Fish Digestive Capacity: Definition and Methods
Abstract
1. Introduction
Nutrient Origin and Early Digestive Ontogeny in Teleosts
- Catalog historical and current uses of the term digestive capacity.
- Synthesize the analytical approaches employed to quantify it.
- Propose a physiologically grounded definition based on hydrolytic potential.
- Recommend harmonized assay procedures applicable across teleost species (and other taxa) and their environmental contexts.
2. Digestive Capacity in Fish
2.1. Systematic Search
2.2. Operational Definitions: Digestive Capacity
2.3. Taxonomic and Ontogenetic Coverage
2.4. Enzymes Assessed
2.4.1. α-Amylase
2.4.2. Chymotrypsin
2.4.3. Leucine–Alanine Peptidase and Leucine Aminopeptidase
2.4.4. Lipase
2.4.5. Acid and Alkaline Phosphatases
2.4.6. Pepsin-like Acid Protease
2.4.7. Total Alkaline Protease
2.4.8. Trypsin
3. Discussion—Interpreting Digestive Capacity Measurements
3.1. Toward a Standard Unit
3.2. Ionic Environment Matters
3.3. Temperature—The Most Neglected Variable
3.4. pH—An Equally Critical Parameter
3.5. Gaps in Enzyme Coverage
3.6. Closing the Gaps
3.7. Digestive Capacity Determination Incorporating Extract Theoretical Volume (ETV)
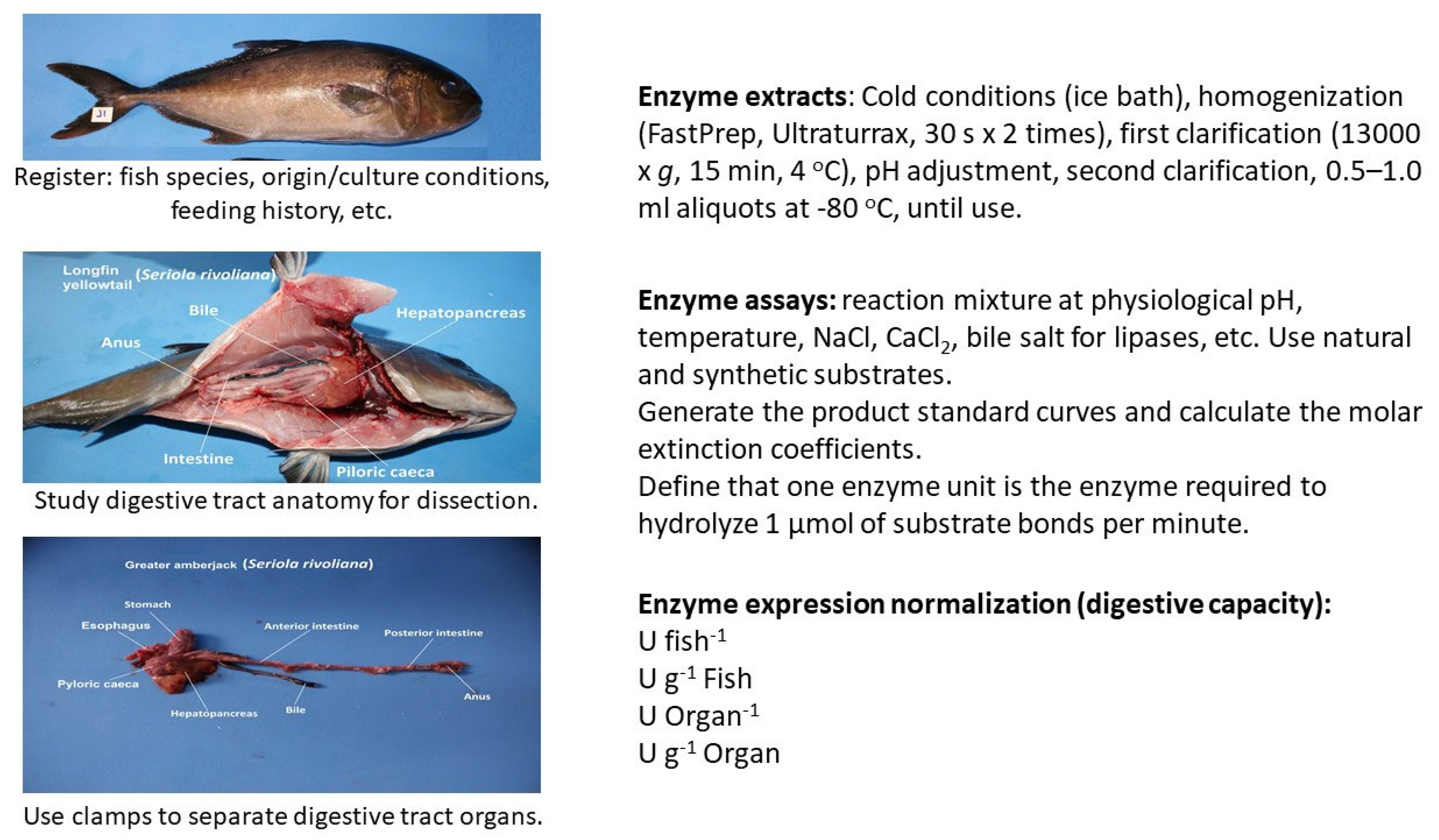
- 1.
- Sampling Through a Standardized Method
- 2.
