1. Introduction
The cherry barb (
Puntius titteya) is a small, omnivorous cyprinid native to Sri Lanka and widely cultured in the ornamental aquarium trade. Its vibrant coloration, peaceful temperament, and adaptability to varied water conditions make it a popular choice for aquarists and a reliable production species for commercial aquaculture producers. Spawning in native populations can be triggered by rain events, which encourage proliferation of zooplankton, supplying ample feed for fish larvae [
1]. These conditions are often mimicked in aquaculture practices to stimulate captive reproduction. In Florida, USA, commercial production of
P. titteya has historically relied on extensive pond-based systems. However, as land and water availability become increasingly limited and costly, commercial operations have begun to employ recirculating aquaculture systems (RAS), which offer improved environmental control, year-round output, and support intensive stocking densities [
2,
3]. Unlike pond systems, RAS rely entirely on external feed inputs to support larval growth, making early diet formulation and delivery critically important. This shift highlights the need for reliable, cost-effective alternatives to live feeds such as
Artemia spp. nauplii (herein referred to as
Artemia), which have been a staple of first-feeding hatchery protocols.
A critical bottleneck in ornamental fish production occurs during the first feeding stage of the larval period. Success during this window has long-term implications for survival, growth, and final production yield. Traditionally, live Artemia have been used as an effective feed for numerous species of ornamental fishes, and they are part of the standard diet in P. titteya larviculture protocols. Investigations into alternative diets are motivated by Artemia’s labor-intensive decapsulation, daily hatching, and culture maintenance requirements, all of which diminish operational efficiency. Additionally, the nutritional quality and hatch rate of Artemia are notoriously variable, contributing to inconsistent outcomes in larviculture.
Efforts to reduce
Artemia dependency have therefore intensified [
4], focusing on enhanced microparticulate diets (MDs) and other alternative feeds. MDs are a convenient, nutritionally appropriate alternative to live feeds; however, their lack of intrinsic movement often fails to evoke feeding responses from larval fish, resulting in poor ingestion of these static particles [
5]. In efforts to overcome this challenge, amino acid-derived feed attractants (FAs), have been investigated to improve palatability and stimulate feeding behavior in fish.
The use of L-isomers of free amino acids as feeding stimulants has been widely documented across teleost species, particularly in those of commercial importance [
6]. Studies have demonstrated their efficacy in rainbow trout (
Salmo gairdneri) [
7], turbot (
Scophthalmus maximus) and Dover sole (
Solea solea) [
8], weatherfish (
Misgurnus anguilicaudatus) and yellowtail jack (
Seriola quinqueradiata) [
9,
10], and European eel (
Anguilla anguilla) [
11]. In gilthead seabream larvae (
Sparus aurata), L-arginine, L-alanine, L-glycine and betaine were found to be functional feeding stimulants [
12]. These compounds not only enhanced feeding behavior but stimulated digestive enzyme activity [
5]. In some cyprinids, such as butter catfish, (
Ompok bimaculatus), betaine increased growth while L-tryptophan improved survival [
13]. Similar survival and growth benefits have been reported in rohu (
Labeo rohita) [
14] and Mrigal carp (
Cirrhinus mrigala) [
15].
Other microdiets such as liquid
Artemia replacement (LA) products offer inert, shelf-stable options that may serve as effective substitutes for live
Artemia if proven effective. These diets are marketed for use in shrimp aquaculture as an alternative for live
Artemia [
16]. Manufacturers of two commercially available products, Cargill and Zeigler, both advertise these products as mimicking key characteristics of
Artemia, such as color and buoyancy, while providing an optimal and consistent nutrient profile. These diets are available in different size gradients suitable for finfish larvae with small mouth gapes. Their applicability as a diet for ornamental marine finfish larvae; however, warrants further investigation, especially regarding digestibility, nutrient utilization and expected performance specific to formulation and species.
In recent years, assessments of larval diet performance have expanded beyond growth and survival to include physiological indicators of larval quality. Parameters such as RNA/DNA ratios and digestive enzyme activity provide deeper insights into the effects of early nutrition on digestion and overall growth potential. Expanding upon the work of Kolkovski [
5], Zambonino Infante and Cahu [
17], Lipscomb et al., [
15,
18], and Murray et al., [
19,
20], this study uses these parameters to evaluate the effects of experimental diets beyond conventional endpoints. The inclusion of these physiological endpoints is particularly relevant when testing manufactured diets such as MDs and LA, which exhibit more structurally complex protein and lipid profiles compared to live prey such as
Artemia. Effective utilization of these diets requires coordinated upregulation of digestive enzymes to achieve adequate hydrolysis of macronutrients. Without sufficient enzymatic response, ingestion alone does not ensure nutrient assimilation, potentially limiting growth even in the presence of adequate feed intake.
RNA/DNA ratios provide an additional metric to better characterize larval quality, reflecting the balance between protein synthesis and cellular growth processes that depend on successful nutrient assimilation [
21]. A deeper understanding of larval physiological responses to these diets enables the design of feeding protocols tailored to key developmental milestones in digestive capacity, thereby facilitating more effective transitions away from live feeds.
