1. Introduction
The ciliary marginal zone (CMZ) is a stem cell niche found in teleost fish and amphibians that generates retinal tissue as the eye and visual field grows, even in adulthood [
1,
2]. The CMZ is an annulus of cells found at the periphery of the retina. Retinal stem cells at the lateral edge of the annulus go through asymmetric division and generate a population of retinal progenitors that finally differentiate as a clonal wedge that includes all mature retinal cell types. CMZ-generated cells sequentially express neurogenic and neurodifferentiation genes implicated in central retinal development and other neural stem cell niches [
3,
4]. Regulation of the CMZ is still not fully understood, though its proximity to other eye structures, including the retinal pigmented epithelium (RPE), retinal vasculature, and lens, suggests coordinated growth by secreted proteins and/or cell–cellcell-cell contact.
While present in most clades of aquatic poikilothermic vertebrates, the CMZ has been lost in homeothermic terrestrial vertebrates [
2]. However, it has been established that the nonpigmented ciliary epithelium in mammals and birds does have neural stem cell potential [
4,
5,
6]. There has thus been an ongoing effort to understand the regulation of retinal stem cell populations in fish and amphibians to perhaps provide novel strategies for repairing retinal injury and treating degenerative retinal diseases in humans.
Forward genetics using zebrafish has been successfully used to uncover novel regulatory genes and pathways underlying several developmental processes [
7,
8,
9]. In forward genetics approaches, male zebrafish are exposed to a mutagen and are bred to normal females to generate F
1 fish that can be screened for dominant mutations. Outcrossing the F
1 fish generates F
2 mutant lines that can be screened for recessive mutations after sibling in-crosses. In a previous study, we used mutagenized zebrafish that were screened for recessive mutations affecting eye morphology and function [
10]. Similarly to a later, more extensive screen by another group [
11], we looked for small eyes that showed relatively normal central retinal histology but lacked a CMZ. In the present study, we characterize one of these eye mutants that we called “
