The Age and Growth of One Population of Diaphus watasei (Jordan & Starks, 1904) in the South China Sea
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish Collection
2.2. Fish Biology
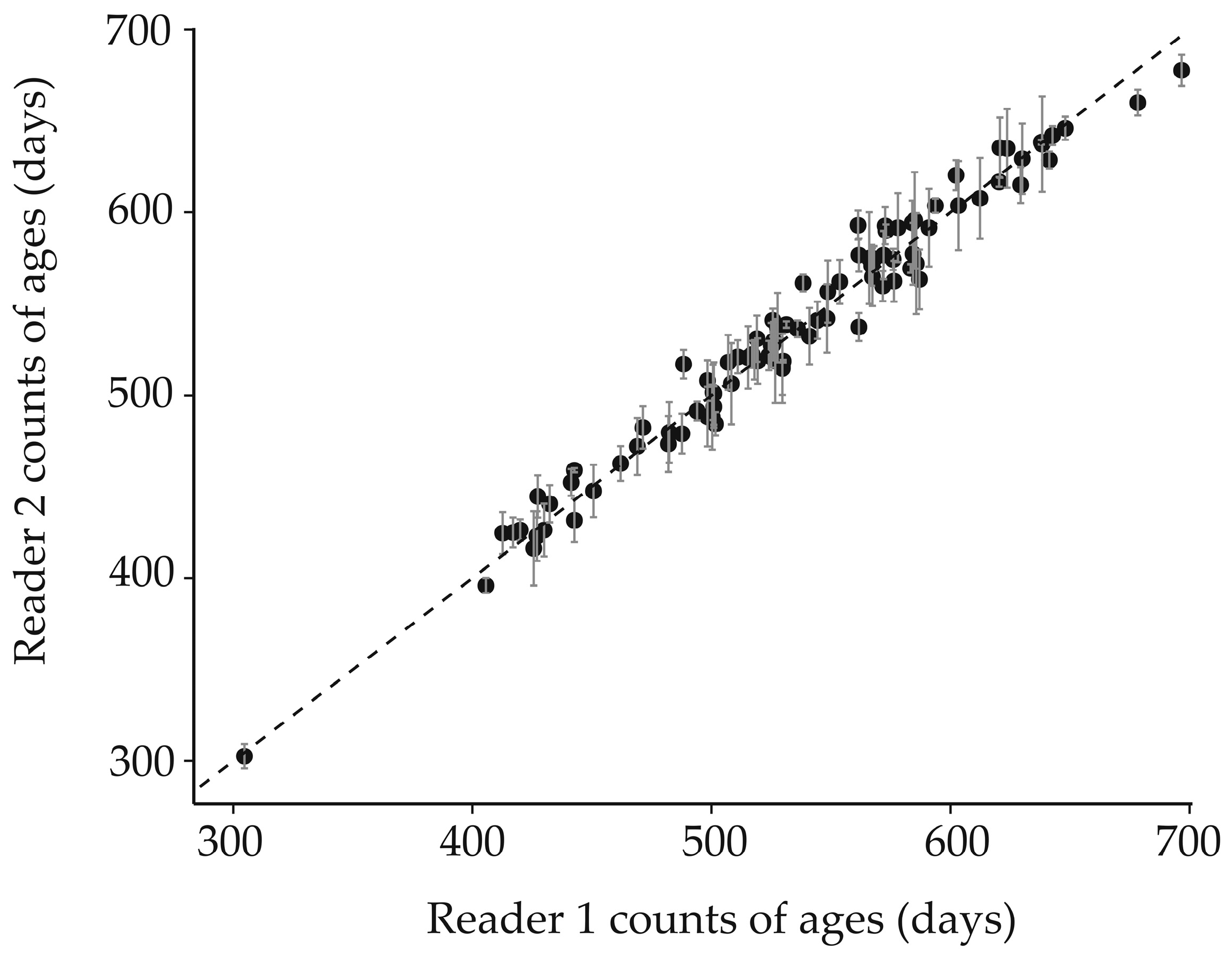
2.3. Otolith Acquisition and Age Estimation
2.4. Selection and Application of Our Model
3. Results
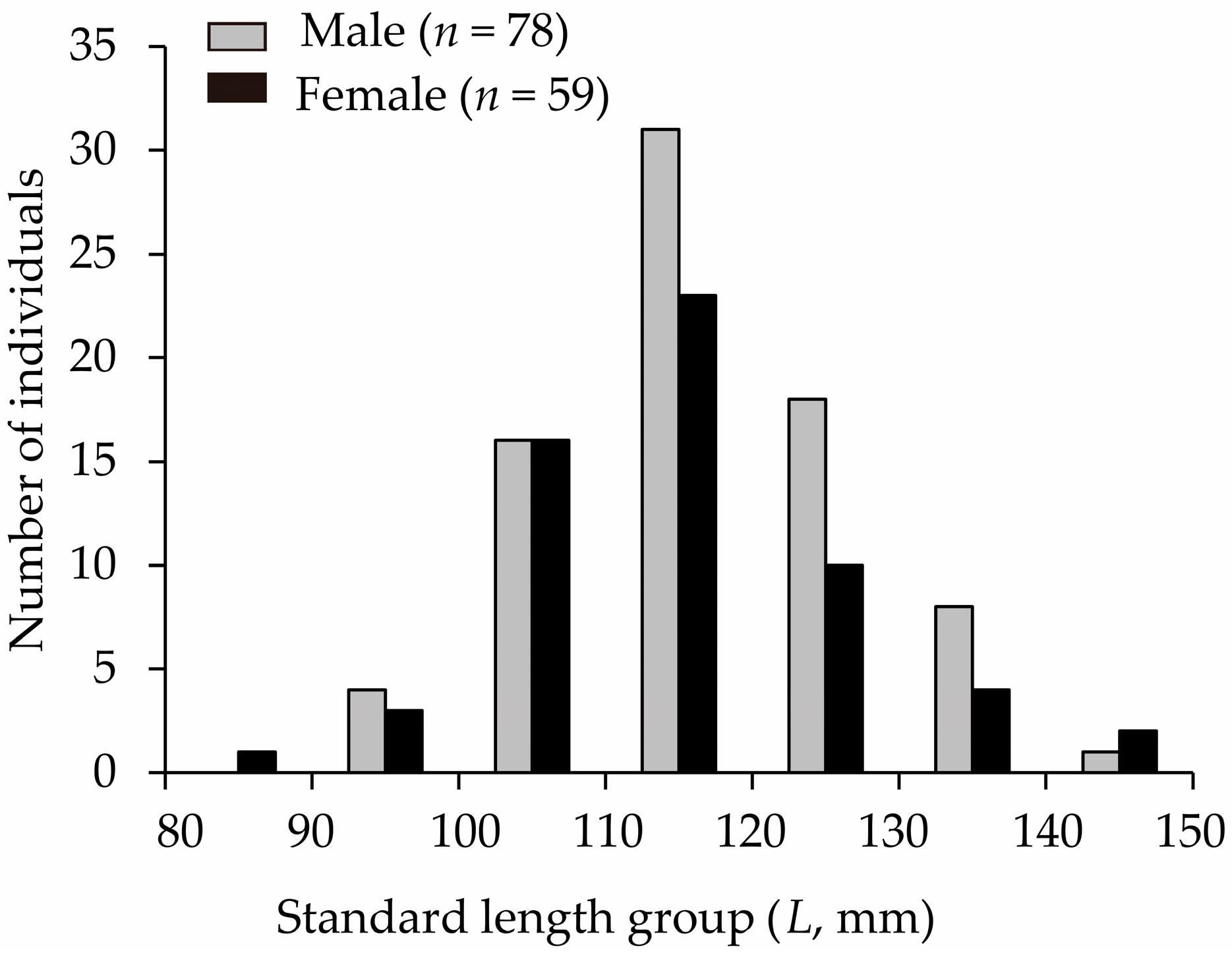
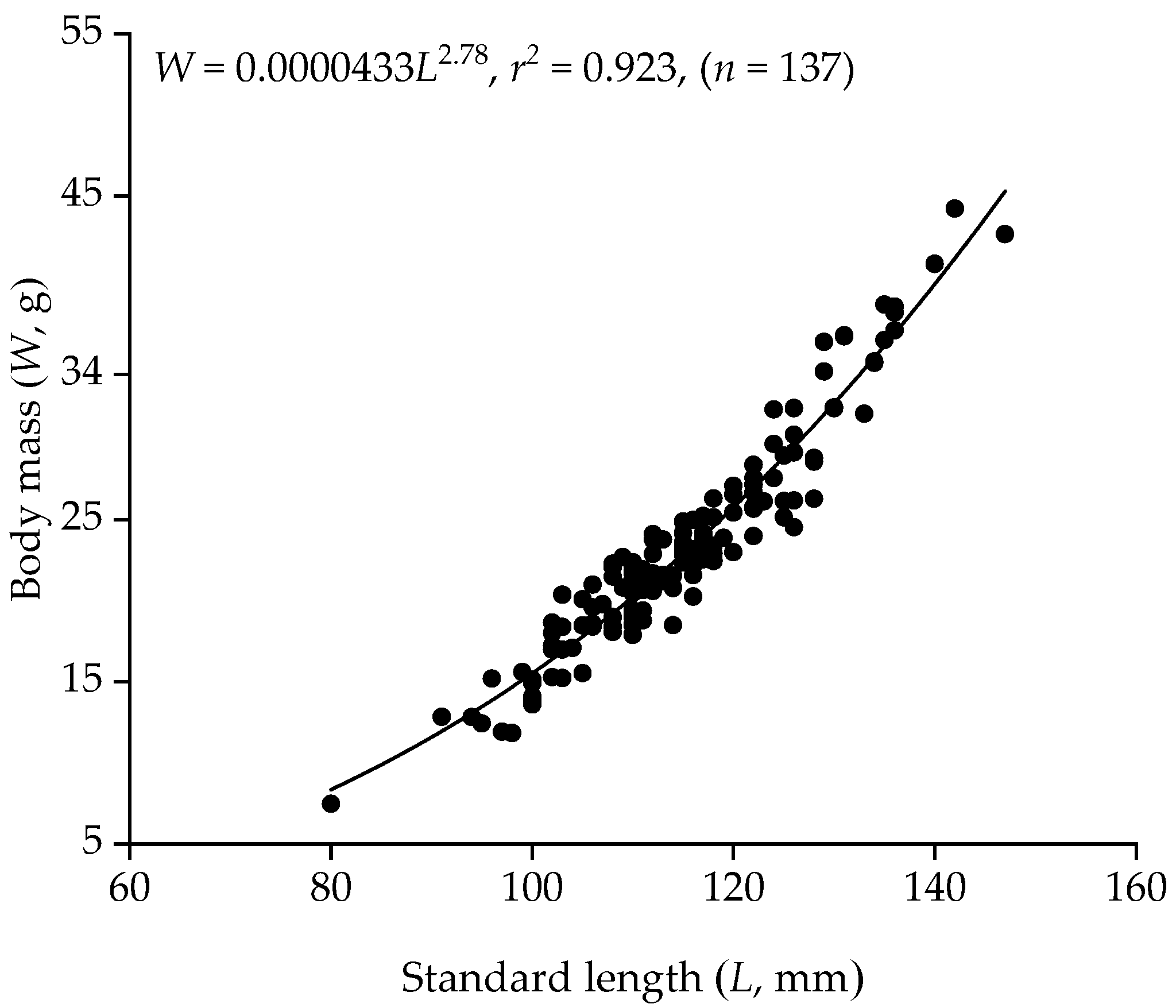
3.1. Body Size
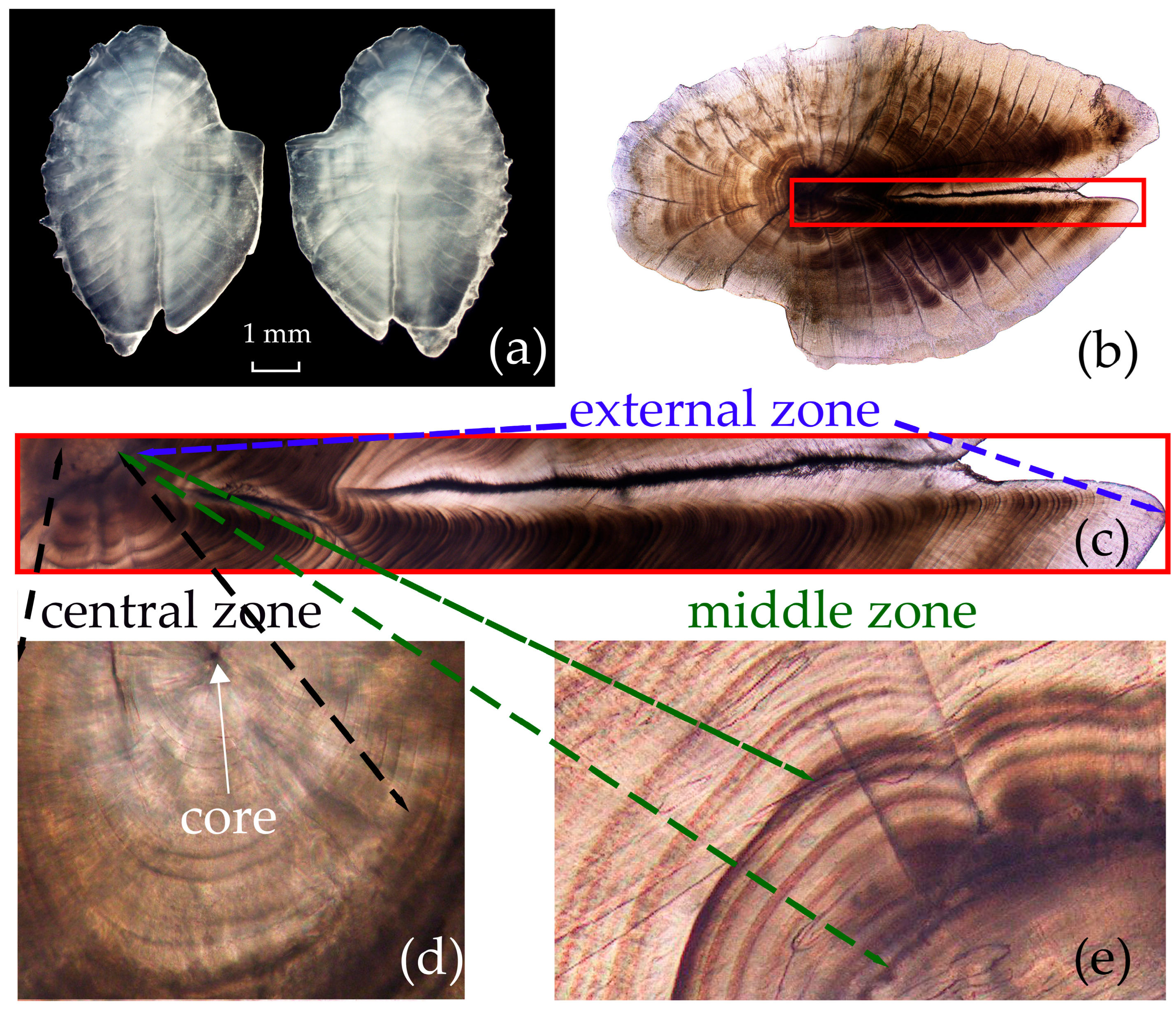
3.2. Otolith Microstructure
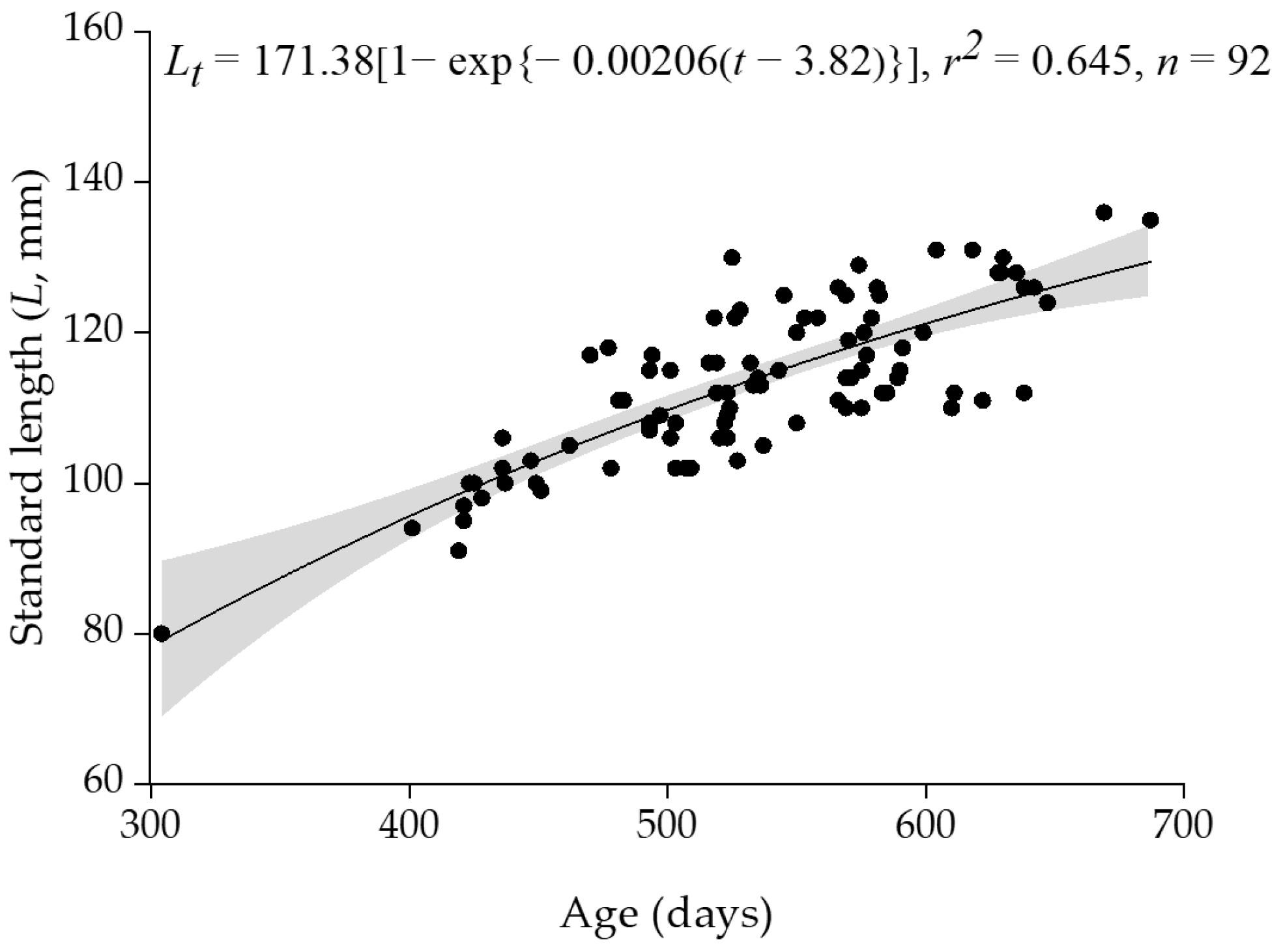
3.3. Age and Growth
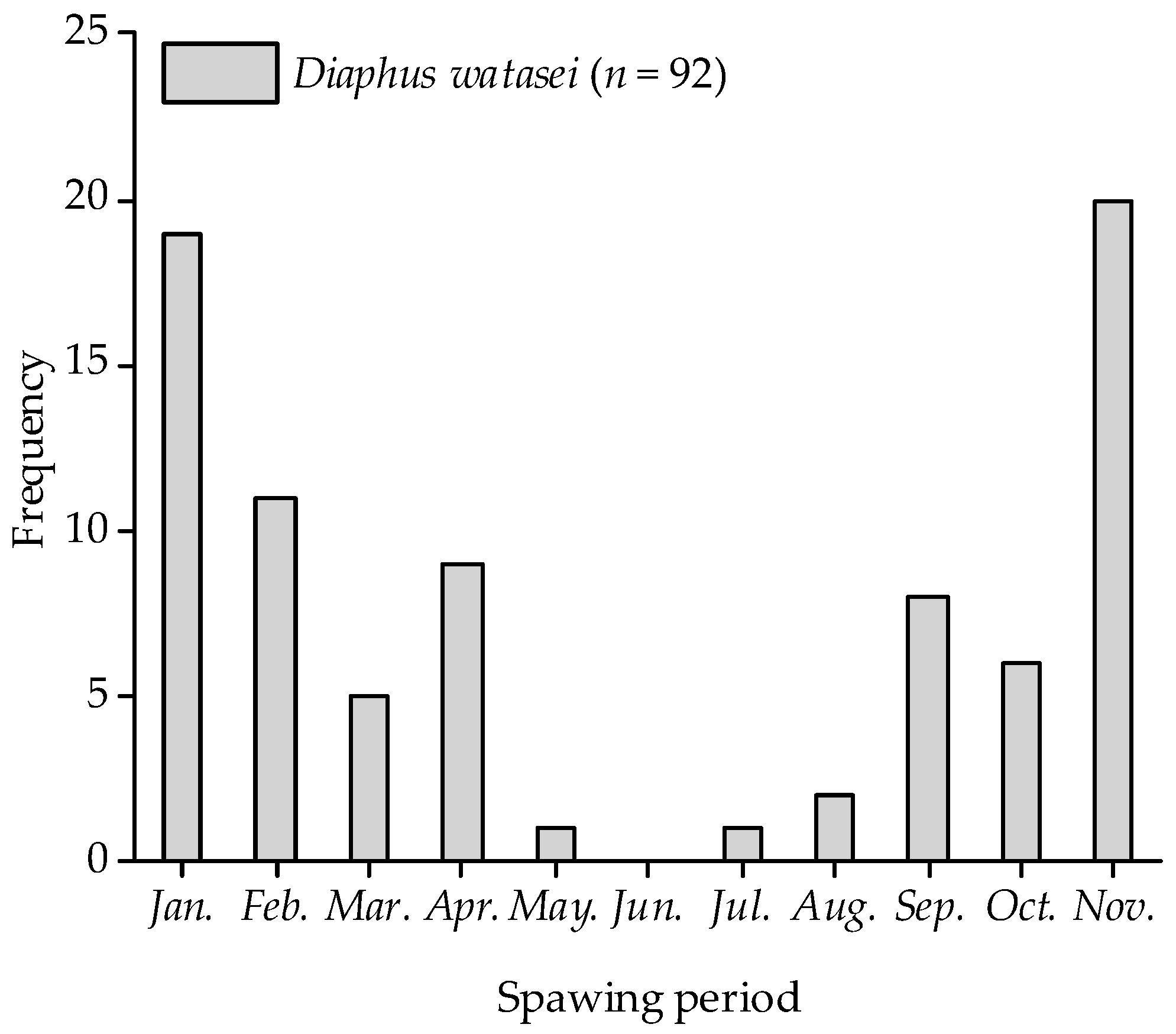
3.4. Spawning Period
4. Discussion
4.1. Sex Ratios
4.2. Biology and Growth
4.3. Predicted Spawning Time
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gjøsaeter, J.; Kawaguchi, K. A Review of the World Resources of Mesopelagic Fish. FAO Fisheries Technical Paper; Food and Agriculture Organization of the United Nations: Rome, Italy, 1980; Volume 193, pp. 1–151. Available online: https://books.google.ca/books?id=Zw0A_veImj8C&printsec=frontcover#v=onepage&q&f=false (accessed on 1 May 2024).
- Klevjer, T.A.; Irigoien, X.; Rostad, A.; Fraile-Nuez, E.; Benitez-Barrios, V.M.; Kaartvedt, S. Large Scale Patterns in Vertical Distribution and Behaviour of Mesopelagic Scattering Layers. Sci. Rep. 2016, 6, 19873. [Google Scholar] [CrossRef]
- Proud, R.; Handegard, N.O.; Kloser, R.J.; Cox, M.J.; Brierley, A.S. From Siphonophores to Deep Scattering Layers: Uncertainty Ranges for the Estimation of Global Mesopelagic Fish Biomass. ICES J. Mar. Sci. 2018, 76, 718–733. [Google Scholar] [CrossRef]