The production of P. titteya in Florida is estimated to exceed 60,000 juveniles monthly; therefore, any improvements in survival or feed efficiency could be increasingly beneficial at scale. By evaluating both attractant-enhanced MDs and live feed replacement strategies, this study seeks to provide ornamental hatcheries with evidence-based tools to enhance performance, reduce costs, and minimize operational complexity. Moreover, by incorporating larval quality indicators alongside traditional growth and survival metrics, this work contributes to a more comprehensive understanding of how early dietary manipulations shape aquaculture protocol outcomes.
The success of top-dressed MDs containing individual and combined feed attractants, and two commercial LA diets relative to a live Artemia reference diet, was assessed for use in larval feeding protocols for P. titteya. The goal was to identify scalable feeding strategies that reduce live feed inputs, support successful first feeding, and contribute to more efficient ornamental fish hatchery protocols.
2. Materials and Methods
All animal husbandry and experimental methods were approved by the University of Florida’s Institutional Animal Care and Use Committee (IACUC protocol # IACUC202400000140).
2.1. General Broodstock Husbandry and Spawning
Cherry barb larvae were produced through controlled spawning of conditioned adult broodstock. Males and females were conditioned separately in concrete holding tanks and fed Zeigler Tropical Flake (45.0% crude protein, 7.0% crude fat; Zeigler Bros., Inc., Gardners, PA, USA) twice daily, supplemented every other day with frozen bloodworms (Glycera dibranchiate; 3.5% crude protein, 0.3% crude fat; Hikari USA, Hayward, CA, USA) and trimmed beef heart processed on-site. For each spawning attempt, between 35 and 42 breeding pairs were placed in individual 10-L plastic aquaria, each filled with 4 L of reverse-osmosis (RO) filtered water reconstituted by adding 0.5 L of filtered well water to achieve a final hardness of approximately 102.6 mg/L as CaCO3. 1.5 mL of CaribSea® Instant Amazon™ Blackwater Extract (CaribSea Inc., Cape Coral, FL, USA) was added to each tank to simulate humic stream conditions. Spawning tanks were placed on shelving units in a temperature-regulated facility maintained at 25.6 °C (78 °F), with a photoperiod of 12 h light and 12 h darkness. Spawning typically occurred at the beginning or end of the daytime cycles, and from each spawning attempt, 22 to 31 viable clutches were collected. After hatch, broodstock were placed back into their original tanks and larvae were kept static with light aeration until stocked into experimental tanks. Larvae from successful spawns were pooled and stocked into experimental tanks at 5 days post-hatch (DPH).
2.2. General Expermental Design
The experimental system was comprised of transparent 3-L polycarbonate tanks arranged in a multi-tiered rack system. Each tank was fitted with a 400 μm mesh screen at the rear to allow for flow-through water exchange while preventing larval escape. Well water, degassed and filtered through a sand bed, was continuously supplied at a target flow rate of 2.5 mL/s. Aeration was provided via a central air pump system, with each tank utilizing a fused silica airstone (1.5 cm × 2.5 cm) to maintain gentle surface agitation while minimizing turbulence. Larvae were stocked at 75 individuals per tank, with five replicate tanks per treatment for all experiments. Experimental diets were introduced beginning on 6 DPH, consistent with commercial protocols for first feeding of P. titteya Experimental tanks were siphoned three times weekly to remove uneaten feed and organic debris.
2.3. Water Quality Monitoring
Water quality parameters were monitored at the beginning and end of each trial. Temperature and dissolved oxygen (DO) were recorded using a YSI ProSolo handheld meter (YSI Inc., Yellow Springs, OH, USA), while pH was measured using an EcoSense pH100A meter (YSI Inc.). Total ammonia-nitrogen (TAN), nitrite-nitrogen (NO2-N), alkalinity, and hardness were quantified using a Hach FF-1A Fish Farmer Water Quality Test Kit following manufacturer’s procedures (Hach Company, Loveland, CO, USA).
2.4. Feed Attractant Diet Preparation
Given the absence of species-specific data for optimal MDs in P. titteya larviculture protocols, a mixture of three commercially available larval feeds was used in equal proportions: Golden Pearls, 100–200 µm (60% protein, 8% fat; Brine Shrimp Direct, Ogden, UT, USA), Otohime A2 (53% protein, 8% fat; Marubeni Nisshin Feed Co. Ltd., Tokyo, Japan), and Z Plus, size 3 (50% protein, 15% fat; Ziegler Bros Inc., Gardners, PA, USA). To prepare the diets for the three separate individual FA experiments, MDs and one FA powder (L-alanine—A2627-100G; or betaine—B2629-100G; or L-tryptophan—T0254-25G; Sigma-Aldrich, St. Louis, MO, USA) were weighed and combined, then hydrated with RO filtered water to create a homogenous paste. The combination diet received all three FAs (L-alanine—0.5%, betaine—0.25%, L-tryptophan—0.25%). Each mixture was then dried by spreading onto aluminum foil sheets and refrigerating at 1 °C for 48 h. Once dried, the feed was milled using a standard coffee grinder, sifted through a 250 µm mesh sieve, and re-pulverized with a mortar and pestle to ensure uniform particle size. Microscopic evaluation via ImageJ software (version 1.54d; NIH, Bethesda, MD, USA) was used to verify particle dimensions suitable for larval P. titteya larval mouth gape. Control diets (0.0%) underwent identical processing without the addition of any FA.
