Histopathological and Molecular Insights into Grass Carp Kidney Responses to Co-Infection with Aeromonas hydrophila and Aeromonas veronii
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Bacteria
2.2. Co-Infection and Sampling
2.3. Tissue Bacterial Loading Assay
2.4. Histopathological Analysis
2.5. Transcriptome Analysis of Kidney Tissues
2.6. Statistical Analysis
3. Results
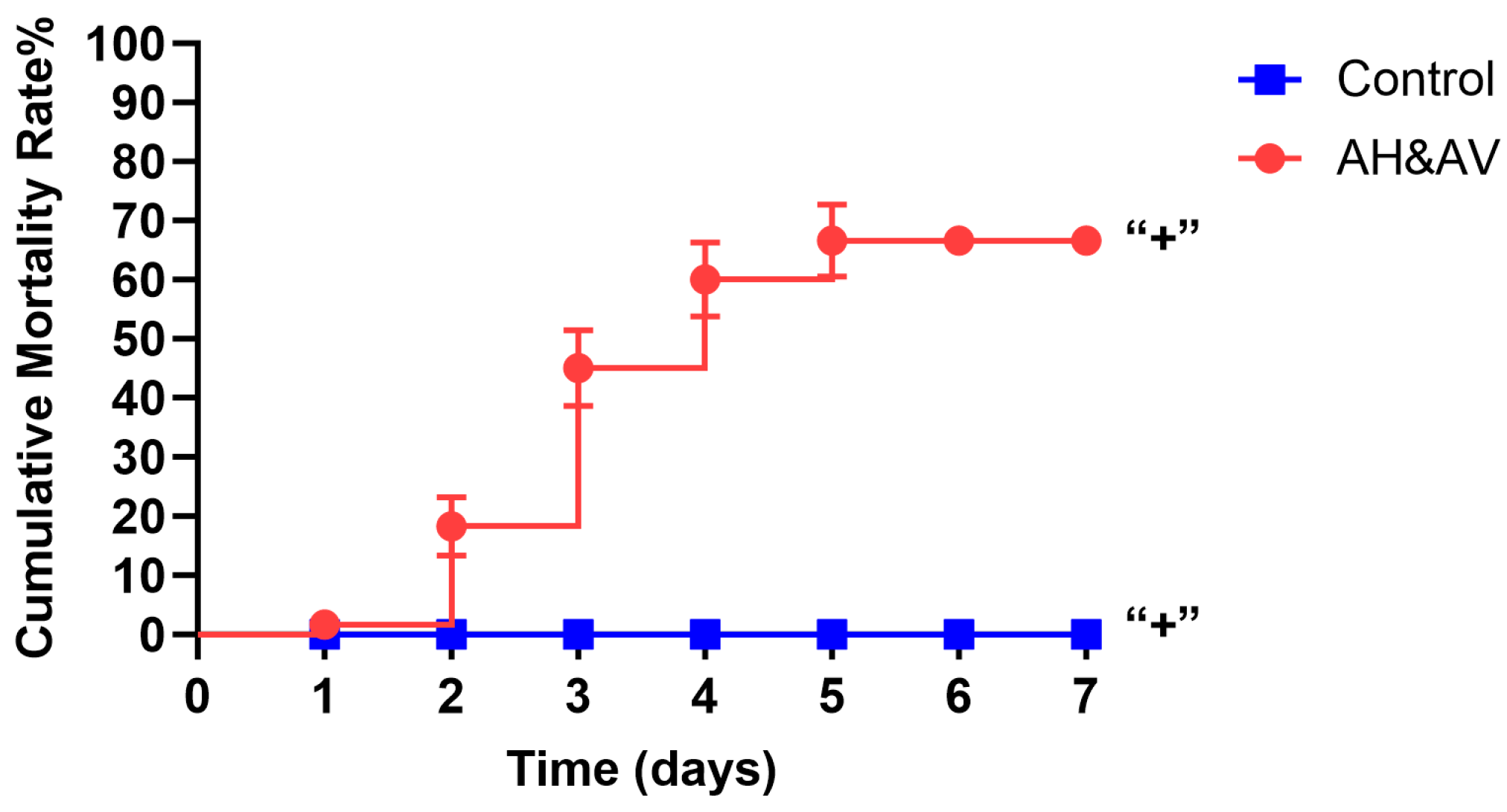
3.1. Cumulative Mortality Percent
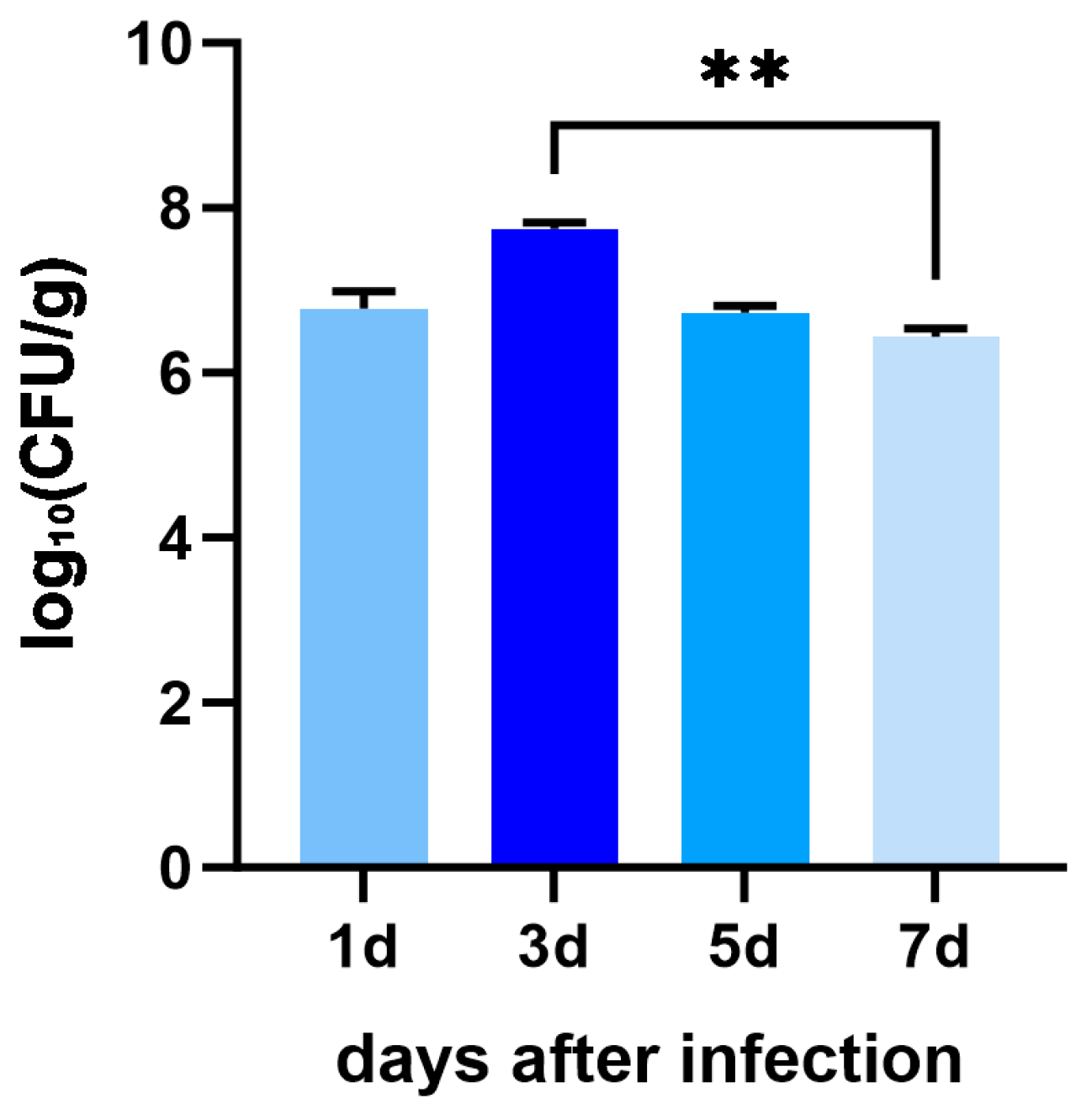
3.2. Bacterial Load
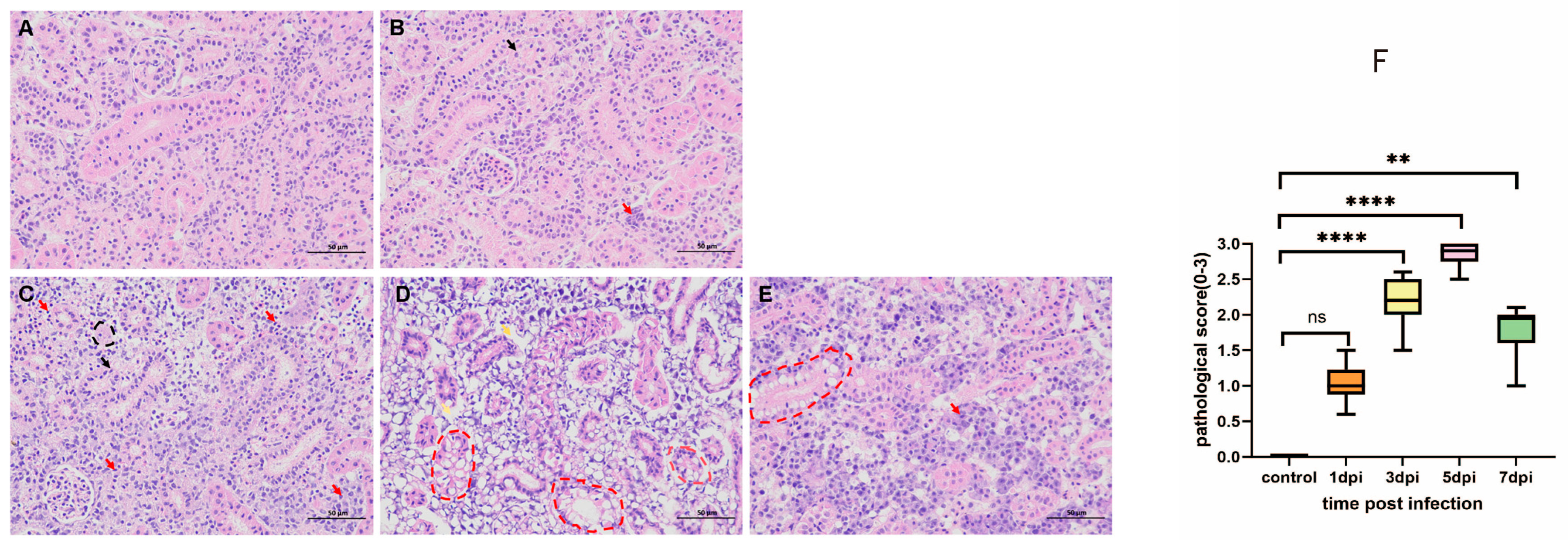
3.3. Kidney Histopathology
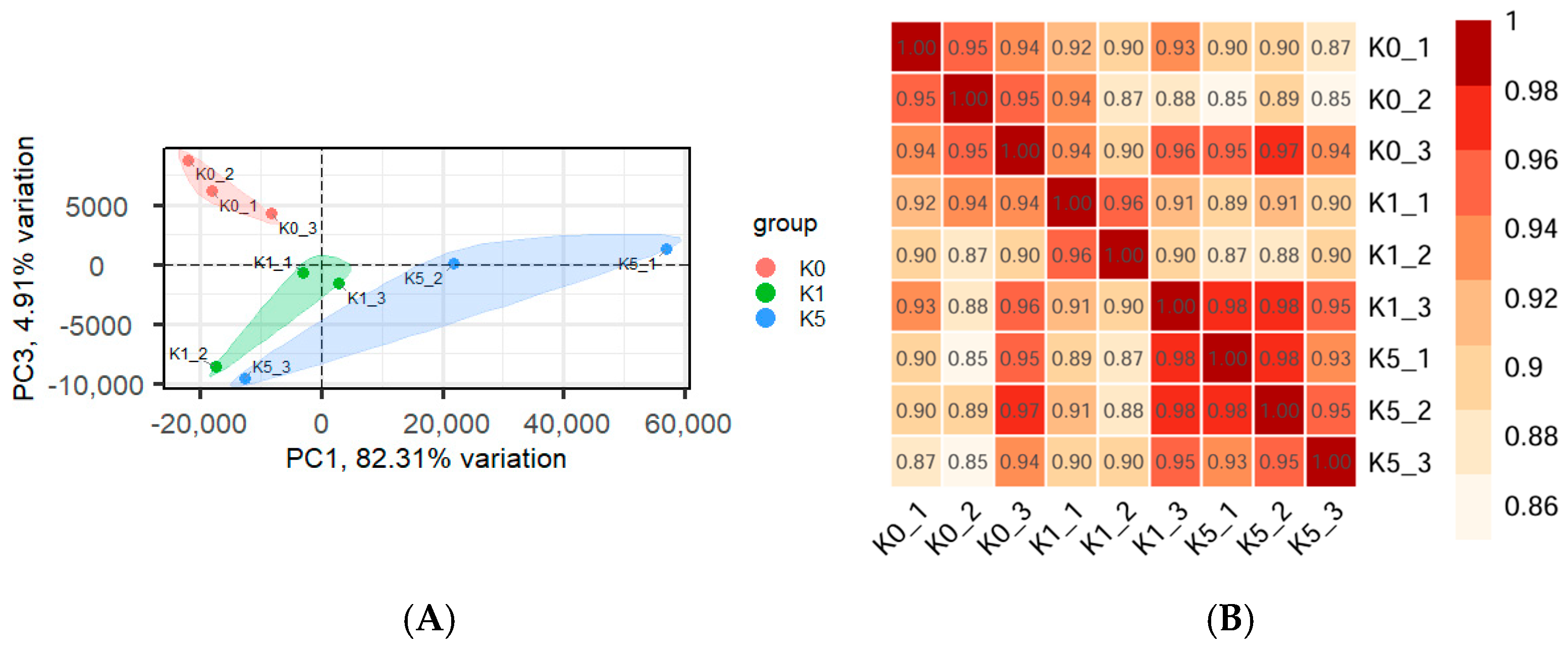
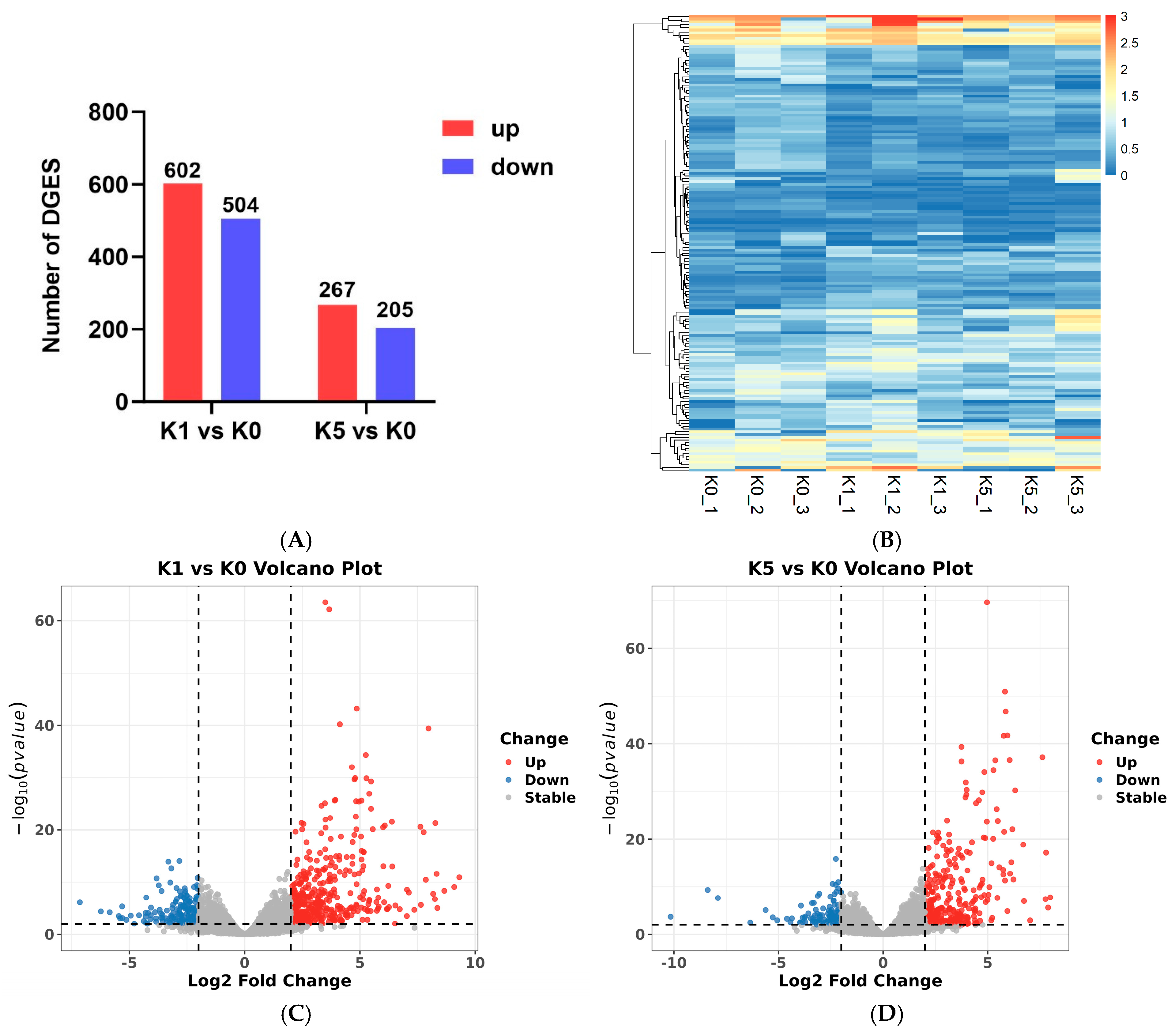
3.4. Transcriptome
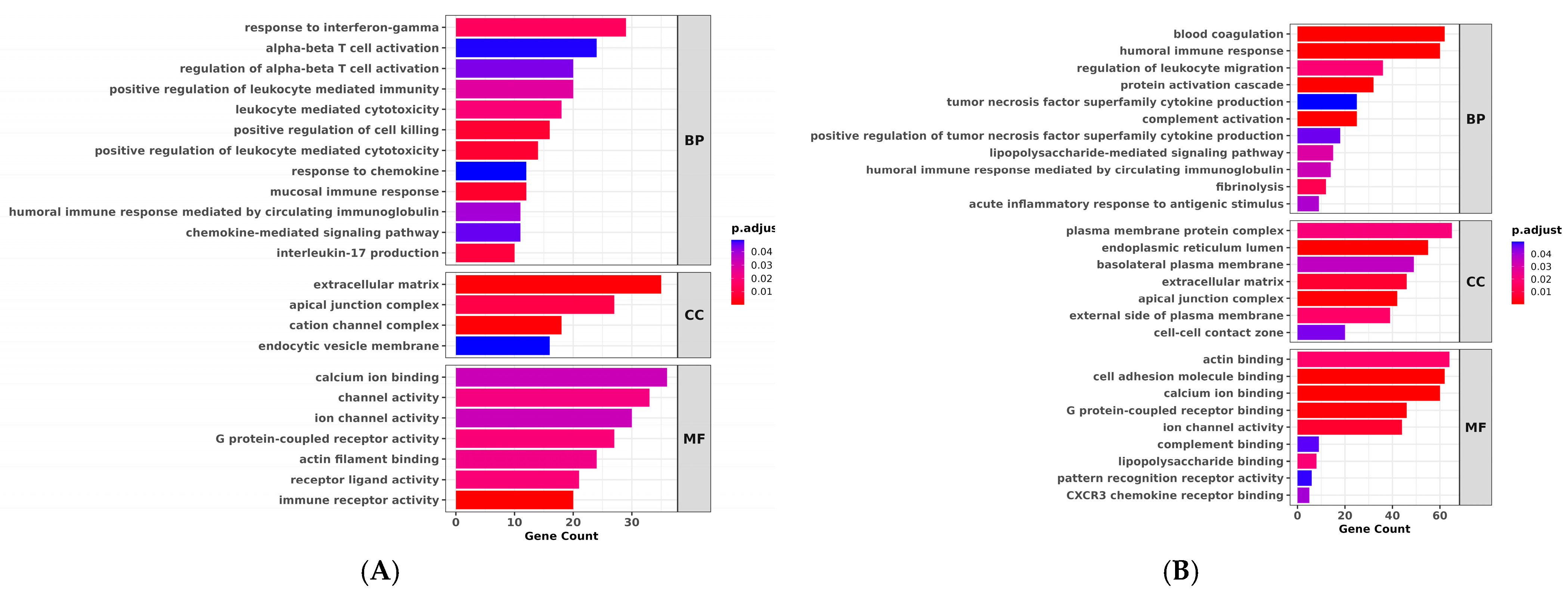
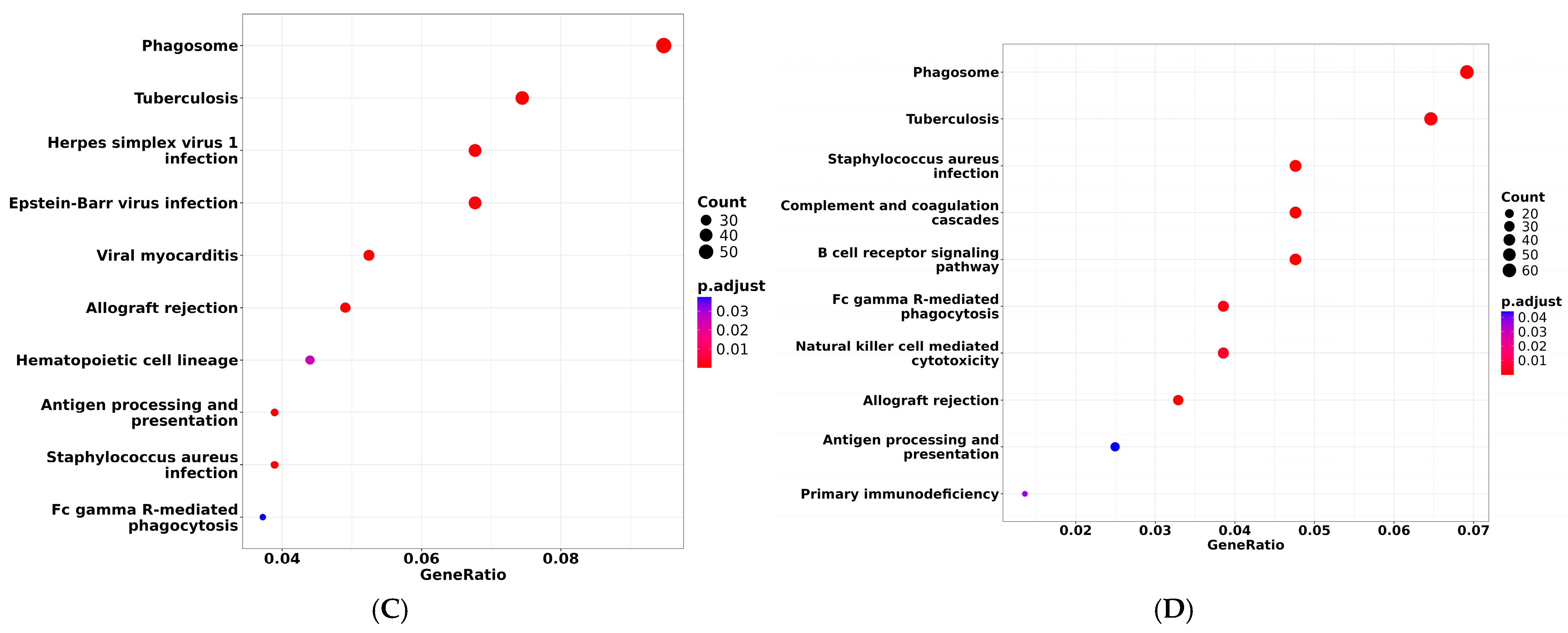
3.5. DEGs and Enrichment Analysis
3.6. Protein–Protein Interaction Networks
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, X.-G.; Shen, H.-Y.; Gu, Z.-j.; Cheng, G.; Wang, J.; Zhu, H. The Environmental Impact and Development Direction of Grass Carp, Ctenopharyngodon Idella, Aquaculture. J. World Aquac. Soc. 2023, 54, 1354–1366. [Google Scholar] [CrossRef]
- Xie, C.; Li, J.; Li, D.; Shen, Y.; Gao, Y.; Zhang, Z. Grass Carp: The Fish That Feeds Half of China. In Aquaculture in China; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 93–115. ISBN 978-1-119-12075-9. [Google Scholar]
