1. Introduction
In a previous study [
1], we expanded on the modern synthesis of evolutionary theory [
2,
3,
4,
5,
6,
7,
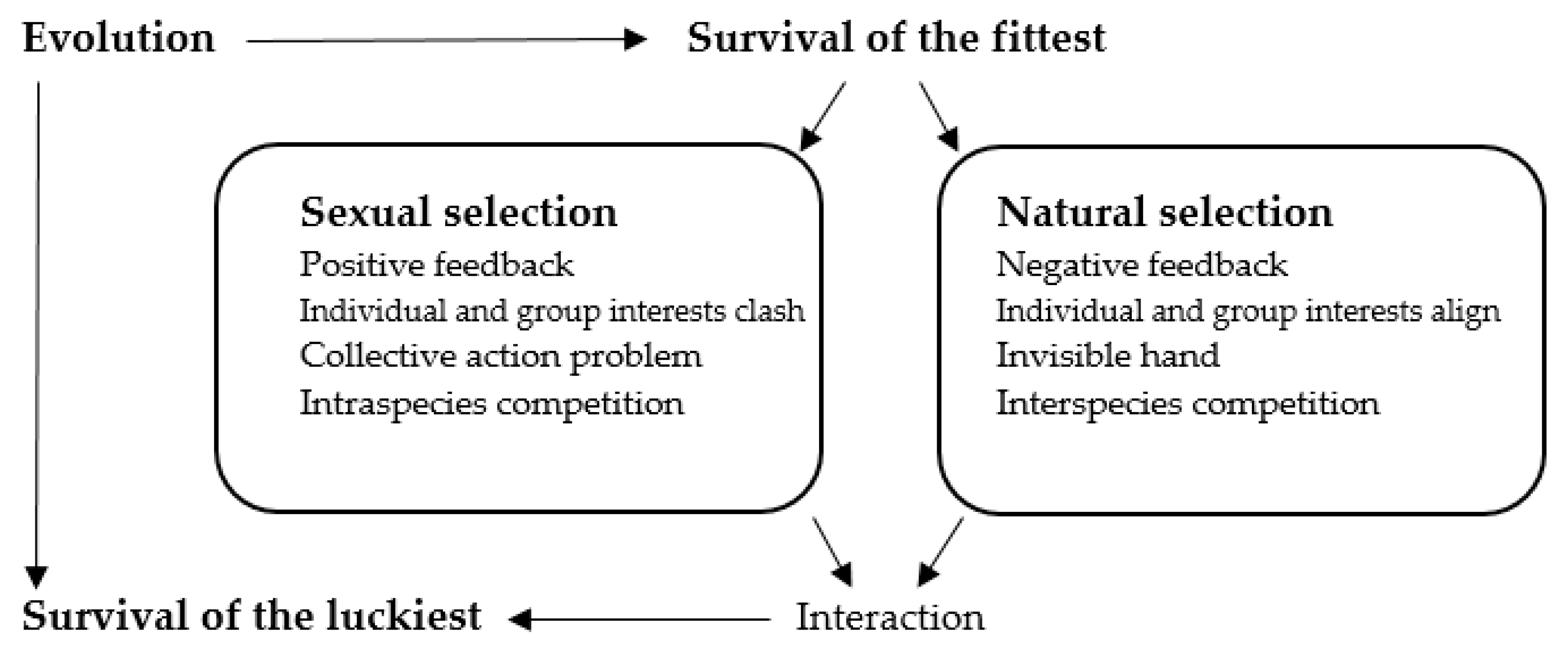
8], often metaphorically described by the concept of “survival of the fittest”. We proposed a complementary perspective: “survival of the luckiest”. The theory addresses the overlooked interaction between natural and sexual selection, suggesting an increased role of randomness in evolution. While natural selection operates via negative feedback, favoring traits that enhance survival in interspecies competition, sexual selection drives positive feedback, amplifying traits that are advantageous in intraspecies mate competition. These opposing dynamics often result in outcomes where luck—unpredictable factors—overrides pure fitness as the determinant of evolutionary success.
Sexual and natural selection follow distinct feedback dynamics [
1]. Sexual selection, as described by R.A. Fisher [
3], operates through positive feedback: once a trait becomes preferred, its exaggeration can intensify across generations, resembling an evolutionary arms race. This escalating dynamic reflects the amplification of initial variation, which is characteristic of positive feedback systems [
9]. In contrast, natural selection tends to function through negative feedback: advantageous mutations may spread, but their effects eventually stabilize at the population level, aligning individual gains with broader species fitness [
10].
A key example illustrating this approach involves two male frogs competing to mate through elaborate signaling displays. Although one frog’s enhanced signaling makes it a favorite in sexual selection, this trait simultaneously increases its visibility to predators, resulting in its demise. Consequently, the runner-up, less fit in terms of sexual selection, survives and reproduces due to sheer luck. While all evolutionary outcomes involve some degree of contingency, this example illustrates how the interaction between natural and sexual selection creates conflicting fitness pressures, increasing the unpredictability of which traits are ultimately retained. This interplay reveals how survival can hinge on compounded contingencies arising from trade-offs between distinct selective forces.
In the frog example, we use the term “luck” to describe the outcome of such trade-offs. However, it is important to clarify what we mean by randomness in this and related contexts. Some patterns in evolution result from intrinsically stochastic processes, such as genetic drift or epimutations, while others only appear to be random due to the complex interaction of multiple deterministic forces. This distinction—between actual randomness and apparent randomness—has been emphasized in paleobiology by Gould, Raup, and the MBL group, and is explored in depth by Cornette and Lieberman [
11]. In their analysis of marine diversity and extinction patterns across the Phanerozoic, they show that the accumulation of evolutionary events often conforms statistically to a random walk, not necessarily because the processes are inherently random, but because the number and diversity of mechanisms involved can obscure causal signals. This perspective is foundational to our framework, which treats “luck” as shorthand for contingent outcomes arising from interactions between natural and sexual selection, development, and environmental variability—none of which imply foresight or teleology, but whose joint effects may yield patterns indistinguishable from randomness at broader scales.