2.5. Feed Attractant Experimental Design
To evaluate the effects of individual FAs, L-alanine, betaine, and L-tryptophan were each tested in separate experiments (ALA, BET, and TRY, respectively) at inclusion levels of 0.0%, 0.25%, 0.5%, and 1.0% (by dry weight) when top-dressed onto an MD. Tanks (
n = 5/treatment) received 11 mg of treatment diet twice daily for seven days. At the end of the seven-day period all larvae were sampled. Following the completion of these individual inclusion trials, a subsequent combination experiment (COM) was conducted using all three FAs concomitantly and at the most effective inclusion levels identified previously (L-alanine—0.5%, betaine—0.25%, L-tryptophan—0.25%), to assess whether synergistic effects could be observed. This combination diet was processed using the same top-dressing and preparation method as the individual FA diets and was administered for 21 days to allow for developmental sampling of digestive enzyme activity. Within this COM experiment, two sampling times were selected, as well as a midpoint sub-sample (4 larvae) at 10 days if possible, and all remaining larvae at the endpoint sample at 21 days, as described below in
Section 2.7.
2.6. Liquid Artemia Replacement Trial
A trial was conducted to evaluate two brands of LA diets against an Artemia reference diet. This experiment is hereafter referred to as LA BRAND. Tanks were fed one of five diets (n = 5/treatment): 100% Liqualife® (LL) size Z-M (3.0% protein, 2.0% fat, by wet weight; Cargill Inc., Minneapolis, MN, USA), 100% EZ Artemia Ultra (EZ) size 1 (12.0% protein, 4.5% fat, by wet weight; Zeigler Bros., Inc., Gardners, PA, USA), 50:50 LL and Artemia (LL + ART), and 50:50 EZ and Artemia (EZ + ART), or a 100% Artemia reference diet. Decapsulated Artemia cysts were hatched daily (INVE Aquaculture, Salt Lake City, UT, USA) and instar I-II nauplii were used at each feeding. Liquid diets were diluted in RO water to achieve a working concentration of 600 particles/mL. Diluted stock solutions of 50 mL were prepared twice weekly. Tanks were fed twice daily for 14 days receiving 600 particles per tank for 100% replacement treatments and 300 particles per tank for 50% replacement treatments. After 14 days, all larvae were sampled for further evaluation.
2.7. Larval Sampling Protocol
At the end of each experimental trial, all remaining P. titteya were collected from their respective tanks and euthanized in a bath containing 300 mg/L buffered tricaine methanesulfonate (Syncaine® MS-222; Syndel USA, Ferndale, WA, USA). All individuals were manually counted to determine treatment-level survival. For length measurements, a subsample of ten larvae per tank were photographed on a Sedgewick Rafter cell using a Jenoptik ProgRes® C5 digital camera affixed to a dissection microscope (Jenoptik AG, Jena, Germany). Standard length (SL) was measured to the nearest 0.01 mm using ImageJ image analysis software (National Institutes of Health, Bethesda, MD, USA).
The coefficient of variation (CV) for SL was calculated on a per-tank basis using the formula:
For the FA COM experiment at the 10-day midpoint (15 DPH), tanks with greater than 15 larvae remaining per tank were sampled, harvesting two larvae for digestive enzyme (lipase and trypsin) analysis and two for RNA/DNA analysis. At the 21-day endpoint (26 DPH), all tanks were harvested, and a mean of 3 ± 1 larvae were preserved for digestive enzyme analyses, due to low survival no RNA/DNA samples could be preserved. All collected samples were placed into sterile 1.5 mL microcentrifuge tubes, flash-frozen, and stored at −80 °C until analysis. Larvae designated for nucleic acid analysis were preserved in RNAlater™ (Thermo Fisher Scientific, Waltham, MA, USA) prior to freezing to ensure RNA integrity.
2.8. Digestive Enzyme Activity Assays
Digestive enzyme activity was quantified via spectrophotometric methods using a 96-well microplate, following protocols originally developed by Faulk et al. [
22] and later modified by Lipscomb et al. [
23] and Murray et al. [
18]. Samples were thawed on ice and homogenized individually in sterile 1.5 mL microcentrifuge tubes. Each sample was homogenized in 400 µL of homogenization buffer (20 mM Tris-HCl, 1 mM EDTA, pH 7.5). Following homogenization, samples were centrifuged at 1700×
g for 10 min at 4 °C. Sample tubes were promptly placed into an aluminum cooling block on ice for subsequent procedures.