- Siamudaala, V.; Basiita, R.K.; Hang’ombe, B.M.; Delamare-Deboutteville, J.; Kakwasha, K.; Chimatiro, S.K.; Yabe, J.; Ndashe, K.; Chadag, M.V.; Siamudaala, V. Emerging and Re-Emerging Fish Diseases and Pathogens in an Environment of the Expanding Aquaculture in Zambia. Preprints 2025, 2025040354. [Google Scholar] [CrossRef]
- Dayana Senthamarai, M.; Rajan, M.R.; Bharathi, P.V. Current Risks of Microbial Infections in Fish and Their Prevention Methods: A Review. Microb. Pathog. 2023, 185, 106400. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Rasmussen-Ivey, C.R.; Moen, F.S.; Fernández-Bravo, A.; Lamy, B.; Beaz-Hidalgo, R.; Khan, C.D.; Castro Escarpulli, G.; Yasin, I.S.M.; Figueras, M.J.; et al. A Global Survey of Hypervirulent Aeromonas Hydrophila (vAh) Identified vAh Strains in the Lower Mekong River Basin and Diverse Opportunistic Pathogens from Farmed Fish and Other Environmental Sources. Microbiol. Spectr. 2023, 11, e03705–e03722. [Google Scholar] [CrossRef]
- Behera, B.K.; Parida, S.N.; Kumar, V.; Swain, H.S.; Parida, P.K.; Bisai, K.; Dhar, S.; Das, B.K. Aeromonas Veronii Is a Lethal Pathogen Isolated from Gut of Infected Labeo Rohita: Molecular Insight to Understand the Bacterial Virulence and Its Induced Host Immunity. Pathogens 2023, 12, 598. [Google Scholar] [CrossRef]
- Tyagi, A.; Sharma, C.; Srivastava, A.; Naveen Kumar, B.T.; Pathak, D.; Rai, S. Isolation, Characterization and Complete Genome Sequencing of Fish Pathogenic Aeromonas veronii from Diseased Labeo rohita. Aquaculture 2022, 553, 738085. [Google Scholar] [CrossRef]
