Spatial Ecology of the Population of Reef Manta Rays (Mobula alfredi) in New Caledonia Using Satellite Telemetry 2—Vertical Behaviour
Abstract
1. Introduction
2. Materials and Methods
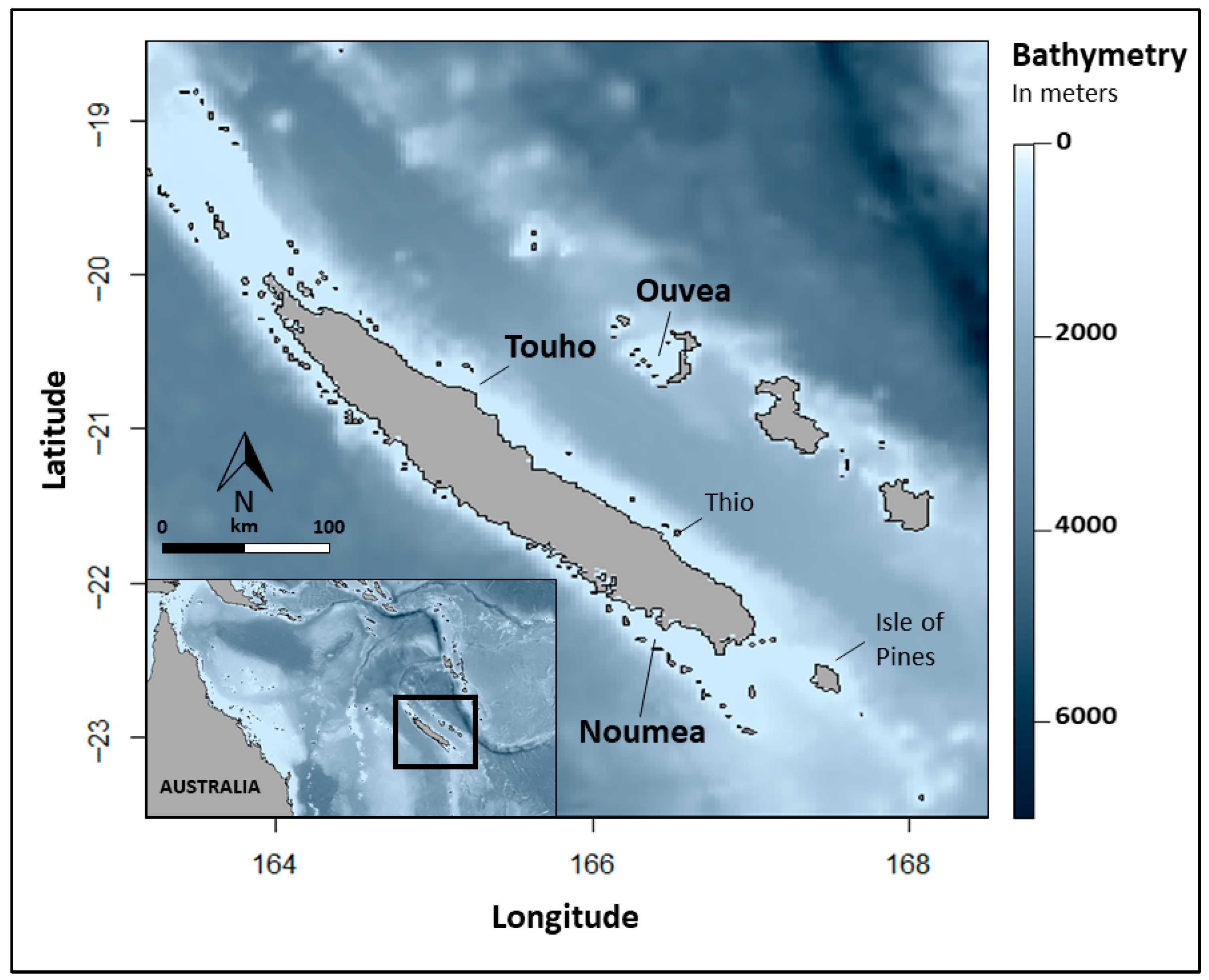
2.1. Study Sites
2.2. Tagging
2.3. Data Analysis
2.3.1. Data Treatment and Analysis of the Overall Dive Activity
2.3.2. Dive Profile Analysis
2.4. Statistical Analysis
3. Results
3.1. Tagging
3.2. Overall Dive Activity (From Transmitted Data, n = 19)
3.2.1. Depth Distribution
3.2.2. Temperature
3.2.3. Sex Comparison
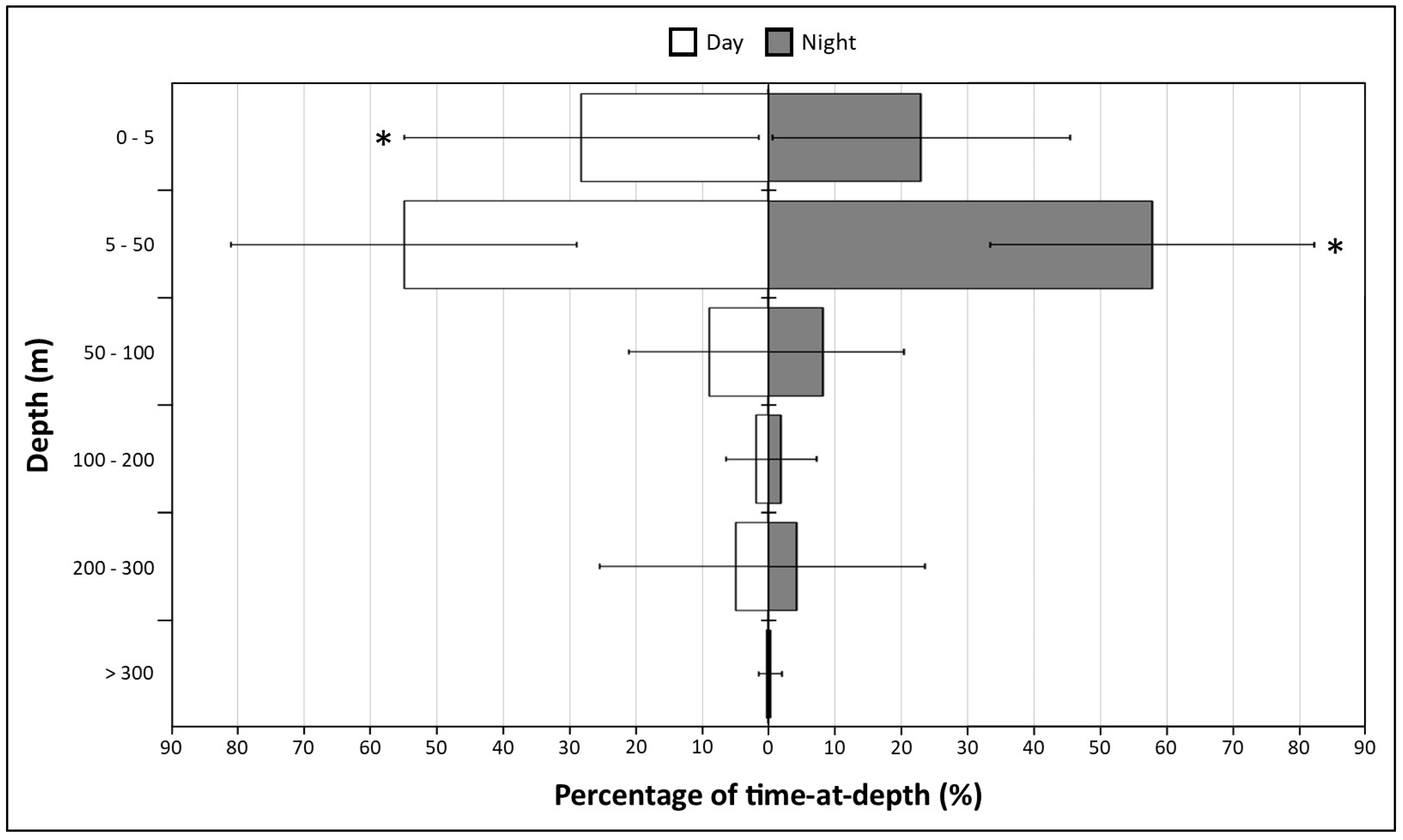
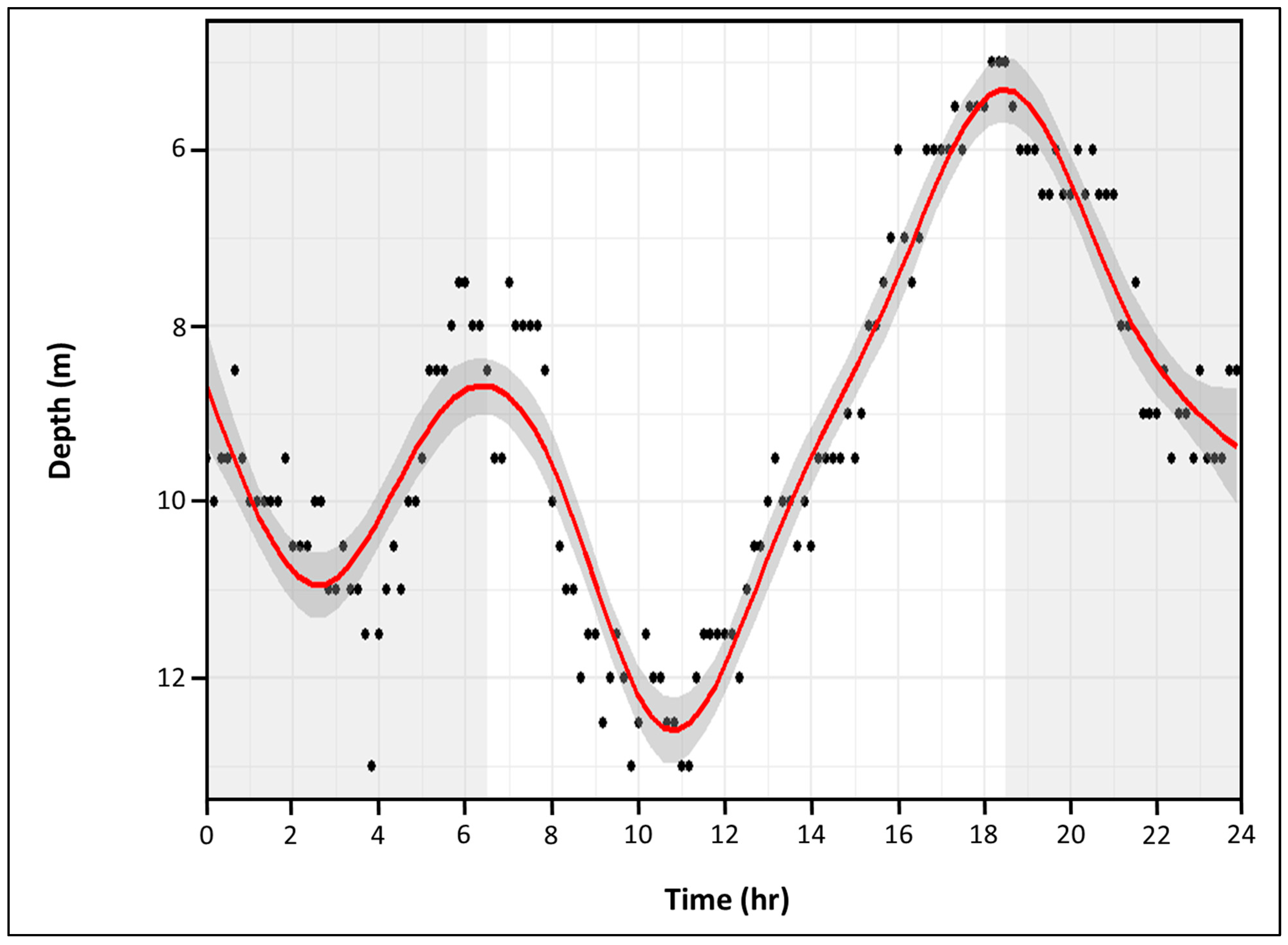
3.2.4. Diel Comparison
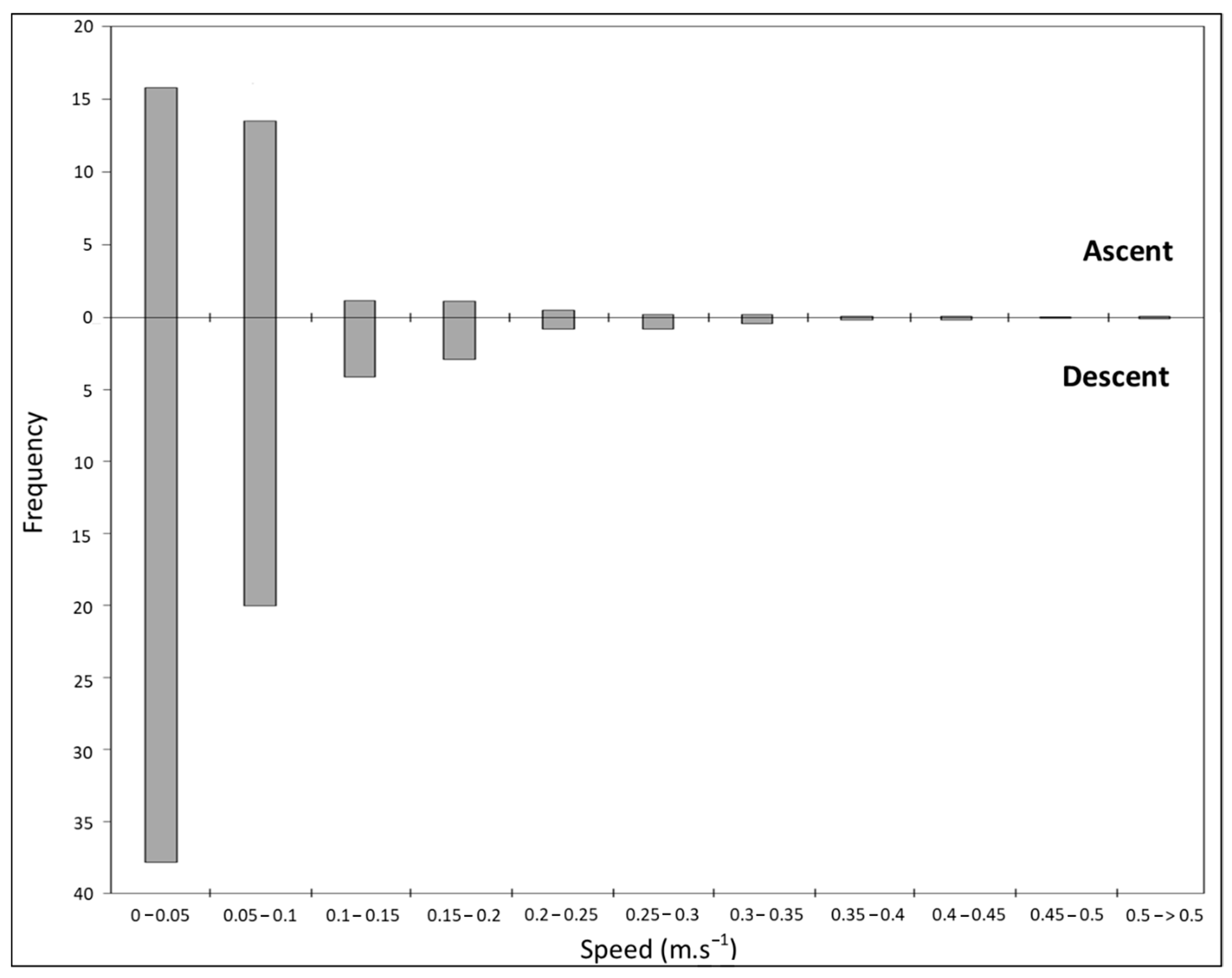
3.3. Dive Profile Analysis (From Archived Data, n = 3 Recovered Tags)
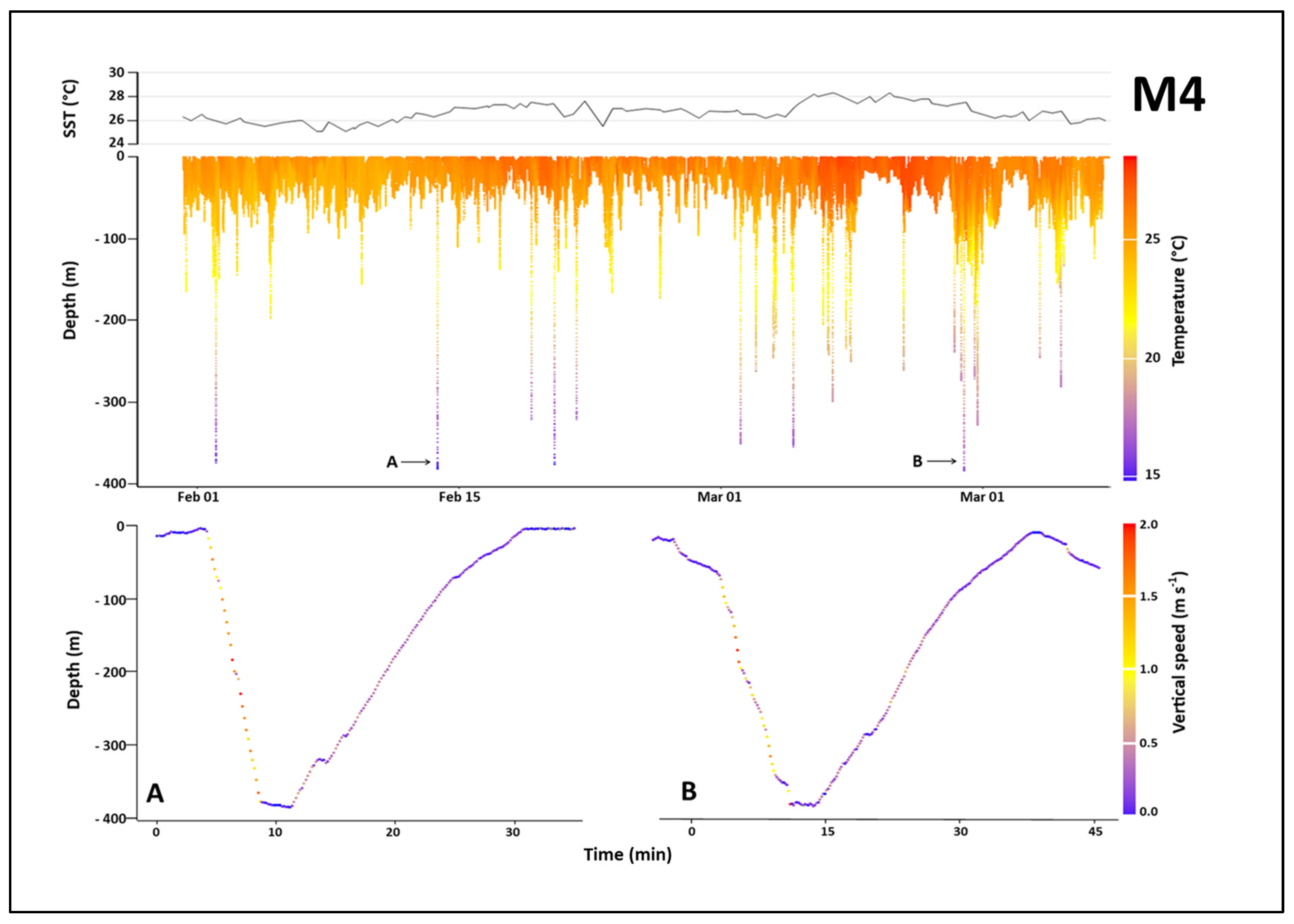
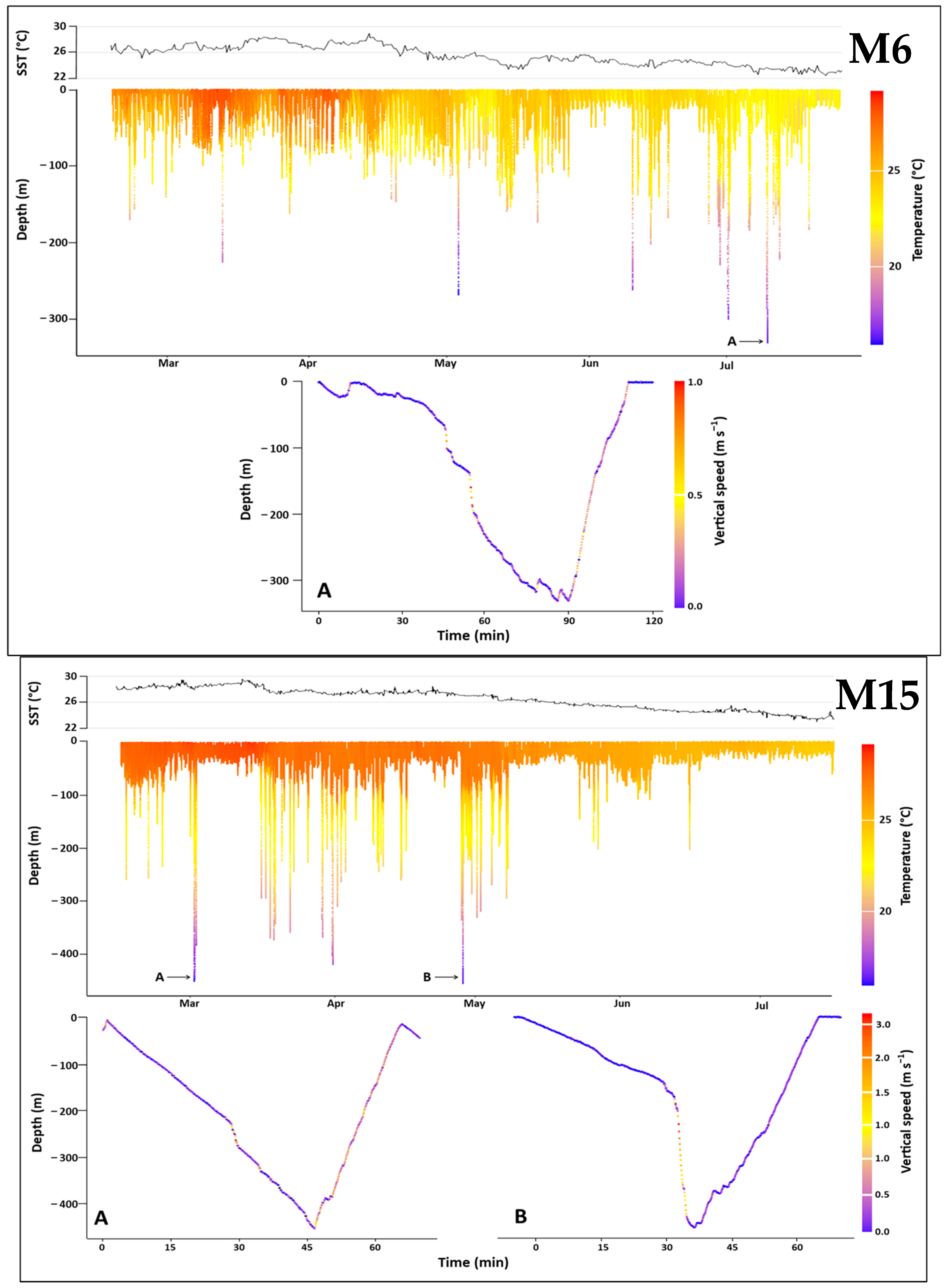
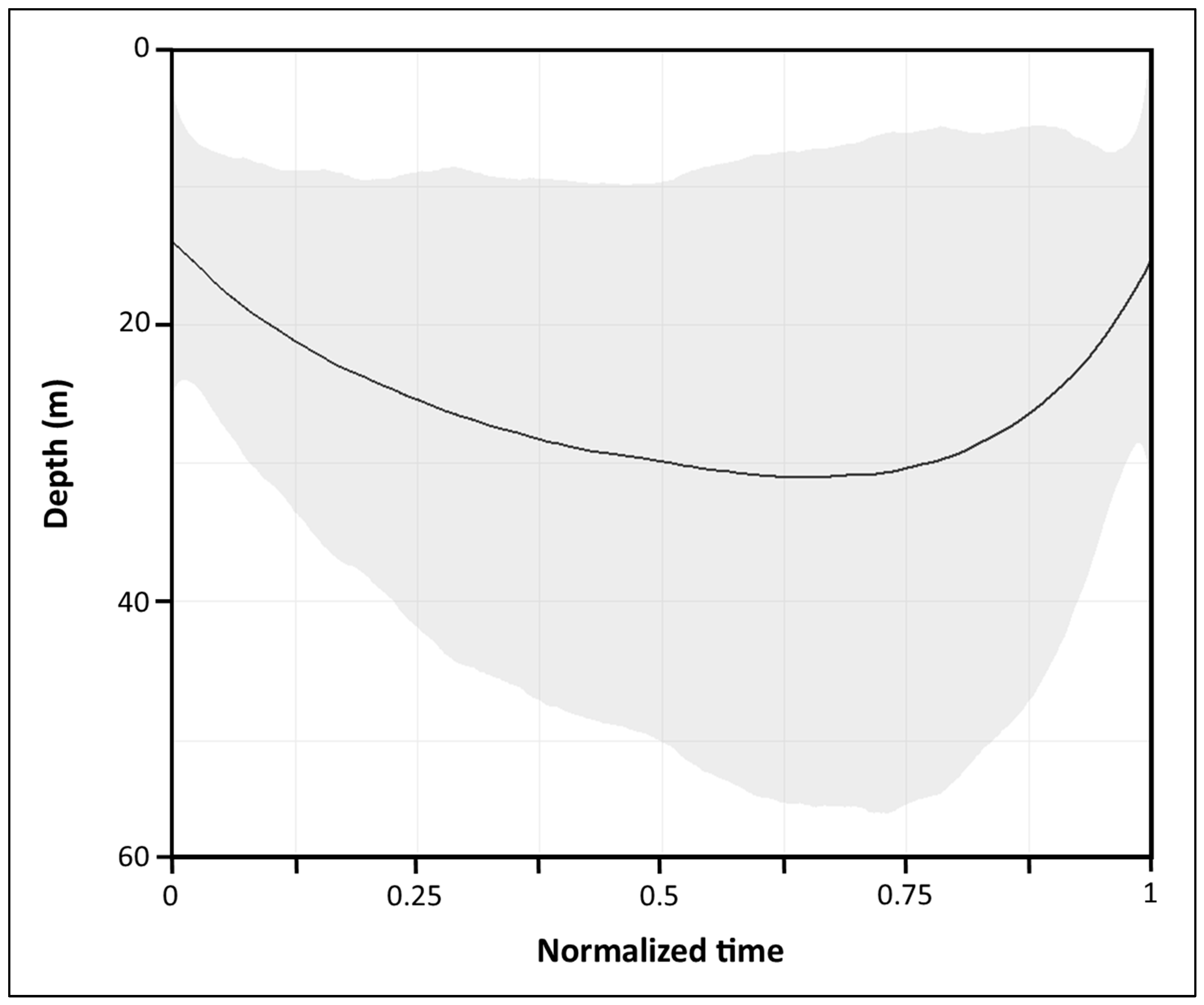
3.3.1. Overall Dive Profiles
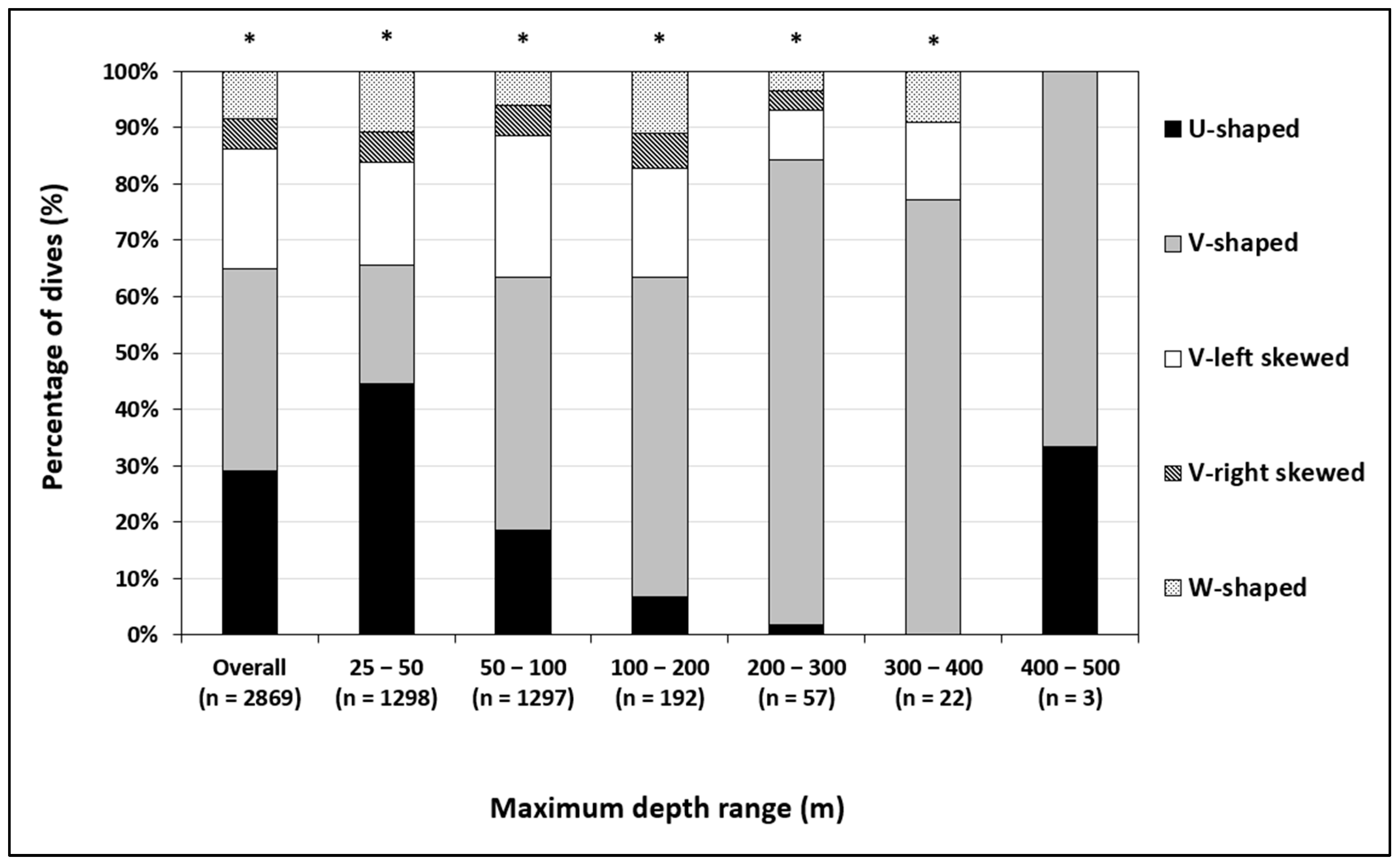
3.3.2. Dive Shape Dynamics
3.3.3. Deepest Dives
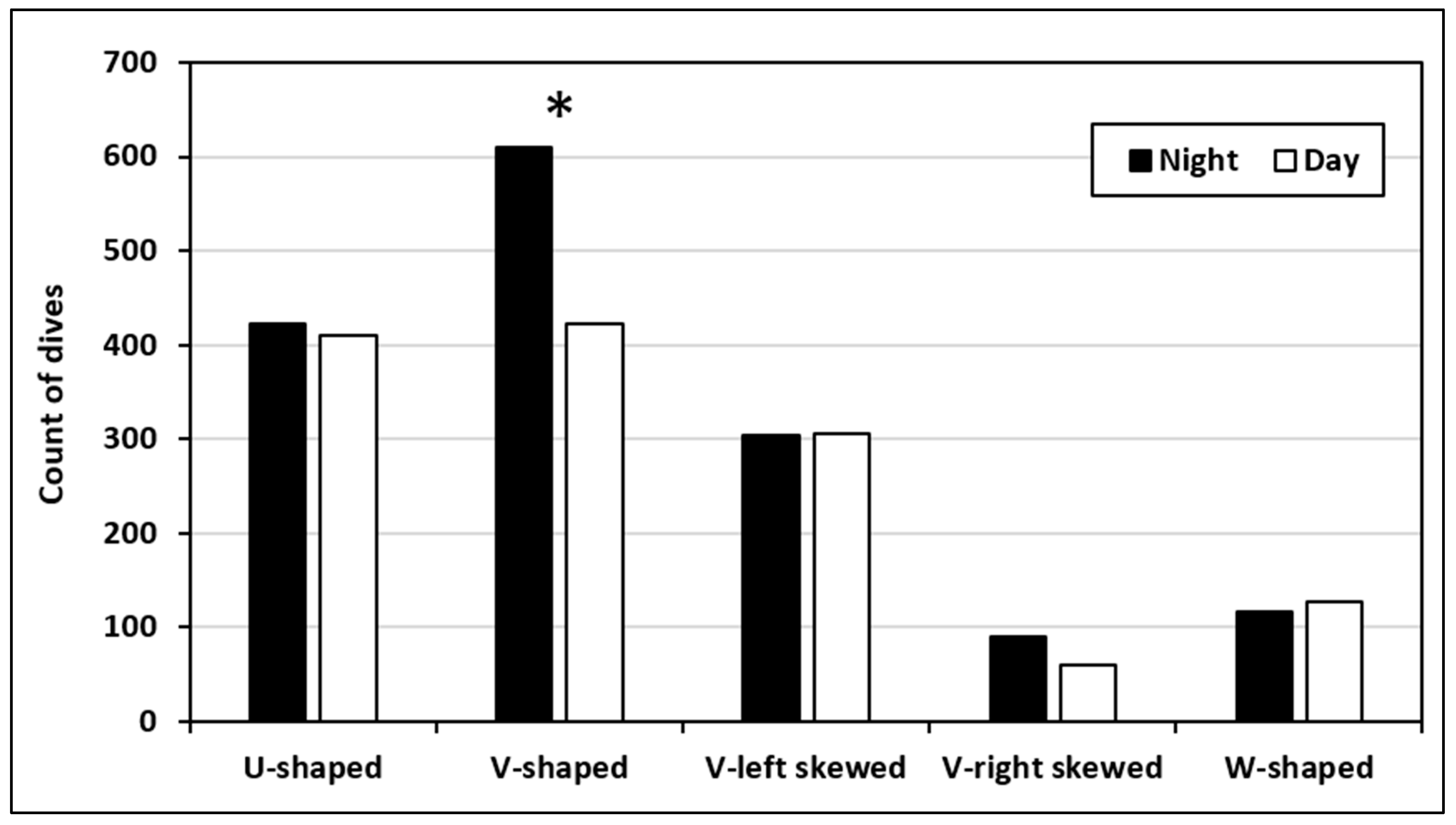
3.3.4. Diel Comparison
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chatzievangelou, D.; Bahamon, N.; Martini, S.; Del Rio, J.; Riccobene, G.; Tangherlini, M.; Danovaro, R.; De Leo, F.C.; Pirenne, B.; Aguzzi, J. Integrating diel vertical migrations of bioluminescent deep scattering layers into monitoring programs. Front. Mar. Sci. 2021, 8, 661809. [Google Scholar] [CrossRef]
- Shipley, O.N.; Matich, P.; Hussey, N.E.; Brooks, A.M.; Chapman, D.; Frisk, M.G.; Guttridge, A.E.; Guttridge, T.L.; Howey, L.A.; Kattan, S.; et al. Energetic connectivity of diverse elasmobranch populations—Implications for ecological resilience. Proc. R. Soc. B. 2023, 290, 20230262. [Google Scholar] [CrossRef]
- Williams, J.J.; Papastamatiou, Y.P.; Caselle, J.E.; Bradley, D.; Jacoby, D.M.P. Mobile marine predators: An understudied source of nutrients to coral reefs in an unfished atoll. Proc. Biol. Sci. 2018, 285, 20172456. [Google Scholar] [CrossRef]
- Bandara, K.; Varpe, Ø.; Wijewardene, L.; Tverberg, V.; Eiane, K. Two hundred years of zooplankton vertical migration research. Biol. Rev. 2021, 96, 1547–1589. [Google Scholar] [CrossRef]
- Andrzejaczek, S.; Gleiss, A.C.; Pattiaratchi, C.B.; Meekan, M.G. Patterns and drivers of vertical movements of the large fishes of the epipelagic. Rev. Fish Biol. Fish. 2019, 29, 335–354. [Google Scholar] [CrossRef]
- Klinard, N.V.; Mull, C.G.; Heithaus, M.R.; MacNeil, M.A. Defining ecological roles of sharks on coral reefs. Biol. Rev. 2025. [Google Scholar] [CrossRef]