Lipase activity was measured by loading 10 µL of each sample supernatant into a 96-well plate (FisherBrand, Cat. No. 21-377-203, Waltham MA, USA) in triplicate. The assay blank (homogenization buffer) and standard (30 µg/mL lipase) were also included in triplicate. A freshly prepared substrate solution composed of 0.5 M Tris-HCl, 6 mM sodium taurocholate, (1 M NaCl [pH 7.40], and 0.35 mM 4-nitrophenyl-n-caproate) was pre-warmed to 30 °C and dispensed into each well (200 µL) using a multichannel pipette. Enzymatic hydrolysis was monitored at 400 nm, and activity was reported in units (U), defined as micromoles of substrate hydrolyzed per minute per milligram of protein (U/mg protein).
Trypsin activity was measured by adding 20 µL of supernatant per well, also in triplicate, to a pre-chilled 96-well plate. Each plate included triplicate aliquots of an assay blank and a 15 µg/mL trypsin standard. To each well, 100 µL of substrate buffer (50 mM Tris-acetate, 20 mM CaCl2, pH 8.20), containing 1 mM Nα-benzoyl-DL-arginine p-nitroanilide, was added at 30 °C. Absorbance at 410 nm was recorded every 45 s over a 30-min incubation period. Trypsin activity was calculated as the rate of p-nitroanilide release (µmol/min), standardized to total protein content.
2.9. Nucleic Acid Quantification
Quantification of total RNA and DNA was conducted using a fluorometric method adapted from Caldarone [
24]. Preserved larval samples were thawed on ice and transferred into sterile 1.5 mL microcentrifuge tubes. Samples were then homogenized in 150 µL of 1% sarcosyl in Tris-EDTA buffer using a multihead vortexer, agitated continuously for 60 min. A homogenization control was concurrently prepared by diluting 2% sarcosyl to 75 µL. Once tissues were dissolved, 1.35 mL of additional Tris-EDTA buffer was added to each tube. Samples were then inverted manually 40 times to mix, followed by centrifugation at 14,000×
g for 15 min at room temperature.
The resulting supernatants were transferred in duplicate (75 µL per well) into a 96-well microplate. Each assay included appropriate blanks, homogenate controls, and RNA and DNA standards prepared in 0.1% sarcosyl to ensure values fell within the linear detection range of the fluorometer. Fluorescent detection was achieved using ethidium bromide, added to each well in 75 µL aliquots from a working solution (32 µL of 1 mg/mL stock diluted in 16 mL Tris-EDTA). Plates were gently vortexed for 15 min before the first fluorescence measurement (excitation 525 nm, emission 600 nm).
Following the initial read, 7.5 µL of RNase was added to each well, and the plate was vortexed for 20 min prior to a second fluorescence measurement. To complete DNA quantification, 7.5 µL of DNase enzyme solution was then added per well. The DNase buffer consisted of 0.1 M MgCl2, 0.08 M CaCl2, and 1 U/µL DNase enzyme in Tris-EDTA. Plates were vortexed again for 15 min and incubated at 37 °C for 1 h. After cooling to room temperature for 30 min, a final fluorescence reading was taken. Final concentrations of RNA and DNA were calculated using the standard curves generated from each plate and reported as μg of RNA/DNA per μg protein. All microplate assays were read using a BioTek spectrophotometer (Synergy HTX Multi-Mode Microplate Reader; BioTek Instruments, Inc., Winooski, VT, USA).
2.10. Total Protein Quantification
Total protein concentration was measured using the Pierce™ Bradford Protein Assay Kit (Thermo Fisher Scientific, Cat. No. 23200, Waltham MA, USA). Supernatants from each sample processed for digestive enzyme activity and RNA/DNA ratios were analyzed for protein concentration. Samples were measured in duplicate in a 96-well plate at 595 nm, with protein concentrations determined from a bovine serum-albumin (BSA; Thermo Fisher Scientific, Waltham, MA, USA) standard curve.
2.11. Data Analysis
Survival, SL, CV, digestive enzyme activity, and RNA/DNA data were analyzed across all feeding trials using standardized statistical procedures consistent with previous larval diet evaluations [
17,
20,
23]. Larval survival was modeled using generalized linear mixed models (GLMMs) with a binomial distribution, treating survival status as a binary response (alive vs. dead). Treatment group was included as a fixed effect, and replicate tank was incorporated as a random effect to account for potential tank effects. Where suitable, post hoc comparisons of estimated marginal means (emmeans) were performed on the logit scale using Tukey-adjusted pairwise tests (α ≤ 0.05).
One-way analysis of variance (ANOVA) or a Kruskal–Wallis test was used to evaluate SL, digestive enzyme activity, and RNA/DNA results. Assumptions of normality and homogeneity of variance were evaluated using Shapiro–Wilk and Levene’s test, respectively. When data failed to satisfy parametric assumptions, Kruskal–Wallis rank-sum tests were used in place of ANOVA, followed by Dunn’s post hoc test. When RNA/DNA data failed to meet these assumptions, data were log-transformed. Significant differences were indicated by α ≤ 0.05.