no marginal zone” (
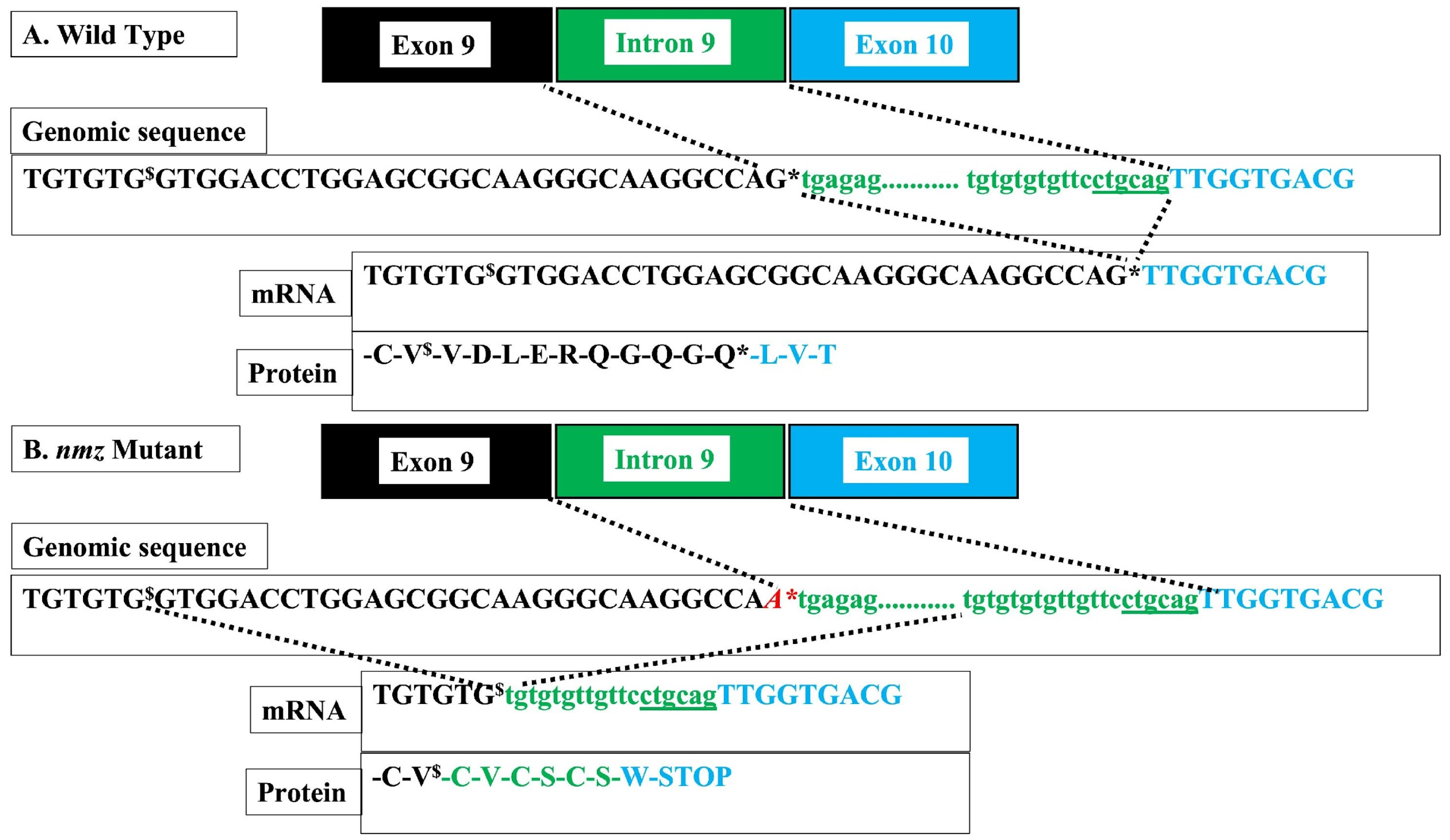
nmz). We mapped the mutation and identified the likely mutation underlying
nmz through genomic sequencing. The implicated gene codes for the damage-specific DNA binding protein-1 (ddb-1) were determined. Complementation analysis using a distinct ddb-1 mutant fish line,
m863, isolated in another laboratory [
12], was used to verify the same defective gene in
nmz.
The ddb-1 protein binds to damaged DNA and initiates repair [
13,
14]. The ddb-1 protein also complexes with Cullin 4 A and associated factors to form a ubiquitin ligase complex called CRL4. The CRL4 complex ubiquitinates several target proteins, marking them for proteasome degradation. In this way, ddb-1 plays a critical role regulating target protein stability and progression progress through the cell cycle. Among potential CRL4 targets is the Tp53 protein, a transcriptional activator of several genes that regulate cell cycle progression and apoptosis [
15,
16,
17]. Several cell stressors downregulate CRL4 activity, which coincides with increased Tp53 levels. The increased Tp53 triggers cell cycle arrest and/or cell death, depending on the developmental context [
12,
18,
19]. Similarly to what has been found by others [
12], our results suggest that ddb-1 also plays a role in CMZ neurogenesis. However, certain caveats of using forward genetics in zebrafish for this application necessitate caution in drawing any firm conclusions. Our study also provides additional support for the well-established model of CMZ neurogenesis and perhaps reveals certain nuances that add to this model.
2. Methods
2.1. Fish Husbandry and Mutagenesis
Zebrafish were maintained in the facility at the University of North Dakota according to standard Institutional Animal Care and Use Committee recommendations (IACUC2307-0047 and -48). Fish were raised and maintained in stand-alone fish racks (Aquatic Habitats, Pentair Aquatics, Apopka, FL, USA). The fish were fed twice daily and kept in a constant 14-h-on, 10-h off, light cycle. All behavioral and visual experiments were conducted in the main facility room, which was maintained at 28 °C, where the home tanks were kept.