- Nhinh, D.T.; Le, D.V.; Van, K.V.; Huong Giang, N.T.; Dang, L.T.; Hoai, T.D. Prevalence, Virulence Gene Distribution and Alarming the Multidrug Resistance of Aeromonas Hydrophila Associated with Disease Outbreaks in Freshwater Aquaculture. Antibiotics 2021, 10, 532. [Google Scholar] [CrossRef]
- Liu, W.; Li, M.; Xue, M.; Zhou, Y.; Jiang, N.; Meng, Y.; Liu, Y.; Jiang, J.; Liao, X.; Fan, Y. Identification of Aeromonas Veronii as the Pathogen Associated with Massive Mortality in Bronze Gudgeon (Coreius heterodon). Animals 2024, 14, 2440. [Google Scholar] [CrossRef]
- Abdella, B.; Shokrak, N.M.; Abozahra, N.A.; Elshamy, Y.M.; Kadira, H.I.; Mohamed, R.A. Aquaculture and Aeromonas Hydrophila: A Complex Interplay of Environmental Factors and Virulence. Aquacult Int. 2024, 32, 7671–7681. [Google Scholar] [CrossRef]
- Okon, E.M.; Okocha, R.C.; Taiwo, A.B.; Michael, F.B.; Bolanle, A.M. Dynamics of Co-Infection in Fish: A Review of Pathogen-Host Interaction and Clinical Outcome. Fish Shellfish Immunol. Rep. 2023, 4, 100096. [Google Scholar] [CrossRef] [PubMed]
- Kotob, M.H.; Gorgoglione, B.; Kumar, G.; Abdelzaher, M.; Saleh, M.; El-Matbouli, M. The impact of Tetracapsuloides bryosalmonae and Myxobolus cerebralis co-infections on pathology in rainbow trout. Parasites Vectors 2017, 10, 442. [Google Scholar] [CrossRef]
