Effect of Dietary Proline on the Growth Performance, Collagen Deposition, and Texture Quality of Sea Cucumbers’ Body Wall (Apostichopus japonicus)
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Experimental Diets and Feeding Experiment
2.3. Sample Collection
2.4. Feed Composition Analysis
2.5. Collagen Contents Analysis
2.6. Amino Acid Contents Analysis
2.7. Histological Staining of the Body Wall
2.8. Characterization of Water Absorption of the Body Wall
2.9. Texture Profile Analysis (TPA)
2.10. Real-Time PCR (RT-PCR) of Collagen Synthesis-Related Genes
2.11. Calculations and Statistical Analysis
3. Results
3.1. Growth Performance
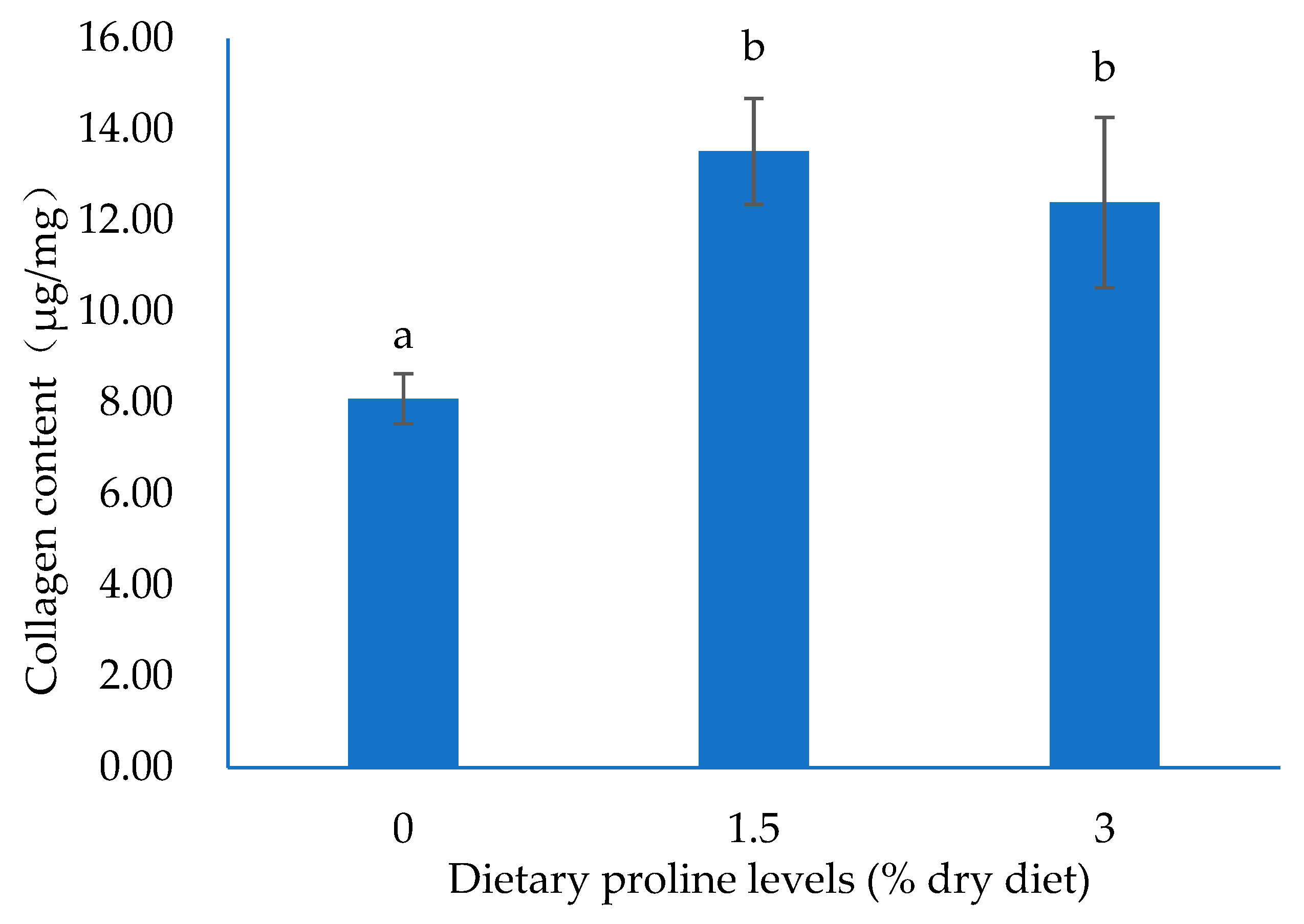
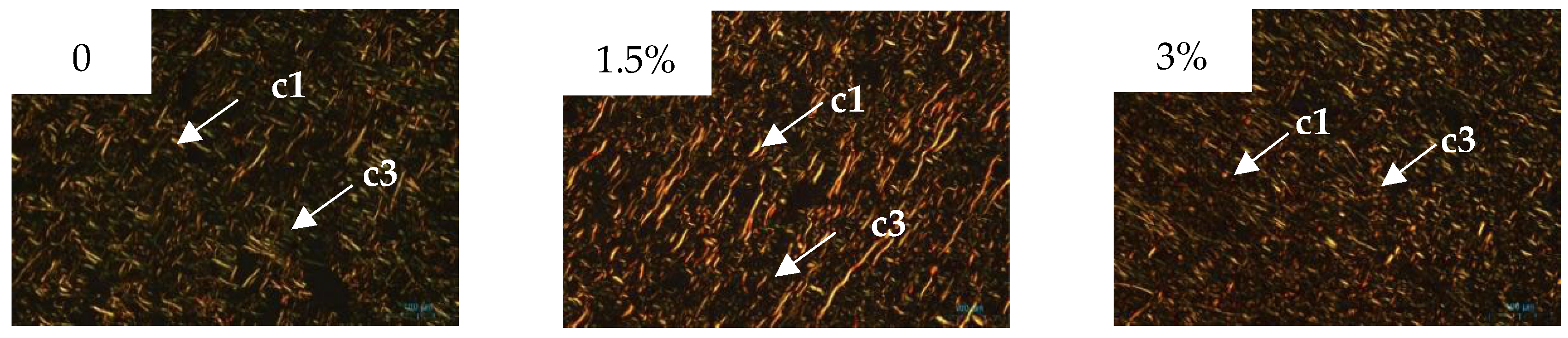
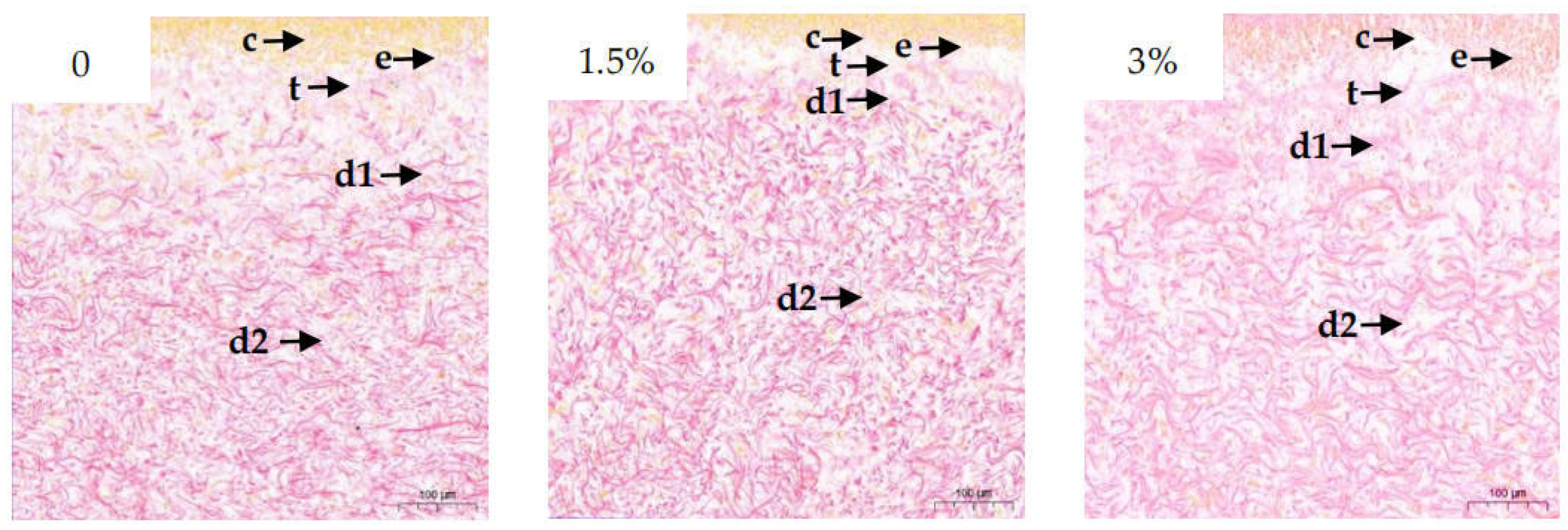
3.2. Collagen Content and Structure
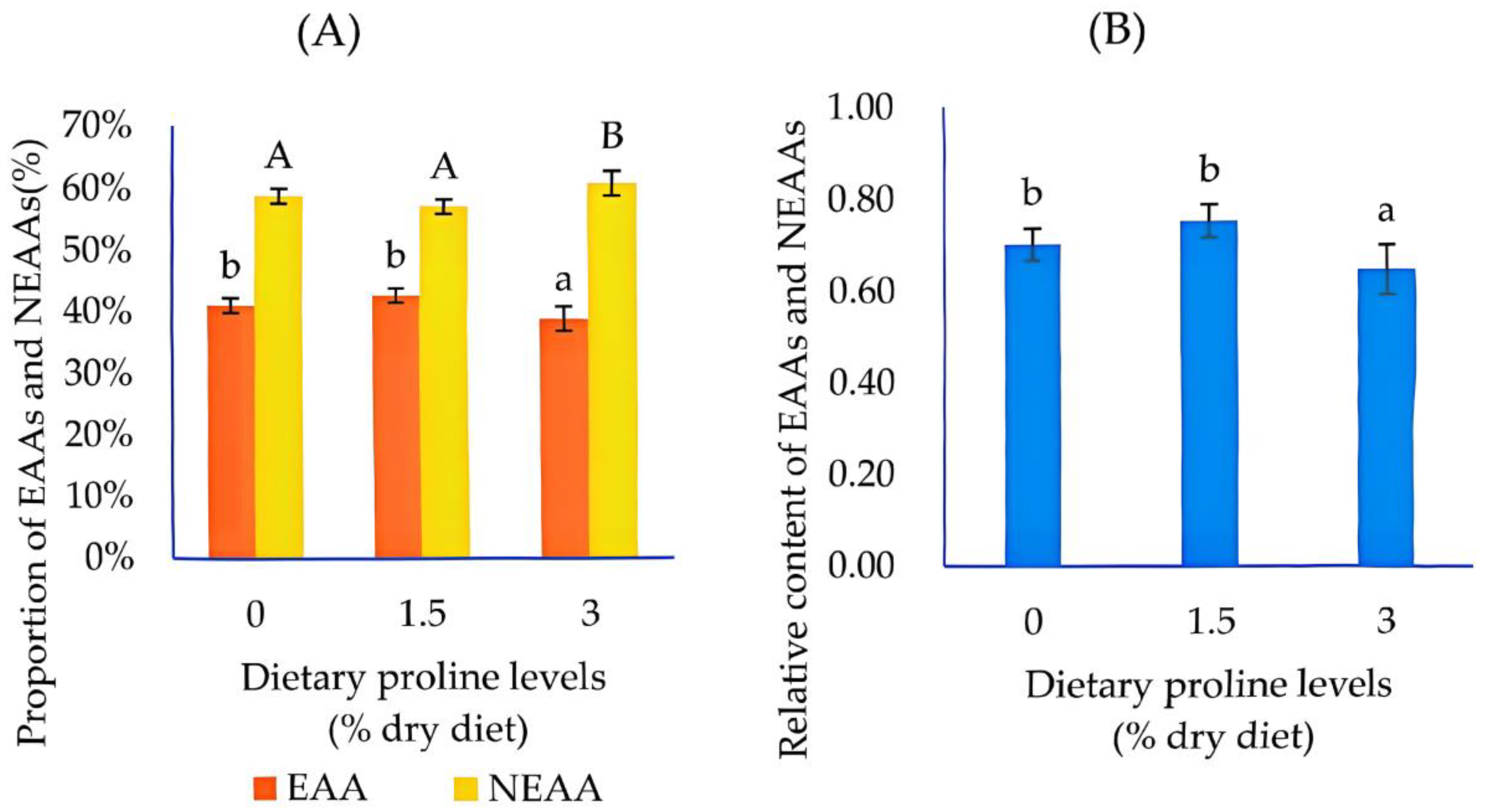
3.3. Amino Acid Profile
3.4. Organoleptic Quality
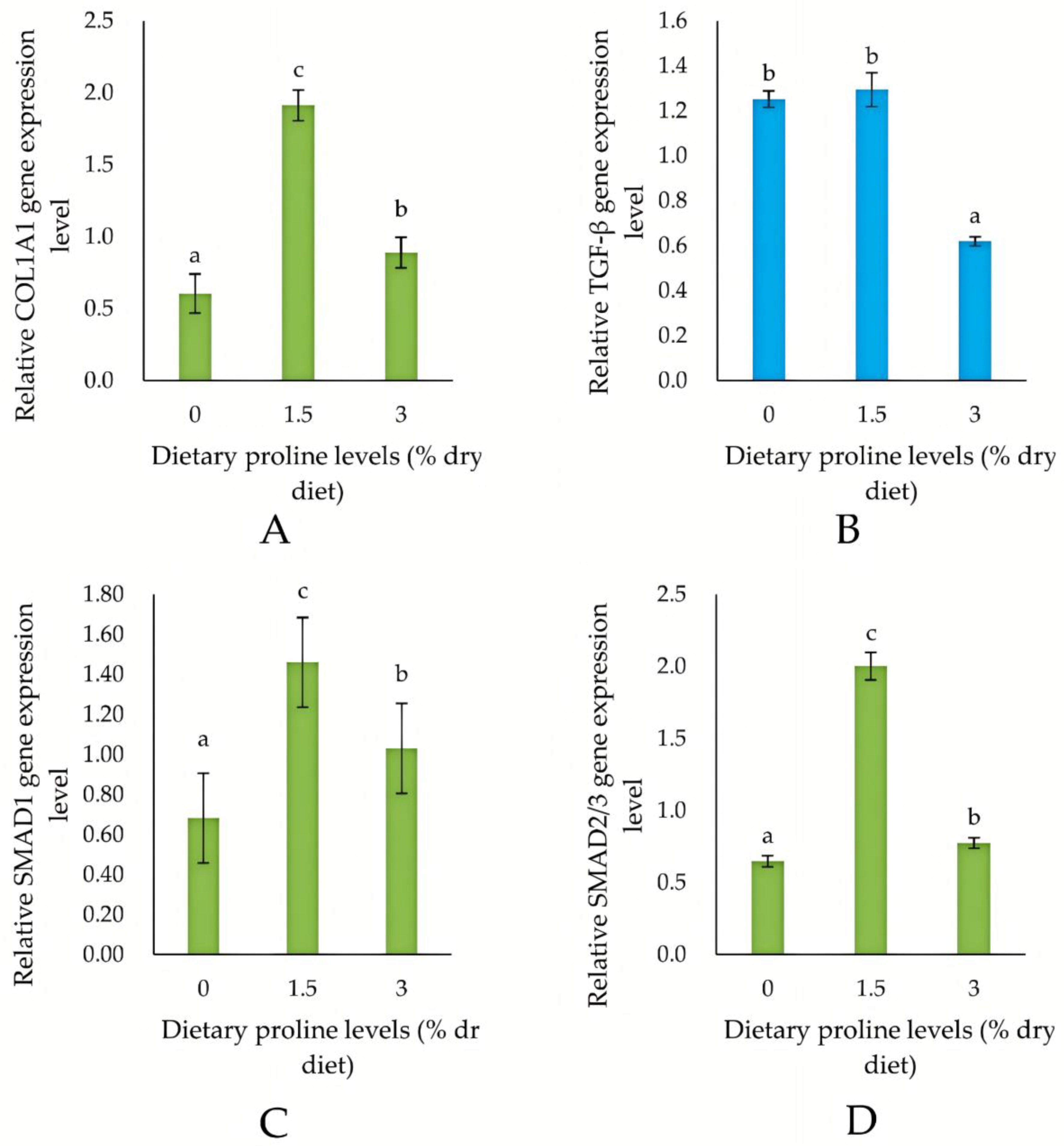
3.5. Expression of Collagen Synthesis-Related Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, G.; Dong, S.; Tian, X.; Gao, Q.; Wang, F.; Xu, K. Life Cycle Assessment of Different Sea Cucumber (Apostichopus japonicus Selenka) Farming Systems. J. Ocean Univ. China 2015, 14, 1068–1074. [Google Scholar] [CrossRef]
- Wang, G.-D.; Li, C.-Y.; Hu, M.; Xu, T.; Xu, K.-F. Current Situation Analysis and Development Suggestions of the Apostichopus japonicus Culture Industry in Shandong Province. Isr. J. Aquac.-Bamidgeh 2022, 74, 1–9. [Google Scholar] [CrossRef]
- Ru, X.; Feng, Q.; Zhang, S.; Liu, S.; Zhang, L.; Yang, H. Eco-Friendly Method for Rearing Sea Cucumber (Apostichopus japonicus) Larvae. Aquac. Res. 2022, 53, 3759–3766. [Google Scholar] [CrossRef]
- Xia, S.; Wang, X. Chapter 19—Nutritional and Medicinal Value. In Developments in Aquaculture and Fisheries Science; Yang, H., Hamel, J.-F., Mercier, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 39, pp. 353–365. [Google Scholar]
- Guo, Z.; Meng, X.; Zhang, Y.; Wu, D.; Zhang, R.; Zhao, X.; Ren, T.; Han, Y. Replacing Sea Mud with Attachment of Suspension Cage Can Improve Growth and Gut Health for Sea Cucumber Apostichopus Japonicus. Front. Mar. Sci. 2024, 11. [Google Scholar] [CrossRef]
- Kobayashi, M.; Msangi, S.; Batka, M.; Vannuccini, S.; Dey, M.M.; Anderson, J.L. Fish to 2030: The Role and Opportunity for Aquaculture. Aquac. Econ. Manag. 2015, 19, 282–300. [Google Scholar] [CrossRef]