The
nmz mutant was originally generated via an ethyl-nitroso-urea (ENU) mutagenesis and screening experiment at Harvard University, as described previously [
10]. The
nmz mutation was mapped to LG 18 on the MGH panel using simple sequence length polymorphism (SSLP) analysis, which has been described by others [
20,
21]. The
m863 mutant was a generous gift from the Driever Laboratory in Freiberg Germany [
12].
2.2. Morphological Analysis, Histology, Electron Microscopy (EM), BrdU Labeling and Immunohistochemistry
Zebrafish larvae were imaged and morphologically measured using a Leica dissecting microscope (M165FC), Leica camera (DFC310FX), and Leica LAS4.1.0 image analysis software suite (Leica Microsystems, Heerbrugg, Switzerland). Histology and electron microscopy (EM) were conducted as described previously [
22,
23]. Briefly, 5 dpf larvae were anesthetized in tricaine and placed in a primary fixative (1% paraformaldehyde and 4% glutaraldehyde dissolved in PBS with 3% sucrose and 0.15 mM CaCl
2). After rinsing, the fixed larvae were dehydrated in an ethanol series followed by propylene oxide and then embedded in Epon-Araldite resin (Electron Microscopy Sciences, Hatfield, PA, USA). For light microscope examination, 1 µm sections were cut on an ultramicrotome and stained with 1% methylene blue and 1% azure blue buffered in borax. Sections were visualized on a Leica compound microscope (DM500B), Leica camera (DFC480) and the Leica LAS3.7 image analysis software suite (Leica Microsystems, Heerbrugg, Switzerland). For EM, secondary fixation with 1% osmium tetroxide was used after primary fixation. After rinsing, 80 nm sections were cut on an ultramicrotome, mounted onto grids, and further treated with lead citrate and uranyl acetate. Sections were visualized on the Hitachi transmission EM in the Harvard University core facility. For BrdU labeling, 3 dpf larvae were incubated for 24 h in 10 mM BrdU. The fish were rinsed at 4 dpf and allowed to grow for another 24 h before fixation and immunolabeling with an anti-BrdU antibody (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and the 1D1 antibody that labels rod photoreceptors (a generous gift from Dr. James Fadool at Florida State University).
2.3. Genomic and Transcriptomic Sequencing and Bioinformatics
A single adult male fish heterozygous for nmz and a single male homozygous wild-type sibling were sacrificed, and the livers were removed. Genomic DNA was purified from the liver tissue using a DNeasy kit (Qiagen, Germantown, MD, USA). The purified DNA was then sent to Admera Health Biopharma Services (South Plainfield, NJ, USA) through the Genohub platform. Admera performed quality control using gDNA TapeStation (Agilent Technologies, Santa Clara, CA, USA), library preparation using a KAPA Hyper Prep kit (Roche Technologies, Basel, Switzerland), and Illumina sequencing, the latter resulting in 80 million bidirectional reads of 150 bp. Admera also performed bioinformatic analysis, including read mapping and identification of single-nucleotide polymorphisms (SNPs) that varied between the two fish using BWA-MEM (v.0.7.17) software. Analysis using GATK HaplotypeCaller (v4.2.3.0) software focused on impactful SNPs affecting coding sequences, start sites, stop sites, and splice sites, particularly those around the mapping location of nmz on linkage group 18. All analyses were performed using the Danio rerio GRCz11 reference genome.
