Mechanism of Biphasic Activation of NLRP3 Inflammasome in the Fat Greenling (Hexagrammos otakii) Under Hypoxic Stress: From Inflammatory Defense to Pyroptosis Execution
Abstract
1. Introduction
2. Materials and Methods
2.1. Management of Fish
2.2. Grouping and Sampling of Experiments
2.3. Sequence Analysis of HoNLRP3
2.4. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.5. IL-1β Content Detection
2.6. Western Blot
2.7. Colocalization of Tissue Immunofluorescence
2.8. Co-Immunoprecipitation
2.9. Measurement of LDH Activity
2.10. Histopathological Analysis
2.11. Statistical Analysis
3. Results
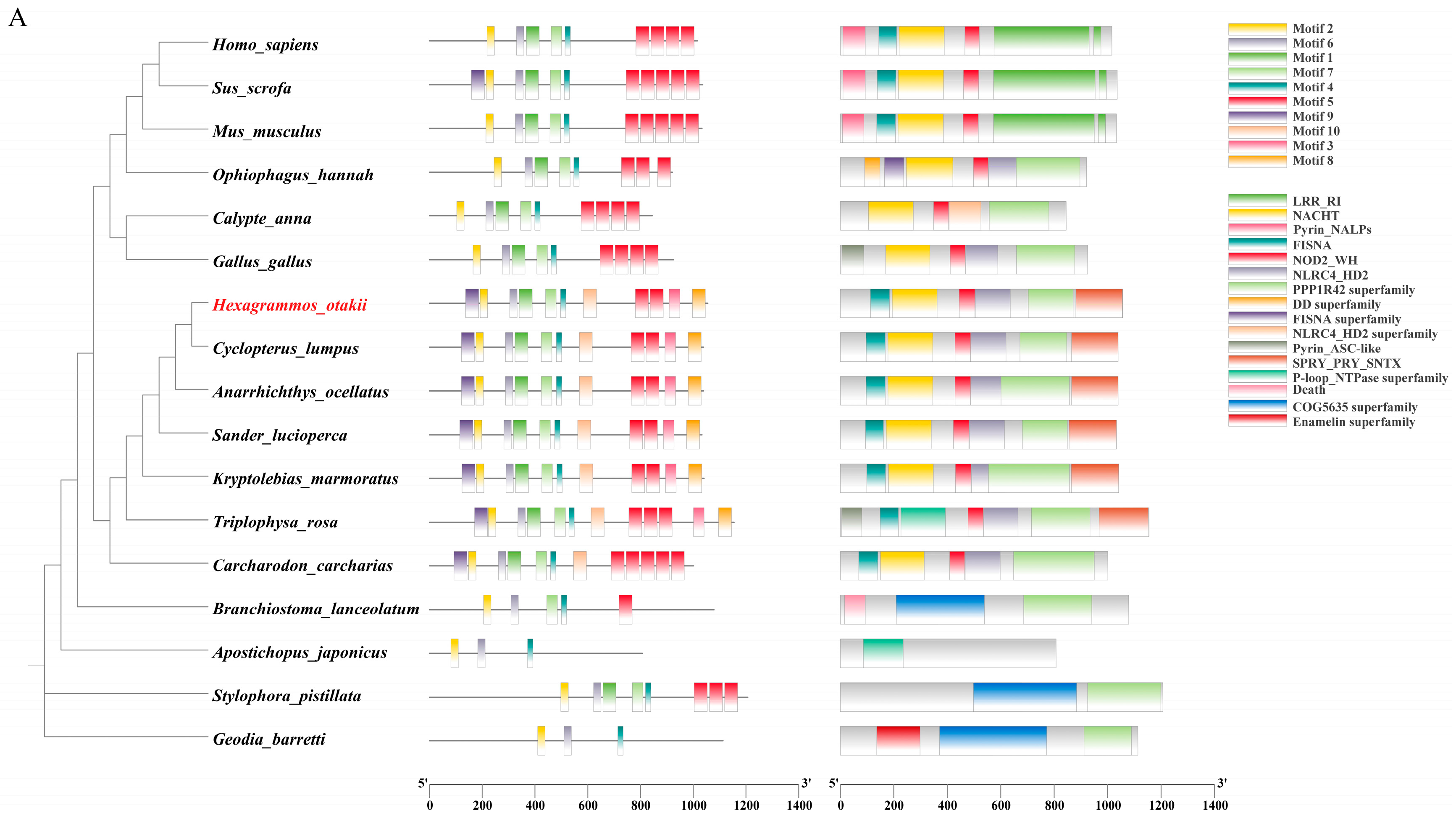
3.1. Molecular Characteristics and Phylogeny Analysis of HoNLRP3
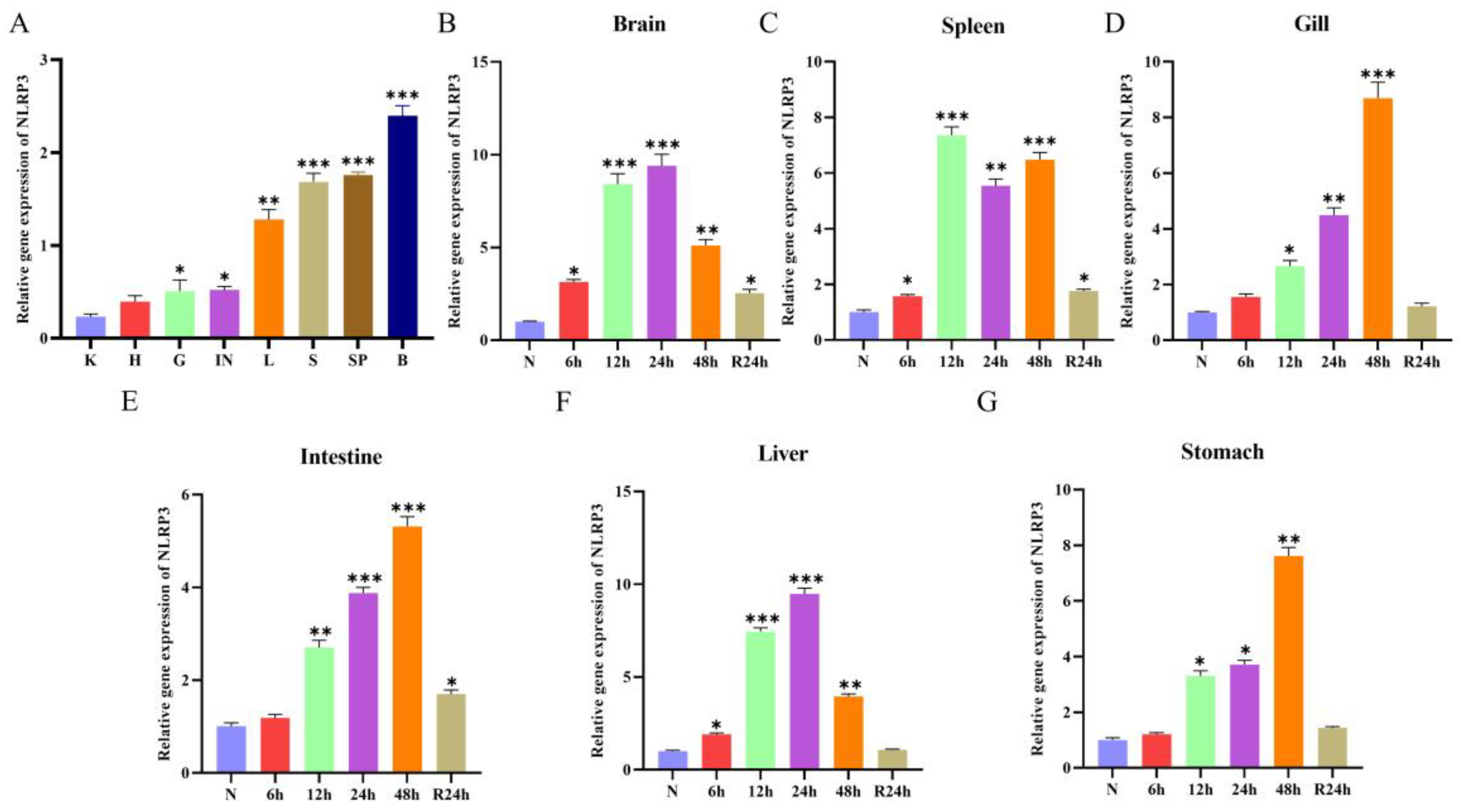
3.2. Tissue Distribution of NLRP3 and Response to Hypoxic Stress
3.3. Dynamics of Gene and Protein Expression of the HIF-1α
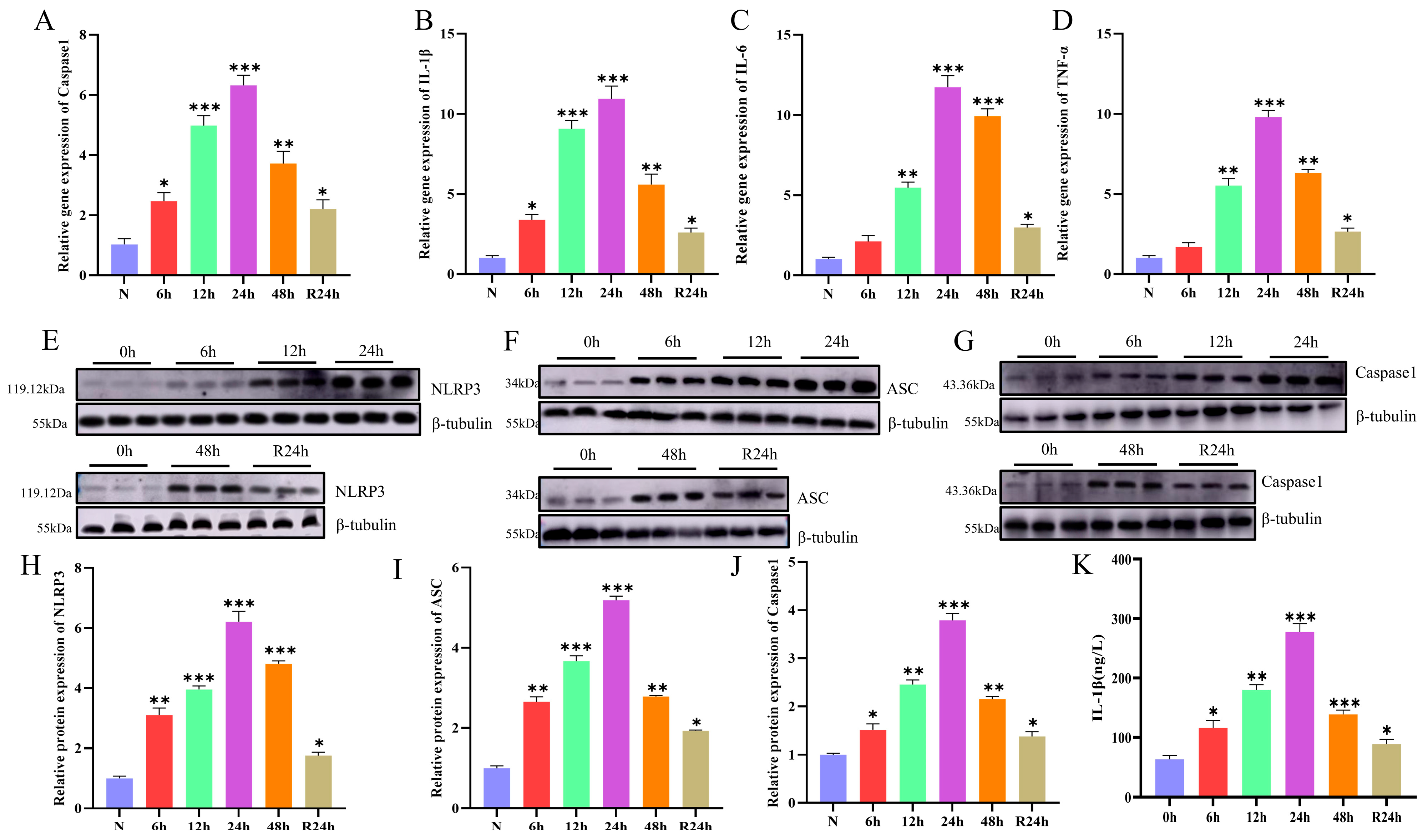
3.4. NLRP3 Inflammasome Activation Promotes Inflammatory Signaling
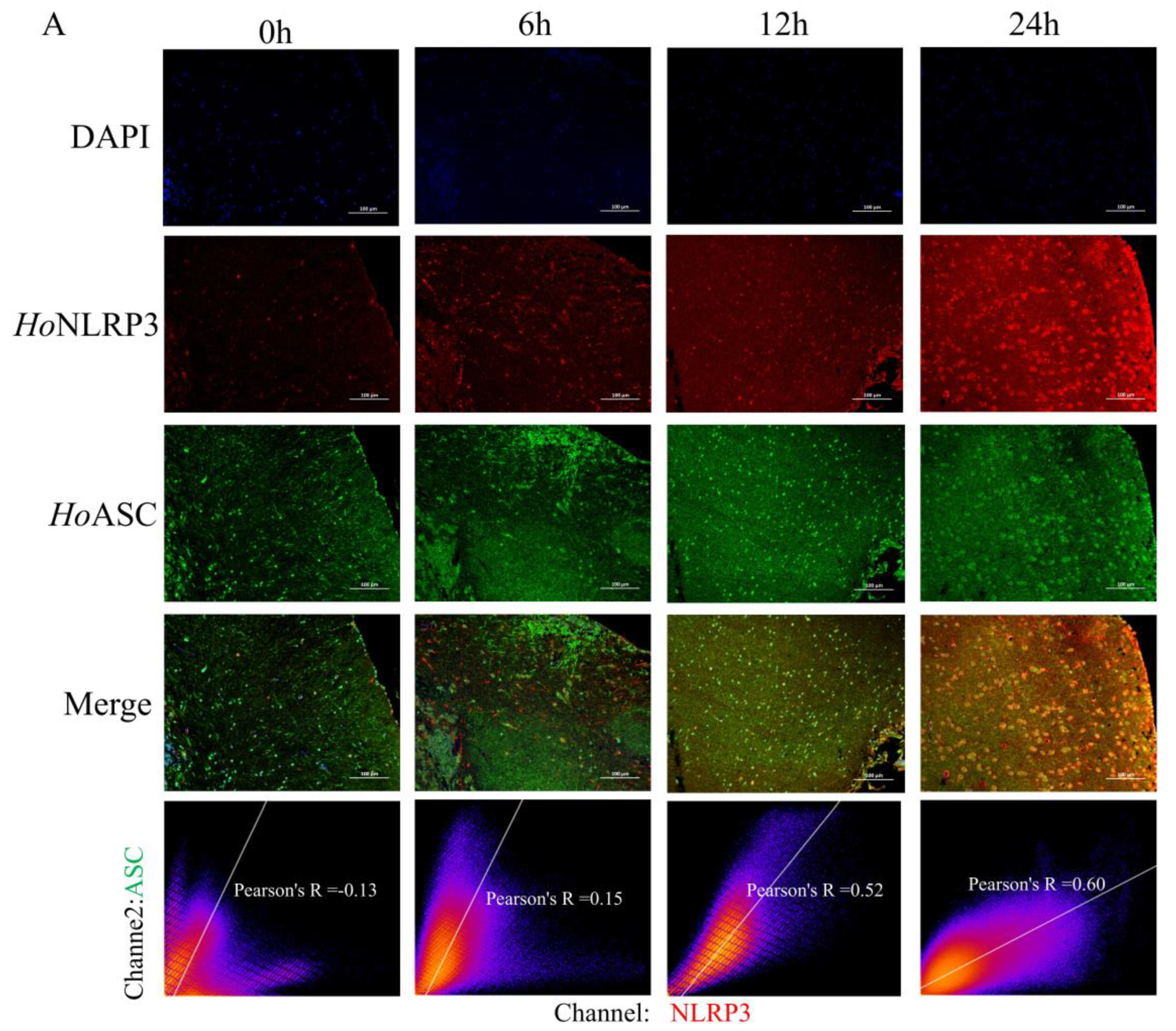
3.5. The Formation of Inflammasomes
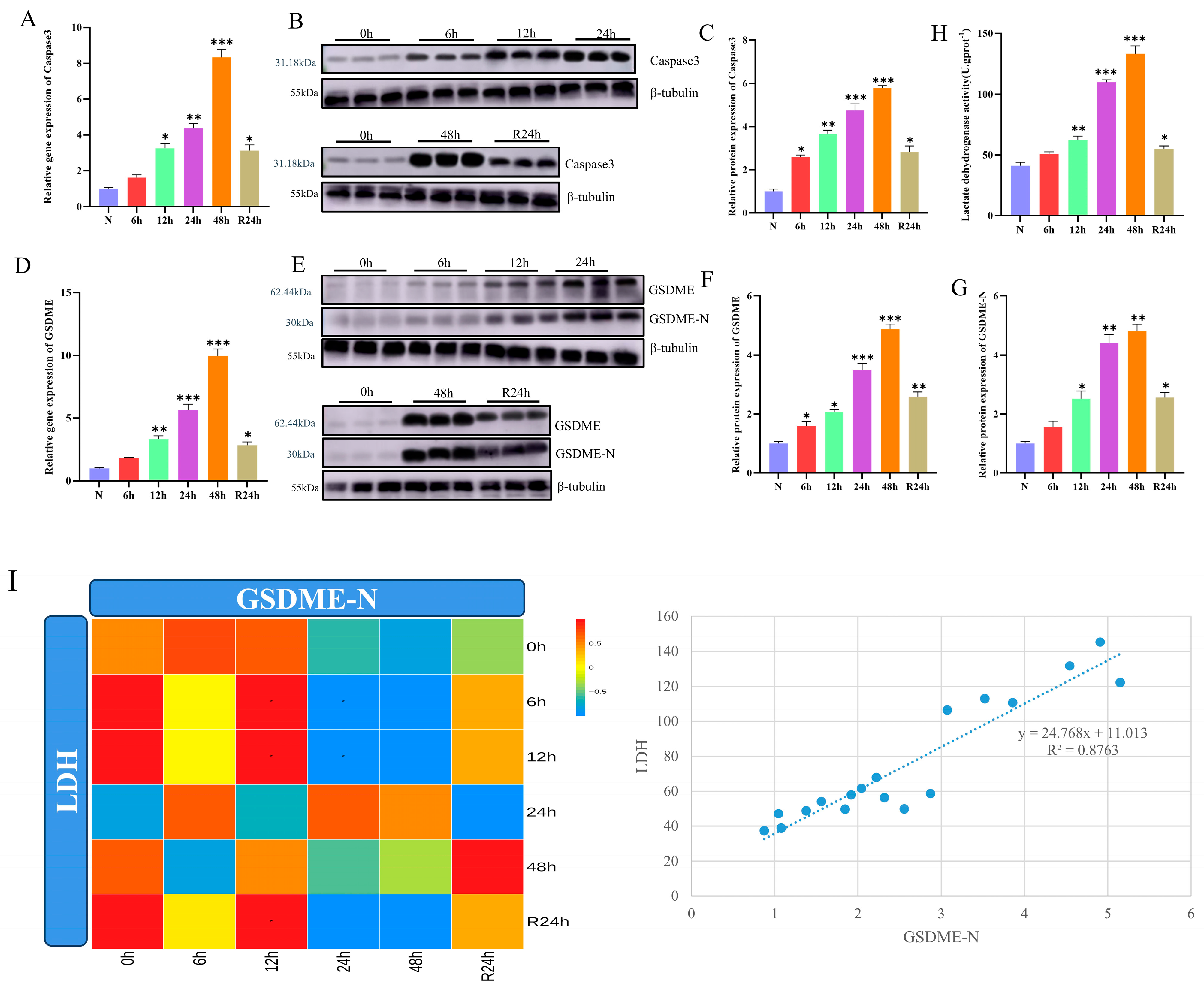
3.6. Late Hypoxia Triggers Caspase3/GSDME-Mediated Pyroptosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lima, A.R.; Booms, E.M.; Lopes, A.R.; Martins-Cardoso, S.; Novais, S.C.; Lemos, M.F.; Ribeiro, L.; Castanho, S.; Candeias-Mendes, A.; Pousão-Ferreira, P. Early life stage mechanisms of an active fish species to cope with ocean warming and hypoxia as interacting stressors. Environ. Pollut. 2024, 341, 122989. [Google Scholar] [CrossRef]
- Wood, C.M.; Eom, J. The osmorespiratory compromise in the fish gill. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2021, 254, 110895. [Google Scholar] [CrossRef]
- Aaskov, M.L.; Nelson, D.; Lauridsen, H.; Huong, D.T.T.; Ishimatsu, A.; Crossley, D.A., 2nd; Malte, H.; Bayley, M. Do air-breathing fish suffer branchial oxygen loss in hypoxic water? Proc. Biol. Sci. 2023, 290, 20231353. [Google Scholar] [CrossRef]
- Johnston, W.; Adil, S.; Cao, C.; Nipu, N.; Mennigen, J.A. Fish models to explore epigenetic determinants of hypoxia-tolerance. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2025, 302, 111811. [Google Scholar] [CrossRef]
- Cerra, M.C.; Filice, M.; Caferro, A.; Mazza, R.; Gattuso, A.; Imbrogno, S. Cardiac hypoxia tolerance in fish: From functional responses to cell signals. Int. J. Mol. Sci. 2023, 24, 1460. [Google Scholar] [CrossRef]