The survival of the luckiest approach integrates the ideas of positive and negative feedback in evolutionary dynamics [
9]. Sexual selection’s positive feedback amplifies traits through intraspecies mate competition, while natural selection’s negative feedback stabilizes populations through interspecies pressures. These combined mechanisms produce a system where “equilibrium” is not optimal and evolution often rewards the lucky rather than the fittest individuals. This perspective disrupts teleological interpretations of evolution, asserting that randomness—not just fitness—is central to evolutionary outcomes.
Figure 1 encapsulates our survival of the luckiest perspective on evolution.
In contexts like perfect competition, Adam Smith’s “invisible hand” describes how individual self-interest aligns with group welfare through negative feedback mechanisms, ensuring that the broader good emerges unintentionally [
9,
10,
12]. However, as Darwin observed, individual incentives can sometimes clash with group interests [
11,
13]. For example, in sexual selection, traits like the peacock’s extravagant tail illustrate how competitive dynamics can lead to a collective action problem, where individual pursuit of advantage imposes costs on the group [
1,
14]. This tension between alignment and conflict underpins the evolutionary dynamics illustrated in
Figure 1.
The survival of the luckiest perspective [
1] reconciles the role of chance with long-standing principles in evolutionary biology, such as frequency-dependent selection. While positive frequency-dependent selection promotes the prevalence of common traits, and negative frequency-dependent selection fosters genetic diversity by favoring rare traits, both mechanisms operate within a stochastic framework. Random environmental changes and genetic drift further reinforce the significance of luck.
In essence, the theory of survival of the luckiest is not a departure from the modern synthesis of evolutionary theory, but rather an extension that emphasizes the interplay between natural and sexual selection. By integrating these distinct yet interconnected dynamics, the theory aligns fully with Darwinian principles, underscoring evolution as a process shaped by both deterministic and stochastic factors. The acknowledgment of chance as a driving force complements Darwin’s insights, enriching our understanding of evolution, while remaining grounded in the framework of descent with modification.
While our previous paper [
1] introduced the theory of survival of the luckiest as a complementary perspective to the modern synthesis of evolutionary theory, it primarily focused on outlining the conceptual framework. What remains unexplored, however, is the theory’s capacity to address identified mechanisms that challenge the modern synthesis. These mechanisms offer an opportunity to showcase the explanatory power of our new perspective.
The justification for our research stems from the need to address recent challenges to the modern synthesis. The “extended evolutionary synthesis” [
15,
16,
17] represents a significant shift in evolutionary thought by emphasizing developmental processes, environmental influences, and extragenetic inheritance alongside natural selection. We propose that the survival of the luckiest framework—by linking natural and sexual selection—offers an interpretation of the modern synthesis that accommodates this contrasting evidence. By considering evolution in its entirety, this approach extends and reinforces the core principles of the modern synthesis, while addressing its critics. We analyze the evidence supporting the extended synthesis through the lens of the survival of the luckiest framework, presenting it as an alternative that preserves the foundational tenets of the modern synthesis.
The modern synthesis asserts that evolutionary adaptations arise through the gradual natural selection of randomly occurring DNA mutations [
6]. In contrast, the extended synthesis seeks to provide a broader framework, placing equal emphasis on the role of an organism’s development and its interaction with environmental conditions in shaping traits and guiding evolutionary trajectories. The extended synthesis suggests that development plays a central role in evolution, complementing natural selection rather than being subordinate to it [
16]. Over the past decade, the extended synthesis has gained attention and remains a topic of intense debate within the field [
17].
Rather than limiting inquiry to advantageous genetic mutations, the extended synthesis emphasizes the role of developmental processes and structural organization in shaping how traits emerge and contribute to fitness. One line of evidence often cited in support of this view is the repeated appearance of traits such as smaller brains, curly tails, floppy ears, and flat muzzles in domesticated animals—features collectively referred to as the “domestication syndrome” [
18]. These traits appear across a wide range of species subjected to similar selective pressures, and their consistent co-occurrence suggests underlying developmental biases that may constrain or guide evolutionary outcomes. Wilkins et al. [
18] propose that changes in neural crest cell behavior during development can account for this suite of traits, offering a mechanistic explanation not easily captured by models relying solely on random mutation and selection. Experimental evidence from long-term domestication studies, such as the Russian farm-fox experiment [
19], supports this hypothesis by showing that selection for behavioral traits can produce correlated morphological changes. In light of such findings, proponents of the extended synthesis argue that incorporating developmental constraints and biases offers a more comprehensive framework for understanding recurring evolutionary patterns [
16].
Moreover, the extended synthesis expands the concept of inheritance beyond genes to include extragenetic factors that influence evolution [
16]. Traits can be transmitted across generations through mechanisms such as social learning, epigenetic modifications, and environmental construction. For instance, epigenetic marks formed during stressful experiences can be passed down, affecting the behavior and physiology of offspring. Some organisms, like dung beetles, actively alter their environment to support the development of future generations. These examples are believed to demonstrate the fundamental role of extragenetic processes in influencing both development and evolution.
Next, we review the evidence often cited in support of the extended evolutionary synthesis [
16,
17]. Applying the survival of the luckiest framework, we provide alternative explanations for this evidence, aiming to reinforce the modern synthesis through a more comprehensive interpretation of evolutionary dynamics.
2. Understanding Evidence and Alternatives
We draw on evidence, primarily from Lala et al. [
17], that the extended synthesis considers cases that are inadequately addressed by the modern synthesis. By examining these cases through the lens of the survival of the luckiest framework, we aim to show how this perspective preserves the foundational principles of the modern synthesis, while providing alternative interpretations to those of the extended synthesis. We consider phenotypic plasticity, plasticity-led evolution, facilitated variation, domesticated animals, cultural transmission and social learning, niche construction, genome reorganization and natural genetic engineering, developmental bias and parallel evolution, epigenetic inheritance and environmental influence, inclusive inheritance, genetic accommodation, evolvability, constructive neutral evolution, exaptation and co-option, genetic drift and population bottlenecks, symbiosis and mutualism, and horizontal gene transfer.
2.1. Phenotypic Plasticity
Phenotypic plasticity refers to the ability of an organism’s phenotype to respond and adapt to environmental cues, triggering signaling pathways or modulating cellular outputs. This adaptability allows genetically similar individuals to exhibit remarkably different traits when exposed to varying conditions [
20,
21].