- Tixier, P.; Lea, M.A.; Hindell, M.A.; Welsford, D.; Mazé, C.; Gourguet, S.; Arnould, J.P.Y. When large marine predators feed on fisheries catches: Global patterns of the depredation conflict and directions for coexistence. Fish Fish. 2021, 22, 31–53. [Google Scholar] [CrossRef]
- Hughes, B.B.; Beheshti, K.M.; Tinker, M.T.; Angelini, C.; Endris, C.; Murai, L.; Anderson, S.C.; Espinosa, S.; Staedler, M.; Tomoleoni, J.A.; et al. Top-predator recovery abates geomorphic decline of a coastal ecosystem. Nature 2024, 626, 111–118. [Google Scholar] [CrossRef]
- Anderson, R.C.; Adam, M.S.; Goes, J.I. From monsoons to mantas: Seasonal distribution of Manta alfredi in the Maldives. Fish. Oceanogr. 2011, 20, 104–113. [Google Scholar] [CrossRef]
- Germanov, E.S.; Marshall, A.D. Running the gauntlet: Regional movement patterns of Manta alfredi through a complex of parks and fisheries. PLoS ONE 2014, 9, e110071. [Google Scholar] [CrossRef] [PubMed]
- Jaine, F.R.A.; Rohner, C.A.; Weeks, S.J.; Couturier, L.I.E.; Bennett, M.B.; Townsend, K.A.; Richardson, A.J. Movements and habitat use of reef manta rays off eastern Australia: Offshore excursions, deep diving and eddy affinity revealed by satellite telemetry. Mar. Ecol. Prog. Ser. 2014, 510, 73–86. [Google Scholar] [CrossRef]
- Jaine, F.R.; Couturier, L.I.; Weeks, S.J.; Townsend, K.A.; Bennett, M.B.; Fiora, K.; Richardson, A.J. When giants turn up: Sighting trends, environmental influences and habitat use of the manta ray Manta alfredi at a coral reef. PLoS ONE 2012, 7, e46170. [Google Scholar] [CrossRef]
- Couturier, L.I.; Dudgeon, C.L.; Pollock, K.H.; Jaine, F.R.A.; Bennett, M.B.; Townsend, K.A.; Weeks, S.J.; Richardson, A.J. Population dynamics of the reef manta ray Manta alfredi in eastern Australia. Coral Reefs 2014, 33, 329–342. [Google Scholar] [CrossRef]
- Deakos, M.H.; Baker, J.D.; Bejder, L. Characteristics of a manta ray Manta alfredi population off Maui, Hawaii, and implications for management. Mar. Ecol. Prog. Ser. 2011, 429, 245–260. [Google Scholar] [CrossRef]
- Whitney, J.L.; Coleman, R.R.; Deakos, M.H. Genomic evidence indicates small island-resident populations and sex-biased behaviors of Hawaiian reef Manta Rays. BMC Ecol. Evol. 2023, 23, 31. [Google Scholar] [CrossRef] [PubMed]