Transcriptomic analysis was conducted comparing 5 dpf homozygous nmz larvae to homozygous wild-type larvae bred from homozygous wild-type siblings in the same background. Small-eye fish were combined from multiple crosses. Three distinct sets of crosses were used to generate biological replicates. Similarly, wild-type crosses were used to generate 3 biological replicates for analysis. Total RNA was purified using the RNeasy kit (Qiagen, Germantown, MD, USA). The RNA was shipped to the Next Generation Sequencing Core Facility at the Oklahoma Medical Research Foundation (Oklahoma City, OK, USA), through the Genohub platform. The Oklahoma team performed QC analysis using Kapa qPCR (Roche Technologies, Basel, Switzerland) and the Agilent Tape station (Agilent Technologies, Santa Clara, CA, USA), and prepared polyA-selected libraries using Illumina TruSeq RNA kits (LC Sciences, Houston, TX, USA). HiSeq Rapid Run Illumina (Illumina, San Diego, CA, USA) sequencing was performed and provided approximately 30 million 50 bp single reads per sample. The Oklahoma facility also mapped and annotated the results using the Danio rerio GRCz10 genome as the reference. The Epigenetic Core Facility at the University of North Dakota used the DESeq2 workflow to provide the list of differentially expressed (DE) genes included in this paper.
2.4. Cocaine-Induced Conditioned Place Preference (CPP) and Optokinetic Response (OKR)
We used CPP to assess the dopaminergic function of adult fish from the
nmz and
m863 background. The procedure was conducted as described previously [
24,
25]. Briefly, sibling fish 8–10 months of age were housed individually for the duration of a three-day test period. On day 1, the fish were transferred to a three-chambered tank and videoed for 10 min using a Top Scan camera array and imaging analysis software suite (CleverSys, Reston, VA, USA). Their preference for each of the three chambers was recorded. The fish were isolated and conditioned in the rear chamber with no drug for 30 min. The next-day baseline preference for all compartments was recorded for 10 min, followed by a second conditioning trial in the rear compartment with or without 10 mg/L cocaine. On the third day, the final preference for all compartments was recorded for untreated and cocaine-treated fish. CPP was determined by subtracting the baseline preference for the rear compartment measured on day 2 from the final preference on day 3.
OKR analysis of adult fish aged 8–10 months old was performed as described by others [
26,
27]. Fish were temporarily anesthetized in 150 µM tricaine and restrained in a sponge submerged in a clear beaker of fish water. A computer screen was set up such that the fish was at a 45° angle to the screen with the right eye 11 cm from the screen. A program was written in MatLab (version R2023b) to generate a sinusoidal wave grating of vertical black bars on a white background. The grating moved across the screen from the nasal to the temporal visual fields of the right eye. We used two different black bar widths, 2 mm and 8 mm, based on extensive trial and error, but the calculated spatial frequency for both was 0.87 cycles/deg. The grating moved at 0.8 cycles/sec with the illumination at 3500 lux. The goal was to measure the robustness of the response as a measure of visual acuity and perhaps, to a lesser degree, contrast sensitivity. Three 30 s videos were recorded for each fish. The first was a baseline video with no bands cycling across the visual field. The second video recorded the response to the thin bands (2 mm) cycling across the visual field. The third video captured the response to the thicker band (8 mm). Videos were scored by a minimum of three investigators blind to the fish and video trial. Investigators scored the number of track–saccade cycles during the video to measure the robustness of the response. The three observations for each fish and trial were divided by the video lengths and collated to obtain saccades/second.
After CPP and OKR testing, fish were sacrificed to collect brain RNA for future experiments and a tail clip to genotype individuals. Taqman assays were designed to genotype individual 5 dpf larvae and fin clips taken from adult fish (Life Technologies, reference AN9H6FX for nmz, and ANT2VDY for m863). Individual larvae were anesthetized in tricaine and then transferred to a proteinase K digestion mix (200 mM NaCl, 10 mM Tris, pH 8.0, 5 mM EDTA, 0.1% SDS and 200 µg/mL proteinase K). After 24 h, the samples were heat-inactivated and diluted. The Taqman assays were performed according to company specifications using a RealTime PCR thermocycler (Biorad, Hercules, CA, USA). To test the genotypes of hybrid larvae, as well as the specificity and efficacy of the Taqman assays, several samples collected were tested with both assays.