- Sobradillo, B.; Boyra, B.; Martinez, G.; Carrera, U.; Peña, P.; Irigoien, M. Target Strength and Swimbladder Morphology of Mueller’s Pearlside (Maurolicus muelleri). Sci. Rep. 2019, 9, 17311. [Google Scholar] [CrossRef]
- Caiger, P.; Lefebvre, E.; Llopiz, L.S.; Growth, J.K. Growth and Reproduction in Mesopelagic Fishes: A Literature Synthesis. ICES J. Mar. Sci. 2021, 78, 765–781. [Google Scholar] [CrossRef]
- Mcginnis, R.F. Biogeography of Lanternfishes (Myctophidae) South of 30° S. Antarct. Res. Ser. 1974, 35, 110. [Google Scholar] [CrossRef]
- Catul, V.; Gauns, M.; Karuppasamy, P.K. A Review on Mesopelagic Fishes Belonging to Family Myctophidae. Rev. Fish Biol. Fish. 2011, 21, 339–354. [Google Scholar] [CrossRef]
- Wilson, R.W.; Millero, F.J.; Taylor, J.R.; Walsh, P.J.; Christensen, V.; Jennings, S.; Grosell, M. Contribution of Fish to the Marine Inorganic Carbon Cycle. Science 2009, 323, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Schwarzhans, W. A Comparative Morphological Study of the Recent Otoliths of the Genera Diaphus, Idiolychnus and Lobianchia (Myctophidae). Palaeo Ichthyol. 2013, 13, 41–82. [Google Scholar] [CrossRef]
- Tian, H.; Jiang, Y.; Zhang, J.; Xu, S.; Chen, Z.; Zhu, J. Age and Growth of Diaphus brachycephalus in the South China Sea Using Sagittal Otolith Microstructure. Fishes 2022, 7, 169. [Google Scholar] [CrossRef]