- Setyawan, E.; Sianipar, A.B.; Erdmann, M.; Fischer, A.M.; Haddy, J.A.; Beale, C.S.; Sarah, A.L.; Mambrasar, R. Site fidelity and movement patterns of reef manta rays (Mobula alfredi: Mobulidae) using passive acoustic telemetry in northern Raja Ampat, Indonesia. Nat. Conserv. Res. 2018, 3, 17–31. [Google Scholar] [CrossRef]
- Lassauce, H.; Chateau, O.; Wantiez, L. Spatial ecology of the population of reef manta rays, Mobula alfredi (Krefft, 1868), in New Caledonia using satellite telemetry 1- Horizontal behaviour. Fishes 2023, 8, 328. [Google Scholar] [CrossRef]
- Lassauce, H.; Dudgeon, C.L.; Armstrong, A.J.; Wantiez, L.; Carroll, E.L. Evidence of fine-scale genetic structure for reef manta rays Mobula alfredi in New Caledonia. Endang. Species Res. 2022, 47, 249–264. [Google Scholar] [CrossRef]
- Rohner, C.A.; Venables, S.K.; Knochel, A.M.; Rambahiniarison, J.M.; Marillac, V.; Cardon, C.; Scholten, N.; Pierce, S.J.; Kiszka, J.J. Movements and habitat use of reef manta rays around the Mozambique Channel Island of Mayotte, Southwestern Indian Ocean. Environ. Biol. Fishes 2025, 108, 937–955. [Google Scholar] [CrossRef]
- Marshall, A.; Barreto, R.; Carlson, J.; Fernando, D.; Fordham, S.; Francis, M.P.; Herman, K.; Jabado, R.W.; Liu, K.M.; Pacoureau, N.; et al. Mobula alfredi (amended version of 2019 assessment). The IUCN Red List of Threatened Species. 2022; e.T195459A214395983. Available online: https://www.iucnredlist.org/species/195459/214395983 (accessed on 9 September 2025).
- Marshall, A.D.; Bennett, M.B. Reproductive ecology of the reef manta ray Manta alfredi in southern Mozambique. J. Fish Biol. 2010, 77, 169–190. [Google Scholar] [CrossRef]
- O’Malley, M.P.; Townsend, K.A.; Hilton, P.; Heinrichs, S.; Stewart, J.D. Characterization of the trade in manta and devil ray gill plates in China and Southeast Asia through trader surveys. Aquat. Conserv. Mar. Freshw. Ecosyst. 2017, 27, 394–413. [Google Scholar] [CrossRef]
- O’Malley, M.P.; Lee-Brooks, K.; Medd, H.B. The global economic impact of manta ray watching tourism. PLoS ONE 2013, 8, e65051. [Google Scholar] [CrossRef]
- Venables, S.; McGregor, F.; Brain, L.; van Keulen, M. Manta ray tourism management, precautionary strategies for a growing industry: A case study from the Ningaloo Marine Park, Western Australia. Pac. Conserv. Biol. 2016, 22, 295–300. [Google Scholar] [CrossRef]
- Ward-Paige, C.A.; Davis, B.; Worm, B. Global population trends and human use patterns of Manta and Mobula rays. PLoS ONE 2013, 8, e74835. [Google Scholar] [CrossRef]