- Identify and Isolate Digestive Organs
- 3.
- Prepare Enzymatic Extracts
- 4.
- Measure Crude Extract Activity (U mL−1)
- Determine the change in absorbance per minute.
- Correct to a 1 cm light path.
- Convert absorbance to μmol of product released, using the molar extinction (absorption) coefficient, derived from a standard curve of the reaction product.
- One unit is the amount of enzyme that catalyzes the conversion of 1 μmol of substrate per minute under specified assay conditions.
- Calculate unit mL−1.
- 5.
- Calculate Extract Theoretical Volume (ETV)
- SV = solvent volume (mL) used for homogenization;
- TM = tissue mass (g);
- %TH = () = tissue moisture content (%).
- 6.
- Compute Total Units (TU)
- 7.
- Calculate Digestive Capacity (DC)
4. Proposal for an Approach Framework to Quantify Digestive Capacity (DC)
4.1. Extract Theoretical Volume (ETV)
4.2. Digestive Capacity (DC)
4.3. Enzyme Activity Expression
4.4. Digestive Processing (DP)
5. Kit-Based Versus Custom Assays—Strengths, Pitfalls, and Recommendations
6. Integrative Technical–Biological Insights Leading to Recommendations
- Match ionic conditions. Adjust assay buffers with NaCl and CaCl2 concentrations that reproduce the species-specific ionic environment of the digestive tract.
- Calibrate in the identical buffer. Construct chromophore standard curves in the same ion-balanced buffer, using the same microplate, wavelength, and incubation temperature as the enzymatic reaction.
- Report absolute units. Express activity as µmol bonds hydrolyzed per minute. Scale results beyond specific activity (U mg−1 protein) to total activity (U fish−1, U organ−1, or U g−1 organ), ensuring biological relevance and enabling robust cross-study comparisons.
7. Conclusions
7.1. Operational Definition
7.2. Generalized Concept
7.3. Minimum Reporting Checklist
- Biological context:
- Species and developmental stage;
- Habitat or culture conditions (water temperature, pH, salinity);
- Sample size, mean body mass, and recent feeding status (including feed information).
- Sampling details:
- Time of slaughter relative to last feeding;
- Organs collected, residual feed status, organ wet mass.
- Extract preparation:
- Homogenization equipment and conditions—type of water (distilled or mili-Q) or buffer composition (including physiological NaCl and CaCl2);
- Solvent volume and tissue moisture used to calculate theoretical extract volume.
- Enzyme assay specifics:
- Substrate and concentration;
- Ionic additives (the proper bile and concentration for lipases) and final buffer pH;
- Incubation temperature (physiological) and duration;
- Detection wavelength (absorbance/fluorescence)—indicates whether the method is kinetic or endpoint;
- Chromophore/fluorescent standard curve and molar extinction coefficient;
- Explicit unit definition (µmol product min−1);
- Indicate the units’ calculation formulas.
7.4. General Guidance
- How to assess the digestive capacity of fish
- Which enzyme activities need to be measured
- Which digestive enzyme in which tissue is being examined
- What the patterns of activity variation in these digestive enzymes in different tissues are
8. Way Forward
- Establish a universal benchmark for digestive enzyme assays across taxa and laboratories.
- Bridge the gap between in vitro enzymatic rates and whole-animal catalytic potential.
- Advance toward reliable estimations of digestive capacity during the complete process of digestion (from ingestion to egestion) of a meal.
- Strengthen physiological relevance, enabling more robust applications in nutritional physiology, ecological comparisons, and aquaculture innovation.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zavala-Leal, I.; Dumas-Lapage, S.; Peña-Martínez, R. Organogénesis durante el periodo larval en peces. CICIMAR Oceánides 2011, 26, 19–30. [Google Scholar] [CrossRef]
- Reyes-Mero, B.M.; Santana-Piñeros, A.M.; Muñoz-Chumo, L.G.; Cruz-Quintana, Y.; Gisbert, E. Yolk Absorption Rate and Mouth Development in Larvae of Dormitator latifrons (Perciformes: Eleotridae). Fishes 2022, 7, 375. [Google Scholar] [CrossRef]
- Gawlicka, A.; Parent, B.; Horn, M.H.; Ross, N.; Opstad, I.; Torrissen, O.J. Activity of digestive enzymes in yolk-sac larvae of Atlantic halibut (Hippoglossus hippoglossus): Indication of readiness for first feeding. Aquaculture 2000, 184, 303–314. [Google Scholar] [CrossRef]