- Prokofiev, A.M.; Emelyanova, O.R.; Orlov, A.M.; Orlova, S.Y. A New Species of Diaphus Associated with Seamounts of the Emperor Chain, North-Western Pacific Ocean (Teleostei: Myctophiformes: Myctophidae). J. Mar. Sci. Eng. 2022, 10, 65. [Google Scholar] [CrossRef]
- Nafpaktitis, B.G.; Robertson, D.A.; Paxton, J.R. Four New Species of the Lanternfish Genus Diaphus (Myctophidae) from the Indo-Pacific. N. Z. J. Mar. Freshw. Res. 1995, 29, 335–344. [Google Scholar] [CrossRef]
- Braga, A.C.; Costa, P.A.S.; Nunan, G.W. First Record of the Firebrow Lanternfish Diaphus adenomus (Myctophiformes: Myctophidae) from the South Atlantic. J. Fish Biol. 2008, 73, 296–301. [Google Scholar] [CrossRef]
- Gibbs, R.H.; Krueger, W.H. Biology of Midwater Fishes of the Bermuda Ocean Acre; Smithsonian Contributions to Zoology; Smithsonian Institution Press: Washington, DC, USA, 1987; Volume 452, pp. 1–187. [Google Scholar] [CrossRef]
- Sassa, C.; Tanaka, H.; Ohshimo, S. Comparative Reproductive Biology of Three Dominant Myctophids of the Genus Diaphus on the Slope Region of the East China Sea. Deep-Sea Res. Part I 2016, 115, 145–158. [Google Scholar] [CrossRef]
- Kawaguchi, K.; Shimizu, H. Taxonomy and Distribution of the Lanternfishes, Genus Diaphus (Pisces, Myctophidae) in the Western Pacific, Eastern Indian Oceans and the Southeast Asian Seas. Bull. Ocean. Res. Inst. 1978, 10, 1–145. [Google Scholar]
- Sebastine, M.; Bineesh, K.K.; Abdussamad, E.M.; Pillai, N.G.K. Myctophid Fishery along the Kerala Coast with Emphasis on Population Characteristics and Biology of the Headlight Fish, Diaphus watasei Jordan & Starks, 1904. Indian J. Fish. 2013, 60, 7–11. [Google Scholar]