- Rohner, C.A.; Pierce, S.J.; Marshall, A.D.; Weeks, S.J.; Bennett, M.B.; Richardson, A.J. Trends in sightings and environmental influences on a coastal aggregation of manta rays and whale sharks. Mar. Ecol. Prog. Ser. 2013, 482, 153–168. [Google Scholar] [CrossRef]
- Croll, D.A.; Dewar, H.; Dulvy, N.K.; Fernando, D.; Francis, M.P.; Galván-Magaña, F.; Hall, M.; Heinrichs, S.; Marshall, A.; Mccauley, D.; et al. Vulnerabilities and fisheries impacts: The uncertain future of manta and devil rays. Aquat. Conserv. Mar. Freshw. Ecosyst. 2016, 26, 562–575. [Google Scholar] [CrossRef]
- Lawson, J.M.; Fordham, S.V.; O’Malley, M.P.; Davidson, L.N.; Walls, R.H.; Heupel, M.R.; Stevens, G.; Fernando, D.; Budziak, A.; Simpfendorfer, C.A.; et al. Sympathy for the devil: A conservation strategy for devil and manta rays. PeerJ 2017, 5, e3027. [Google Scholar] [CrossRef] [PubMed]
- McGregor, F.; Richardson, A.J.; Armstrong, A.J.; Armstrong, A.O.; Dudgeon, C.L. Rapid wound healing in a reef manta ray masks the extent of vessel strike. PLoS ONE 2019, 14, e0225681. [Google Scholar] [CrossRef]
- Andrzejaczek, S.; Lucas, T.C.; Goodman, M.C.; Hussey, N.E.; Armstrong, A.J.; Carlisle, A.; Coffey, D.M.; Gleiss, A.C.; Huveneers, C.; Jacoby, D.M.; et al. Diving into the vertical dimension of elasmobranch movement ecology. Sci. Adv. 2022, 8, eabo1754. [Google Scholar] [CrossRef]
- Graham, R.T.; Witt, M.J.; Castellanos, D.W.; Remolina, F.; Maxwell, S.; Godley, B.J.; Hawkes, L.A. Satellite tracking of manta rays highlights challenges to their conservation. PLoS ONE 2012, 7, e36834. [Google Scholar] [CrossRef] [PubMed]
- Lefort, K.J.; Hussey, N.E.; Jones, J.M.; Johnson, K.F.; Ferguson, S.H. Satellite-tracked sperm whale migrates from the Canadian Arctic to the subtropical western North Atlantic. Mar. Mamm. Sci. 2022, 38, 1242–1248. [Google Scholar] [CrossRef]
- Braun, C.D.; Della Penna, A.; Arostegui, M.C.; Afonso, P.; Berumen, M.L.; Block, B.A.; Brown, C.A.; Fontes, J.; Furtado, M.; Gallagher, A.J.; et al. Linking vertical movements of large pelagic predators with distribution patterns of biomass in the open ocean. Proc. Natl. Acad. Sci. USA 2023, 120, e2306357120. [Google Scholar] [CrossRef]
- Robichaud, J.A.; Haley, A.L.; LaRochelle, L.; Dello Russo, J.; Zhang, J.; Cunningham, K.E.; Lawson, L.; Bergman, J.N.; Jolin, E.; Madden, J.C.; et al. Global trends in aquatic animal satellite telemetry studies. Environ. Rev. 2025, 33, 1–19. [Google Scholar] [CrossRef]
