, 337–346. [Google Scholar] [CrossRef]
- Zhi, T.; Li, X.; Sadiq, F.A.; Mao, K.; Gao, J.; Mi, S.; Liu, X.; Deng, W.; Chitrakar, B.; Sang, Y. Novel antioxidant peptides from protein hydrolysates of scallop (Argopecten irradians) mantle using enzymatic and microbial methods: Preparation, purifcation, identifcation and characterization. LWT 2022, 164, 113636. [Google Scholar] [CrossRef]
- Ismail, T.; Hegazi, E.; Dawood, M.A.; Nassef, E.; Bakr, A.; Paray, B.A.; Van Doan, H. Using of betaine to replace fsh meal with soybean or/and corn gluten meal in Nile tilapia (Oreochromis niloticus) diets: Histomorphology, growth, fatty acid, and glucose-related gene expression traits. Aquac. Rep. 2020, 17, 100376. [Google Scholar] [CrossRef]
- Siddik, M.A.B.; Julien, B.B.; Islam, S.M.M.; Francis, D.S. Fermentation in aquafeed processing: Achieving sustainability in feeds for global aquaculture production. Rev. Aquac. 2024, 16, 1244–1265. [Google Scholar] [CrossRef]
- Li, S.; Ding, G.; Song, F.; Sang, C.; Wang, A.; Chen, N. Comparison of dehulled, fermented and enzyme-treated soybean meal in diets for largemouth bass, Micropterus salmoides: Effects on growth performance, feed utilization, immune response and intestinal morphology. Anim. Feed. Sci. Technol. 2020, 267, 114548. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Cui, M.; Zhang, M.; Xu, J.; Zhang, Z.; Sui, Z.; Wang, L.; Zhang, C.; Li, C.; et al. Comparison of the performance of raw and Lactobacillus paracasei fermented soybean meal in diets for turbot (Scophthalmus maximus L.): Growth, intestinal morphology, apoptosis, tight junction, and microbiota. Aquac. Rep. 2022, 24, 101184. [Google Scholar] [CrossRef]
- Xu, C.; Liu, W.; Zhang, D.; Liu, J.; Zheng, X.; Zhang, C.; Yao, J.; Zhu, C.; Chi, C. Effects of partial fish meal replacement with two fermented soybean meals on the growth of and protein metabolism in the Chinese mitten crab (Eriocheir sinensis). Aquac. Rep. 2020, 17, 100328. [Google Scholar] [CrossRef]
- Wang, Z.; Dong, W.X.; Qiao, F.; Du, Z.Y.; Zhang, M.L. Comparison of Pediococcus pentosaceus YC64-fermented soybean meal and raw soybean meal in diets for Nile tilapia: Growth performance, feed utilization, and intestine health. Aquac. Rep. 2024, 38, 102321. [Google Scholar] [CrossRef]
- Du, Z.; Li, Y. Review and perspective on bioactive peptides: A roadmap for research, development, and future opportunities. J. Agric. Food Res. 2022, 9, 100353. [Google Scholar] [CrossRef]
- Gao, R.; Yu, Q.; Shen, Y.; Chu, Q.; Chen, G.; Fen, S.; Yang, M.; Yuan, L.; McClements, D.J.; Sun, Q. Production, bioactive properties, and potential applications of fish protein hydrolysates: Developments and challenges. Trends Food Sci. Technol. 2021, 110, 687–699. [Google Scholar] [CrossRef]
- Abdollahpour, H.; Falahatkar, B. Improve growth performance in Sterlet Sturgeon Acipenser ruthenus larvae subsequent thyroxine injection to brood stock. In Proceedings of the 8th International Symposium on Sturgeons, Vienna, Austria, 10–15 September 2017. [Google Scholar]
- Lam, T.J. Thyroxine enhances larval development and survival in Sarotherodon (Tilapia) mossambicus Ruppell. Aquaculture 1980, 21, 287–291. [Google Scholar] [CrossRef]