The blind Mexican cavefish has emerged as a key model system in evolutionary developmental biology and evolutionary ecology, offering insights into the interplay between developmental processes and evolutionary mechanisms [
22,
23,
24,
25,
26,
27].
Astyanax mexicanus is notable for its two distinct populations: river-dwelling fish with normal eyesight and pigmentation, and cave-dwelling fish that are eyeless and have reduced pigmentation. Beyond these well-known traits, cavefish exhibit enhancements in nonvisual sensory systems, changes in bone structure, increased fat storage, and altered behaviors. Importantly, troglomorphic traits such as eye loss and depigmentation have evolved independently across multiple cavefish populations, including separate lineages of
Astyanax, highlighting repeated convergence under similar environmental pressures. This pattern of parallel evolution has been cited as evidence in support of developmental constraints and bias emphasized by the extended synthesis, but it may also reflect the statistical dynamics of phenotypic emergence under the survival of the luckiest framework, where certain variants become recurrently fixed through overlapping selective filters.
Proponents of the extended synthesis argue that these traits are interconnected through shared developmental mechanisms and regulatory networks, which guide evolution along specific trajectories. Experiments seem to demonstrate the role of phenotypic plasticity in shaping cavefish adaptations: environmental stress, such as darkness, activates developmental pathways leading to blindness and increased fat storage. According to this perspective, developmental processes shape evolutionary pathways by biasing the kinds of phenotypic variation that are most readily produced, thereby influencing which traits are likely to emerge and persist under selection ([
17], chs. 7 & 12).
The survival of the luckiest framework interprets these adaptations as outcomes of contingent interactions between environmental pressures and pre-existing phenotypic variation. Traits such as blindness and increased fat storage are not predetermined by developmental programs, but emerge from a broad array of heritable variants, generated through stochastic genetic mutations, environmentally responsive yet probabilistic epigenetic modifications, and developmental plasticity. When environmental conditions shift, some of these variants may confer survival advantages—such as enhanced energy efficiency or improved nonvisual sensory systems—and are subsequently retained through natural selection. In addition, sexual selection may amplify or maintain such traits by favoring cues associated with robustness or developmental stability under stress, not because individuals anticipate future environments, but because mate choice often relies on perceivable proxies of condition. This process does not require cognitive evaluation or foresight; rather, it reflects the alignment of evolved sensory biases with traits that happen to co-occur with fitness-relevant outcomes. Importantly, this framework avoids anthropomorphism and teleology by emphasizing that both natural and sexual selection act on present variation. The convergence of these selection regimes on certain traits, such as nonvisual sensory enhancement, increases the likelihood that some variants will persist under novel environmental pressures—even if the majority do not. This statistical dynamic enables adaptation without invoking rational judgment, predictive foresight, or improbable serendipity.
Therefore, in the absence of predation and under resource limitations, natural selection favors energy-saving traits like increased fat storage and blindness. Simultaneously, sexual selection dynamics in cave populations may shift to emphasize nonvisual sensory cues in mate choice. Traits that improve navigation and communication in darkness could offer a reproductive advantage, reinforcing their prevalence. This perspective frames adaptations as chance-driven developments that are later reinforced by selection dynamics. It emphasizes individual-level advantages without relying on complex group or kin selection mechanisms or developmental processes.
Evolutionary developmental biology has revealed the surprising versatility of regulatory genes, such as Pax6, which plays a central role in eye development. Studies have shown that Pax6 is present in a wide range of species, including some without eyes, suggesting that this gene was co-opted as a regulatory element in eye development through exaptive processes—where existing genetic components acquire new functions without being originally selected for them [
6,
28]. Proponents of the extended synthesis interpret these findings as evidence of developmental bias and the inherent plasticity of developmental pathways, which create structured opportunities for evolutionary innovation. Thus, such regulatory genes act as developmental constraints, guiding the direction of evolutionary change and explaining the repeated appearance of similar traits, such as eyes, across unrelated lineages.
The survival of the luckiest framework offers an alternative perspective by emphasizing the role of contingency and stochasticity in the evolutionary use of regulatory genes. The presence of Pax6 in eyeless species can be interpreted not as a teleological signal of future function, but as a byproduct of ancestral regulatory architecture—one that, in certain lineages, became associated with eye development through historically contingent processes. This association depended on genetic and developmental compatibility, but its fixation was influenced by selective pressures favoring vision in particular environments. The repeated evolution of eyes across taxa reflects a combination of developmental possibilities and selective retention, shaped by both structural constraints and chance. In this view, evolutionary innovation does not follow a predetermined path, but emerges when existing genetic configurations intersect, by chance, with environments where they provide an advantage. Far from implying foresight, this account stresses the probabilistic nature of evolutionary change, grounded in the interaction between variable traits and shifting selective landscapes.
The case of
Astyanax mexicanus illustrates how the survival of the luckiest framework integrates stochastic processes with developmental plasticity and selective pressures. While the extended synthesis prioritizes developmental mechanisms—suggesting that evolution follows where development leads [
17]—this alternative framework underscores the fundamental role of randomness. Beyond cavefish adaptations, the interaction between stochasticity, developmental plasticity, and selection extends to broader evolutionary processes, including sexual reproduction and the coevolution of sexes.
In this broader context, the survival of the luckiest framework can offer an alternative perspective on sexual reproduction, anisogamy, and the coevolution of sexes [
29], incorporating developmental processes alongside stochastic influences. Whereas the modern synthesis focuses on adaptive explanations for traits such as parental care, sex roles, and mate choice, the survival of the luckiest framework shows how random genetic variations, stochastic events, and developmental plasticity intersect to shape these dynamics. For instance, developmental processes may create pathways that facilitate the emergence of specific traits, such as nonrandom female mating preferences, which might initially arise from chance associations between male traits and reproductive success. These traits can then become fixed through the interplay of natural and sexual selection, with developmental mechanisms reinforcing evolutionary trajectories shaped by random environmental pressures.