- Nichols, R.C.; Cade, D.E.; Kahane-Rapport, S.; Goldbogen, J.; Stimpert, A.; Nowacek, D.; Read, A.J.; Johnston, D.W.; Friedlaender, A.S. Intra-seasonal variation in feeding rates and diel foraging behaviour in a seasonally fasting mammal, the humpback whale. R. Soc. Open Sci. 2022, 9, 211674. [Google Scholar] [CrossRef] [PubMed]
- Gould, L.A.; Manning, A.D.; McGinness, H.M.; Hansen, B.D. A review of electronic devices for tracking small and medium migratory shorebirds. Anim. Biotelemetry 2024, 12, 11. [Google Scholar] [CrossRef]
- Renshaw, S.; Hammerschlag, N.; Gallagher, A.J.; Lubitz, N.; Sims, D.W. Global tracking of shark movements, behaviour and ecology: A review of the renaissance years of satellite tagging studies, 2010–2020. J. Exp. Mar. Biol. Ecol. 2023, 560, 151841. [Google Scholar] [CrossRef]
- Haywood, J.C.; Fuller, W.J.; Godley, B.J.; Margaritoulis, D.; Shutler, J.D.; Snape, R.T.; Widdicombe, S.; Zbinden, J.A.; Broderick, A.C. Spatial ecology of loggerhead turtles: Insights from stable isotope markers and satellite telemetry. Divers. Distrib. 2020, 26, 368–381. [Google Scholar] [CrossRef]
- Meyers, M.M.; Francis, M.P.; Erdmann, M.; Constantine, R.; Sianipar, A. Movement patterns of whale sharks in Cenderawasih Bay, Indonesia, revealed through long-term satellite tagging. Pac. Conserv. Biol. 2020, 26, 353–364. [Google Scholar] [CrossRef]
- Watanabe, Y.Y.; Papastamatiou, Y.P. Biologging and biotelemetry: Tools for understanding the lives and environments of marine animals. Annu. Rev. Anim. Biosci. 2023, 11, 247–267. [Google Scholar] [CrossRef]
- Braun, C.D.; Skomal, G.B.; Thorrold, S.R.; Berumen, M.L. Diving behavior of the reef manta ray links coral reefs with adjacent deep pelagic habitats. PLoS ONE 2014, 9, e88170. [Google Scholar] [CrossRef]
- Andrzejaczek, S.; Schallert, R.J.; Forsberg, K.; Arnoldi, N.S.; Cabanillas-Torpoco, M.; Purizaca, W.; Block, B.A. Reverse diel vertical movements of oceanic manta rays off the northern coast of Peru and implications for conservation. Ecol. Solut. Evid. 2021, 2, e12051. [Google Scholar] [CrossRef]
- Lassauce, H.; Chateau, O.; Erdmann, M.V.; Wantiez, L. Diving behavior of the reef manta ray (Mobula alfredi) in New Caledonia: More frequent and deeper nighttime diving to 672 meters. PLoS ONE 2020, 15, e0228815. [Google Scholar] [CrossRef]
- Beale, C.S.; Runtuboy, F.; Sianipar, A.; Beer, A.J.; Erdmann, M.; Setyawan, E.; Green, L.; Duffy, C.A.; Andrzejaczek, S.; Block, B.A.; et al. Deep diving behaviour in oceanic manta rays and its potential function. Front. Mar. Sci. 2025, 12, 1630451. [Google Scholar] [CrossRef]