- Ma, Q.; Luo, Y.; Zhong, J.; Limbu, S.M.; Li, L.Y.; Chen, L.Q.; Qiao, F.; Zhang, M.L.; Lin, Q.; Du, Z.Y. Hypoxia tolerance in fish depends on catabolic preference between lipids and carbohydrates. Zool. Res. 2023, 44, 954–966. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Bratton, D.L.; Colgan, S.P. Targeting hypoxia signalling for the treatment of ischaemic and inflammatory diseases. Nat. Rev. Drug Discov. 2014, 13, 852–869. [Google Scholar] [CrossRef]
- Yuan, X.; Ruan, W.; Bobrow, B.; Carmeliet, P.; Eltzschig, H.K. Targeting hypoxia-inducible factors: Therapeutic opportunities and challenges. Nat. Rev. Drug Discov. 2024, 23, 175–200. [Google Scholar] [CrossRef]
- Cyran, A.M.; Zhitkovich, A. HIF1, HSF1, and NRF2: Oxidant-responsive trio raising cellular defenses and engaging immune System. Chem. Res. Toxicol. 2022, 35, 1690–1700. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, K.; Wang, D.; Wang, Y.; Lu, H.; Zhao, H.; Xing, M. Polystyrene microplastics-induced cardiotoxicity in chickens via the ROS-driven NF-κB-NLRP3-GSDMD and AMPK-PGC-1α axes. Sci. Total Environ. 2022, 840, 156727. [Google Scholar] [CrossRef]
- Yu, Q.; Shi, H.; Ding, Z.; Wang, Z.; Yao, H.; Lin, R. The E3 ubiquitin ligase TRIM31 attenuates NLRP3 inflammasome activation in Helicobacter pylori-associated gastritis by regulating ROS and autophagy. Cell Commun. Signal 2023, 21, 1. [Google Scholar] [CrossRef]
- Li, J.; Lin, Q.; Shao, X.; Li, S.; Zhu, X.; Wu, J.; Mou, S.; Gu, L.; Wang, Q.; Zhang, M.; et al. HIF1α-BNIP3-mediated mitophagy protects against renal fibrosis by decreasing ROS and inhibiting activation of the NLRP3 inflammasome. Cell Death Dis. 2023, 14, 200. [Google Scholar] [CrossRef]
- Fu, J.; Wu, H. Structural mechanisms of NLRP3 inflammasome assembly and activation. Annu. Rev. Immunol. 2023, 41, 301–316. [Google Scholar] [CrossRef]
- Zheng, S.; Que, X.; Wang, S.; Zhou, Q.; Xing, X.; Chen, L.; Hou, C.; Ma, J.; An, P.; Peng, Y.; et al. ZDHHC5-mediated NLRP3 palmitoylation promotes NLRP3-NEK7 interaction and inflammasomes activation. Mol. Cell 2023, 83, 4570–4585.e7. [Google Scholar] [CrossRef]
- Sayaf, K.; Battistella, S.; Russo, F.P. NLRP3 inflammasome in acute and chronic liver diseases. Int. J. Mol. Sci. 2024, 25, 4537. [Google Scholar] [CrossRef]
- Wang, H.; Ma, L.; Su, W.; Liu, Y.; Xie, N.; Liu, J. NLRP3 inflammasome in health and disease (Review). Int. J. Mol. Med. 2025, 55, 48. [Google Scholar] [CrossRef]