2.2. Plasticity-Led Evolution
Proponents of the extended synthesis note that development is highly responsive to environmental cues, which can activate signaling pathways or influence cellular outputs. As seen, this adaptability, referred to as phenotypic plasticity, enables genetically similar individuals to exhibit remarkably different traits under varying circumstances [
20,
21]. Thus, developmental processes not only guide evolutionary adaptation, but also drive taxonomic diversity and the appearance of evolutionary novelties. Plasticity-led evolution is therefore a mechanism whereby phenotypic plasticity allows organisms to respond adaptively to environmental changes, with genetic evolution subsequently stabilizing these traits. For instance, an organism might develop a morphological or behavioral trait to address a specific environmental challenge, and over time, genetic changes may assimilate these traits, embedding them within the population. As a result, phenotypic plasticity is a significant force in adaptive evolution, enabling populations to respond rapidly to changing conditions [
16,
17,
20].
From the survival of the luckiest framework, plasticity-led evolution can be viewed as a process shaped by randomness and the contingent interplay between natural and sexual selection. While phenotypic plasticity provides a flexible response to environmental challenges, the initial expression and success of plastic traits depend heavily on stochastic factors. For instance, only individuals with the right developmental potential and who randomly encounter favorable environmental conditions can successfully display adaptive plastic traits. These traits are subsequently filtered by natural selection based on their contribution to survival.
The interplay with sexual selection further shapes plasticity-led evolution. Traits that emerge through plastic responses to environmental pressures may also affect reproductive success. For example, a morphological adaptation that improves survival in a harsh environment might simultaneously enhance an individual’s attractiveness as a mate by signaling resilience or resource acquisition capabilities. This dual reinforcement by natural and sexual selection amplifies the prevalence of such traits in the population. Over time, genetic changes may stabilize these traits, but their initial emergence and spread are contingent on the luck of environmental interactions and mating dynamics.
2.3. Phenotypic Plasticity and Facilitated Variation
Phenotypic plasticity is a defining feature of development, enabling living organisms to modify their shapes and properties in response to environmental changes. For instance, temperature fluctuations can alter protein folding, which, in turn, determines whether specific genes are expressed, while changes in pressure can influence the assembly and dynamics of cellular molecules.
Natural selection influences both the extent and responsiveness of phenotypic plasticity, shaping these traits alongside the physical properties of cells and molecules. A compelling example is found in Bichir fish (
Polypterus spp.), which, when raised on land, develop enhanced walking abilities compared to those reared in water. These fish undergo structural changes in their bones and muscles, improving their terrestrial movement in a way that parallels adaptations seen in the fossil record. Supporters of the extended synthesis argue that experimental evidence suggests that developmental plasticity played a pivotal role in facilitating the evolutionary transition to terrestrial locomotion [
17].
The extended synthesis interprets processes like exploratory behavior in vascular systems as instances of facilitated variation, where resilient developmental mechanisms enable minor genetic changes to produce important, coordinated phenotypic innovations. These mechanisms contribute to evolution by providing adaptive solutions that do not rely solely on genetic mutations. In this view, developmental plasticity allows organisms to generate and refine functional phenotypes, which are later honed through selection, potentially expediting evolutionary change [
16].
Proponents of the extended synthesis challenge the notion of organisms as genetically programmed, arguing that environmental induction plays a greater role in evolution than random mutation. They emphasize the need to consider both selection and developmental mechanisms when explaining adaptive evolution [
20]. This stands in sharp contrast to the perspective of the survival of the luckiest.
From the survival of the luckiest framework, developmental plasticity and facilitated variation can be understood as mechanisms that enhance the chances of survival in unpredictable environments. The ability of vascular systems to expand into oxygen-deficient areas, for instance, reflects not only the organism’s adaptive potential, but also the role of randomness in shaping these outcomes. Chance interactions between environmental stressors and developmental pathways determine which individuals successfully stabilize functional phenotypes. While natural selection refines these traits over generations, their initial emergence often depends on stochastic events and developmental flexibility.
Furthermore, natural and sexual selection interact in shaping these processes. Traits emerging from phenotypic plasticity, such as enhanced physiological responses, can improve both survival and reproductive success. For instance, individuals with superior vascular adaptations may signal greater robustness or resource efficiency to potential mates, increasing their chances of reproductive success. This dual reinforcement ensures that traits influenced by phenotypic plasticity are not only preserved for survival, but also amplified through mate selection.
This interpretation differs from that of the extended synthesis by emphasizing the random interplay of environmental conditions, developmental flexibility, and the combined forces of natural and sexual selection, rather than treating developmental processes as predetermined drivers of evolution. The survival of the luckiest framework incorporates phenotypic plasticity into a broader evolutionary perspective, suggesting the role of randomness and dual selective pressures in shaping phenotypic innovations and evolutionary trajectories.
2.4. Domesticated Animals
Domesticated animals have long been central to understanding evolutionary processes, even though their evolution is predominantly shaped by artificial selection. While domestication involves deliberate human intervention, it does not exclude the operation of natural and sexual selection within the domesticated context. Natural selection persists in the form of environmental pressures that domesticated animals face, such as disease resistance or adaptation to captivity. Similarly, sexual selection may operate indirectly through human preferences, which act as a proxy for mate choice, favoring traits that humans find desirable or practical. These dynamics suggest that domestication is not a complete departure from evolutionary principles, but rather an altered environment where natural, sexual, and artificial selection interact. This interplay provides fertile ground for exploring how developmental processes, stochastic factors, and selective pressures combine to shape the traits observed in domesticated species.
The extended synthesis explains the consistent appearance of traits such as smaller brains, curly tails, white patches, and flat muzzles in domesticated animals through a developmental lens. It argues that these traits are not random, but are shaped by developmental biases rooted in shared genetic and cellular mechanisms. Specifically, these features are linked to the neural crest, an embryonic cell type that plays a crucial role in the development of these traits [
18]. The repeated evolution of such traits across different species and time periods supposedly reflects the inherent constraints and pathways of development, which make certain traits more likely to arise together. Thus, understanding these developmental processes is key to predicting how traits originate, the directions evolution might take, and the pace at which it occurs [
16].
Wilkins et al. [
18] proposed that the traits collectively known as the domestication syndrome—such as docility, reduced brain size, altered pigmentation, and changes in morphology—can be largely traced to mild deficits in neural crest cell development. The initial target of human selection, docility, may be linked to a slowed pace of neural development, resulting in reduced fear–startle responses. These changes, initiated by mild neurocristopathy, are hypothesized to involve a large genetic target, due to the vast number of genes implicated in neural crest cell biology. While this explanation offers a cohesive developmental–neurobiological pathway, certain traits, like curly tails, remain unaccounted for. Moreover, the genetic and epigenetic underpinnings of the domestication syndrome require further investigation, including the identification of specific genes and loci contributing to these traits across multiple species. Progress in genetic mapping of domesticated species like rats, foxes, and dogs has laid a foundation for testing these hypotheses.