- Zhang, C.; Guo, H. Age, Growth and Feeding Habit of Watase’s Lanternfish Diaphus watasei (Pisces: Myctophidae) in the East China Sea. Fish. Sci. 2024, 90, 555–564. [Google Scholar] [CrossRef]
- Vipin, P.M.; Pradeep, K.; Ravi, R.; Fernandez, J.T.; Remesan, M.P.; Madhu, V.; Boopendranath, M.R. First Estimate of the Length–Weight Relationship of Diaphus watasei Jordan and Starks, 1904 Caught off the Southwest Coast of India. Asian Fish. Sci. 2011, 24, 453–455. [Google Scholar] [CrossRef]
- Rajamoorthy, K.; Pradeep, K.; Anandan, R.; Libin, B.; Sankar, T.V.; Lakshmanan, P.T. Biochemical Composition of Myctophid Species Diaphus watasei and Myctophum obtusirostre Caught from Arabian Sea. Soc. Fish. Technol. 2013, 50, 41–44. [Google Scholar]
- Gong, Y.; Yang, Y.; Kong, X.; Zhang, J.; Jiang, Y.; Chen, Z.; Yan, L.; Zhang, K. Preliminary Study on the Fishery-Relevant Biology of Diaphus watasei in the Continental Slope of the Northern South China Sea. South China Fish. Sci. 2018, 25, 1091–1101. (In Chinese) [Google Scholar] [CrossRef]
- Lombardi-Carlson, L.A.; Andrews, A.H. Age Estimation and Lead-Radium Dating of Golden Tilefish, Lopholatilus chamaeleonticeps. Environ. Biol. Fishes 2015, 98, 1787–1801. [Google Scholar] [CrossRef]
- Carlström, D. A Crystallographic Study of Vertebrate Otoliths. Biol. Bull. 1963, 125, 441–463. [Google Scholar] [CrossRef]