- Vasudevan, S.O.; Behl, B.; Rathinam, V.A. Pyroptosis-induced inflammation and tissue damage. Semin. Immunol. 2023, 69, 101781. [Google Scholar] [CrossRef]
- Xu, J.; Núñez, G. The NLRP3 inflammasome: Activation and regulation. Trends Biochem. Sci. 2023, 48, 331–344. [Google Scholar] [CrossRef]
- Toldo, S.; Abbate, A. The role of the NLRP3 inflammasome and pyroptosis in cardiovascular diseases. Nat. Rev. Cardiol. 2024, 21, 219–237. [Google Scholar] [CrossRef]
- Abderrazak, A.; Syrovets, T.; Couchie, D.; El Hadri, K.; Friguet, B.; Simmet, T.; Rouis, M. NLRP3 inflammasome: From a danger signal sensor to a regulatory node of oxidative stress and inflammatory diseases. Redox Biol. 2015, 4, 296–307. [Google Scholar] [CrossRef]
- Tourkochristou, E.; Aggeletopoulou, I.; Konstantakis, C.; Triantos, C. Role of NLRP3 inflammasome in inflammatory bowel diseases. World J. Gastroenterol. 2019, 25, 4796. [Google Scholar] [CrossRef]
- Ma, X.; Jiao, J.; Aierken, M.; Sun, H.; Chen, L. Hypoxia Inducible Factor-1α through ROS/NLRP3 pathway regulates the mechanism of acute ischemic stroke microglia scorching mechanism. Biologics 2023, 17, 167–180. [Google Scholar] [CrossRef]
- Wu, X.; Gong, L.; Xie, L.; Gu, W.; Wang, X.; Liu, Z.; Li, S. NLRP3 deficiency protects against intermittent hypoxia-induced neuroinflammation and mitochondrial ROS by promoting the PINK1-Parkin pathway of mitophagy in a murine model of sleep apnea. Front. Immunol. 2021, 12, 628168. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, M.; Ji, X.; Xia, Q.; Jiang, L.; Zhao, L. Acrylamide induces neurotoxicity in zebrafish (Danio rerio) via NLRP3-mediated pyroptosis. Sci. Total Environ. 2023, 896, 165208. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, Y.; Chen, Q.; Shan, S.; Yang, G.; Li, H. The function of NLRP3 in anti-infection immunity and inflammasomes assembly of common carp (Cyprinus carpio L.). Fish Shellfish Immunol. 2024, 145, 109367. [Google Scholar] [CrossRef]
- Song, Z.; Zou, J.; Wang, M.; Chen, Z.; Wang, Q. A comparative review of pyroptosis in mammals and fish. J. Inflamm. Res. 2022, 15, 2323–2331. [Google Scholar] [CrossRef]
- Hao, K.; Yuan, L.; Yu, C.; Xu, H.; Sun, L. Paralichthys olivaceus GSDME-mediated pyroptosis is regulated by multiple caspases in different manners. Fish Shellfish Immunol. 2024, 155, 110002. [Google Scholar] [CrossRef]