From the perspective of the survival of the luckiest, these observations can be interpreted differently, through the role of stochastic factors in shaping domestication outcomes. Traits like docility and reduced fear responses may have emerged as random genetic variations in individuals subjected to the novel and artificial environment of domestication. While mild neurocristopathy might create a predisposition for certain traits, their fixation depends on chance interactions between genetic variation, environmental pressures, and human preferences acting as selective agents. Thus, the consistent emergence of domestication traits across species is not solely the result of deterministic developmental pathways, but also a product of randomness in the breeding environment, genetic drift, and pleiotropy. For instance, traits like curly tails, which lack a direct developmental explanation, may persist due to genetic linkage with more directly advantageous traits like docility.
Therefore, traits like tameness, the primary target of selective breeding, are associated with other phenotypic changes due to pleiotropy or genetic linkage. While the developmental pathways involving the neural crest may play a role, the consistent emergence of these traits is also heavily influenced by random genetic mutations and stochastic factors within the breeding environment. The traits are not the inevitable result of developmental biases, but are contingent outcomes shaped by chance interactions between genetic variation and selective pressures.
Zeder [
30] posed critical questions about the pathways to animal domestication, exploring differences in the behavioral, physiological, and morphological responses of animals subjected to varying domestication processes, including commensal, prey, and directed routes. For instance, she considered the variability in tameness and cranial morphology among species that followed the commensal pathway, such as dogs, pigs, and cats, versus those domesticated through the prey or directed routes, such as sheep and horses. Zeder emphasized the need to identify distinctive genetic markers and archeological evidence that could differentiate these pathways and their respective selective pressures. Further, she questioned the varying capacities for feralization across domesticates, noting that animals with commensal origins may revert more effectively to feral behaviors.
From the survival of the luckiest perspective, the diversity of domestication outcomes can be interpreted as a consequence of stochastic interactions between genetic variation, environmental pressures, and human intervention. Traits such as tameness or specific morphological changes, rather than being preordained outcomes of a particular domestication pathway, emerge as contingent results of random genetic mutations and chance alignments with human preferences or environmental conditions. For instance, the inconsistent presence of cranial changes across species may reflect the randomness inherent in genetic drift or pleiotropy, rather than deterministic developmental pathways. Similarly, the variable feralization capacities of commensal versus prey domesticates could arise from the stochastic nature of behavioral and physiological traits that were inadvertently selected during domestication. This framework shifts the focus from deterministic genetic signatures to the role of chance in shaping domestication processes, emphasizing that the observed variability reflects the interplay of randomness and human-mediated selective pressures.
Furthermore, this perspective suggests the unpredictable impact of domestication on environmental and biodiversity outcomes. While the extended synthesis might attribute modern domestication traits to developmental constraints and genetic pathways, survival of the luckiest underlines the role of unpredictable events in shaping traits that align with human needs, often at ecological costs. Thus, understanding the interplay between randomness, environmental factors, and human intervention can inform conservation efforts, improve animal welfare in captivity, and mitigate the biodiversity impacts of domestication practices.
Domesticated animal populations often face genetic bottlenecks and inbreeding from controlled breeding, reducing genetic diversity and increasing vulnerability to inbreeding depression. These factors are critical for the sustainability and resilience of these species, requiring careful genetic management. In connection to this, Frankham [
31] reviewed the evidence on the role of genetic factors in increasing extinction risk, particularly focusing on inbreeding depression, loss of genetic diversity, and mutation accumulation. His findings suggest that inbreeding depression and reduced genetic diversity elevate extinction risk in laboratory populations of naturally outbreeding species. Similar patterns have been observed in wild populations, with case studies and computer simulations supporting the conclusion that genetic factors contribute significantly to extinction risk. Importantly, Frankham argued that most species persist long enough for genetic factors to impact their survival, emphasizing that mutation accumulation plays a smaller role, due to the extensive time required for its effects to manifest. He concluded that ignoring genetic factors leads to an underestimation of extinction risk and the development of potentially ineffective recovery strategies.
From the perspective of the survival of the luckiest framework, extinction risk can also be viewed through the lens of stochastic processes, considering the interplay between random environmental changes and genetic predispositions. While genetic factors, such as inbreeding depression and reduced diversity, undeniably contribute to extinction risk, chance events—such as sudden habitat loss or unpredictable environmental fluctuations—play an equally critical role. For example, a population with relatively high genetic diversity may still face extinction if it encounters an abrupt environmental shift that exceeds its adaptive capacity. Thus, genetic factors interact with these stochastic pressures, amplifying or mitigating their effects based on chance alignments between genetic predispositions and environmental challenges.
Moreover, extinction risk cannot be fully understood without considering the random factors influencing survival and reproduction at an individual level. Populations with reduced genetic diversity may be particularly vulnerable to stochastic mortality events, such as disease outbreaks or extreme weather, where individuals lucky enough to possess advantageous traits survive by chance. This perspective suggests the need to integrate both deterministic genetic factors and stochastic environmental processes into conservation strategies. By acknowledging the role of randomness, the survival of the luckiest framework complements Frankham’s findings, offering a broader understanding of extinction dynamics and advocating for recovery strategies that address both genetic vulnerabilities and the unpredictable challenges faced by threatened populations. While domesticated animals are typically abundant and protected under human care, their reliance on controlled environments and selective breeding increases their risk in the face of unexpected challenges. Their reduced genetic diversity, a consequence of breeding for specific traits, makes them less adaptable to novel diseases, environmental shifts, or disruptions in human management. Moreover, many domesticated species exist in managed populations, with limited opportunities for natural selection to increase resilience, leaving them disproportionately vulnerable to stochastic mortality events compared to their wild counterparts.