- MacKenzie, D.S.; VanPutte, C.M.; Leiner, K.A. Nutrient regulation of endocrine function in fish. Aquaculture 1998, 161, 3–25. [Google Scholar] [CrossRef]
- Hajirezaee, S.; Ramezani, S.; Ahani, S. Betaine and the probiotic, Lactobacillus rhamnosus in the diet of the Common carp, Cyprinus carpio: Effects on growth, digestive enzyme activities, antioxidant system, humoral and mucosal immunity and resistance to Streptococcus iniae. Aquac. Rep. 2024, 38, 102282. [Google Scholar] [CrossRef]
- Jemil, I.; Nasri, R.; Abdelhedi, O.; Aristoy, M.-C.; Salem, R.B.S.-B.; Kallel, C.; Marrekchi, R.; Jamoussi, K.; ElFeki, A.; Hajji, M.; et al. Beneficial effects of fermented sardinelle protein hydrolysates on hypercaloric diet induced hyperglycemia, oxidative stress and deterioration of kidney function in wistar rats. J. Food Sci. Technol. 2017, 54, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.P.; Zhuang, P.; Zhang, L.Z.; Liu, J.Y.; Duan, M.; Zhao, F.; Yan, W.G. Effects of water temperature on metabolic enzyme and antioxidase activities in juvenile Chinese sturgeon (Acipenser sinensis). Acta Hydrobiol. Sin. 2012, 36, 137–142, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Luo, Y.W.; Ai, Q.H.; Mai, K.S.; Zhang, W.B.; Xu, W.; Zhang, Y.J. Effects of dietary rapeseed meal on growth performance, digestion and protein metabolism in relation to gene expression of juvenile cobia (Rachycen troncanadum). Aquaculture 2012, 368–369, 109–116. [Google Scholar] [CrossRef]
- Sharif, M.; Zafar, M.H.; Aqib, A.I.; Saeed, M.; Farag, M.R.; Alagawany, M. Single cell protein: Sources, mechanism of production, nutritional value and its uses in aquaculture nutrition. Aquaculture 2021, 531, 735885. [Google Scholar] [CrossRef]
- Maas, R.M.; Verdegem, M.C.; Stevens, T.L.; Schrama, J.W. Effect of exogenous enzymes (phytase and xylanase) supplementation on nutrient digestibility and growth performance of Nile tilapia (Oreochromis niloticus) fed different quality diets. Aquaculture 2020, 529, 735723. [Google Scholar] [CrossRef]
- Hendam, B.M.; Munir, M.B.; Eissa, M.E.; El-Haroun, E.; van Doan, H.; Chung, T.H.; Eissa, E.-S.H. Effects of water additive probiotic, Pediococcus acidilactici on growth performance, feed utilization, hematology, gene expression and disease resistance against Aspergillus flavus of Nile tilapia (Oreochromis niloticus). Anim. Feed. Sci. Technol. 2023, 303, 115696. [Google Scholar] [CrossRef]
- Siddik, M.A.B.; Howieson, J.; Fotedar, R. Beneficial effects of tuna hydrolysate in poultry by-product meal diets on growth, immune response intestinal health and disease resistance to Vibrio harveyi in juvenile barramundi, Lates calcarifer. Fish Shellfish Immunol. 2019, 89, 61–70. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, Z.; Cai, J. Effects of dietary fish meal replacement by fermented moringa (Moringa oleifera Lam.) leaves on growth performance, nonspecific immunity and disease resistance against Aeromonas hydrophila in juvenile gibel carp (Carassius auratus gibelio var. CAS III). Fish Shellfish Immunol. 2020, 102, 430–439. [Google Scholar] [CrossRef]
- Knoop, K.A.; Newberry, R.D. Goblet cells: Multifaceted players in immunity at mucosal surfaces. Mucosal Immunol. 2018, 11, 1551–1557. [Google Scholar] [CrossRef]