- Saito, M.; Kunisaki, N.; Urano, N.; Kimura, S. Collagen as the Major Edible Component of Sea Cucumber (Stichopus japonicus). J. Food Sci. 2002, 67, 1319–1322. [Google Scholar] [CrossRef]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The Extracellular Matrix at a Glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef]
- To, V.-A.; Liou, C.-H. Taurine Supplementation Enhances the Replacement Level of Fishmeal by Soybean Concentrate in Diets of Juvenile Pacific White Shrimp (Litopenaeus vannamei Boone, 1931). Aquac. Res. 2021, 52, 3771–3784. [Google Scholar] [CrossRef]
- Rong, H.; Zhang, Y.; Hao, M.; Lin, F.; Zou, W.; Zhang, H.; Yu, C.; Yu, J.; Shi, Q.; Aweya, J.J.; et al. Effect of Hydroxyproline Supplementation on Growth Performance, Body Composition, Amino Acid Profiles, Blood-Biochemistry and Collagen Synthesis of Juvenile Chu’s Croaker (Nibea coibor). Aquac. Res. 2020, 51, 1264–1275. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Burghardt, R.C.; Johnson, G.A.; Kim, S.W.; Knabe, D.A.; Li, P.; Li, X.; McKnight, J.R.; Satterfield, M.C.; et al. Proline and Hydroxyproline Metabolism: Implications for Animal and Human Nutrition. Amino Acids 2011, 40, 1053–1063. [Google Scholar] [CrossRef]
- Albaugh, V.L.; Mukherjee, K.; Barbul, A. Proline Precursors and Collagen Synthesis: Biochemical Challenges of Nutrient Supplementation and Wound Healing. J. Nutr. 2017, 147, 2011–2017. [Google Scholar] [CrossRef]
- Rong, H.; Xia, Y.; Wang, X.; Hu, Q.; Jia, D.; Wu, X.; Kong, L.; Bi, B. Effects of dietary proline on growth, body composition and antioxidant capacity of Chu’ s croaker (Nibea coibor). J. Shanghai Ocean Univ. 2023, 32, 89–97. [Google Scholar]
- Rong, H.; Yang, Y.; Wang, X.; Yan, H.; Gao, Y.; Bi, B.; Shi, Q.; Kong, L. Effects of Proline on Collagen Deposition and Related Gene Expression in Swim Bladder of Chu’ s Croaker (Nibea coibor). Chin. J. Anim. Nutr. 2022, 34, 3184–3193. [Google Scholar]
- Deng, Z.; Song, W.; Rui, Q.; Shi, C.; Zhang, J.; Li, C. Effects of Proline on Growth Performance, Antioxidant Capacity and Resistance to Aeromonas hydrophila Infection of Pelodiscus sinensis. Chin. J. Anim. Nutr. 2023, 35, 3213–3227. [Google Scholar]
- Salo, A.M.; Myllyharju, J. Prolyl and Lysyl Hydroxylases in Collagen Synthesis. Exp. Dermatol. 2021, 30, 38–49. [Google Scholar] [CrossRef]