- Andréfouët, S.; Cabioch, G.; Flamand, B.; Pelletier, B. A reappraisal of the diversity of geomorphological and genetic processes of New Caledonian coral reefs: A synthesis from optical remote sensing, coring and acoustic multibeam observations. Coral Reefs 2009, 28, 691–707. [Google Scholar] [CrossRef]
- Armstrong, A.J.; Armstrong, A.O.; McGregor, F.; Richardson, A.J.; Bennett, M.B.; Townsend, K.A. Satellite tagging and photographic identification reveal connectivity between two UNESCO world heritage areas for reef manta rays. Front. Mar. Sci. 2020, 7, 725. [Google Scholar] [CrossRef]
- Musyl, M.K.; Domeier, M.L.; Nasby-Lucas, N.; Brill, R.W.; McNaughton, L.M.; Swimmer, J.Y.; Lutcavage, M.S.; Wilson, S.G.; Galuardi, B.; Liddle, J.B. Performance of pop-up satellite archival tags. Mar. Ecol. Prog. Ser. 2011, 433, 1–28. [Google Scholar] [CrossRef]
- D’Antonio, B.; Ferreira, L.C.; Meekan, M.; Thomson, P.G.; Lieber, L.; Virtue, P.; Power, C.; Pattiaratchi, C.B.; Brierley, A.S.; Sequeira, A.M.M.; et al. Links between the three-dimensional movements of whale sharks (Rhincodon typus) and the bio-physical environment off a coral reef. Mov. Ecol. 2024, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Braun, C.D.; Skomal, G.B.; Thorrold, S.R.; Berumen, M.L. Movements of the reef manta ray (Manta alfredi) in the Red Sea using satellite and acoustic telemetry. Mar. Biol. 2015, 162, 2351–2362. [Google Scholar] [CrossRef]
- Peel, L.R.; Stevens, G.M.; Daly, R.; Keating Daly, C.A.; Collin, S.P.; Nogués, J.; Meekan, M.G. Regional movements of reef manta rays (Mobula alfredi) in Seychelles waters. Front. Mar. Sci. 2020, 7, 558. [Google Scholar] [CrossRef]
- Jewell, O.J.D.; Chapple, T.K.; Jorgensen, S.J.; Kanive, P.; Moxley, J.H.; Tweedley, J.R.; Anderson, S.; Block, B.A.; Gleiss, A.C. Diverse habitats shape the movement ecology of a top marine predator, the white shark Carcharodon carcharias. Ecosphere 2024, 15, e4825. [Google Scholar] [CrossRef]
- Watanabe, Y.Y.; Nakamura, I.; Chiang, W.-C. Behavioural thermoregulation linked to foraging in blue sharks. Mar. Biol. 2021, 168, 161. [Google Scholar] [CrossRef]
- Queiroz, N.; Vila-Pouca, C.; Couto, A.; Southall, E.J.; Mucientes, G.; Humphries, N.E.; Sims, D.W. Convergent foraging tactics of marine predators with different feeding strategies across heterogeneous ocean environments. Front. Mar. Sci. 2017, 4, 239. [Google Scholar] [CrossRef]