Natural and sexual selection also interact to shape the traits observed in domesticated animals. While tameness is the primary trait targeted during selective breeding, sexual selection likely plays a reinforcing role. In a domesticated environment, humans act as agents of both natural and sexual selection, favoring animals that exhibit not only behavioral traits like tameness, but also secondary traits perceived as desirable, such as a smaller size or appealing coloration. For example, traits like white patches or curly tails, though not directly related to survival, may inadvertently influence human preferences, effectively acting as proxies in a form of artificial sexual selection. These traits could then become linked with tameness through genetic or developmental pathways, further cementing their prevalence.
Trut et al. [
19] reviewed the evolutionary trajectory of domestic animals, with a focus on the initial stages of domestication and their impact on subsequent evolutionary processes. Using the domestication of dogs (
Canis familiaris) as a foundational example, they emphasized the rapid acceleration of phenotypic variation driven by both unconscious and later deliberate selection of behavioral traits by humans. This process was exemplified through a longitudinal experiment on silver foxes (
Vulpes vulpes) selected for tameability. Over generations, the foxes exhibited behavioral, morphological, and physiological changes, such as reduced fearfulness, altered coat patterns, and changes in reproductive timing, paralleling traits observed in domesticated dogs. The study suggests the role of neurospecific regulatory genes and their developmental and molecular genetic mechanisms as drivers of these transformations. Trut et al. concluded that the selection for behavioral traits played a central causative role in the evolutionary shifts seen during domestication.
The survival of the luckiest can offer an alternative perspective on the observations made by Trut et al., suggesting the interplay between randomness, genetic predispositions, and selective pressures. While the selection for tameability was deliberate, the associated morphological and physiological changes could be understood as contingent outcomes of random genetic variations that have happened to align with human preferences or environmental conditions in domesticated settings. For instance, traits like altered coat patterns or changes in reproductive timing may not have been directly targeted, but may have emerged as byproducts of genetic linkage or pleiotropy, subsequently reinforced by selective pressures.
Moreover, the nature of the genetic changes underlying domestication is stochastic. The diversity observed in early domesticated species, such as dogs and foxes, may reflect the interplay between chance genetic mutations and the environmental conditions of human-managed habitats. Traits like docility, while beneficial for coexistence with humans, may have initially arisen in a subset of individuals by chance, and only later become fixed through selective breeding. This randomness challenges deterministic interpretations of domestication as solely the product of human intent or developmental constraints.
Traits that adapt for survival in human-managed environments are also influenced by sexual selection, as reflected in human esthetic and practical preferences. Their emergence results from stochastic genetic variation, selective pressures, and the interplay of natural and sexual selection. This perspective challenges the deterministic implications of the extended synthesis, proposing that traits arise not from fixed developmental constraints, but through randomness and context-dependent evolutionary processes.
2.5. Cultural Transmission and Social Learning
In alignment with the extended synthesis, Jablonka and Lamb [
32] asserted that induced and acquired changes play a key role in evolution, challenging the gene-centered perspective of the modern synthesis, which attributes adaptation exclusively to natural selection acting on random DNA variations. They suggested that heredity extends beyond genes, identifying four dimensions of inheritance that influence evolution: genetic, epigenetic (non-DNA cellular transmission of traits), behavioral, and symbolic (transmission through language and other forms of symbolic communication). This marks a significant divergence from the modern synthesis.
Advocates of the extended synthesis argue that cultural transmission and learning are now widely acknowledged as essential drivers of animal behavior and adaptation, and this challenges the conventional focus on genetic inheritance. Numerous species rely on social learning and imitation to transmit critical information across generations, including feeding techniques, predator recognition, migratory pathways, and even mate and breeding site choices [
33,
34].
Proponents of the extended synthesis cite humpback whales (
Megaptera novaeangliae) as an example of cultural transmission shaping adaptive strategies. Their foraging behaviors, such as bubble-net feeding in the northeast Pacific and lobtail feeding in the Gulf of Maine, are seen as learned innovations, rather than products of genetic mutations or natural selection. These behaviors, shared across individuals and generations, allow whales to exploit locally abundant prey. In addition, each population develops unique songs that evolve too rapidly to be solely attributed to genetic changes. Both their songs and foraging strategies are thought to be transmitted culturally, independent of inherited genetic variation [
17]. Similarly, Mojave Desert woodrats (
Neotoma lepida) adapt to a toxic diet by acquiring detoxifying microbiomes through behavioral mechanisms, such as consuming soil and feces in their environment. Moreover, socially transmitted traits, such as tool use taught by primates or song learning in birds, are also forms of non-genetic inheritance. These behaviors create new selective pressures and act as adaptive responses to environmental challenges, working alongside natural selection to shape evolutionary outcomes.
The survival of the luckiest framework can offer a different explanation for these phenomena. Behaviors such as bubble-net feeding in whales or the dietary adaptations of woodrats can be understood as emergent outcomes of chance interactions between individual experiences, environmental conditions, and survival pressures. The transmission of epigenetic marks can be seen as a mechanism that increases the chances of survival under specific circumstances, driven by random environmental triggers. These events do not necessarily represent a directed path of evolution, but are instead shaped by the interplay of luck, adaptability, and selective pressures.
Extragenetic inheritance is not an independent driver of evolution, but is a tool that organisms use to navigate unpredictable environments. The passing down of learned behaviors, microbial associations, or epigenetic modifications increases the odds of survival for subsequent generations facing similar conditions. However, the appearance and persistence of these traits depend heavily on chance environmental changes and the random success of individuals who first adopt these adaptations. Thus, rather than displacing the modern synthesis, the survival of the luckiest framework incorporates extragenetic phenomena into a broader narrative that emphasizes the role of randomness in shaping evolutionary trajectories.
The interplay between natural and sexual selection can also influence the persistence and transmission of behaviors and traits in whales and desert woodrats. For instance, in whales, bubble-net feeding strategies not only maximize survival by improving hunting efficiency, but may also influence mate selection. Whales showing greater skill or innovation in foraging could gain an advantage in sexual selection if these traits signal fitness or resource acquisition potential to potential mates. Similarly, in desert woodrats, the transmission of gut microorganisms through maternal feces improves the offspring’s ability to digest toxic plants, providing a survival advantage. However, the quality of these microbial communities could indirectly influence mate selection by affecting the physical health of individuals, creating a feedback loop between survival and reproductive success. This dynamic interplay ensures that traits benefiting survival under natural selection are further filtered by sexual selection. Therefore, this perspective contrasts with the deterministic tone of the extended synthesis, which views cultural transmission as an active evolutionary force. Instead, the survival of the luckiest framework emphasizes the contingent nature of these behaviors.