- Xu, H.; Qin, K.; Hao, K.; Yuan, Z.; Sun, L. Pufferfish gasdermin Ea is a significant player in the defense against bacterial pathogens. Mar. Life Sci. Technol. 2024, 6, 462–474. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiao, D.; Zhang, J.; Gao, F.; Pei, C.; Li, C.; Kong, X. Activation mechanism of CcGSDMEb-1/2 and regulation for bacterial clearance in common carp (Cyprinus carpio). J. Immunol. 2023, 211, 658–672. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, J.; Qiao, D.; Gao, F.; Gu, Y.; Jiang, X.; Zhu, L.; Kong, X. CcGSDMEa functions the pore-formation in cytomembrane and the regulation on the secretion of IL-lβ in common carp (Cyprinus carpio haematopterus). Front. Immunol. 2022, 13, 1110322. [Google Scholar] [CrossRef]
- Zhan, Y.; Gao, D.; Peng, L.; Cui, D.; Li, G.; Cao, S.; Chen, Y.; Xue, Z.; Wang, W. Hypoxia induces pyroptosis and inflammation in the liver of fat greenling (Hexagrammos otakii). Comp. Immunol. Rep. 2024, 6, 200146. [Google Scholar] [CrossRef]
- Gao, D.; Wu, Y.; Zhan, Y.; Peng, L.; Zhao, L.; Cao, S.; Xue, Z.; Wang, W. Chronic hypoxia drives the occurrence of ferroptosis in liver of fat greening (Hexagrammos otakii) by activating HIF-1α and promoting iron production. Ecotoxicol. Environ. Saf. 2024, 285, 117135. [Google Scholar] [CrossRef]
- Yang, Z.; Lin, P.; Chen, B.; Zhang, X.; Xiao, W.; Wu, S.; Huang, C.; Feng, D.; Zhang, W.; Zhang, J. Autophagy alleviates hypoxia-induced blood-brain barrier injury via regulation of CLDN5 (claudin 5). Autophagy 2021, 17, 3048–3067. [Google Scholar] [CrossRef]
- Li, J.Y.; Wang, Y.Y.; Shao, T.; Fan, D.D.; Lin, A.F.; Xiang, L.X.; Shao, J.Z. The zebrafish NLRP3 inflammasome has functional roles in ASC-dependent interleukin-1β maturation and gasdermin E-mediated pyroptosis. J. Biol. Chem. 2020, 295, 1120–1141. [Google Scholar] [CrossRef]
- Chen, H.; Ding, S.; Tan, J.; Yang, D.; Zhang, Y.; Liu, Q. Characterization of the Japanese flounder NLRP3 inflammasome in restricting Edwardsiella piscicida colonization in vivo. Fish Shellfish Immunol. 2020, 103, 169–180. [Google Scholar] [CrossRef]
- Morimoto, N.; Kono, T.; Sakai, M.; Hikima, J.I. Inflammasomes in teleosts: Structures and mechanisms that induce pyroptosis during bacterial infection. Int. J. Mol. Sci. 2021, 22, 4389. [Google Scholar] [CrossRef]
- Deng, N.; Zhao, Y.; Xu, J.; Ouyang, H.; Wu, Z.; Lai, W.; Lu, Y.; Lin, H.; Zhang, Y.; Lu, D. Molecular characterization and functional study of the NLRP3 inflammasome genes in Tetraodon nigroviridis. Fish Shellfish Immunol. 2022, 131, 570–581. [Google Scholar] [CrossRef]