2.5. Statistical Analysis
GraphPad Prism (version.10.5.1 Bost, MA, USA) was used for statistical analysis in these experiments. Eye size and body length were measured from homozygous nmz, m863, and nmz/m863 hybrids. The mutant larvae were compared to wild-type siblings using type 2, two-tailed t-tests. Double-blind-assessed OKR scores (saccades/sec) for nmz and m863 adult fish were compared after genotyping, using one-way ANOVA with Bonferroni correction to compare all conditions. For CPP, the second baseline score of percent time spent in the back compartment after conditioning with no drug was compared to the final conditioning trial with or without drug. Fish were genotyped after CPP and OKR testing. As luck would have it, only three heterozygous fish from the m863 background were in the untreated CPP group. A minimum of 18 were compared for each of the other groups. Similarly, 6 fish were untreated wild type from the nmz background, but there was a minimum of 14 in the other groups. One-way ANOVA with Bonferroni’s correction was used to compare test groups from each background. OKR scores were compared for each background using repeated measures ANOVA with Bonferroni’s correction. A minimum of six fish were used for the m863 background and a minimum of eighteen were used for each group in the nmz background.
The DESeq2 tool was used to generate a table of DE genes. Base means were calculated along with the log base2 fold change in expression in
nmz mutant fish relative to wild-type fish. A standard deviation was calculated for the log base2 fold change. Dividing the change by the standard deviation generated a Wald test statistic (stat in
Table 1 and in
Supplementary Table S1). A standard
p-value and a
p-value adjusted for the false discovery rate using the Benjamini–Hochberg method (p-adj) were used. The stat numbers were negative if the gene was downregulated in
nmz and positive if it was upregulated in
nmz. Functional clustering was performed using the DAVID Bioinformatics website tool (final access date 1 August 2025 National Institutes of Health,
https://davidbioinformatics.nih.gov/) with default medium standards. We included all DE genes with an adjusted
p value lower than 1.0 × 10
−3, or 601 in total (listed in
Supplementary Table S1). The DAVID Bioinformatics website was able to assign 495 of these 601 genes for functional clustering.
4. Discussion
The Ddb-1 gene encodes a protein with central roles in regulating UV-damage repair of DNA and cell cycle progression [
13,
14]. The Ddb-1 protein complexes with Cullin 4A and its associated factors to form CRL4, which functions as an E3 ubiquitin ligase. There is evidence that CRL4 regulates activation of the Tp53, which in turn regulates cell cycle progression and apoptosis [
28,
29,
30]. Normally, Tp53 levels are low because the Mdm2-mediated ubiquitin ligase complex rapidly targets it for degradation [
16]. Cellular stress initiated by chemical or UV insult inhibits Mdm2 activity by a number of mechanisms and thus increases Tp53 stability. Since Tp53 is a transcription factor that autoregulates, the increased stability also increases Tp53 transcript levels. In turn, Tp53 regulates the expression of many target genes that can initiate cell cycle arrest and/or apoptosis. There is evidence that some forms of the CRL4 complex act similarly to Mdm2 and regulate Tp53 stability by targeting it for degradation [
9]. There is also evidence that CRL4 can regulate Mdm2 activity indirectly by controlling ribosome biosynthesis [
28,
29]. Certain ribosomal proteins bind to and inhibit the Mdm2 ubiquitination of Tp53, thus increasing its stability. This may explain the observation by many groups that loss of Ddb-1, and presumably, as a result, CRL4 function, leads to an increase in Tp53 expression, with wide-ranging developmental effects [
12,
18,
19].