In line with our survival of the luckiest approach, Koonin [
35] argued that evolutionary processes are profoundly shaped by stochasticity and contingency, rather than being strictly deterministic. He identified the key role of random events, such as genetic drift, horizontal gene transfer, and environmental fluctuations, in influencing evolutionary outcomes. While Koonin did not explicitly address the survival of the luckiest approach’s emphasis on the interplay between natural and sexual selection in amplifying the role of chance, his framework aligned with its core principles. He suggested that the apparent complexity of evolutionary systems often arises from the interaction of random processes, rather than from targeted genetic or epigenetic changes. For instance, the transmission of traits through mechanisms such as microbiome acquisition or cultural behaviors can be attributed to unpredictable ecological interactions, rather than predetermined evolutionary pathways. Thus, adaptations may emerge not only through cumulative selection, but also through fortuitous circumstances that influence survival and reproductive success.
2.6. Niche Construction
Niche construction refers to the process by which organisms actively modify their external environments, creating developmental biases that supposedly influence their own evolution and that of their offspring. While mainstream biology does not typically regard niche construction as a distinct evolutionary process, it accepts the important role of organism–environment interactions in evolutionary causation [
36]. Examples of niche construction include animals building nests, burrows, or pupation chambers, as well as plants altering soil properties through the secretion of exudates and allelopaths. Often, these modifications serve to regulate the developmental conditions experienced by offspring, such as buffering them from predators or thermal fluctuations, perhaps shaping their evolutionary trajectories [
36,
37,
38].
A related concept is reciprocal causation, where A causes B, and B, in turn, causes A, creating a cyclical relationship. This concept suggests that developing organisms are not merely the products of evolution, but are also active contributors to evolutionary processes [
16]. Reciprocal causation supposedly influences the evolution of morphology and other aspects of the phenotype [
39]. Through continuous cycles of interaction, developmental processes have both shaped and been shaped by evolution through natural selection. In this feedback loop, natural selection sculpts developmental processes, while those same processes channel and direct evolutionary trajectories [
16,
17]. Thus, reciprocal causation suggests that organisms and their environments coevolve, with niche construction actively shaping selective pressures [
16].
Odling-Smee et al. [
38] observed that niche construction actively modifies biotic and abiotic components of the external environment, which fundamentally alter sources of natural selection. They argued that niche construction generates evolutionary feedback on a scale previously underestimated. In addition, niche construction plays a pivotal role in ecology by facilitating ecosystem engineering and influencing the flow of energy and nutrients within ecosystems. Thus, niche construction and ecological inheritance represent key evolutionary processes that complement traditional mechanisms.
The extended synthesis interprets the behavior of dung beetles as an example of niche construction [
16,
38]. The dung beetle’s practice of creating nutrient-rich dung balls with added microbes is seen as a developmental mechanism that directly impacts the growth, morphology, and eventual evolution of their offspring. By shaping the environment in which larvae develop, dung beetles supposedly play an active role in directing evolutionary processes.
In contrast, when examining the relationship between variation, fitness, and inheritance, the modern synthesis can explain the adaptive fit between dung beetles and their environments in straightforward terms: mutations alter the beetles’ brood ball processing in ways that enhance fitness. Brood ball processing is considered a proximate mechanism, and fitness differences explain adaptation.
However, proponents of the extended synthesis argue that the underlying causation is far more complex. For instance, the fitness contributions of certain dung beetle traits, such as offspring body size and developmental time, are influenced by phenotypic variation in the niche-constructing behaviors of mothers and larvae. Here, inheritance is tightly linked to phenotypic variation and fitness differences, as the brood ball—a parental effect—is ecologically inherited by the larvae. At the same time, phenotypic variation is itself shaped by inheritance, since beetles develop within the constructed environment of the brood ball, which influences both their traits and the relationships among those traits. Through these interactions, developmental mechanisms bias phenotypic variation, inheritance, and fitness, creating a more intricate causal network. Advocates of the extended synthesis contend that attributing the complementarity between beetles and their environments solely to fitness differences leads to an incomplete understanding of adaptation [
17].
Nevertheless, the survival of the luckiest framework can offer an alternative explanation: while constructing dung balls undoubtedly benefits offspring development, the emergence and persistence of this behavior are outcomes of random environmental pressures and survival challenges faced by ancestral populations. The initial adoption of dung ball construction may have arisen through chance variations in behavior that provided a marginal survival advantage. Over time, selective pressures favored individuals engaging in this behavior, but its origin and propagation are deeply tied to randomness and the unpredictable interplay between individual actions and environmental conditions. Therefore, the dung beetle’s niche construction is a behavior that enhances survival in a specific context, rather than a deterministic driver of evolution. The size, shape, and development of the beetles, which are influenced by their dung balls, are not preordained outcomes of this behavior, but are shaped by the random interactions of genetic variation, environmental constraints, and chance survival events. As a result, the survival of the luckiest framework integrates niche construction as an important but contingent element of evolution. This reinforces the modern synthesis by framing these behaviors within a broader narrative of stochastic processes and selective pressures.
The interplay between natural and sexual selection also shapes the dung beetle’s niche construction behavior. While the creation of nutrient-rich dung balls improves offspring survival by providing a controlled developmental environment, this behavior may also influence sexual selection dynamics. For example, male dung beetles that build larger or higher-quality dung balls may secure a reproductive advantage by signaling their superior resource-gathering abilities, thereby attracting more mates. Similarly, females may select mates based on the quality of the dung balls, as these directly impact the survival and fitness of their offspring. This interaction between natural selection, which favors offspring survival, and sexual selection, which favors traits that improve reproductive success, creates a feedback loop that amplifies the evolutionary importance of dung ball construction. By incorporating this dual selective pressure, the survival of the luckiest framework shows how the behavior’s persistence is not solely a product of niche construction, but also a result of the chance alignment of environmental conditions, individual actions, and reproductive dynamics.