- Yúfera, M.; Moyano, F.J.; Martínez-Rodríguez, G. The digestive function in developing fish larvae and fry. From molecular gene expression to enzymatic activity. In Emerging Issues in Fish Larvae Research; Yúfera, M., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 51–86. [Google Scholar] [CrossRef]
- Zambonino-Infante, J.L.; Gisbert, E.; Sarasquete Navarro, I.; Gutiérrez, J.; Cahu, C.L. Ontogeny and physiology of the digestive system of marine fish larvae. In Feeding and Digestive Functions of Fishes, 1st ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 281–348. [Google Scholar]
- Rønnestad, I.; Yúfera, M.; Ueberschär, B.; Ribeiro, L.; Sæle, Ø.; Boglione, C. Feeding behaviour and digestive physiology in larval fish: Current knowledge, and gaps and bottlenecks in research. Rev. Aquacult. 2013, 5 (Suppl. S1), S59–S98. [Google Scholar] [CrossRef]
- Nazemroaya, S.; Nematollahi, M.A.; Yazdanparast, R.; Farahmand, H.; Rezaie, A.; Zabayeh Najafabadi, M. Pepsinogen expression during larval development of a Persian Gulf Sparid, Sobaity. Aquaculture 2020, 523, 735131. [Google Scholar] [CrossRef]
- Asgari, R.; Rafiee, G.; Eagderi, S.; Noori, F.; Agh, N.; Poorbagher, H.; Gisbert, E. Ontogeny of the digestive enzyme activities in hatchery produced Beluga (Huso huso). Aquaculture 2013, 416–417, 33–40. [Google Scholar] [CrossRef]
- Yúfera, M.; Darias, M.J. The onset of exogenous feeding in marine fish larvae. Aquaculture 2007, 268, 53–63. [Google Scholar] [CrossRef]
- Volkoff, H.; Rønnestad, I. Effects of temperature on feeding and digestive processes in fish. Temperature 2020, 7, 307–320. [Google Scholar] [CrossRef]
- Navarro-Guillén, C.; Gilannejad, N.; Pérez-Hilario, D.; Martínez-Rodríguez, G.; Yúfera, M. Gut transit of daily consecutive meals in greater amberjack juveniles reared at different temperatures. Aquaculture 2023, 567, 739224. [Google Scholar] [CrossRef]
- Navarro-Guillén, C.; Perera, E.; Pérez-Hilario, D.; Martos-Sitcha, J.A.; Molina-Roque, L.; Gregorio, S.F.; Fonseca, F.; Fuentes, J.; Yúfera, M. Digestive and metabolic consequences of on-growing greater amberjack (Seriola dumerili) juveniles at different temperatures. In-vivo and ex-vivo assessment. Aquaculture 2025, 598, 742011. [Google Scholar] [CrossRef]
- Gisbert, H.; Nolasco, H.; Solovyev, M. Towards the standardization of brush border purification and intestinal alkaline phosphatase quantification in fish with notes on other digestive enzymes. Aquaculture 2018, 487, 102–108. [Google Scholar] [CrossRef]
- Nolasco-Soria, H.; Nolasco-Alzaga, H.R.; Gisbert, E. The importance of pepsin-like acid protease quantification in aquaculture studies: A revision of available procedures and presentation of a new protocol for its assessment. Rev. Aquac. 2020, 12, 1928–1943. [Google Scholar] [CrossRef]
- Nolasco-Soria, H. Improving and standardizing protocols for alkaline protease quantification in fish. Rev. Aquac. 2020, 13, 43–65. [Google Scholar] [CrossRef]
- Nolasco-Soria, H. Amylase quantification in aquaculture fish studies: A revision of most used procedures and presentation of a new practical protocol for its assessment. Aquaculture 2021, 538, 736536. [Google Scholar] [CrossRef]
- Nolasco-Soria, H.; Moyano-López, F.; Vega-Villasante, F.; Del Monte, A.; Espinoza-Chaurand, D.; Gisbert, E. Lipase and Phospholipase Activity Methods for Marine Organisms. In Lipases and Phospholipases: Methods and Protocols, Methods in Molecular Biology; Georgina, S., Ed.; Chapter 7; Humana Press: New York, NY, USA, 2018; Volume 1835. [Google Scholar] [CrossRef]
- Nolasco-Soria, H. Fish digestive lipase quantification methods used in aquaculture studies. Front. Aquac. 2023, 2, 1225216. [Google Scholar] [CrossRef]
- Navarro-Guillén, C.; Yúfera, M. Understanding rhythms in the digestive functionality of fish gut. J. Exp. Biol. 2025, 228, jeb249942. [Google Scholar] [CrossRef] [PubMed]
- Labuda, J.; Bowater, R.; Fojta, M.; Gauglitz, G.; Glatz, Z.; Hapala, I.; Havliš, J.; Kilar, F.; Kilar, A.; Malinovská, L.; et al. Terminology of bioanalytical methods (IUPAC Recommendations 2018). Pure Appl. Chem. 2018, 90, 1121–1198. [Google Scholar] [CrossRef]
- Nolasco-Soria, H.; Alvarez-González, C.A.; Tovar-Ramírez, D.; González-Bacerio, J.; del Monte-Martínez, A.; Vega-Villasante, F. Optimization of classical lipase activity assays for fish digestive tract samples. Fishes 2024, 9, 261. [Google Scholar] [CrossRef]
- Boyd, C.E. Typical chemical characteristics of full-strength seawater. Aquac. Advocate. 2020. Available online: https://www.globalseafood.org/advocate/typical-chemical-characteristics-of-full-strength-seawater/ (accessed on 28 July 2025).