- Acevedo, W.; Morán-Figueroa, R.; Vargas-Chacoff, L.; Morera, F.J.; Pontigo, J.P. Revealing the salmo salar NLRP3 inflammasome: Insights from structural modeling and transcriptome analysis. Int. J. Mol. Sci. 2023, 24, 4556. [Google Scholar] [CrossRef]
- Shi, Z.M.; Jing, J.J.; Xue, Z.J.; Chen, W.J.; Tang, Y.B.; Chen, D.J.; Qi, X.Y.; Huang, L.; Zou, Y.Q.; Wu, X.Z.; et al. Stellate ganglion block ameliorated central post-stroke pain with comorbid anxiety and depression through inhibiting HIF-1α/NLRP3 signaling following thalamic hemorrhagic stroke. J. Neuroinflam. 2023, 20, 82. [Google Scholar] [CrossRef]
- Jia, Y.; Gao, Y.; Wan, J.; Gao, Y.; Li, J.; Guan, C. Altered physiological response and gill histology in black rockfish, Sebastes schlegelii, during progressive hypoxia and reoxygenation. Fish Physiol. Biochem. 2021, 47, 1133–1147. [Google Scholar] [CrossRef]
- Ding, W.; Cao, L.; Cao, Z.; Bing, X. Gill and brain transcriptomic analysis of mandarin fish (Siniperca chuatsi) reveals hypoxia-induced mitochondrial dysfunction and modulation of metabolism. Comp. Biochem. Physiol. Part. D Genom. Proteom. 2025, 53, 101367. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, H.; Ge, J.; Li, L.; Luo, J.; He, K.; Yan, H.; Zhang, X.; Tahir, R.; Luo, W.; et al. Chronic hypoxia and Cu2+ exposure induce gill remodeling of largemouth bass through endoplasmic reticulum stress, mitochondrial damage and apoptosis. Aquat. Toxicol. 2023, 255, 106373. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Gong, W.; Hou, Y.; Ren, J.; Duan, C.; Zhang, H.; Nie, X.; Li, J. Aspirin exposure coupled with hypoxia interferes energy metabolism, antioxidant and autophagic processes and causes liver injury in estuarine goby Mugilogobius chulae. J. Hazard. Mater. 2024, 476, 135071. [Google Scholar] [CrossRef]
- Zheng, M.; Kanneganti, T.D. The regulation of the ZBP1-NLRP3 inflammasome and its implications in pyroptosis, apoptosis, and necroptosis (PANoptosis). Immunol. Rev. 2020, 297, 26–38. [Google Scholar] [CrossRef]
- He, Y.; Li, Z.; Xu, T.; Luo, D.; Chi, Q.; Zhang, Y.; Li, S. Polystyrene nanoplastics deteriorate LPS-modulated duodenal permeability and inflammation in mice via ROS drived-NF-κB/NLRP3 pathway. Chemosphere 2022, 307 Pt 1, 135662. [Google Scholar] [CrossRef]
- Shah, B.; Solanki, N. Aegeline attenuates TNBS-induced colitis by suppressing the NF-ƙB-mediated NLRP3 inflammasome pathway in mice. Inflammopharmacology 2024, 32, 2589–2599. [Google Scholar] [CrossRef]