- Hemaiswarya, S.; Raja, R.; Ravikumar, R.; Carvalho, I.S. Mechanism of action of probiotics. Braz. Arch. Biol. Technol. 2013, 56, 113–119. [Google Scholar] [CrossRef]
- Das, P.; Mandal, S.; Khan, A.; Manna, S.K.; Ghosh, K. Distribution of extracellular enzyme-producing bacteria in the digestive tracts of 4 brackish water fish species. Turk. J. Zool. 2014, 38, 79–88. [Google Scholar] [CrossRef]
| Feed Ingredient (g kg−1) | Control | Different Fishmeal Replacement Levels with Fermented By-Product (%) | |||
|---|---|---|---|---|---|
| 25 | 50 | 75 | 100 | ||
| Fishmeal a | 200 | 150 | 100 | 50 | 0 |
| Fermented by-product α | 0 | 57.5 | 115.5 | 173.2 | 231.0 |
| Soybean meal a | 270 | 270 | 270 | 270 | 270 |
| Wheat gluten a | 145 | 145 | 145 | 145 | 145 |
| Wheat flour a | 127 | 127 | 127 | 127 | 127 |
| Corn flour a | 127 | 127 | 127 | 127 | 127 |
| Fish oil a | 40 | 40 | 40 | 40 | 40 |
| Soybean oil a | 40 | 40 | 40 | 40 | 40 |
| Mineral supplement b* | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 |
| Vitamin supplement b** | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 |
| Carboxymethyl cellulose (CMC) c | 36 | 28.3 | 20.5 | 12.8 | 5 |
| Chemical composition (g kg−1) d | |||||
| Dry matter | 921.3 | 928.7 | 928.7 | 930.6 | 929.3 |
| Crude protein | 377.1 | 377.1 | 377.1 | 377.1 | 377.1 |
| Crude fat | 100.8 | 100.7 | 100.7 | 100.7 | 100.7 |
| Crude fiber | 35 | 35 | 34.7 | 34.6 | 34.5 |
| Nitrogen-free extract | 413.8 | 412.9 | 412.3 | 412.0 | 411.8 |
| Ash | 73.3 | 74.3 | 75.2 | 75.6 | 75.9 |
| Control | Fishmeal Replacement Levels with Fermented by-Products (%) | ||||
|---|---|---|---|---|---|
| 25 | 50 | 75 | 100 | ||
| Initial weight (g) | 2.83 ± 0.03 | 2.81 ± 0.05 | 2.81± 0.07 | 2.83 ± 0.06 | 2.85 ± 0.06 |
| Final weight (g) | 12.46 ± 0.10 a | 13.16 ± 0.20 b | 13.40 ± 0.32 b | 14.35 ± 0.31 c | 14.24 ± 0.27 c |
| Weight gain (g) | 347.27 ± 1.93 a | 368.93 ± 4.27 b | 376.31± 4.31 c | 407.66 ± 0.85 e | 399.72 ± 1.41 d |
| Condition factor (%) | 2.44 ± 0.19 | 2.50 ± 0.38 | 2.23 ± 0.43 | 2.22 ± 0.07 | 2.29 ± 0.30 |
| Protein efficiency ratio | 1.94 ± 0.03 a | 2.14 ± 0.01 b | 2.30 ± 0.03 c | 2.76 ± 0.02 d | 2.89 ± 0.02 e |
| Hepatosomatic index (%) | 2.88 ± 1.06 | 2.31 ± 0.56 | 2.36 ± 0.67 | 2.50 ± 1.00 | 2.70 ± 1.10 |
| Viscerosomatic index (%) | 8.85 ± 2.38 b | 7.68 ± 1.63 ab | 6.93 ± 1.40 a | 6.89 ± 1.35 a | 6.70 ± 1.68 a |
| Control | Fishmeal Replacement Levels with Fermented By-Products (%) | ||||
|---|---|---|---|---|---|
| 25 | 50 | 75 | 100 | ||
| Dry matter (%) | 95.53 ± 0.11 a | 95.63 ± 0.06 a | 95.77 ± 0.15 ab | 95.90 ± 0.3 ab | 96.10 ± 0.36 b |
| Crude protein (%) | 57.33 ± 0.06 a | 57.87 ± 0.06 b | 58.43 ± 0.15 c | 59.87 ± 0.4 d | 60.50 ± 0.36 e |
| Crude lipid (%) | 9.13 ± 0.20 e | 8.73 ± 0.06 d | 8.40 ± 0.10 c | 7.93 ± 0.15 b | 7.50 ± 0.10 a |
| Ash (%) | 6.80 ± 0.10 a | 7.40 ± 0.10 b | 7.80 ± 0.10 c | 8.60 ± 0.10 d | 8.87 ± 0.06 e |
| Carbohydrates (%) | 26.73 ± 0.23 d | 26.00 ± 0.17 c | 25.37 ± 0.15 b | 23.60 ± 0.6 a | 23.13 ± 0.29 a |
| Control | Fishmeal Replacement Levels with Fermented By-Products (%) | ||||
|---|---|---|---|---|---|
| 25 | 50 | 75 | 100 | ||
| Triiodothyronine (ng mL−1) | 1.33 ± 0.01 a | 1.40 ± 0.02 b | 1.60 ± 0.02 c | 1.87 ± 0.01 d | 1.85 ± 0.01 d |