- Schaber, M.; Gastauer, S.; Cisewski, B.; Hielscher, N.; Janke, M.; Peña, M.; Sakinan, S.; Thorburn, J. Extensive oceanic mesopelagic habitat use of a migratory continental shark species. Sci. Rep. 2022, 12, 2047. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, C.T.; Pérez-Jorge, S.; Prieto, R.; Oliveira, C.; Tobeña, M.; Scheffer, A.; Silva, M.A. Dive behavior and activity patterns of fin whales in a migratory habitat. Front. Mar. Sci. 2022, 9, 875731. [Google Scholar] [CrossRef]
- Schwarz, J.F.L.; Mews, S.; DeRango, E.; Langrock, R.; Piedrahita, P.; Páez-Rosas, D.; Krüger, O. Individuality counts: A new comprehensive approach to foraging strategies of a tropical marine predator. Oecologia 2021, 195, 313–325. [Google Scholar] [CrossRef]
- Florko, K.R.N.; Shuert, C.R.; Cheung, W.W.L.; Ferguson, S.H.; Jonsen, I.D.; Rosen, D.A.S.; Sumaila, U.R.; Tai, T.C.; Yurkowski, D.J.; Auger-Méthé, M. Linking movement and dive data to prey distribution models: New insights in foraging behaviour and potential pitfalls of movement analyses. Mov. Ecol. 2023, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Braun, C.D.; Arostegui, M.C.; Thorrold, S.R.; Papastamatiou, Y.P.; Gaube, P.; Fontes, J.; Afonso, P. The functional and ecological significance of deep diving by large marine predators. Annu. Rev. Mar. Sci. 2022, 14, 129–159. [Google Scholar] [CrossRef] [PubMed]
- Vedor, M.; Mucientes, G.; Hernández-Chan, S.; Rosa, R.; Humphries, N.; Sims, D.W.; Queiroz, N. Oceanic diel vertical movement patterns of blue sharks vary with water temperature and productivity to change vulnerability to fishing. Front. Mar. Sci. 2021, 8, 688076. [Google Scholar] [CrossRef]
- Hounslow, J.L.; Fossette, S.; Byrnes, E.E.; Whiting, S.D.; Lambourne, R.N.; Armstrong, N.J.; Tucker, A.D.; Richardson, A.R.; Gleiss, A.C. Multivariate analysis of biologging data reveals the environmental determinants of diving behaviour in a marine reptile. R. Soc. Open Sci. 2022, 9, 211860. [Google Scholar] [CrossRef]
- Couturier, L.I.; Rohner, C.A.; Richardson, A.J.; Marshall, A.D.; Jaine, F.R.; Bennett, M.B.; Townsend, K.A.; Weeks, S.J.; Nichols, P.D. Stable isotope and signature fatty acid analyses suggest reef manta rays feed on demersal zooplankton. PLoS ONE 2013, 8, e77152. [Google Scholar] [CrossRef]
- Burgess, K.B.; Couturier, L.I.; Marshall, A.D.; Richardson, A.J.; Weeks, S.J.; Bennett, M.B. Manta birostris, predator of the deep? Insight into the diet of the giant manta ray through stable isotope analysis. R. Soc. Open Sci. 2016, 3, e160717. [Google Scholar] [CrossRef]