Enzyme assay results were excluded from analysis when technical or biological replication fell below the required minimum (n < 3) for a particular treatment. As such, only datasets meeting threshold replication standards were included in the final statistical comparisons reported.
Water quality data were evaluated across treatment groups within each experimental trial. Parameters assessed included temperature (°C), DO (mg/L), pH, total alkalinity (mg/L CaCO3), and hardness (mg/L CaCO3). TAN and NO2-N concentrations remained below detection thresholds throughout all trials and therefore were not statistically analyzed.
All analyses were performed using R software (version 4.2.1; R Core Team, 2022). Survival results are reported as mean proportion ± standard error of the proportion (SEₚ), while all other data are presented as mean ± standard deviation (SD), unless otherwise stated.
4. Discussion
In this study, the application of amino acid-based FAs to MDs resulted in positive outcomes for survival and growth in
P. titteya larvae. Notably, moderate inclusion levels of L-alanine and L-tryptophan were associated with improved survival, suggesting their potential role in stimulating feed recognition and ingestion. However, the effects varied by inclusion level and compound. Survival plateaued with increasing betaine, while increased inclusion of L-tryptophan led to a decline in survival. This nonlinearity echoes electrophysiological findings in red sea bream (
Pagrus major), where olfactory responses to L-alanine increased between 10
−3 and 10
−2 M but plateaued beyond that range [
25]. These findings illuminate the importance of attractant dosages to balance efficacy and prevent oversaturation.
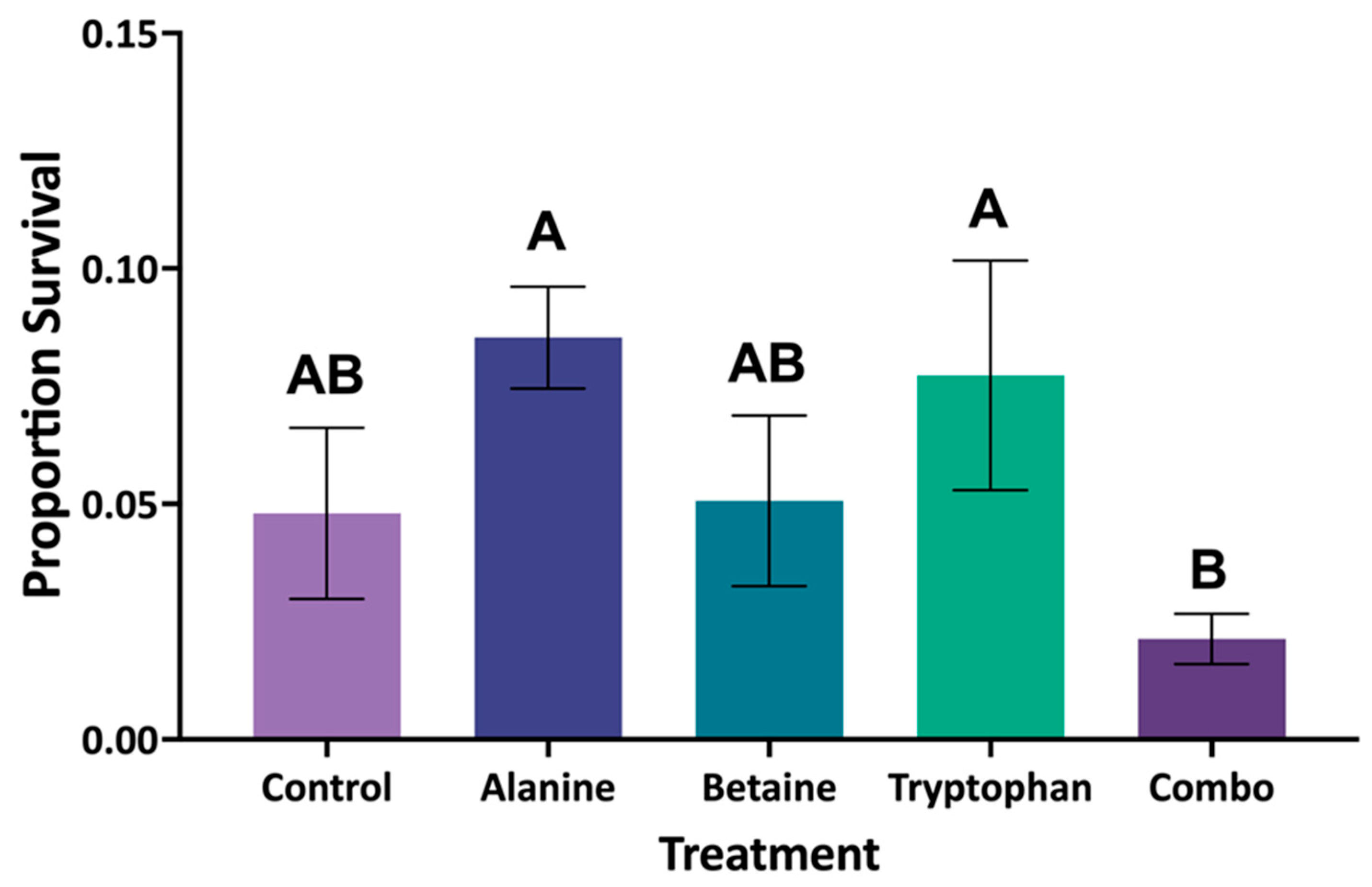
The FA COM experiment demonstrated that combining multiple FAs can be counterproductive. The combination diet resulted in the lowest mean survival across all treatment groups with similar outcomes to the control (no FA) and betaine groups, while the L-alanine and L-tryptophan treatments responded positively, mirroring outcomes of the individual FA inclusion rate experiments and suggesting the possibility of antagonistic sensory interactions. Although previous studies have shown that synergistic effects of FA combinations provide utility in juvenile genetically improved farmed tilapia (GIFT) (
Oreochromis sp.) [
26], these results were not observed in
P. titteya. The absence of improved survival, growth, or larval quality metrics in response to combination FA treatments may reflect receptor-level suppression driven by competitive binding among amino acids. Fish chemoreceptors, particularly those detecting neutral L-alpha-amino acids (which possess a short, unbranched, and uncharged side chain), are non-specific, meaning structurally similar compounds often interact with overlapping receptor sites [