- Leask, A.; Abraham, D.J. TGF-Beta Signaling and the Fibrotic Response. FASEB J. 2004, 18, 816–827. [Google Scholar] [CrossRef]
- Hillege, M.M.G.; Galli Caro, R.A.; Offringa, C.; de Wit, G.M.J.; Jaspers, R.T.; Hoogaars, W.M.H. TGF-β Regulates Collagen Type I Expression in Myoblasts and Myotubes via Transient Ctgf and Fgf-2 Expression. Cells 2020, 9, 375. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, R.; Yang, H.; Li, R.; Ding, J.; Chang, Y.; Zuo, R. Vitamin E Regulates the Collagen Contents in the Body Wall of Sea Cucumber (Apostichopus japonicus) via Its Antioxidant Effects and the TGF-β/Smads Pathway. Antioxidants 2024, 13, 847. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis (OMA), 16th ed.; AOAC International: Arlington, VA, USA, 1995. [Google Scholar]
- AOAC International. Official Methods of Analysis (OMA), 17th ed.; AOAC International: Arlington, VA, USA, 2002. [Google Scholar]
- Dong, X.; Liu, W.; Song, X.; Lin, X.; Yu, D.; Yu, C.; Zhu, B. Characterization of Heat-Induced Water Adsorption of Sea Cucumber Body Wall. J. Food Sci. 2019, 84, 92–100. [Google Scholar] [CrossRef]
- Zuo, R.; Li, M.; Ding, J.; Chang, Y. Higher Dietary Arachidonic Acid Levels Improved the Growth Performance, Gonad Development, Nutritional Value, and Antioxidant Enzyme Activities of Adult Sea Urchin (Strongylocentrotus intermedius). J. Ocean Univ. China 2018, 17, 932–940. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Church, D.D.; Hirsch, K.R.; Park, S.; Kim, I.-Y.; Gwin, J.A.; Pasiakos, S.M.; Wolfe, R.R.; Ferrando, A.A. Essential Amino Acids and Protein Synthesis: Insights into Maximizing the Muscle and Whole-Body Response to Feeding. Nutrients 2020, 12, 3717. [Google Scholar] [CrossRef]
- Wu, G. Functional Amino Acids in Growth, Reproduction, and Health. Adv. Nutr. 2010, 1, 31–37. [Google Scholar] [CrossRef]
- Xie, S.-W.; Tian, L.-X.; Li, Y.-M.; Zhou, W.; Zeng, S.-L.; Yang, H.-J.; Liu, Y.-J. Effect of Proline Supplementation on Anti-Oxidative Capacity, Immune Response and Stress Tolerance of Juvenile Pacific White Shrimp, Litopenaeus vannamei. Aquaculture 2015, 448, 105–111. [Google Scholar] [CrossRef]
- Taziki, T.; Jafari, V.; Mazandarani, M.; Hoseinifar, S.H. Effects of Dietary L-Proline and L-Alanine on Growth Performance, and Flesh Quality of Common Carp (Cyprinus carpio) Juveniles. Ann. Anim. Sci. 2023, 23, 195–204. [Google Scholar] [CrossRef]