- Zheng, X.; Qiu, J.; Gao, N.; Jiang, T.; Li, Z.; Zhang, W.; Gong, Y.; Hong, Z.; Hong, H. Paroxetine attenuates chondrocyte pyroptosis and inhibits osteoclast formation by inhibiting NF-κB pathway activation to delay osteoarthritis progression. Drug Des. Dev. Ther. 2023, 17, 2383–2399. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, J.; Lin, C.; Zhu, Q.; Wu, L. Impact of Caspase3/GSDME-mediated pyroptosis on tumor immune microenvironment and clinical prognosis across multiple cancers. Cancer Manag. Res. 2024, 16, 1663–1683. [Google Scholar] [CrossRef]
- Tang, J.; Bei, M.; Zhu, J.; Xu, G.; Chen, D.; Jin, X.; Huang, J.; Dong, J.; Shi, L.; Xu, L.; et al. Acute cadmium exposure induces GSDME-mediated pyroptosis in triple-negative breast cancer cells through ROS generation and NLRP3 inflammasome pathway activation. Environ. Toxicol. Pharmacol. 2021, 87, 103686. [Google Scholar] [CrossRef]
- Liu, X.; Lieberman, J. Inflammasomes-independent pyroptosis. Curr. Opin. Immunol. 2024, 88, 102432. [Google Scholar] [CrossRef]
- Zheng, X.; Wan, J.; Tan, G. The mechanisms of NLRP3 inflammasome/pyroptosis activation and their role in diabetic retinopathy. Front. Immunol. 2023, 14, 1151185. [Google Scholar] [CrossRef]
- Shah, B.; Solanki, N. Ameliorative effect of nodakenin in combating TNBS-induced ulcerative colitis by suppressing NF-ƙB-mediated NLRP3 inflammasome pathway. Naunyn Schmiedebergs Arch. Pharmacol. 2025, 398, 673–686. [Google Scholar] [CrossRef]
- Drakul, M.; Tomić, S.; Bekić, M.; Mihajlović, D.; Vasiljević, M.; Rakočević, S.; Đokić, J.; Popović, N.; Bokonjić, D.; Čolić, M. Sitagliptin induces tolerogenic human dendritic cells. Int. J. Mol. Sci. 2023, 24, 6829. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Wang, H.; Zhang, X.; Chen, Y.; Chen, G. Microglial pyroptosis in hippocampus mediates sevolfurane-induced cognitive impairment in aged mice via ROS-NLRP3 inflammasome pathway. Int. Immunopharmacol. 2023, 116, 109725. [Google Scholar] [CrossRef]
- Han, X.; Xu, T.; Fang, Q.; Zhang, H.; Yue, L.; Hu, G.; Sun, L. Quercetin hinders microglial activation to alleviate neurotoxicity via the interplay between NLRP3 inflammasome and mitophagy. Redox Biol. 2021, 44, 102010. [Google Scholar] [CrossRef]