Elimination of Ddb-1 in mice results in lethality by E12.5 [
18]. More selective deletion in the brain was still lethal and the investigators reported elimination of proliferating populations of neural progenitors along with evidence of extensive apoptosis [
18]. The effect of Ddb-1 elimination on these neural precursors was partially mitigated by suppression of Tp53. Similarly, fetal hematopoiesis was found to be dependent on Ddb-1 in mice [
19]. In that study, selective silencing of Ddb-1 led to a rise in the Tp53-mediated apoptosis of hematopoietic precursors. In the study characterizing the ddb-1 mutation in the
m863 zebrafish line, the investigators also reported a similar connection to tp53 signaling and hypothesized that this led to genomic instability, cell cycle arrest, and apoptosis in neural stem cell niches, including the CMZ, and dopaminergic systems of the brain [
12]. Our findings for
nmz are consistent with this connection of ddb-1 elimination with tp53-directed cell cycle disruption and apoptosis in the CMZ.
Regulation of cell cycle progression and apoptosis by Tp53 is very complex and involves activation of distinct sets of effectors (recently reviewed in [
16,
17]). Apoptosis is stimulated by Tp53 in two general pathways, called intrinsic and extrinsic. Tp53 drives the intrinsic pathway by increasing the expression of BH-3 proteins, including Puma and Noxa. These inhibit Bcl-2, thus activating the Bax/Bak cascade of protein interactions, which leads to caspase-mediated cell destruction. The extrinsic pathway involves the binding of ligands to so-called death receptors on the cell membrane. Ligand binding activates a signaling cascade and induces the formation of an intracellular death-inducing-signaling-complex (DISC). The DISC recruits Casp8, which then initiates a further cascade of caspase activation that leads to cell death. The central role of Tp53 in this process involves the increasing expression of death receptors like the Fas and Tnf-a receptors as well as intracellular components like Casp8. Tp53 stimulates the expression of other genes including Gadd45 and Cdk1 (p21), which can arrest the cell cycle at the G2/M stage and trigger apoptosis [
31,
32].
In the
nmz mutants, the absence of ddb-1 stimulates tp53 expression in a manner similar to that described in other studies, including the work with the
m863 zebrafish mutant [
12,
18,
19]. As reported by others, the loss of ddb-1 results in the appearance of apoptotic cells, as indicated by TUNEL assays in the zebrafish brain and eye, including the CMZ [
12]. We saw several tp53-regulated genes that were transcriptionally upregulated in
nmz, including gadd45a and cdkn1a (p21), tnfrsf (tnfa receptor), and casp8. Caution must be exercised here, because the tp53 pathway is also regulated extensively post-transcriptionally, but this combination of gadd45a and cdkn1a upregulation has been most closely associated with cell cycle arrest at the G2/M boundary, while tnfrsf and casp8 are linked to the extrinsic apoptotic pathway [
16,
17]. It is tempting to speculate that crosstalk between the two tp53-regulated processes results from the loss of ddb-1 in the CMZ of the
nmz mutants. However, this transcriptomic analysis was on whole embryos, and without further CMZ-specific validation of these tp53-regulated genes, strong conclusions must be viewed with caution.