- Aksnes, A.; Mundheim, H.; Toppe, J.; Albrektsen, S. The Effect of Dietary Hydroxyproline Supplementation on Salmon (Salmo salar L.) Fed High Plant Protein Diets. Aquaculture 2008, 275, 242–249. [Google Scholar] [CrossRef]
- Nagaoka, I.; Tsuruta, A.; Yoshimura, M. Chondroprotective Action of Glucosamine, a Chitosan Monomer, on the Joint Health of Athletes. Int. J. Biol. Macromol. 2019, 132, 795–800. [Google Scholar] [CrossRef]
- Sipilä, K.H.; Drushinin, K.; Rappu, P.; Jokinen, J.; Salminen, T.A.; Salo, A.M.; Käpylä, J.; Myllyharju, J.; Heino, J. Proline Hydroxylation in Collagen Supports Integrin Binding by Two Distinct Mechanisms. J. Biol. Chem. 2018, 293, 7645–7658. [Google Scholar] [CrossRef]
- Lin, F.; Rong, H.; Lin, J.; Yuan, Y.; Yu, J.; Yu, C.; You, C.; Wang, S.; Sun, Z.; Wen, X. Enhancement of Collagen Deposition in Swim Bladder of Chu’s Croaker (Nibea coibor) by Proline: View from In-Vitro and In-Vivo Study. Aquaculture 2020, 523, 735175. [Google Scholar] [CrossRef]
- Cao, S.; Xiao, Y.; Huang, R.; Zhao, D.; Xu, W.; Li, S.; Tang, J.; Qu, F.; Jin, J.; Xie, S.; et al. Dietary Supplementation with Hydroxyproline Enhances Growth Performance, Collagen Synthesis and Muscle Quality of Carassius auratus Triploid. Front. Physiol. 2022, 13. [Google Scholar] [CrossRef]
- Zhang, K.; Ai, Q.; Mai, K.; Xu, W.; Liufu, Z.; Zhang, Y.; Peng, M. Effects of Dietary Hydroxyproline on Growth Performance, Body Composition, Hydroxyproline and Collagen Concentrations in Tissues in Relation to Prolyl 4-Hydroxylase α(I) Gene Expression of Juvenile Turbot, Scophthalmus maximus L. Fed High Plant Protein Diets. Aquaculture 2013, 404–405, 77–84. [Google Scholar] [CrossRef]
- Shi, M.; Li, H.; Chen, T.; Huang, B.; Li, X.; Dong, X.; Chi, S.; Yang, Q.; Liu, H.; Deng, J.; et al. Effects of Hydroxyproline Supplementation in Low Fish Meal Diets on Collagen Synthesis, Myofiber Development and Muscular Texture of Juvenile Pacific White Shrimp (Litopenaeus vannamei). Anim. Nutr. 2024, 17, 428–437. [Google Scholar] [CrossRef]
- Barbul, A. Proline Precursors to Sustain Mammalian Collagen Synthesis12. J. Nutr. 2008, 138, 2021S–2024S. [Google Scholar] [CrossRef]
- Li, P.; Wu, G. Roles of Dietary Glycine, Proline, and Hydroxyproline in Collagen Synthesis and Animal Growth. Amino Acids 2017, 50, 29–38. [Google Scholar] [CrossRef]