- Qiu, W.Q.; Ai, W.; Zhu, F.D.; Zhang, Y.; Guo, M.S.; Law, B.Y.; Wu, J.M.; Wong, V.K.; Tang, Y.; Yu, L.; et al. Polygala saponins inhibit NLRP3 inflammasome-mediated neuroinflammation via SHP-2-Mediated mitophagy. Free Radic. Biol. Med. 2022, 179, 76–94. [Google Scholar] [CrossRef]
- Choudhry, H.; Harris, A.L. Advances in hypoxia-inducible factor biology. Cell Metab. 2018, 27, 281–298. [Google Scholar] [CrossRef]
- Qi, R.; Xie, Y.; Zhang, X.; Jiang, S.; Liu, X.; Xie, W.; Jia, X.; Bade, R.; Liu, Y.; Gong, K. Possible involvement of DNA methylation in TSC1 gene expression in neuroprotection induced by hypoxic preconditioning. Oxidative Med. Cell. Longev. 2022, 2022, 9306097. [Google Scholar] [CrossRef]
- Kim, J.; Lee, H.; Yi, S.-J.; Kim, K. Gene regulation by histone-modifying enzymes under hypoxic conditions: A focus on histone methylation and acetylation. Exp. Mol. Med. 2022, 54, 878–889. [Google Scholar] [CrossRef]
- Lai, K.P.; Tam, N.; Wang, S.Y.; Lin, X.; Chan, T.F.; Au, D.W.T.; Wu, R.S.S.; Kong, R.Y.C. Hypoxia causes sex-specific hepatic toxicity at the transcriptome level in marine medaka (Oryzias melastigma). Aquat. Toxicol. 2020, 224, 105520. [Google Scholar] [CrossRef]
- Das, T.; Soren, K.; Yerasi, M.; Kumar, A.; Chakravarty, S. Revealing sex-specific molecular changes in hypoxia-ischemia induced neural damage and subsequent recovery using zebrafish model. Neurosci. Lett. 2019, 712, 134492. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Song, Y.; Zhang, G.; Zhang, K.; Yin, S.; Ji, J. Multi-omics analysis identifies sex-specific hepatic protein-metabolite networks in yellow catfish (Pelteobagrus fulvidraco) exposed to chronic hypoxia. Int. J. Biol. Macromol. 2024, 268, 131892. [Google Scholar] [CrossRef] [PubMed]




| Gene | Primer | Primer Sequence (5′-3′) | Product Length (bp) | Tm |
|---|---|---|---|---|
| HIF1-α | HIF-1α-F | GCTGGGTGACATAAGAGAGATG | 112 | 55.1 |
| HIF-1α-R | TGAAGGCAGCAGAAGTATGG | 54.8 | ||
| NLRP3 | NLRP3-F | CCCAGTCCAGAGTGAACTTATG | 96 | 55.5 |
| NLRP3-R | CACCTGGAAGAGAGATACCAATC | 54.6 | ||
| Gasdermin-E | Gasdermin-E-F | ACAAGTCGTTGAGGGTGAAG | 128 | 54.8 |
| Gasdermin-E-R | CATCCATGTGTCCCGAGTAAA | 54.4 | ||
| Caspase3 | Caspase3-F | GTCGATGCTGACCAAAGAGA | 114 | 54.6 |
| Caspase3-R | CCACCTCACACACACATACA | 54.6 | ||
| Caspase1 | Caspase1-F | TTTGGCCGCAGGGTAAATA | 108 | 54.1 |
| Caspase1-R | GCTGCAGAGCAACAGATAGA | 54.7 | ||
| IL-1β | IL-1β-F | GAGGAATGCTCGAAGCTGAA | 118 | 54.9 |
| IL-1β-R | GGCACTTCACGGACTCAAA | 55.4 | ||
| IL-6 | IL-6-F | GTCTGTATCTGGCCGTGATATG | 103 | 55.1 |