- Istvánovics, V.; Honti, M.; Clement, A.; Kravinszkaja, G.; Pósfai, M.; Torma, P. Chloride and sodium budgets of a shallow freshwater lake—Current status and the impact of climate change. Sci. Total Environ. 2024, 957, 177616. [Google Scholar] [CrossRef]
- Sabapathy, U.; Teo, L.H. Some properties of the intestinal proteases of the rabbitfish, Siganus canaliculatus (Park). Fish Physiol. Biochem. 1995, 14, 215–221. [Google Scholar] [CrossRef]
- Fernández, I.; Moyano, F.J.; Díaz, M.; Martínez, T. Characterization of α-amylase activity in five species of Mediterranean sparid fishes (Sparidae, Teleostei). J. Exp. Mar. Biol. Ecol. 2001, 262, 1–12. [Google Scholar] [CrossRef]
- Nolasco, H.; Moyano-López, F.; Vega-Villasante, F. Partial characterization of pyloric-duodenal lipase of gilthead seabream (Sparus aurata). Fish Physiol. Biochem. 2011, 37, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Rueda-López, S.; Martínez-Montaño, E.; Viana, M.T. Biochemical characterization and comparison of pancreatic lipases from the Pacific bluefin tuna (Thunnus orientalis), totoaba (Totoaba macdonaldi), and striped bass (Morone saxatilis). J. World Aquac. Soc. 2017, 48, 156–165. [Google Scholar] [CrossRef]
- Nurhayati, T.; Abdullah, A.; Rahmawati, S.; Kurniawan, R. Characteristics of trypsin isolated from intestines of different fish and correlation toward trypsin activity. Squalen Bull. Mar. Fish. Postharvest Biotechnol. 2024, 19, 131–143. [Google Scholar] [CrossRef]
- Zhou, L.; Budge, S.M.; Ghaly, A.E.; Brooks, M.S.; Dave, D. Extraction, purification and characterization of fish chymotrypsin: A review. Am. J. Biochem. Biotechnol. 2011, 7, 104–123. [Google Scholar] [CrossRef]
- Yúfera, M.; Moyano, F.J.; Astola, A.; Pousão-Ferreira, P.; Martínez-Rodríguez, G. Acidic digestion in a teleost: Postprandial and circadian pattern of gastric pH, pepsin activity, and pepsinogen and proton pump mRNAs expression. PLoS ONE 2012, 7, e33687. [Google Scholar] [CrossRef]
- dos Santos, V.C.W.; da Costa Marques, M.E.; de Araújo Tenório, H.; de Miranda, E.C.; Vieira Pereira, J.H. Purification and characterization of trypsin from Luphiosilurus alexandri pyloric cecum. Biochem. Biophys. Rep. 2016, 8, 29–33. [Google Scholar] [CrossRef]
- Champasri, C.; Phetlum, S.; Pornchoo, C. Diverse activities and biochemical properties of amylase and proteases from six freshwater fish species. Sci. Rep. 2021, 11, 5727. [Google Scholar] [CrossRef]
- Cara, B.; Moyano, F.J.; Zambonino, J.L.; Fauvel, C. Trypsin and chymotrypsin as indicators of nutritional status of post-weaned sea bass larvae. J. Fish Biol. 2007, 70, 1798–1808. [Google Scholar] [CrossRef]
- Monge-Ortiz, R.; Martínez-Llorens, S.; Lemos-Neto, M.J.; Falcó-Giaccaglia, S.L.; Pagán, M.J.; Godoy-Olmos, S.; Jover-Cerdá, M.; Tomás-Vidal, A. Growth, sensory and chemical characterization of Mediterranean yellowtail (Seriola dumerili) fed diets with partial replacement of fish meal by other protein sources. Aquac. Rep. 2020, 18, 100466. [Google Scholar] [CrossRef]
- Shao, Y.; Lin, A.H-M. Improvement in the quantification of reducing sugars by miniaturizing the Somogyi-Nelson assay using a microtiter plate. Food Chem. 2018, 240, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Harris, T.K.; Keshwani, M.M. Measurement of Enzyme Activity. In Methods in Enzymology; Burgess, R.R., Deutscher, M.P., Eds.; Academic Press: Cambridge, MA, USA, 2009; Volume 463, pp. 57–71. [Google Scholar] [CrossRef]
- Jiao, F.; Zhang, L.; Limbu, S.M.; Yin, H.; Xie, Y.; Yang, Z.; Shang, Z.; Kong, L.; Rong, H. A comparison of digestive strategies for fishes with different feeding habits: Digestive enzyme activities, intestinal morphology, and gut microbiota. Ecol. Evol. 2023, 13, e10499. [Google Scholar] [CrossRef] [PubMed]
- Gilannejad, N.; de las Heras, V.; Martos-Sitcha, J.A.; Moyano, F.J.; Yúfera, M.; Martínez-Rodríguez, G. Ontogeny of expression and activity of digestive enzymes and establishment of gh/igf1 Axis in the omnivorous fish Chelon labrosus. Animals 2020, 10, 874. [Google Scholar] [CrossRef] [PubMed]