- Stewart, J.D.; Hoyos-Padilla, E.M.; Kumli, K.R.; Rubin, R.D. Deep-water feeding and behavioral plasticity in Manta birostris revealed by archival tags and submersible observations. Zoology 2016, 119, 406–413. [Google Scholar] [CrossRef]
- Klöcker, C.A.; Bjelland, O.; Ferter, K.; Arostegui, M.C.; Braun, C.D.; da Costa, I.; Cidade, T.; Queiroz, N.; Sims, D.W.; Junge, C. Basking sharks of the Arctic Circle: Year-long, high-resolution tracking data reveal wide thermal range and prey-driven vertical movements across habitats. Anim. Biotelemetry 2025, 13, 15. [Google Scholar] [CrossRef]
- Siders, Z.A.; Westgate, A.J.; Bell, K.R.; Koopman, H.N. Highly variable basking shark (Cetorhinus maximus) diving behavior in the lower Bay of Fundy, Canada. Front. Mar. Sci. 2022, 9, 976857. [Google Scholar] [CrossRef]
- Arrowsmith, L.M.; Sequeira, A.M.M.; Pattiaratchi, C.B.; Meekan, M.G. Water temperature is a key driver of horizontal and vertical movements of an ocean giant, the whale shark Rhincodon typus. Mar. Ecol. Prog. Ser. 2021, 679, 101–114. [Google Scholar] [CrossRef]
- Armstrong, A.O.; Armstrong, A.J.; Jaine, F.R.; Couturier, L.I.; Fiora, K.; Uribe-Palomino, J.; Weeks, S.J.; Townsend, K.A.; Bennett, M.B.; Richardson, A.J. Prey density threshold and tidal influence on reef manta ray foraging at an aggregation site on the Great Barrier Reef. PLoS ONE 2016, 11, e0153393. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lassauce, H.; Chateau, O.; Wantiez, L. Spatial Ecology of the Population of Reef Manta Rays (Mobula alfredi) in New Caledonia Using Satellite Telemetry 2—Vertical Behaviour. Fishes 2025, 10, 545. https://doi.org/10.3390/fishes10110545
Lassauce H, Chateau O, Wantiez L. Spatial Ecology of the Population of Reef Manta Rays (Mobula alfredi) in New Caledonia Using Satellite Telemetry 2—Vertical Behaviour. Fishes. 2025; 10(11):545. https://doi.org/10.3390/fishes10110545
Chicago/Turabian StyleLassauce, Hugo, Olivier Chateau, and Laurent Wantiez. 2025. "Spatial Ecology of the Population of Reef Manta Rays (Mobula alfredi) in New Caledonia Using Satellite Telemetry 2—Vertical Behaviour" Fishes 10, no. 11: 545. https://doi.org/10.3390/fishes10110545
APA StyleLassauce, H., Chateau, O., & Wantiez, L. (2025). Spatial Ecology of the Population of Reef Manta Rays (Mobula alfredi) in New Caledonia Using Satellite Telemetry 2—Vertical Behaviour. Fishes, 10(11), 545. https://doi.org/10.3390/fishes10110545