- Chandrarathna, H.P.S.U.; Nikapitiya, C.; Dananjaya, S.H.S.; Wijerathne, C.U.B.; Wimalasena, S.H.M.P.; Kwun, H.J.; Heo, G.-J.; Lee, J.; De Zoysa, M. Outcome of Co-Infection with Opportunistic and Multidrug Resistant Aeromonas hydrophila and A. Veronii in Zebrafish: Identification, Characterization, Pathogenicity and Immune Responses. Fish Shellfish Immunol. 2018, 80, 573–581. [Google Scholar] [CrossRef]
- Erfanmanesh, A.; Beikzadeh, B.; Mohseni, F.A.; Nikaein, D.; Mohajerfar, T. Ulcerative Dermatitis in Barramundi Due to Coinfection with Streptococcus Iniae and Shewanella Algae. Dis. Aquat. Org. 2019, 134, 89–97. [Google Scholar] [CrossRef]
- Long, A.; Garver, K.A.; Jones, S.R.M. Synergistic Osmoregulatory Dysfunction during Salmon Lice (Lepeophtheirus salmonis) and Infectious Hematopoietic Necrosis Virus Co-Infection in Sockeye Salmon (Oncorhynchus nerka) Smolts. J. Fish Dis. 2019, 42, 869–882. [Google Scholar] [CrossRef] [PubMed]
- Crumlish, M.; Thanh, P.C.; Koesling, J.; Tung, V.T.; Gravningen, K. Experimental Challenge Studies in Vietnamese Catfish, Pangasianodon hypophthalmus (Sauvage), Exposed to Edwardsiella ictaluri and Aeromonas hydrophila. J. Fish Dis. 2010, 33, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Dinçtürk, E.; Tanrıkul, T.T. Yersinia Ruckeri and Pseudomonas Fluorescens Co-Infection in Rainbow Trout (Oncorhynchus Mykiss Walbaum, 1792). Aquac. Res. 2021, 52, 4858–4866. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, X.; Li, J. Microbial Load, Serum Enzyme Activity and Histopathological Alterations Induced by Aeromonas Hydrophilain in Experimentally Challenged Grass Carp. 2022. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4187626 (accessed on 25 August 2025).
- Lv, W.; Zhou, Z.; Xie, L.; Wang, X.; Zhou, Y.; Gui, L.; Xu, X.; Shen, Y.; Li, J.; Qiu, J. Pathological and Molecular Characterization of Grass Carp Co-Infected with Two Aeromonas Species. Animals 2025, 15, 263. [Google Scholar] [CrossRef]
- Ayala-Soldado, N.; Mora-Medina, R.; Molina-López, A.M.; Lora-Benítez, A.J.; Moyano-Salvago, R. Evaluation of the Effectiveness of Eugenol and MS-222 as Anesthetics in Zebrafish in Repeated Exposures and Post-Anesthesia Behaviour. Animals 2024, 14, 2418. [Google Scholar] [CrossRef]
- Vila, J.; Ruiz, J.; Gallardo, F.; Vargas, M.; Soler, L.; Figueras, M.J.; Gascon, J. Aeromonas Spp. and Traveler’s Diarrhea: Clinical Features and Antimicrobial Resistance. Emerg. Infect. Dis. 2003, 9, 552–555. [Google Scholar] [CrossRef]
- Slaoui, M.; Bauchet, A.-L.; Fiette, L. Tissue Sampling and Processing for Histopathology Evaluation. In Drug Safety Evaluation: Methods and Protocols; Gautier, J.-C., Ed.; Springer: New York, NY, 2017; pp. 101–114. ISBN 978-1-4939-7172-5. [Google Scholar]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An Ultra-Fast All-in-One FASTQ Preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A