| Digestive Capacity Definition | Studies | References | % |
|---|---|---|---|
| The activity of digestive enzymes. | 19 | 4, 5, 7, 15, 16, 17, 18, 26, 33, 34, 42, 43, 49, 51, 52, 57, 61, 64, 69 | 30.6 |
| No definition, but the activity of digestive enzymes. | 18 | 6, 8, 10, 19, 27, 40, 44, 48, 54, 55, 56, 58, 59, 60, 63, 65, 66, 70 | 29.0 |
| The anatomical properties. | 3 | 1, 9, 29 | 4.8 |
| No definition, but anatomical properties. | 4 | 2, 30, 35, 37 | 6.5 |
| The anatomical properties and the activity of digestive enzymes. | 6 | 13, 22, 23, 45, 46, 68, | 9.7 |
| No definition, but the anatomical properties and the activity of digestive enzymes. | 4 | 14, 24, 39, 47 | 6.5 |
| No definition, but the intestine upregulated the interactome. | 1 | 20 | 1.6 |
| The in vivo digestibility (apparent digestibility of diet protein) a digestive capacity assay. | 1 | 36 | 1.6 |
| No definition, but in vivo digestibility (apparent digestibility coefficient). | 1 | 3 | 1.6 |
| The gene expression of digestive enzymes (pepsin, trypsin, chymotrypsin, amylase, and phospholipase A2). | 1 | 50 | 1.6 |
| No definition, but gene expression (genes related to protein, fat, and carbohydrate digestion, absorption pathways, and pancreatic secretion). | 1 | 11 | 1.6 |
| No definition, but viscerosomatic index, hepatosomatic index, and the expression of intestine transport genes. | 1 | 25 | 1.6 |
| The gastric evacuation rate (the amount of meal remaining in the stomach) as a measure of digestive capacity. | 1 | 28 | 1.6 |
| The use of residual nutrients on their biological performance as a digestive capacity. | 1 | 38 | 1.6 |
| Species | Environment: Milieu | Preferred Temperature | References | % |
|---|---|---|---|---|
| Acanthopagrus latus | Marine; freshwater; brackish; demersal | 21.7–28.5, mean 27.4 °C | 48, 65 | 3.2 |
| Acipenser baerii | Freshwater; brackish; demersal; | 1 °C–19 °C (Environm.) | 69 | 1.6 |
| Amphiprion ocellaris | Marine; reef-associated; non-migratory | 26.2–29.3, mean 28.7 °C | 22 | 1.6 |
| Argyrosomus regius | Marine; brackish; benthopelagic; oceanodromous | 13.3–19.4, mean 15.3 °C | 58 | 1.6 |
| Carassius auratus gibelio | Freshwater; brackish; benthopelagic | 10 °C–20 °C | 54 | 1.6 |
| Centropomus viridis | Marine; demersal. Tropical | 22.3–29.1, mean 26.3 °C | 61 | 1.6 |
| Channa argus | Freshwater; benthopelagic. Subtropical | 4 °C–22 °C | 39 | 1.6 |
| Ctenopharyngodon idella | Freshwater; brackish; benthopelagic; potamodromous | 0 °C–35 °C | 42 | 1.6 |
| Cyprinus carpio | Freshwater; brackish; benthopelagic | 3 °C–35 °C | 4, 6, 8, 17, 37, 49, 57 | 11.3 |
| Danio rerio | Freshwater; benthopelagic | 18 °C–24 °C | 11 | 1.6 |
| Epinephelus coioides | Marine; brackish; reef-associated | 24.4–29.1, mean 28.1 °C | 47 | 1.6 |
| Epinephelus fuscoguttatus♀×lanceolatus♂ | Marine; reef-associated | 25.4–29.1, mean 28.2 °C (E. fuscoguttatus), 24.3–29.1, mean 28.1 °C (E. lanceolatus) | 63, 66 | 3.2 |
| Gadus morhua | Marine; brackish; benthopelagic; oceanodromous | 0.5–10.3, mean 6.6 °C | 45 | 1.6 |
| Gymnocorymbus ternetzi | Freshwater; pelagic | 20 °C–26 °C | 68 | 1.6 |
| Ictalurus punctatus | Freshwater; demersal | 10 °C–32 °C | 29 | 1.6 |
| Larimichthys crocea | Marine; brackish; benthopelagic; oceanodromous | 20.8–24.7, mean 22.8 °C | 23 | 1.6 |
| Lateolabrax maculatus | Marine; freshwater; brackish; reef-associated; catadromous | 12.7–26.3, mean 22.4 °C | 24, 25 | 3.2 |
| Megalobrama amblycephala | Freshwater; benthopelagic | 10 °C–20 °C | 34 | 1.6 |
| Micropterus salmoides | Freshwater; benthopelagic | 10 °C–32 °C | 1, 15, 40, 55 | 6.5 |
| Mugil cephalus | Marine; freshwater; brackish; benthopelagic; catadromous | 11.3–27.9, mean 23.2 °C | 38 | 1.6 |
| Oncorhynchus mykiss | Marine; freshwater; brackish; benthopelagic; anadromous | 10 °C–24 °C | 26, 30, 36 | 4.8 |
| Oreochromis niloticuss | Freshwater; brackish; benthopelagic; potamodromous | 14 °C–33 °C | 33, 64 | 3.2 |
| Paracheirodon innesi | Freshwater; pelagic | 20 °C–26 °C | 13 | 1.6 |