- D’Iglio, C.; Natale, S.; Albano, M.; Savoca, S.; Famular, S.; Gervasi, C.; Lanteri, G.; Panarello, G.; Spano, N.; Capillo, G. Otolith Analyses Highlight Morpho-Functional Differences of Three Species of Mullet (Mugilidae) from Transitional Water. Sustainability 2022, 14, 398. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Chen, Z.; Jiang, Y.; Xu, S. Age and Growth of Ceratoscopelus warmingii (Myctophidae) in the South China Sea Based on Sagittal Otolith Microstructure. Mar. Biol. Res. 2022, 17, 733–743. [Google Scholar] [CrossRef]
- Gartner, J.V. Life Histories of Three Species of Lanternfishes (Pisces: Myctophidae) from the Eastern Gulf of Mexico. Mar. Biol. 1991, 111, 11–20. [Google Scholar] [CrossRef]
- Giragosov, V.; Ovcharov, O.P. Age and Growth of the Lantern Fish Myctophum nitidulum (Myctophidae) from the Tropical Atlantic. J. Appl. Ichthyol. 1992, 32, 34–42. [Google Scholar]
- Hosseini-Shekarabi, S.; Valinassab, P.; Bystydzienska, T.; Linkowski, Z. Age and Growth of Benthosema pterotum (Alcock, 1890) (Myctophidae) in the Oman Sea. J. Appl. Ichthyol. 2014, 31, 51–56. [Google Scholar] [CrossRef]
- Zenteno, J.I.; Bustos, C.A.; Landaeta, M.F. Larval Growth, Condition and Fluctuating Asymmetry in the Otoliths of a Mesopelagic Fish in an Area Influenced by a Large Patagonian Glacier. Mar. Biol. Res. 2014, 10, 504–514. [Google Scholar] [CrossRef]
- Campana, S.E.; Annand, M.C.; McMillan, J.I. Graphical and statistical methods for determining the consistency of age determinations. Trans. Am. Fish. Soc. 1995, 124, 131–138. [Google Scholar] [CrossRef]