- Tong, F.; Bai, J.; Tang, Z.; Li, C.; Liu, S.; Wei, Z. Replacing Fish Meal with Hydrolyzed Collagen Derived from Fish By-Products Improved Muscle Quality and Glycolipid Metabolism of Triploid Crucian Carp. Foods 2023, 12, 1235. [Google Scholar] [CrossRef]
- Wolinska-Kennard, K.; Schönberger, C.; Fenton, A.; Sahin, A.W. Mouthfeel of Food and Beverages: A Comprehensive Review of Physiology, Biochemistry, and Key Sensory Compounds. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70223. [Google Scholar] [CrossRef]
- Zhang, Y.; Pandiselvam, R.; Zhu, H.; Su, D.; Wang, H.; Ai, Z.; Kothakota, A.; Khaneghah, A.M.; Liu, Y. Impact of Radio Frequency Treatment on Textural Properties of Food Products: An Updated Review. Trends Food Sci. Technol. 2022, 124, 154–166. [Google Scholar] [CrossRef]
- Li, X.; Bickerdike, R.; Lindsay, E.; Campbell, P.; Nickell, D.; Dingwall, A.; Johnston, I.A. Hydroxylysyl Pyridinoline Cross-Link Concentration Affects the Textural Properties of Fresh and Smoked Atlantic Salmon (Salmo salar L.) Flesh. J. Agric. Food Chem. 2005, 53, 6844–6850. [Google Scholar] [CrossRef]
- Wang, M.; Feng, L.; Wu, P.; Liu, Y.; Ren, H.; Jin, X.; Zhou, X.; Jiang, W. Regulation of Muscle Springiness and Hardness: The Role of Myo-Inositol in Enhancing Fish Flesh Texture. Food Chem. X 2025, 29, 102788. [Google Scholar] [CrossRef]
- Li, S.; Zhou, Y.; Sun, L.; Wang, Y.; Song, S.; Ai, C.; Yang, J. Effects of Storage Method on the Quality of Processed Sea Cucumbers (Apostichopus japonicus). Foods 2022, 11, 4098. [Google Scholar] [CrossRef]
- Wu, B.; Li, N.; Liu, M.; Cheng, K.; Jiang, D.; Yi, Y.; Ma, S.; Yan, B.; Lu, Y. Construction of Human Periodontal Ligament Constitutive Model Based on Collagen Fiber Content. Materials 2023, 16, 6582. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Du, T.; Liu, Y. Biomechanical Characteristics and Analysis Approaches of Bone and Bone Substitute Materials. J. Funct. Biomater. 2023, 14, 212. [Google Scholar] [CrossRef] [PubMed]
- Orgel, J.P.R.O.; Irving, T.C.; Miller, A.; Wess, T.J. Microfibrillar Structure of Type I Collagen In Situ. Proc. Natl. Acad. Sci. USA 2006, 103, 9001–9005. [Google Scholar] [CrossRef] [PubMed]