| Pelteobagrus fulvidraco | Freshwater; demersal | 16 °C–25 °C | 5, 19 | 3.2 |
| Pseudoplatystoma punctifer | Freshwater; demersal. Tropical | Nind | 50 | 1.6 |
| Puntigrus tetrazona | Freshwater; benthopelagic | 20 °C–26 °C | 44 | 1.6 |
| Salmo salar | Marine; freshwater; brackish; benthopelagic; anadromous | 2 °C–9 °C | 20 | 1.6 |
| Sander lucioperca | Freshwater; brackish; pelagic; potamodromous | 6 °C–22 °C | 46 | 1.6 |
| Scophthalmus maximus | Marine; brackish; demersal; oceanodromous | 5.9–11.9, mean 9.4 °C | 18, 51 | 3.2 |
| Seriola dumerili | Marine; reef-associated; oceanodromous | 16.9–29, mean 27.1 °C | 9 | 1.6 |
| Silurus meridionalis | Freshwater; demersal. Subtropical | NInd | 28 | 1.6 |
| Siniperca chuatsi | Freshwater; benthopelagic | 4 °C–22 °C | 16, 27 | 3.2 |
| Sparus aurata | Marine; brackish; demersal | 12.1–21, mean 17.8 °C | 2, 35, 43, 52, 56, 60, 70 | 11.3 |
| Tachysurus fulvidraco | Freshwater; demersal | 16 °C–25 °C | 10 | 1.6 |
| Totoaba macdonaldi | Marine; brackish; benthopelagic | 19.8–26.7, mean 22.5 °C | 3, 7, 14 | 4.8 |
| Vieja melanurus, V. bifasciata | Freshwater; brackish; benthopelagic/freshwater; benthopelagic. Tropical | 24 °C–30 °C (V. melanurus), 26 °C–30 °C (V. bifasciata) | 59 | 1.6 |
| Stage | References | % |
|---|---|---|
| Embryo | 11 | 1.6 |
| Larvae | 9, 13, 22, 23, 33, 44, 46, 48, 50, 52, 56, 68 | 19.4 |
| Postlarvae | 15, 35 | 3.2 |
| Juvenile | 1, 2, 3, 4, 5, 6, 6, 7, 8, 10, 14, 16, 17, 18, 19, 20, 24, 25, 26, 27, 28, 29, 30, 34, 36, 37, 38, 39, 40, 42, 43, 47, 49, 51, 54, 55, 57, 58, 59, 60, 61, 63, 64, 65, 66, 69, 70 | 74.2 |
| Adult | 45 | 1.6 |
| Enzyme Types | References | % |
|---|---|---|
| Amylase, lipase, pepsin (acid protease), and phosphatase (alkaline) | 1 | 1.6 |
| Amylase, lipase, pepsin, and trypsin | 65, 66 | 3.2 |
| Amylase, phosphatase (alkaline), and trypsin | 6 | 1.6 |
| Leucine-aminopeptidase, protease (alkaline), and trypsin | 14 | 1.6 |
| Aminopeptidase (N), amylase, chymotrypsin, lipase, phosphatase (alkaline), and trypsin | 56 | 1.6 |
| Aminopeptidase (N), phosphatase (alkaline), pepsin, and trypsin, | 46 | 1.6 |
| Aminopeptidase (N), amylase, lipase, phosphatase (alkaline), protease (alkaline), and trypsin | 43 | 1.6 |
| Aminopeptidase, amylase, pepsin, phosphatase (acid and alkaline), and trypsin | 52 | 1.6 |
| Amylase, chymotrypsin, lipase, pepsin, and trypsin | 10 | 1.6 |
| Amylase, chymotrypsin, lipase, protease (alkaline), and trypsin | 38, 60 | 3.2 |
| Amylase, leucine-amino peptidase, lipase, and phosphatase (alkaline) | 48 | 1.6 |
| Amylase and lipase | 18, 37 | 3.2 |
| Amylase, lipase, and pepsin | 69 | 1.6 |
| Amylase, lipase, pepsin, and trypsin | 27 | 1.6 |
| Amylase, lipase, phosphatase (alkaline), and protease (alkaline) | 34 | 1.6 |
| Amylase, lipase, and protease (alkaline) | 17, 40, 42, 54, 64 | 8.1 |
| Amylase, lipase, and trypsin | 3, 4, 5, 7, 24, 26, 49, 55, 57, 63 | 16.1 |
| Amylase, lipase, trypsin, and pepsin | 51 | 1.6 |
| Amylase, pepsin, and trypsin, | 39 | 1.6 |
| Amylase, leucine-aminopeptidase, lipase, and trypsin | 23 | 1.6 |
| Chymotrypsin, lipase, pepsin, protease (alkaline), and trypsin | 61 | 1.6 |
| Chymotrypsin, pepsin, protease (alkaline), and trypsin | 8 | 1.6 |
| Chymotrypsin, trypsin, and chymotrypsin/trypsin ratio | 15 | 1.6 |
| Leucine-alanine peptidase and phosphatase (alkaline) | 35 | 1.6 |
| Lipase, pepsin, and trypsin | 13, 68, 70 | 4.8 |
| Lipase and protease (alkaline) | 16, 70 | 3.2 |
| Lipase, protease (alkaline), and trypsin | 58 | 1.6 |
| Lipase and trypsin | 19, 44 | 3.2 |
| Pepsin (not measured, just mentioned) | 45 | 1.6 |
| Phosphatase (alkaline) and trypsin | 25 | 1.6 |
| Protease (acid and alkaline) | 33, 59 | 3.2 |
| Protease (alkaline) and trypsin | 47 | 1.6 |
| Not determined | 2, 9, 11, 20, 28, 29, 30, 36, 50 | 14.5 |
| Substrate or Method (Number of Studies) | Unit | Activity Expression |
|---|---|---|
| ELISA kits (Shanghai Jianglai Industry Co., Ltd., Shanghai, China) (1) | Nind | Specific activity |
| EPS, 37 °C (1) | Nind | Specific activity |
| Kit (Jiancheng Biotech., Nanjing, China) (19) | Nind | Specific activity |