| IL-6-R | ATGACCGTTACCTGGAGTTTG | 54.6 | ||
| TNF-α | TNF-α-F | CTTCTACCAGTACGCACATCC | 121 | 55.3 |
| TNF-α-R | AACACTCAGACAGCCATACAC | 54.6 | ||
| β-actin | β-actin-F | CTGGTCTGGATTGGCTGTGA | 89 | 57.5 |
| β-actin-R | GGAAGGAAGGCTGGAAGAGG | 58.3 |
| Antibody | Catalog No. | Source | Company Information |
|---|---|---|---|
| HIF1-α | RM7162 | Rabbit | Biodragon, Suzhou, China |
| NLRP3 | 68102-1-Ig | Mouse | Proteintech, Wuhan, China |
| ASC | BY2922 | Rabbit | Abways, Shanghai, China |
| Caspase1 | BYab-00584 | Rabbit | Byabscience, Nanjing, China |
| Caspase3 | BD-PT0656 | Rabbit | Biodragon, Suzhou, China |
| GSDME | 13075-1-AP | Rabbit | Proteintech, Wuhan, China |
| IgG | 10284-1-AP | Rabbit | Proteintech, Wuhan, China |
| rabbit IgG | 30000-0-AP | Rabbit | Proteintech, Wuhan, China |
| β-tubulin | GB15140 | Mouse | Servicebio, Wuhan, China |
| Goat Anti-Rabbit | RGAR001 | Goat | Proteintech, Wuhan, China |
| Goat Anti-Mouse | RGAM001 | Goat | Proteintech, Wuhan, China |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Zhao, L.; Zhang, X.; Liu, R.; Gao, D.; Su, J.; Peng, L.; Liu, Y.; Yan, Y.; Xue, Z.; et al. Mechanism of Biphasic Activation of NLRP3 Inflammasome in the Fat Greenling (Hexagrammos otakii) Under Hypoxic Stress: From Inflammatory Defense to Pyroptosis Execution. Fishes 2025, 10, 542. https://doi.org/10.3390/fishes10110542
Wu Y, Zhao L, Zhang X, Liu R, Gao D, Su J, Peng L, Liu Y, Yan Y, Xue Z, et al. Mechanism of Biphasic Activation of NLRP3 Inflammasome in the Fat Greenling (Hexagrammos otakii) Under Hypoxic Stress: From Inflammatory Defense to Pyroptosis Execution. Fishes. 2025; 10(11):542. https://doi.org/10.3390/fishes10110542
Chicago/Turabian StyleWu, Yiting, Ling Zhao, Xinying Zhang, Rangman Liu, Dongxu Gao, Junru Su, Lei Peng, Yuan Liu, Yuqing Yan, Zhuang Xue, and et al. 2025. "Mechanism of Biphasic Activation of NLRP3 Inflammasome in the Fat Greenling (Hexagrammos otakii) Under Hypoxic Stress: From Inflammatory Defense to Pyroptosis Execution" Fishes 10, no. 11: 542. https://doi.org/10.3390/fishes10110542
APA StyleWu, Y., Zhao, L., Zhang, X., Liu, R., Gao, D., Su, J., Peng, L., Liu, Y., Yan, Y., Xue, Z., & Wang, W. (2025). Mechanism of Biphasic Activation of NLRP3 Inflammasome in the Fat Greenling (Hexagrammos otakii) Under Hypoxic Stress: From Inflammatory Defense to Pyroptosis Execution. Fishes, 10(11), 542. https://doi.org/10.3390/fishes10110542