27]. This receptor promiscuity enables both effective and ineffective attractants to compete for the same binding sites [
28]. When an ineffective compound possesses similar affinity but lower FA potential, it can occupy the receptor and prevent the binding and subsequent signaling of a more effective attractant, a phenomenon Hara [
27] describes as suppression. Such functional antagonism likely contributed to the underperformance of the combination treatment. Contrasting outcomes were reported in tiger barb (
Puntius tetrazona), an ecologically similar species. When larval tiger barbs were fed a MD top-dressed with either taurine, or L-tryptophan a significant reduction in survival was observed when compared with the combination treatment, whereas treatments receiving betaine or the combination of all three, were not different from controls [
18]. Species-specific differences in olfaction could explain the differences in these results, supporting the need for further research to refine the use of FAs in cyprinid culture.
Moreover, the reduction in survival may also relate to digestive challenges associated with MD use. From day 10 to day 21, SL increased by ~13%, whereas trypsin activity rose by nearly 5-fold among treatments. This disproportionate increase in enzyme activity relative to growth may reflect the digestive challenge associated with ingestion MDs; however, such enzymatic upregulation is also characteristic of normal early larval development [
29]. Kolkolvski [
5] describes persistent challenges associated with complete replacement of live diets with MDs from first feeding, noting that while some enzymes are present prior to exogenous feeding in most larvae, the complexity of MD profiles is mismatched with larval digestive capabilities.
Despite differences in survival, SL did not vary across FA treatments, and CV values remained low. This uniformity suggests that growth was unaffected by FA inclusion and that individual larvae achieved consistent size outcomes. The absence of SL divergence relative to differences in larval densities across treatments likely reflects low intra-tank competition, a known driver of growth variation [
23]. This pattern implies that feed availability was not a limiting factor and that rations were effectively provided ad libitum across treatments, regardless of the number of surviving larvae. From a production standpoint, size uniformity is highly desirable in ornamental aquaculture, as it simplifies feeding, reduces labor associated with grading, and ensures a consistent and marketable product for shipping and sale [
30].
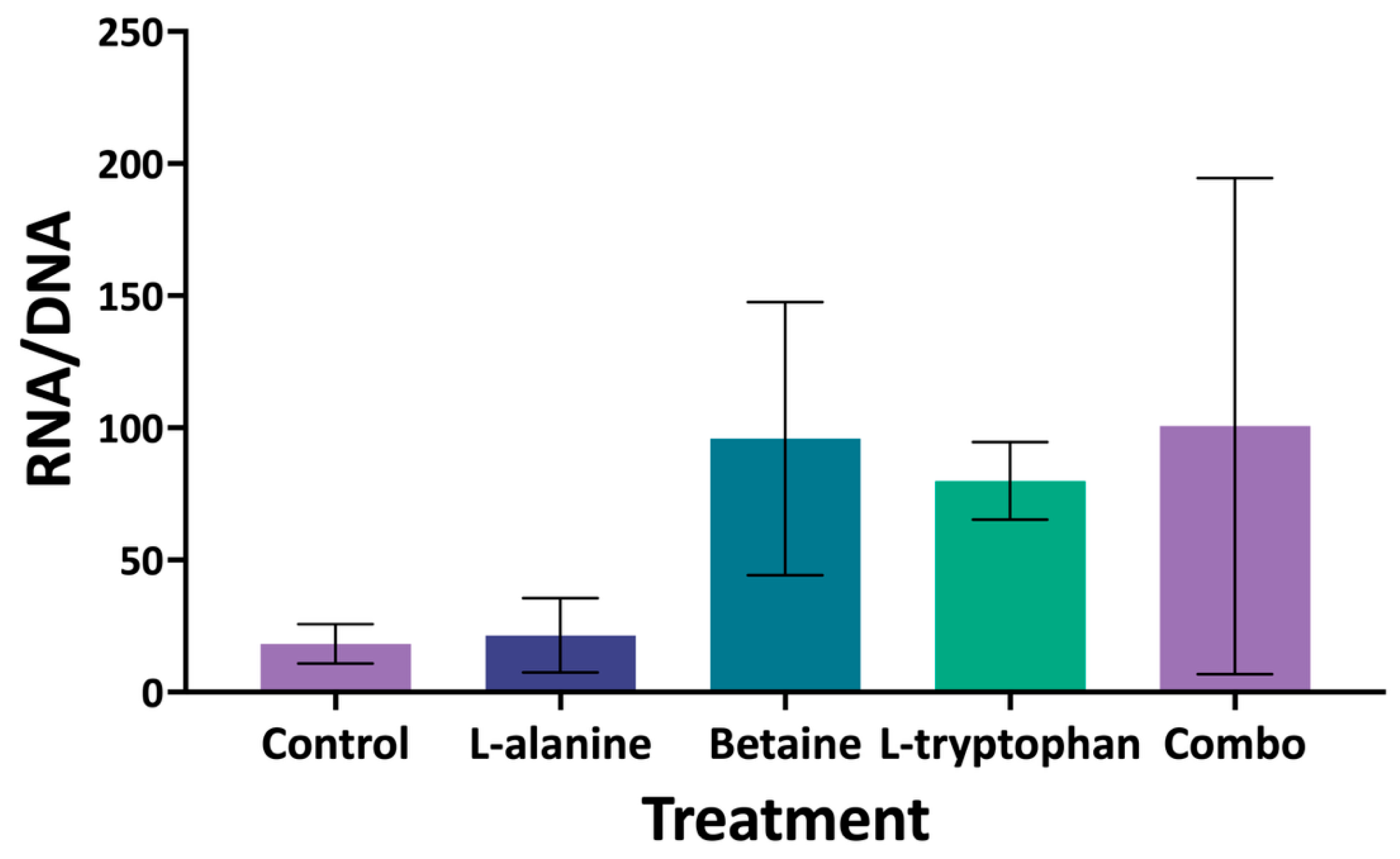