Fast Spliced Aligner with Low Memory Requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A Universal Enrichment Tool for Interpreting Omics Data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]
- Otasek, D.; Morris, J.H.; Bouças, J.; Pico, A.R.; Demchak, B. Cytoscape Automation: Empowering Workflow-Based Network Analysis. Genome Biol. 2019, 20, 185. [Google Scholar] [CrossRef]
- Li, R.; Hao, Y.; Shen, Y.; Gui, L.; Lv, W.; Yuan, L.; Du, B.; Xie, L.; Li, J.; Xu, X. Impact of Cadmium and Diclofenac Exposure on Biochemical Responses, Transcriptome, Gut Microflora, and Growth Performance in Grass Carp (Ctenopharyngodon Idella). Chemosphere 2024, 360, 142428. [Google Scholar] [CrossRef]
- Kotob, M.H.; Menanteau-Ledouble, S.; Kumar, G.; Abdelzaher, M.; El-Matbouli, M. The Impact of Co-Infections on Fish: A Review. Vet. Res. 2016, 47, 98. [Google Scholar] [CrossRef] [PubMed]
- Gorgoglione, B.; Bailey, C.; Fast, M.D.; Bass, D.; Saraiva, M.; Adamek, M.; Noguera, P.; Ciulli, S.; Palikova, M.; Aguirre-Gil, I.; et al. Co-Infections and Multiple Stressors in Fish. Bull. Eur. Assoc. Fish. Pathol. 2020, 40, 4–19. [Google Scholar]
- Banu, A.N.H.; Islam, M.A.; Chowdhury, M.B.R. Bacterial Load in Pond Water and Different Organs of a Indian Major Carp Cirrhinus Mrigala Ham. Bangladesh J. Fish. Res. 2001, 5, 53–58. Available online: http://hdl.handle.net/1834/33169 (accessed on 22 June 2025).