| Kit (Molecular Probes, Eugene, OR, USA), pH 6–9 (1) | RFU (Relative Fluorescence Units) | Total activity (RFU/mg larva dry weight) |
| Kit (NInd) (1) | Nind | Specific activity |
| Kit (Spinreact, Girona, Spain) (2) | 1 μmol product/min at 37 °C | Specific activity |
| Starch (25 °C) (2) | 0.1 Abs U/min | Specific activity |
| Starch (1) | 1 Abs U/min | U/Organ |
| Starch (1) | 1 Abs U | U/g weight? Or U/mg wet fish? |
| Starch (1) | 1 mg starch hydrolyzed/30 min | Specific activity |
| Starch (1) | 1 μmol/min | Specific activity |
| Starch, pH 7.4 (1) | 1 mg starch hydrolyzed/30 min | Specific activity |
| Starch-DNS (2) | Nind | Specific activity |
| Starch-Iodine (1) | 1 mg starch hydrolyzed/30 min | Specific activity |
| Starch-Iodine, 25 °C (1) | 1 mg starch hydrolyzed/30 min | u/Fish, U/mg protein |
| Starch-Somogyi-Nelson (1) | 1 μmol/min | U/L of serum |
| Nind: not indicated, EPS (4,6-Ethylidene-(G7)-1,4-nitrophenyl-(G1)-D-maltoheptaoside) | ||
| Specific activity (U/mg protein or U/g protein) | ||
| Substrate or Method (Number of Studies) | Unit | Activity Expression |
|---|---|---|
| BTEE (1) | 1 Abs U/min | U/Organ |
| BTEE (1) | 1 Abs U | U/g weight? Or U/mg wet fish? |
| BTEE, pH 7.8 (1) | Nind | Specific activity |
| BTEE, pH 7.9 (1) | 1 μmol product/min at 25 °C | Specific activity |
| Kit (Jiancheng Biotech.) (1) | Nind | Specific activity |
| N-Succinyl-Ala-Ala-Phe-7-amido-4-methylcoumarin (1) | RFU (Relative Fluorescence Units) per mg larvae dry weight | Total activity (RFU/mg larva dry weight) |
| SAPNA (1) | Nind | ND |
| Specific activity (U/mg protein or U/g protein) | ||
| SAPNA: N-succinil-Ala-Ala-Pro-Phe p-nitroanilide |
| Substrate or Method (Number of Studies) | Unit | Activity Expression |
|---|---|---|
| Kit (Jiancheng Biotech.) (1) | Nind | Specific activity, U/dL (amylase) |
| Kit (NInd) (1) | Nind | Specific activity |
| L-leucine p-nitroanalide (1) | 1 Abs U/min | U/Organ |
| L-leucine p-nitroanalide (1) | Nind | Specific activity |
| Specific activity (U/mg protein or U/g protein) |
| Substrate or Method (Number of Studies) | Unit | Activity Expression |
|---|---|---|
| 4-methylumbelliferyl butyrate, pH 7.0 (1) | RFU (Relative Fluorescence Units) | Total activity (RFU/mg larva dry weight) |
| DGGR (1,2-o-dilauryl-rac-glycero-3-glutaric acid-(6′-methylresorufin) ester) (1) | 1 μmol FFA/min | Specific activity |
| ELISA kits (Shanghai Jianglai Industry Co., Ltd.) (1) | Nind | Specific activity |
| Kit (Jiancheng Biotech.) (18) | Nind | Specific activity |
| Kit (NInd) (1) | Nind | Specific activity |
| Kit (Spinreact) (1) | Nind | Specific activity |
| Kit (Spinreact), 1-2-O-dilauryl-rac-glycero-3-glutaric acid-(6′-methylresorufin)-ester (1) | 1 μmol product/min at 37 °C | Specific activity |
| pNPCaproate (1) | Nind | U/Fish |
| pNPCaproate (1) | 1 μmol s/min at 30 °C | U/Larvae |
| pNPCaproate, pH 7.4 (1) | 1 μmol product/min at 25 °C | Specific activity |
| PNPCaproate, pH 7.4, 30 °C (1) | 1 μmol product/min | U/Fish |
| pNPCaproate (1) | 1 μmol product/min | U/Fish |
| pNPMyristate (1) | 1 Abs U/min | U/Organ |
| pNPMyristate (2) | Nind | Specific activity |
| pNPMyristate (4) | 1 μmol product/min | U/L of serum |
| pNPMyristate (25 °C, pH 8.5) (2) | 0.1 Abs U/min | Specific activity |
| pNPMyristate, 25 °C, pH 9.0 (1) | 1 μmol pNPM/min | u/Fish, U/mg protein |
| pNPPalmitate (1) | Nind | Specific activity |
| Nind: not indicated; NA: not available | ||
| Specific activity (U/mg protein or U/g protein) |
| Substrate or Method (Number of Studies) | Unit | Activity Expression |
|---|---|---|
| Acid phosphatase | ||
| Kit (Jiancheng Biotech.) (2) | Nind | Specific activity |
| pNP-Phosphate, pH 4.8 | 1 μmol/min | Specific activity |
| Alkaline phosphatase | ||
| 4-methylumbelliferyl phosphate | RFU (Relative Fluorescence Units) | Total activity (RFU/mg larva dry weight) |
| Kit (Jiancheng Biotech.) (4) | Nind | Specific activity |
| Kit (Sigma), pNP-Phosp | 1 μmol product/min at 37 °C | Specific activity |