Our additional observations described herein support previous work elegantly outlining the retinogenic process at the CMZ [
1,
3]. First, cells are generated at the lateral edge of the CMZ, where there is proximity to the future ciliary epithelium, RPE, circumferential vitreal blood vessels, and the lens. Initial expansion of the CMZ is relatively slow, involving asymmetric divisions of retinal stem cells at the very periphery. These are the rx2-positive stem cells described by others [
3]. We speculate that these make up the peripheral-most CMZ population of BrdU-positive cells in our pulse chase experiments (arrow 2 in
Figure 4A). Because of the variation in labeling patterns in the pulse-chase experiments (
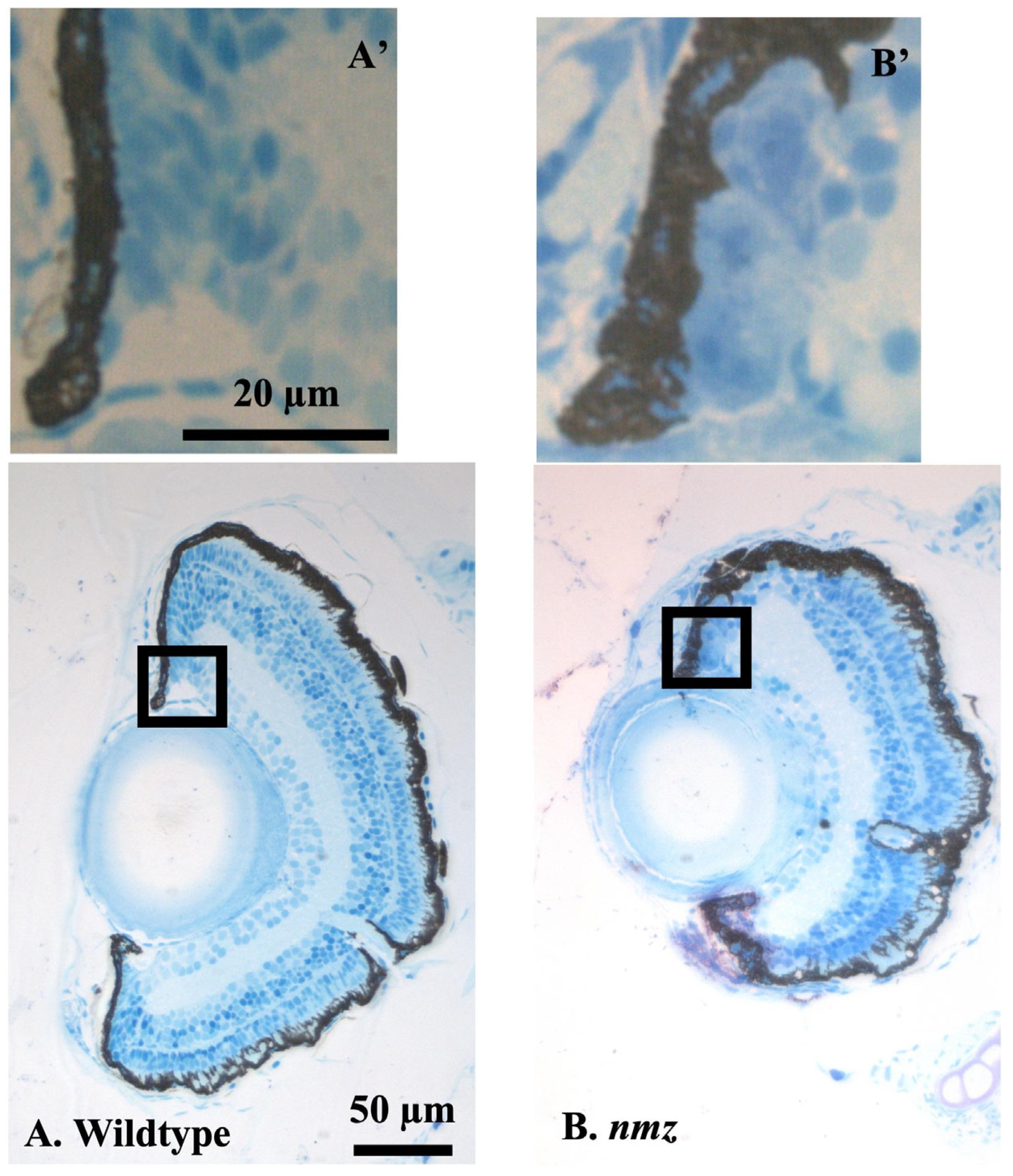
Supplementary Figure S2), we suspect that the labeling of those early stem cells was infrequent, but we also cannot fully exclude tnfaartifacts arising from the angle of sectioning. Looking at the finer histology and electron microscopy in the CMZ, we saw numerous examples where it appeared that the CMZ had retinal stem cells and/or progenitor cells, with elongated nuclei, that also had melanosomes (
Figure 3D and S1C). It is tempting to argue that CMZ retinal stem cells might result from an earlier asymmetric division of a pigmented precursor. It is worth noting that we have noticed an apparent increase in the number of pigmented cells in the CMZ, although we cannot rule out the smaller dimension of the
nmz eye, distorting the RPE and presumptive ciliary epithelium. In this way, the
nmz perhaps falls in the Class 1C mutants described in similar work by another laboratory that screened for CMZ mutants [
11].