- Froese, R. Cube Law, Condition Factor and Weight–Length Relationship: History, Meta-Analysis and Recommendations. J. Appl. Ichthyol. 2006, 22, 241–253. [Google Scholar] [CrossRef]
- Takagi, K.; Yatsu, A.; Moku, M.; Sassa, C. Age and Growth of Lanternfishes, Symbolophorus californiensis and Ceratoscopelus warmingii (Myctophidae), in the Kuroshio–Oyashio Transition Zone. Ichthyol. Res. 2006, 53, 281–289. [Google Scholar] [CrossRef]
- Hulley, P.A. Family Myctophidae. In Smiths’ Sea Fishes; Smith, M.M., Heemstra, P.C., Eds.; Macmillan: Johannesburg, South Africa, 1986; pp. 282–321. [Google Scholar]
- Baby, L.; Sankar, T.V.; Anandan, R. Comparison of Lipid Profile in Three Species of Myctophids from the South West Coast of Kerala, India. Natl. Acad. Sci. Lett. 2014, 37, 33–37. [Google Scholar] [CrossRef]
- López-Pérez, C.; Olivar, M.P.; Hulley, P.A.; Tuset, V.M. Length–Weight Relationships of Mesopelagic Fishes from the Equatorial and Tropical Atlantic Waters: Influence of Environment and Body Shape. J. Fish Biol. 2020, 96, 1388–1398. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, J.; Chen, Z.; Jiang, Y.; Xu, S.; Li, Z.; Wang, X.; Ying, Y.; Zhao, X.; Zhou, M. Age and Growth of Myctophum asperum in the South China Sea Based on Otolith Microstructure Analysis. Deep-Sea Res. Part II 2018, 167, 121–167. [Google Scholar] [CrossRef]
- Karnella, C. Family Myctophidae, Lanternfishes. In Biology of Midwater Fishes of Bermuda Ocean Acre; Gibbs, R.H., Krueger, W.H., Eds.; Smithsonian Contributions to Zoology: Washington, DC, USA, 1987; Volume 452, pp. 51–168. [Google Scholar]