| pNP-Phosphate | Nind | Specific activity |
| pNP-Phosphate | Nind | Specific activity |
| pNP-Phosphate | Nind | Specific activity |
| pNP-Phosphate, 37 °C, pH 8.0 | 1 nmol/min | Total activity (U/mg dry weight) |
| pNP-Phosphate, pH 9.8 | 1 μg/min | Specific activity |
| pNP-Phosphate, pH 9.8 | 1 μmol/min | Specific activity |
| NInd | Nind | Specific activity |
| NInd: not indicated; NA: not available | ||
| Specific activity (U/mg protein or U/g protein) |
| Substrate or Method (Number of Studies) | Unit | Activity Expression |
|---|---|---|
| Hemoglobin (1) | 1 Abs U/min | U/Organ |
| Hemoglobin (3) | Nind | Specific activity |
| Hemoglobin (1) | Nind | U/Fish |
| Hemoglobin (1) | Nind | Specific activity |
| Hemoglobin (1) | 1 μmol/min | U/Fish |
| Hemoglobin, 60 mM HCl, 37 °C (1) | 1 μmol tyrosine/min | U/Fish |
| Hemoglobin, pH 2.0 (1) | 1 μmol tyrosine/min | Specific activity |
| Hemoglobin, pH 2.0 (2) | 1 μg tyrosine/min | U/Fish and U/mg protein |
| Hemoglobin, pH 3.0 (1) | 1 μg tyrosine/min at 37 °C | Specific activity |
| Hemoglobin, pH 3.0 (1) | 1 μmol product/min at 25 °C | Specific activity |
| Kit (Jiancheng Biotech.) (7) | Nind | Specific activity |
| Hemoglobin? (Lowry method) (1) | Nind | Specific activity |
| Nind | Nind | Specific activity |
| Substrate or Method (Number of Studies) | Unit | Activity Expression |
|---|---|---|
| Azocasein (1) | Abs/min | Specific activity |
| Azocasein (1) | 1 μmol azo/min | Specific activity |
| Azocasein, 37 °C (1) | Nind | Specific activity |
| Azocasein, pH 9.0 (1) | NInd | Specific activity |
| Casein (1) | 1 Abs U | U/g weight? Or U/mg wet fish? |
| Casein, pH 9.0 (1) | 1 μmol product/min at 37 °C | Specific activity |
| Casein, pH 9.0 (1) | 1 μg tyrosine/min at 37 °C | Specific activity |
| Casein, pH 9.0 (1) | 1 μmol product/min at 25 °C | Specific activity |
| Casein? Or azocasein? (1) | 1 Abs U/min | U/Organ |
| Kit (Jiancheng Biotech.) (5) | Nind | Specific activity |
| Kit (Spinreact) (1) | Nind | Specific activity |
| Lowry method (1) | Nind | Specific activity |
| Nind | Nind | Specific activity |
| Nind: not indicated; NA: not available | ||
| Specific activity (U/mg protein or U/g protein) |
| Substrate or Method (Number of Studies) | Unit | Activity Expression |
|---|---|---|
| BAPNA (1) | 1 Abs U/min | U/Organ |
| BAPNA (3) | Nind | Specific activity |
| BAPNA (1) | Nind | U/Fish |
| BAPNA (1) | 1 Abs U | U/g weight? Or U/mg wet fish? |
| BAPNA, 37 °C (1) | 1 μmol/min | Specific activity |
| BAPNA, 25 °C, pH 8.2 (1) | 0.1 Abs U/min | Specific activity |
| BAPNA, 25 °C, pH 8.2 (1) | 1 μmol/min | Specific activity |
| BAPNA, pH 7.4 (1) | 1 μmol/min | Specific activity |
| BAPNA, pH 7.5, 30 °C (3) | 1 μmol/min | U/Fish |
| BAPNA, pH 8.2 (1) | Nind | Specific activity |
| BAPNA, 25 °C, pH 9.0 (1) | 1 μmol/min | Specific activity |
| Boc-Gln-Ala-Arg-7- methylcoumarin hydrochloride | RFU (Relative Fluorescence Units) | RFU/mg larva dry weight |
| BTEE or BAPNA? (2) | Nind | Specific activity |
| ELISA kits (Shanghai Jianglai Industry Co., Ltd.) (1) | Nind | Specific activity |
| Kit (Jiancheng Biotech.) (12) | Nind | Specific activity |
| Kit (NInd) | Nind | Specific activity |
| Nind: not indicated; NA: not available | ||
| Specific activity (U/mg protein or U/g protein) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nolasco-Soria, H.; Yúfera, M.; Nolasco-Alzaga, H.R. Fish Digestive Capacity: Definition and Methods. Fishes 2025, 10, 546. https://doi.org/10.3390/fishes10110546
Nolasco-Soria H, Yúfera M, Nolasco-Alzaga HR. Fish Digestive Capacity: Definition and Methods. Fishes. 2025; 10(11):546. https://doi.org/10.3390/fishes10110546
Chicago/Turabian StyleNolasco-Soria, Héctor, Manuel Yúfera, and Héctor R. Nolasco-Alzaga. 2025. "Fish Digestive Capacity: Definition and Methods" Fishes 10, no. 11: 546. https://doi.org/10.3390/fishes10110546
APA StyleNolasco-Soria, H., Yúfera, M., & Nolasco-Alzaga, H. R. (2025). Fish Digestive Capacity: Definition and Methods. Fishes, 10(11), 546. https://doi.org/10.3390/fishes10110546