Similarly, RNA/DNA ratios measured at the 15 DPH sub-sample timepoint in the FA COM experiment did not produce any statistically significant pairwise differences among treatments. This result was likely influenced by the high variability inherent to the assay, which is insensitive relative to other molecular techniques (such as quantitative real-time PCR, or transcriptomic approaches), and prone to error both within and among treatments. Variability may also have been compounded by inter-individual differences in larval condition, which can also contribute to error [
24]. The conservative nature of the Tukey HSD test, designed to minimize type I error, likely reduced the ability to detect treatment effects despite positive trends in the data, such as elevated RNA/DNA ratios in larvae fed the betaine, L-tryptophan, or combination diets. Increased replication, both in number of pooled larvae and number of tanks per treatment, may minimize potential sources of error in future assays and is therefore recommended when using RNA/DNA ratios as a larval quality endpoint to better account for individual variation.
Additionally, no treatment in the FA COM experiment significantly elevated survival; however, there were two diets, L-alanine and L-tryptophan, that achieved numerically higher survival compared to the control diet. These elevations in survival are consistent with the results of the FA dose trials, where L-alanine and L-tryptophan inclusion significantly elevated survival compared to the control. The key difference between the FA dose trials and the FA COM trial duration is that the dose trials lasted 7 days, while the FA COM trial extended to 21 days. Overall survival was reduced from approximately 75–80% in the dose trials to approximately 7–9% survival in the FA COM trial. Overall survival declined markedly from approximately 75–80% in the shorter dose trials to just 7–9% in the longer FA COM trial. This reduction was likely due to the inability of
P. titteya to efficiently digest MD from first feeding, a limitation that only became evident later in development. In larviculture, weaning from live feeds to inert diets typically occurs once the digestive system matures, often marked by the development of a functional stomach and sufficient production of key digestive enzymes that enable efficient digestion of complex diets [
5,
31]. Although cyprinids do not develop stomachs during their lifetime, many species can still be successfully weaned to inert diets. Agastric fish species often possess alternative adaptations for digesting complex diets, such as
P. tetrazona, which has a keratinized pharyngeal jaw structure thought to aid in manual grinding of feeds. Other cyprinids that have successfully been weaned to inert diets include pike silverside (