- Badcock, J.; Araújo, T.M.H. On the Significance of Variation in a Warm Water Cosmopolitan Species, Nominally Ceratoscopelus warmingii (Pisces, Myctophidae). Bull. Mar. Sci. 1988, 42, 16–43. [Google Scholar]
- Badcock, J.; Merrett, N.R. Midwater Fishes in the Eastern North Atlantic—I. Vertical Distribution and Associated Biology in 30° N, 23° W, with Developmental Notes on Certain Myctophids. Prog. Oceanogr. 1976, 7, 3–58. [Google Scholar] [CrossRef]
- Olivar, M.P.; Bernal, A.; Molí, B.; Peña, M.; Balbín, R.; Castellón, A.; Miquel, J.; Massutí, E. Vertical Distribution, Diversity and Assemblages of Mesopelagic Fishes in the Western Mediterranean. Deep-Sea Res. Part I 2012, 62, 53. [Google Scholar] [CrossRef]
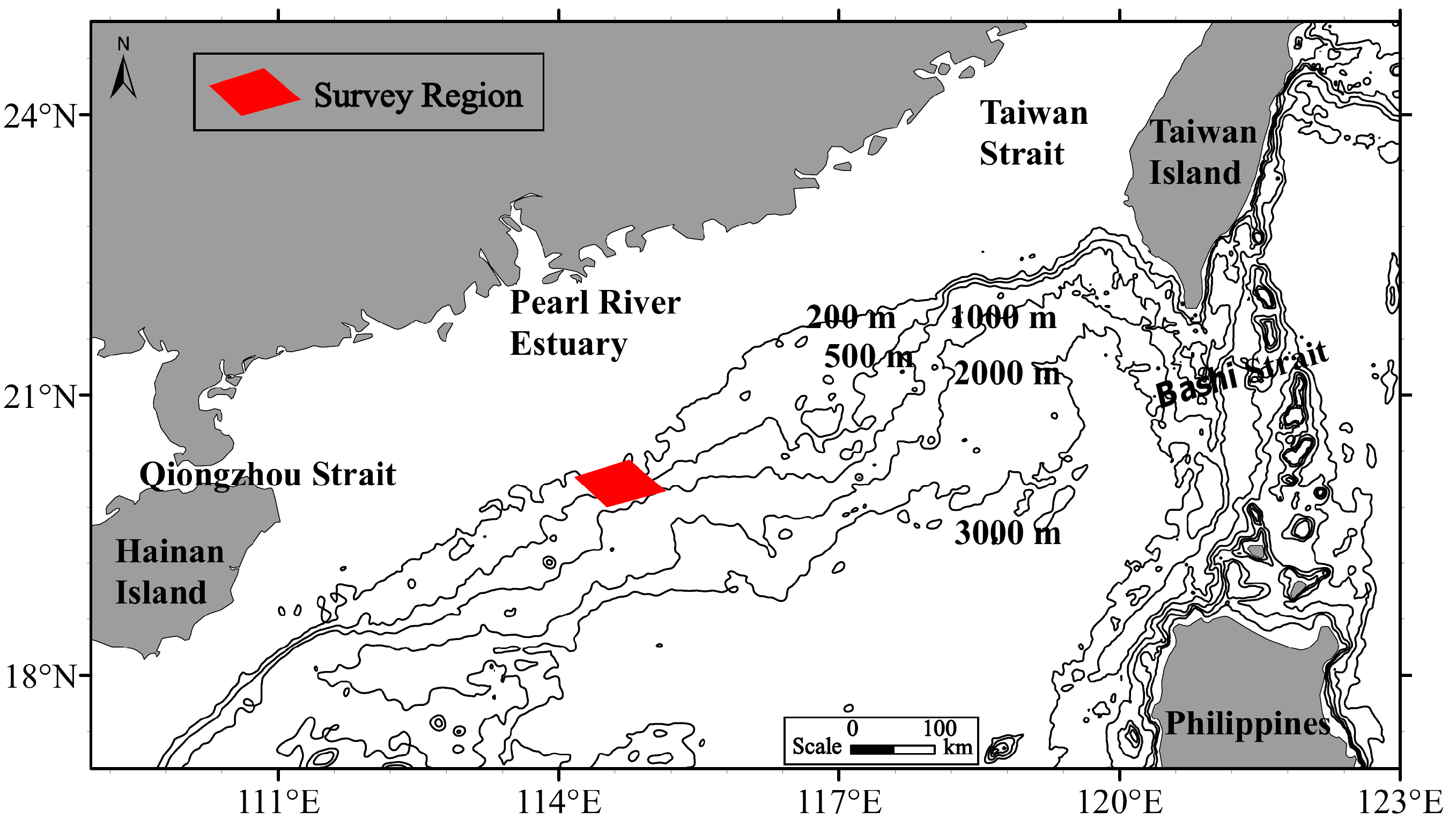
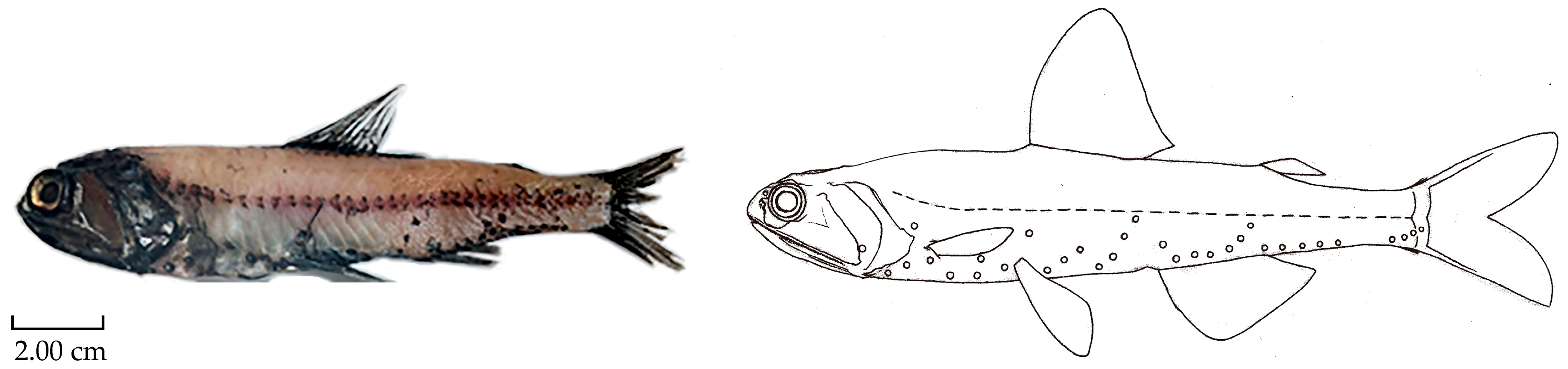

| Sample | D/M/Y | Starting Location | Finishing Location | Trawl Depth | Towing Speed |
|---|---|---|---|---|---|
| Time | Lat. (N)/Long. (E) | Lat. (N)/Long. (E) | (m) | (m/s) | |
| 04:20–05:20 | 17/06/2015 | 19°45.16′/114°08.11′ | 19°33.54′/113°58.92′ | 487–490 | 2.0 |
| 10:46–11:46 | 16/06/2015 | 19°42.30′/114°20.24′ | 19°31.11′/113°53.45′ | 550–560 | 1.9 |
| 20:50–21:50 | 17/06/2015 | 19°53.38′/114°40.75′ | 19°52.15′/114°68.58′ | 465–470 | 1.9 |
| Sex | Number | Standard Length Range (mm) | Mean Standard Length (±SD; mm) | Body Mass Range (g) | Mean Body Mass (±SD; g) |
|---|---|---|---|---|---|