- Rong, H.; Zhang, H.; Ning, L.; Wu, K.; Limbu, S.M.; Shi, Q.; Qin, C.; Wen, X. The Transforming Growth Factor Beta (TGF-β/Smads) Pathway Regulates Collagen Synthesis and Deposition in Swim Bladder of Chu’s Croaker (Nibea coibor) Stimulated by Proline. Aquaculture 2022, 558, 738360. [Google Scholar] [CrossRef]
- Rong, H.; Lin, F.; Limbu, S.M.; Lin, Z.; Bi, B.; Dou, T.; Zhao, L.; Wen, X. Effects of Dietary Proline on Swim Bladder Collagen Synthesis and Its Possible Regulation by the TGF-β/Smad Pathway in Spotted Drum, Nibea diacanthus. Aquac. Nutr. 2020, 26, 1792–1805. [Google Scholar] [CrossRef]
- Meng, X.; Nikolic-Paterson, D.J.; Lan, H.Y. TGF-β: The Master Regulator of Fibrosis. Nat. Rev. Nephrol. 2016, 12, 325–338. [Google Scholar] [CrossRef]
- Devos, H.; Zoidakis, J.; Roubelakis, M.G.; Latosinska, A.; Vlahou, A. Reviewing the Regulators of COL1A1. Int. J. Mol. Sci. 2023, 24, 10004. [Google Scholar] [CrossRef]
- Guo, X.; Wang, X.-F. Signaling Cross-Talk between TGF-β/BMP and Other Pathways. Cell Res. 2009, 19, 71–88. [Google Scholar] [CrossRef]
- Saadat, S.; Noureddini, M.; Mahjoubin-Tehran, M.; Nazemi, S.; Shojaie, L.; Aschner, M.; Maleki, B.; Abbasi-kolli, M.; Rajabi Moghadam, H.; Alani, B.; et al. Pivotal Role of TGF-β/Smad Signaling in Cardiac Fibrosis: Non-Coding RNAs as Effectual Players. Front. Cardiovasc. Med. 2021, 7, 588347. [Google Scholar] [CrossRef]
- Wu, M.; Chen, G.; Li, Y.-P. TGF-β and BMP Signaling in Osteoblast, Skeletal Development, and Bone Formation, Homeostasis and Disease. Bone Res. 2016, 4, 16009. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Lai, Q.; Yao, Z.; Zhou, K.; Niu, D. Proline Metabolism and Its Osmotic Regulatory Role in Enhancing Hypersalinity Tolerance of the Razor Clam (Sinonovacula constricta). Aquaculture 2024, 589, 741019. [Google Scholar] [CrossRef]
- Wang, S.; Lin, R.; Cheng, S.; Tan, M. Water Dynamics Changes and Protein Denaturation in Surf Clam Evaluated by Two-Dimensional LF-NMR T1-T2 Relaxation Technique during Heating Process. Food Chem. 2020, 320, 126622. [Google Scholar] [CrossRef]
- Ge, X.; Wang, H.; Yin, M.; Wang, X. Effect of Different Thawing Methods on the Physicochemical Properties and Microstructure of Frozen Instant Sea Cucumber. Foods 2022, 11, 2616. [Google Scholar] [CrossRef]
- Meena, M.; Divyanshu, K.; Kumar, S.; Swapnil, P.; Zehra, A.; Shukla, V.; Yadav, M.; Upadhyay, R.S. Regulation of L-Proline Biosynthesis, Signal Transduction, Transport, Accumulation and Its Vital Role in Plants during Variable Environmental Conditions. Heliyon 2019, 5. [Google Scholar] [CrossRef]