- Thornton, L.M.; LeSueur, M.C.; Yost, A.T.; Stephens, D.A.; Oris, J.T.; Sellin Jeffries, M.K. Characterization of Basic Immune Function Parameters in the Fathead Minnow (Pimephales promelas), a Common Model in Environmental Toxicity Testing. Fish Shellfish Immunol. 2017, 61, 163–172. [Google Scholar] [CrossRef]
- Sarkar, M.J.A.; Rashid, M.M. Pathogenicity of the Bacterial Isolate Aeromonas Hydrophila to Catfishes, Carps and Perch. J. Bangladesh Agric. Univ. 2012, 10, 157–161. [Google Scholar] [CrossRef]
- Shi, F.; Lu, Z.; Yang, M.; Li, F.; Zhan, F.; Zhao, L.; Li, Y.; Li, Q.; Li, J.; Li, J.; et al. Astragalus Polysaccharides Mediate the Immune Response and Intestinal Microbiota in Grass Carp (Ctenopharyngodon idellus). Aquaculture 2021, 534, 736205. [Google Scholar] [CrossRef]
- Hal, A.M.; El-Barbary, M.I. Gene Expression and Histopathological Changes of Nile Tilapia (Oreochromis niloticus) Infected with Aeromonas hydrophila and Pseudomonas fluorescens. Aquaculture 2020, 526, 735392. [Google Scholar] [CrossRef]
- Perico, L.; Benigni, A.; Casiraghi, F.; Ng, L.F.P.; Renia, L.; Remuzzi, G. Immunity, Endothelial Injury and Complement-Induced Coagulopathy in COVID-19. Nat. Rev. Nephrol. 2021, 17, 46–64. [Google Scholar] [CrossRef]
- Satyam, A.; Graef, E.R.; Lapchak, P.H.; Tsokos, M.G.; Dalle Lucca, J.J.; Tsokos, G.C. Complement and Coagulation Cascades in Trauma. Acute Med. Surg. 2019, 6, 329–335. [Google Scholar] [CrossRef]
- Morgan, B.P. The Complement System:An Overview. In Complement Methods and Protocols; Morgan, B.P., Ed.; Humana Press: Totowa, NJ, USA, 2000; pp. 1–13. ISBN 978-1-59259-056-8. [Google Scholar]
- Manthey, H.D.; Woodruff, T.M.; Taylor, S.M.; Monk, P.N. Complement Component 5a (C5a). Int. J. Biochem. Cell Biol. 2009, 41, 2114–2117. [Google Scholar] [CrossRef] [PubMed]
- West, E.E.; Kemper, C. Complosome—The Intracellular Complement System. Nat. Rev. Nephrol. 2023, 19, 426–439. [Google Scholar] [CrossRef]
- Lasorsa, F.; Rutigliano, M.; Milella, M.; Ferro, M.; Pandolfo, S.D.; Crocetto, F.; Simone, S.; Gesualdo, L.; Battaglia, M.; Ditonno, P.; et al. Complement System and the Kidney: Its Role in Renal Diseases, Kidney Transplantation and Renal Cell Carcinoma. Int. J. Mol. Sci. 2023, 24, 16515. [Google Scholar] [CrossRef]
- Nandi, G. The Complement System. In An Interplay of Cellular and Molecular Components of Immunology; CRC Press: Boca Raton, Fl, USA, 2022; ISBN 978-1-003-28642-4. [Google Scholar]
- Hoppe, C.; Gregory-Ksander, M. The Role of Complement Dysregulation in Glaucoma. Int. J. Mol. Sci. 2024, 25, 2307. [Google Scholar] [CrossRef] [PubMed]
- Mastellos, D.C.; Hajishengallis, G.; Lambris, J.D. A Guide to Complement Biology, Pathology and Therapeutic Opportunity. Nat. Rev. Immunol. 2024, 24, 118–141. [Google Scholar] [CrossRef]
- Noris, M.; Galbusera, M. The Complement Alternative Pathway and Hemostasis. Immunol. Rev. 2023, 313, 139–161. [Google Scholar] [CrossRef] [PubMed]
- Bayly-Jones, C.; Bubeck, D.; Dunstone, M.A. The Mystery behind Membrane Insertion: A Review of the Complement Membrane Attack Complex. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160221. [Google Scholar] [CrossRef]
- Mackman, N. Role of Tissue Factor in Hemostasis, Thrombosis, and Vascular Development. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Wolberg, A.S. Fibrinogen and Fibrin: Synthesis, Structure, and Function in Health and Disease. J. Thromb. Haemost. 2023, 21, 3005–3015. [Google Scholar] [CrossRef] [PubMed]
- Keragala, C.B.; Medcalf, R.L. Plasminogen: An Enigmatic Zymogen. Blood 2021, 137, 2881–2889. [Google Scholar] [CrossRef] [PubMed]