Chirostoma estor) and zebrafish (
Danio rerio) [
32,
33]. Therefore, incorporating a weaning regime centered around the timing of
P. titteya digestive maturity may help increase long-term larval survival, growth, and quality.
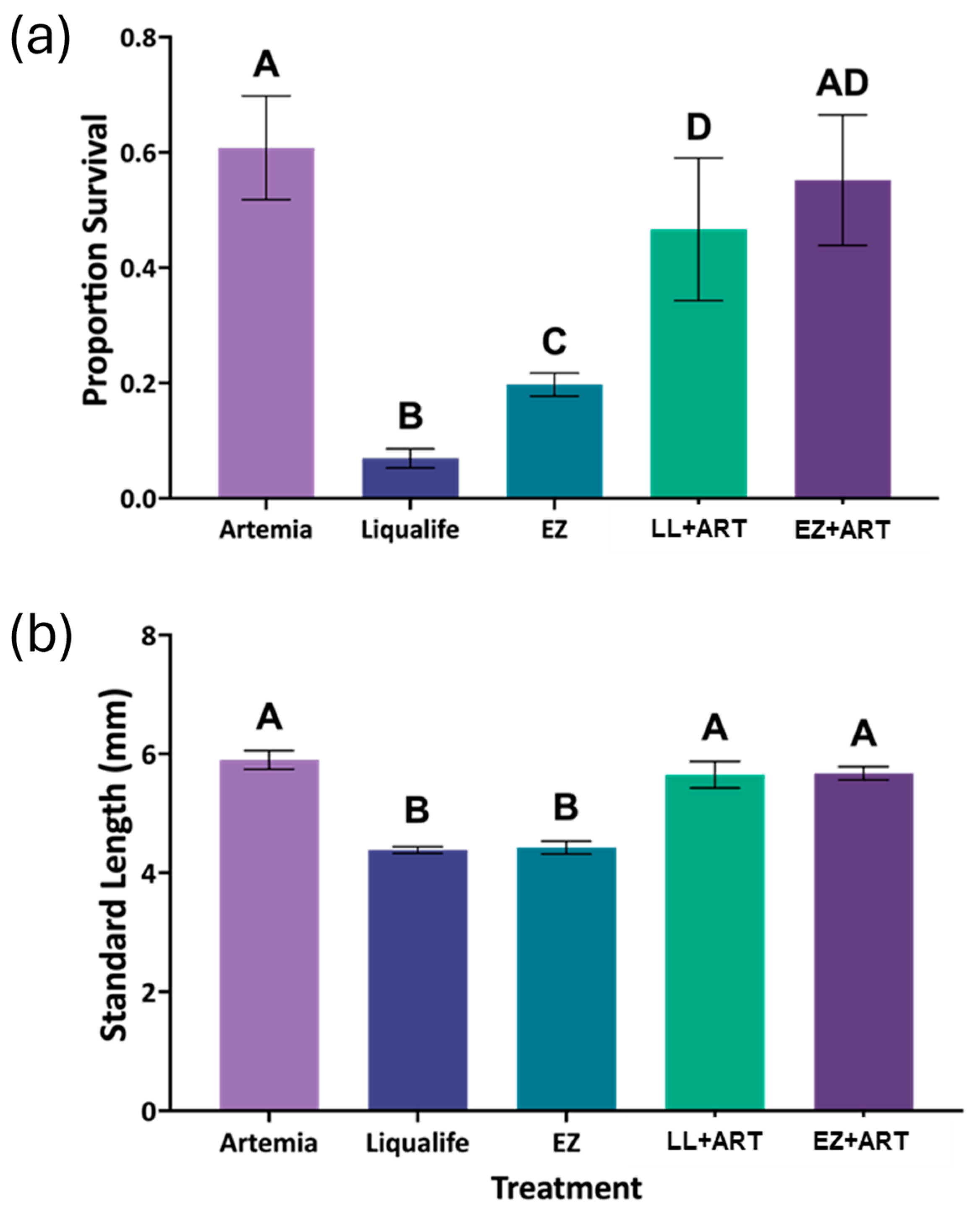
Such consistency in growth was not observed in the LA replacement trials, where suppression of SL and elevated variability indicated broader nutritional or behavioral limitations. Larvae fed exclusively on LL or EZ were 33–35% (approximately 1.4 mm) shorter at the endpoint than those fed live
Artemia, a biologically meaningful difference given a SL of approximately 6 mm at 26 DPH. These reductions may suggest poor ingestion cues, lack of nutrient bioavailability, or limited digestibility, all of which are critical during early larval development [
19,
34]. However, co-feeding strategies with live
Artemia resulted in growth outcomes comparable to the control, indicating that partial, but not full replacement, may offer a viable compromise. This is consistent with findings by Kolkovski et al. [
12], who reported improved survival in larval sea bream (
Sparus auratus) when visual cues from live prey were present during MD feeding, highlighting the role of sensory stimulation in promoting ingestion of inert diets. Increased ingestion alone may not be the sole component of mitigating survival and growth decreases in full vs. partial replacement comparisons. LA diets are composed of complex proteins, which require adequate digestive capability for assimilation. The benefits of co-feeding may also involve enzyme supplementation or improved feed recognition, not just ingestion frequency. However, without prior feed density experiments, it remains unclear whether larvae in the co-feeding treatments were ingesting both diet types or relying primarily on live
Artemia. Such feed density trials are warranted, as they could help define the minimum
Artemia input required to sustain growth while facilitating
Artemia reduction strategies in the aquaculture of
P. titteya.