Slow asymmetric division of the retina stem cells gives rise to a second, faster expansion of the retinal progenitor cells [
1,
3]. These cells express rx2 and pax6a, along with various delta-notch pairs. We suspect that these are the cells we see in the second more centrally located cell cluster after BrdU pulse-chase (1st arrow in
Figure 4A). The retinal progenitors form a wedge or arched stripe [
3] that eventually fully differentiates into all retinal cell types. It is tempting to speculate that these wedges comprise functionally linked circuits representing discrete expansions of the peripheral visual field as the eye grows.
Our data seems to suggest that the ddb-1 mutation affects the rapid secondary expansion, rather than the slow earlier expansion (
Figure 4B), but not necessarily. Others have shown that ddb-1 is ubiquitously expressed in the early zebrafish embryo but becomes more restricted later [
11]. The
nmz and
m863 phenotypes do not start becoming apparent until 3 dpf. The investigators who characterized the
m863 mutant demonstrated that ddb-1 was maternally expressed and argued that this maternal expression rescued any earlier phenotype [
11]. Screening the ddb-1 mutation phenotype probably reflects the tissues normally proliferating rapidly during the maternal to zygotic transition (MZT, [
33]). At the MZT, maternally derived ddb-1 transitions to that exclusively provided by the larval fish. In the mutant lines, cell proliferation and development are essentially arrested by 5 dpf, but structures developed before the transition at 3 dpf look relatively normal. This points to a central caveat of screening for stem cell mutants in this type of experiment. Maternal rescue can mask the early ubiquitous effects of housekeeping genes, making the mutation seem specific for a particular organ or tissue. Finally, we concede that since we conducted transcriptome analysis on whole larvae, rather than the isolated retina, we must view these conclusions with caution.
We tested fish heterozygous for the ddb-1 mutations to see if there were any subtle changes in dopaminergic and visual function. We used CPP to test dopaminergic function. The nmz and m863 lines showed different sensitivities to cocaine. The nmz line, which was insensitive to the drug, is highly inbred, much more so than the m863 line, which had physical traits of multiple zebrafish strains. During the nmz line’s history, they may have lost drug sensitivity randomly through inbreeding. In contrast, the m863 fish behaved normally in the CPP test. The basis for the difference in sensitivity between the two lines was not due to the ddb-1 mutation, as wild-type and heterozygous fish showed no difference in sensitivity.
OKR was used to assess visual function. Again, the mutant lines behaved differently. The nmz fish showed much more spontaneous eye movements, even without any grating stimulus. With higher scrutiny, the baseline eye movements did look less rhythmic than with the moving bars, but since we all scored the responses similarly, we left the results as is. We do not have an explanation except that perhaps the nmz fish do not see very well and the spontaneous eye movements are due to the resulting agitation in a novel situation. Several earlier experiments using a rotating drum with similar spatial and temporal frequencies also indicated poor OKR in the nmz background (unreported observations). Unfortunately, we did not know about the ddb-1 mutation during these earlier experiments and did not genotype the fish at that time. Poorer vision may also have played a role in the low CPP response since the secondary cues in the assay are mostly visual. In contrast, the m863 fish displayed a low background and a high response to the thin bars. We currently believe that the thicker bands require a higher spatial or temporal frequency to elicit a more robust OKR in fish with normal vision. Regardless of the differences between the two lines, the ddb-1 mutations were clearly not related to visual function, as both wild type and heterozygous fish in each background showed very similar responses.