- Rau, J.C.; Beaulieu, L.M.; Huntington, J.A.; Church, F.C. Serpins in Thrombosis, Hemostasis and Fibrinolysis. J. Thromb. Haemost. 2007, 5, 102–115. [Google Scholar] [CrossRef]
- Xu, J.; Fang, J.; Cheng, Z.; Fan, L.; Hu, W.; Zhou, F.; Shen, H. Overexpression of the Kininogen-1 Inhibits Proliferation and Induces Apoptosis of Glioma Cells. J. Exp. Clin. Cancer Res. 2018, 37, 180. [Google Scholar] [CrossRef]
- Hallam, T.M.; Sharp, S.J.; Andreadi, A.; Kavanagh, D. Complement Factor I: Regulatory Nexus, Driver of Immunopathology, and Therapeutic. Immunobiology 2023, 228, 152410. [Google Scholar] [CrossRef]

| Sample | Clean Reads (M) | Mapped Reads (M) | Mapped (%) | GC Content (%) | Q30 (%) |
|---|---|---|---|---|---|
| K0_1 | 47.45 | 42.70 | 89.98 | 43.04 | 95.55 |
| K0_2 | 46.22 | 41.54 | 89.86 | 43.63 | 93.99 |
| K0_3 | 44.59 | 39.31 | 88.16 | 43.60 | 94.66 |
| K1_1 | 45.20 | 40.80 | 90.26 | 44.31 | 94.78 |
| K1_2 | 48.00 | 43.43 | 90.48 | 44.68 | 94.66 |
| K1_3 | 41.07 | 37.05 | 90.22 | 42.98 | 94.15 |
| K5_1 | 47.79 | 43.13 | 90.25 | 43.27 | 94.20 |
| K5_2 | 43.84 | 37.79 | 86.20 | 43.30 | 94.48 |
| K5_3 | 43.53 | 38.56 | 88.59 | 44.25 | 94.54 |
| ID | Description | Abbreviation | Log2FoldChange | |
|---|---|---|---|---|
| K1 vs. K0 | K5 vs. K0 | |||
| Cide__007094-RA | Complement component 5 | c5 | −0.85 | 3.27 |
| Cide__012914-RA | Complement component 8 alpha polypeptide | c8a | 0.75 | 3.80 |
| Cide__012915-RA | Complement component 8 beta polypeptide | c8b | −0.61 | 3.23 |
| Cide__034928-RA | Complement component 8 gamma polypeptide | c8g | −0.36 | 1.93 |
| Cide__007983-RA | Complement component 9 | c9 | −0.59 | 3.28 |
| Cide__032115-RA | Complement factor I | cfi | −0.45 | 2.58 |
| Cide__010271-RA | Coagulation factor X | f10 | −0.01 | 1.30 |
| Cide__003445-RA | Coagulation factor II | f2 | −0.37 | 3.00 |
| Cide__010273-RA | Coagulation factor VII | f7 | −0.60 | 2.08 |
| Cide__005925-RA | Coagulation factor IX | f9 | −0.35 | 3.43 |
| Cide__010004-RA | Fibrinogen alpha | fga | −0.21 | 3.48 |
| Cide__010005-RA | Fibrinogen B beta polypeptide | fgb | −0.33 | 3.74 |
| Cide__009699-RA | Fibrinogen gamma | fgg | −0.42 | 3.66 |
| Cide__005301-RA | Kininogen 1 | kng1 | −0.53 | 3.43 |
| Cide__026768-RA | Plasminogen | plg | −0.52 | 3.11 |
| Cide__005080-RA | Serpin family C member 1 | serpinc1 | −0.38 | 3.68 |
| Cide__019063-RA | Serpin family D member 1 | serpind1 | −0.03 | 3.82 |
| Cide__029826-RA | Serpin family F member 2 | serpinf2 | −0.25 | 2.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Zhu, R.; Xie, L.; Lv, W.; Wang, X.; Wu, M.; Xu, X.; Qiu, J. Histopathological and Molecular Insights into Grass Carp Kidney Responses to Co-Infection with Aeromonas hydrophila and Aeromonas veronii. Fishes 2025, 10, 484. https://doi.org/10.3390/fishes10100484
Zhou Y, Zhu R, Xie L, Lv W, Wang X, Wu M, Xu X, Qiu J. Histopathological and Molecular Insights into Grass Carp Kidney Responses to Co-Infection with Aeromonas hydrophila and Aeromonas veronii. Fishes. 2025; 10(10):484. https://doi.org/10.3390/fishes10100484
Chicago/Turabian StyleZhou, Yifei, Ruijun Zhu, Lingli Xie, Wenyao Lv, Xinyue Wang, Mengzhou Wu, Xiaoyan Xu, and Junqiang Qiu. 2025. "Histopathological and Molecular Insights into Grass Carp Kidney Responses to Co-Infection with Aeromonas hydrophila and Aeromonas veronii" Fishes 10, no. 10: 484. https://doi.org/10.3390/fishes10100484
APA StyleZhou, Y., Zhu, R., Xie, L., Lv, W., Wang, X., Wu, M., Xu, X., & Qiu, J. (2025). Histopathological and Molecular Insights into Grass Carp Kidney Responses to Co-Infection with Aeromonas hydrophila and Aeromonas veronii. Fishes, 10(10), 484. https://doi.org/10.3390/fishes10100484