| Thyroxine (ng mL−1) | 2.26 ± 0.03 a | 2.37 ± 0.02 b | 2.50 ± 0.02 c | 2.90 ± 0.02 d | 2.90 ± 0.03 d |
| Control | Fishmeal Replacement Levels with Fermented By-Products (%) | ||||
|---|---|---|---|---|---|
| 25 | 50 | 75 | 100 | ||
| Lysozyme (mg mL−1) | 2.47 ± 0.01 a | 2.60 ± 0.02 b | 2.77 ± 0.02 c | 2.93 ± 0.01 d | 3.00 ± 0.02 e |
| ACH50 (Unit mL−1) | 3.12 ± 0.01 a | 3.36 ± 0.01 b | 3.60 ± 0.02 c | 3.79 ± 0.03 e | 3.74 ± 0.01 d |
| Total immunoglobulin (mg mL−1) | 2.11 ± 0.01 a | 2.34 ± 0.01 b | 2.46 ± 0.01 c | 2.65 ± 0.01 e | 2.62 ± 0.01 d |
| Control | Fishmeal Replacement Levels with Fermented By-Products (%) | ||||
|---|---|---|---|---|---|
| 25 | 50 | 75 | 100 | ||
| Catalase (Unit L−1) | 1.11 ± 0.02 a | 1.19 ± 0.02 b | 1.37 ± 0.01 c | 1.47 ± 0.02 e | 1.40 ± 0.02 d |
| superoxide dismutase (Unit L−1) | 2.36 ± 0.01 a | 2.40 ± 0.01 b | 2.58 ± 0.01 c | 2.80 ± 0.01 c | 2.70 ± 0.01 d |
| Aspartate aminotransferase (Unit L−1) | 0.97 ± 0.005 a | 1.05 ± 0.025 b | 1.12 ± 0.01 c | 1.16 ± 0.01 d | 1.22 ± 0.03 e |
| Alanine aminotransferase (Unit L−1) | 1.34 ± 0.02 a | 1.38 ± 0.01 b | 1.58 ± 0.01 c | 1.80 ± 0.02 d | 1.79 ± 0.03 d |
| Control | Fishmeal Replacement Levels with Fermented By-Products (%) | ||||
|---|---|---|---|---|---|
| 25 | 50 | 75 | 100 | ||
| Amylase (Unit mg−1 protein) | 1.34 ± 0.005 a | 1.38 ± 0.01 b | 1.44 ± 0.01 c | 1.60 ± 0.02 d | 1.60 ± 0.01 d |
| Protease (Unit mg−1 protein) | 1.66 ± 0.01 a | 1.80 ± 0.02 b | 1.97 ± 0.02 c | 2.16 ± 0.06 d | 2.10 ± 0.01 d |
| Lipase (Unit mg−1 protein) | 1.81 ± 0.01 a | 2.02 ± 0.03 b | 2.27 ± 0.03 c | 2.61 ± 0.10 d | 2.54 ± 0.01 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taghizadeh Tabasi, F.; Safari, O.; Sarkheil, M.; Gord Noshahri, N.; Paolucci, M. Substituting Fishmeal with Bacillus licheniformis-Fermented Fish By-Products Protein Hydrolysates in Nile Tilapia Diet (Oreochromis niloticus): Impacts on Growth Performance, Humoral Immunity, Oxidative Defense, and Digestive Enzymes. Fishes 2025, 10, 556. https://doi.org/10.3390/fishes10110556
Taghizadeh Tabasi F, Safari O, Sarkheil M, Gord Noshahri N, Paolucci M. Substituting Fishmeal with Bacillus licheniformis-Fermented Fish By-Products Protein Hydrolysates in Nile Tilapia Diet (Oreochromis niloticus): Impacts on Growth Performance, Humoral Immunity, Oxidative Defense, and Digestive Enzymes. Fishes. 2025; 10(11):556. https://doi.org/10.3390/fishes10110556
Chicago/Turabian StyleTaghizadeh Tabasi, Faezeh, Omid Safari, Mehrdad Sarkheil, Najme Gord Noshahri, and Marina Paolucci. 2025. "Substituting Fishmeal with Bacillus licheniformis-Fermented Fish By-Products Protein Hydrolysates in Nile Tilapia Diet (Oreochromis niloticus): Impacts on Growth Performance, Humoral Immunity, Oxidative Defense, and Digestive Enzymes" Fishes 10, no. 11: 556. https://doi.org/10.3390/fishes10110556
APA StyleTaghizadeh Tabasi, F., Safari, O., Sarkheil, M., Gord Noshahri, N., & Paolucci, M. (2025). Substituting Fishmeal with Bacillus licheniformis-Fermented Fish By-Products Protein Hydrolysates in Nile Tilapia Diet (Oreochromis niloticus): Impacts on Growth Performance, Humoral Immunity, Oxidative Defense, and Digestive Enzymes. Fishes, 10(11), 556. https://doi.org/10.3390/fishes10110556