| Male | 78 | 91–140 | 116 (±11) | 11.85–40.80 | 23.37 (±6.25) |
| Female | 59 | 80–147 | 114 (±12) | 7.47–44.22 | 23.49 (±7.20) |
| All | 137 | 80–147 | 115 (±11) | 7.47–44.22 | 23.42 (±6.65) |
| Otolith Microstructure | Daily Growth Increments Counting Range | Mean Value (±SD) |
|---|---|---|
| Central zone | 20–37 | 26.8 (±3.8) |
| Middle zone | 8–14 | 11.2 (±1.5) |
| External zone | 273–657 | 496.8 (±68.8) |
| Total | 304–687 | 534.5 (±68.9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Tian, H.; Jiang, Y.; Xu, S.; Zhu, J.; Zhang, J.; Zhang, J.; Chen, Z. The Age and Growth of One Population of Diaphus watasei (Jordan & Starks, 1904) in the South China Sea. Fishes 2025, 10, 538. https://doi.org/10.3390/fishes10110538
Zhang K, Tian H, Jiang Y, Xu S, Zhu J, Zhang J, Zhang J, Chen Z. The Age and Growth of One Population of Diaphus watasei (Jordan & Starks, 1904) in the South China Sea. Fishes. 2025; 10(11):538. https://doi.org/10.3390/fishes10110538
Chicago/Turabian StyleZhang, Kui, Han Tian, Yan’e Jiang, Shannan Xu, Jiangfeng Zhu, Junyi Zhang, Jun Zhang, and Zuozhi Chen. 2025. "The Age and Growth of One Population of Diaphus watasei (Jordan & Starks, 1904) in the South China Sea" Fishes 10, no. 11: 538. https://doi.org/10.3390/fishes10110538
APA StyleZhang, K., Tian, H., Jiang, Y., Xu, S., Zhu, J., Zhang, J., Zhang, J., & Chen, Z. (2025). The Age and Growth of One Population of Diaphus watasei (Jordan & Starks, 1904) in the South China Sea. Fishes, 10(11), 538. https://doi.org/10.3390/fishes10110538