- Geng, S.; Wang, H.; Wang, X.; Ma, X.; Xiao, S.; Wang, J.; Tan, M. A Non-Invasive NMR and MRI Method to Analyze the Rehydration of Dried Sea Cucumber. Anal. Methods 2015, 7, 2413–2419. [Google Scholar] [CrossRef]
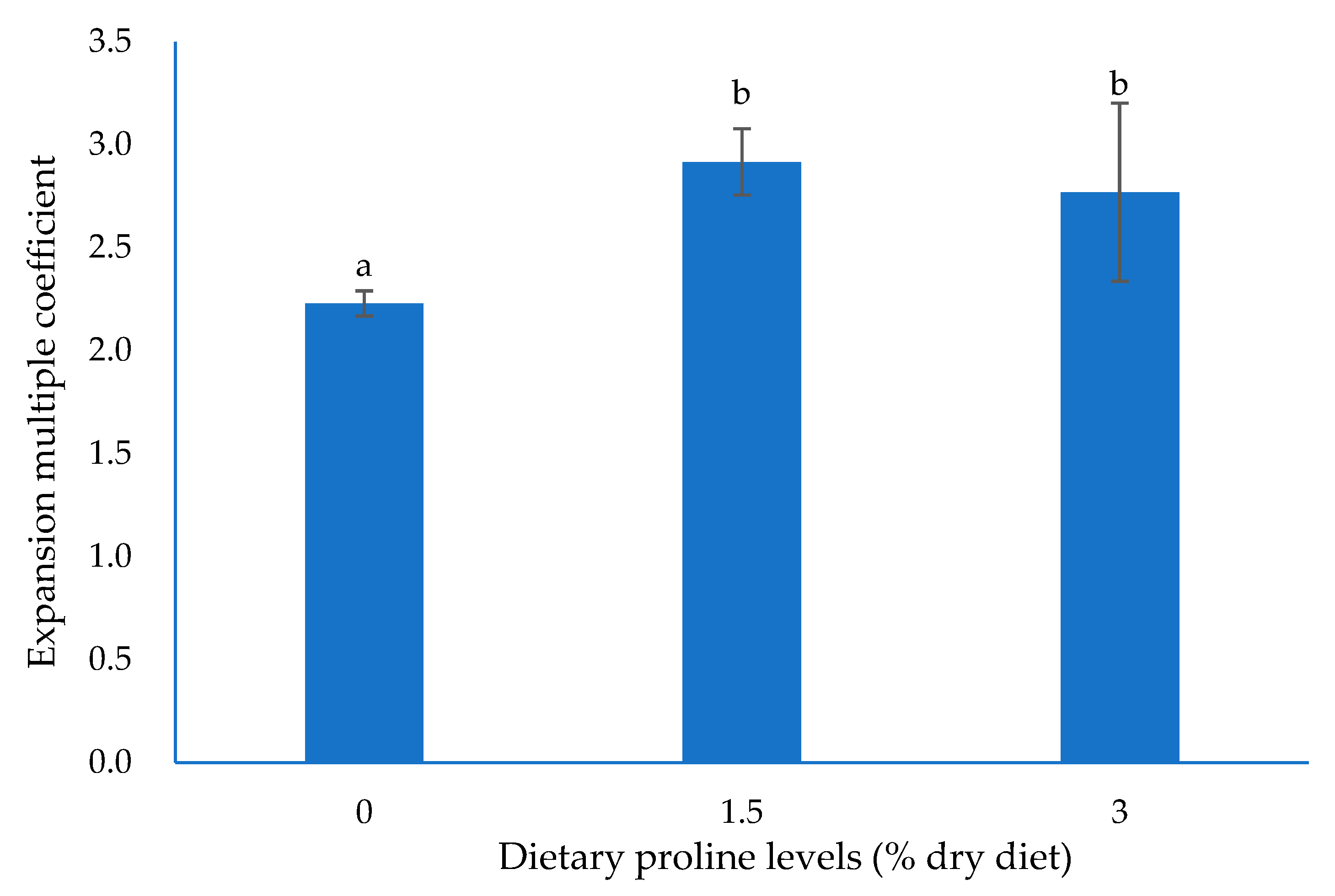
| Ingredient | Dietary Proline Levels (% Dry Diet) | ||||
|---|---|---|---|---|---|
| 0 | 1.5 | 3 | |||
| Skim Fish Meal 1 | 2 | 2 | 2 | ||
| Sargassum Thunbergii Meal 2 | 28 | 28 | 28 | ||
| Fermented Soybean Meal 3 | 12 | 12 | 12 | ||
| Proline | 0 | 1.5 | 3 | ||
| Serine | 3 | 1.5 | 0 | ||
| Vitamin Premix 4 | 0.5 | 0.5 | 0.5 | ||
| Mineral Premix 5 | 0.5 | 0.5 | 0.5 | ||
| Ca(H2PO4)2 6 | 2 | 2 | 2 | ||
| Sea Mud | 52 | 52 | 52 | ||
| Proximate composition | |||||
| Crude Protein (%) | 9.52 | 9.56 | 9.48 | ||
| Crude lipid (%) | 0.12 | 0.11 | 0.13 | ||
| Genes | Gene ID | Annealing Temperature (°C) | Primer Sequences (5′-3′) |
|---|---|---|---|
| Col1A2 1 | c54738.graph_c0 | 56 | F:5′-CGGACTTTTACTTTGGCGTTAT-3′ R:5′-TTTCTGGCGGTCTGCCTAT-3′ |
| TGF-β 2 | BSL78_20284 | 59 | F:5′-ACCGCTCCTCACCCTTTAACAC-3′ R:5′-CCACTAGCACTAAGCAGCATATCAG-3′ |
| SMAD1 3 | BSL78_15508 | 58 | F:5′-GGCATACTCGCAGCAGTCTAAAG-3′ R:5′-AGGTGTCTCATCGGAAAGGTCTAC-3′ |
| SMAD2/3 4 | BSL78_11878 | 59 | F:5′-AGAACCACCACGAACTCAAACATG-3′ R:5′-GCAGACAGCAGCAGGGATAAAC-3′ |
| Dietary Proline Levels (% Dry Diet) | |||
|---|---|---|---|
| 0 | 1.5 | 3 | |
| Survival Rate (%) | 100.00 | 100.00 | 100.00 |
| Initial Body Weight (g) | 30.40 ± 2.03 | 30.02 ± 2.06 | 30.35 ± 3.49 |
| Final Body Weight (g) | 55.54 ± 12.46 | 60.92 ± 11.08 | 66.39 ± 19.85 |
| Weight Growth Rate (%) | 82.20 ± 34.57 a | 101.76 ± 25.09 ab | 115.30 ± 42.34 b |
| Body Wall Weight (g) | 34.53 ± 9.16 a | 40.34 ± 8.85 ab | 42.16 ± 10.89 b |
| Body Wall Index (%) | 69.36 ± 6.37 | 69.80 ± 5.94 | 67.93 ± 6.59 |
| Amino Acid | Dietary Proline Levels (% Dry Diet) | ||
|---|---|---|---|
| 0 | 1.5 | 3 | |
| Aspartic | 8.17 ± 0.47 | 8.52 ± 0.26 | 8.15 ± 0.50 |
| Glutamic | 15.47 ± 0.44 b | 15.63 ± 0.75 b | 14.35 ± 0.62 a |
| Serine | 4.90 ± 0.08 | 4.83 ± 0.31 | 4.69 ± 0.17 |
| Glycine | 11.49 ± 1.66 a | 9.92 ± 0.93 a | 13.96 ± 1.87 b |
| Histidine | 1.99 ± 0.17 b | 2.07 ± 0.14 b | 1.71 ± 0.05 a |
| Argnine | 10.14 ± 0.45 | 9.81 ± 0.53 | 9.57 ± 0.34 |
| Threonine | 5.23 ± 0.19 | 5.15 ± 0.37 | 5.21 ± 0.12 |
| Alanine | 6.76 ± 0.30 a | 6.37 ± 0.18 a | 6.90 ± 0.44 b |
| Proline | 8.67 ± 0.37 a | 8.40 ± 0.50 a | 9.67 ± 0.54 b |
| Tyrosine | 3.40 ± 0.16 b | 3.53 ± 0.12 ab | 3.25 ± 0.09 a |
| Valine | 4.26 ± 0.07 | 4.41 ± 0.04 | 4.36 ± 0.19 |
| Methionine | 1.49 ± 0.44 a | 2.12 ± 0.41 ab | 1.76 ± 0.56 b |
| Isoleucine | 3.76 ± 0.12 b | 3.96 ± 0.10 c | 3.57 ± 0.18 a |
| Leucine | 0.30 ± 0.12 b | 0.29 ± 0.12 b | 0.44 ± 0.18 a |
| Phenylalanine | 3.09 ± 0.12 ab | 3.21 ± 0.08 b | 2.98 ± 0.19 a |
| Lysine | 5.18 ± 0.44 b | 5.74 ± 0.52 b | 4.40 ± 0.64 a |
| Dietary Proline Levels (% Dry Diet) | |||
|---|---|---|---|
| 0 | 1.5 | 3 | |
| Hardness 1 | 2.80 ± 0.08 a | 3.32 ± 0.73 ab | 3.76 ± 0.55 b |
| Springiness 2 | 2.64 ± 0.33 | 2.84 ± 0.29 | 2.78 ± 0.27 |
| Cohesiveness 3 | 0.80 ± 0.05 a | 0.83 ± 0.03 ab | 0.85 ± 0.03 b |
| Chewiness 4 | 5.86 ± 1.03 a | 7.79 ± 2.44 ab | 8.90 ± 1.88 b |
| Gumminess 5 | 2.21 ± 0.16 a | 2.72 ± 0.70 ab | 3.18 ± 0.46 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, R.; Wang, Z.; Liu, H.; Li, R.; Wang, X.; Yang, H.; Ding, J.; Chang, Y.; Zuo, R. Effect of Dietary Proline on the Growth Performance, Collagen Deposition, and Texture Quality of Sea Cucumbers’ Body Wall (Apostichopus japonicus). Fishes 2025, 10, 482. https://doi.org/10.3390/fishes10100482
Xu R, Wang Z, Liu H, Li R, Wang X, Yang H, Ding J, Chang Y, Zuo R. Effect of Dietary Proline on the Growth Performance, Collagen Deposition, and Texture Quality of Sea Cucumbers’ Body Wall (Apostichopus japonicus). Fishes. 2025; 10(10):482. https://doi.org/10.3390/fishes10100482
Chicago/Turabian StyleXu, Rujian, Zitong Wang, Haijing Liu, Ruixue Li, Xianyu Wang, Hongbing Yang, Jun Ding, Yaqing Chang, and Rantao Zuo. 2025. "Effect of Dietary Proline on the Growth Performance, Collagen Deposition, and Texture Quality of Sea Cucumbers’ Body Wall (Apostichopus japonicus)" Fishes 10, no. 10: 482. https://doi.org/10.3390/fishes10100482
APA StyleXu, R., Wang, Z., Liu, H., Li, R., Wang, X., Yang, H., Ding, J., Chang, Y., & Zuo, R. (2025). Effect of Dietary Proline on the Growth Performance, Collagen Deposition, and Texture Quality of Sea Cucumbers’ Body Wall (Apostichopus japonicus). Fishes, 10(10), 482. https://doi.org/10.3390/fishes10100482