Another favorite example cited by proponents of the extended synthesis is earthworms modifying soil to create conditions that enhance their survival, effectively simulating an aquatic environment on land. However, this behavior likely originated as a chance variation in ancestral populations. Individuals that engaged in soil processing may have experienced better survival, particularly in fluctuating moisture conditions. While natural selection eventually reinforced this trait for its survival benefits, its initial emergence was shaped by random environmental pressures and individual actions. In addition, earthworms that create more stable or resource-rich environments could attract more mates or produce healthier offspring, reinforcing the behavior through a feedback loop between survival and reproduction. Thus, although selection gradually reinforces these traits over time, their emergence and continued presence depend on chance and the interplay between environmental conditions and selective pressures.
In summary, in contrast to the extended synthesis, which positions niche construction as an active and deterministic evolutionary process, the survival of the luckiest suggests its contingent nature. The emergence of niche construction behaviors, their ecological impacts, and their evolutionary importance are seen as outcomes of random variations filtered through the dual pressures of natural and sexual selection.
2.7. Genome Reorganization and Natural Genetic Engineering
Shapiro [
40] challenged the modern synthesis by advocating for an information- and systems-based perspective on evolutionary processes. He combined ideas such as symbiogenesis, epigenetics, and saltationism, arguing that evolution is an active, cell-driven process. Regulated by epigenetic mechanisms, this process can prompt rapid and significant changes through pathways including horizontal DNA transfer, interspecific hybridization, whole-genome duplication, symbiogenesis, and extensive genome restructuring.
The extended synthesis interprets phenomena such as genome reorganization during hybridization and the function of natural genetic engineering systems like CRISPR–Cas9 [
41] as evidence of evolutionary mechanisms that extend beyond the gradual selection of random DNA mutations. It argues that these processes—including the incorporation of viral information into bacterial genomes, and the reorganization of genetic material in hybrid species like modern wheat—demonstrate the active and dynamic nature of genome evolution. From this viewpoint, these mechanisms are crucial for understanding how genomes adapt to environmental challenges and opportunities, potentially accelerating evolutionary change and influencing patterns of genetic diversity [
16].
CRISPR, which stands for Clustered Regularly Interspaced Short Palindromic Repeats, is a family of DNA sequences found in bacteria and archaea that functions as an adaptive immune system against viruses. Along with associated proteins such as Cas9, these sequences constitute the CRISPR-Cas system—a natural antiviral defense mechanism that has been repurposed as a precise gene-editing tool in biotechnology and medicine [
41,
42]. CRISPR-Cas9 originates from coevolutionary dynamics between viruses and bacteria. In its natural context, the CRISPR system captures fragments of viral DNA and integrates them into the bacterial genome, effectively creating a genetic record of past infections. This adaptive mechanism enables bacteria to swiftly recognize and eliminate invading viruses during subsequent encounters, illustrating an evolutionary response finely tuned by environmental pressures.
Sexual selection principles, often linked to mate choice and reproduction in complex organisms, also apply to viral and bacterial evolution. Viruses show similar processes through mechanisms such as phage-mediated selection—where bacteriophages compete for bacterial hosts—viral interference, and genetic exchange via recombination and reassortment [
43]. Bacteria also exchange genetic material through conjugation, gene transfer agents, and quorum sensing [
9], a form of chemical communication that coordinates gene sharing within populations [
44]. Although these processes differ from eukaryotic sexual selection, which involves intentional mate choice and sexual reproduction, they influence microbial genetic diversity and coevolution with hosts. Methods like phylogenetics, experimental evolution, and bioinformatics have helped to uncover how these microbial systems drive adaptation and diversification [
43,
44,
45].
The survival of the luckiest framework can view genomic phenomena as outcomes of random events coupled with selective forces. In wheat hybridization, for example, the merging of genetic material from different species is essentially a chance occurrence. The resulting genomic reorganizations may offer adaptive benefits, yet their establishment in a population depends on fluctuating environmental conditions, the viability of hybrid individuals, and the strength of selective pressures. Similarly, the CRISPR–Cas system can be understood as a survival strategy that evolved through random pathogen encounters. Its effectiveness, like that of wheat hybridization, depends on specific selective contexts that favor such defense mechanisms. Overall, genome evolution is shaped by random events, such as hybridization or viral infections, that create opportunities for change. These changes emerge not through a directed process, but as a result of chance interactions working alongside natural selection. This perspective connects genomic phenomena to the modern synthesis by emphasizing both their stochastic origins and their integration into the broader dynamics of adaptation.
Furthermore, natural and sexual selection interact to shape genomic phenomena such as hybridization and natural genetic engineering systems. In hybrid species like modern wheat, genome reorganization not only enhances adaptation to environmental pressures, but also influences reproductive compatibility and success, playing a role in sexual selection. Hybrids with reorganized genomes that improve resource acquisition, stress tolerance, or other survival traits may gain a reproductive advantage by becoming preferred mates. A similar dynamic applies to the CRISPR–Cas9 system, which primarily evolved as a bacterial defense against pathogens. Although natural selection is the main driver, it indirectly boosts reproductive success by helping bacterial populations to survive and outcompete rivals in resource-poor environments. These cases show that random genomic changes—like hybridization or viral interactions—are both shaped and reinforced by natural and sexual selection. Traits that improve survival can also influence mating, creating a cycle that links reproductive success to overall genomic evolution.
2.8. Developmental Bias and Parallel Evolution
Proponents of the extended synthesis argue that the parallel evolution of cavefish populations is best understood through the interconnected nature of multiple traits. Rather than evolving independently, these traits are shaped by interacting developmental mechanisms within shared regulatory networks, similar to those observed in the domestication syndrome. This interaction produces a set of developmentally linked traits, where underlying mechanisms create predictable patterns of trait covariation (developmental biases or genetic channeling). As a result, some traits are indirectly favored when selection acts on others, showing how developmental and evolutionary processes are deeply intertwined [
17].
Thus, developmental bias is viewed as a process that channels phenotypic variation along predictable pathways, enabling parallel evolution. Biases in development, rather than randomness, drive the emergence of specific phenotypes, which are subsequently fine-tuned by natural selection to suit local environmental conditions. Therefore, developmental systems guide evolution by making certain phenotypic outcomes more likely than others.
Likewise, the parallel evolution of cichlid fishes (Cichlidae) in the African Rift Valley lakes appears to be guided by developmental mechanisms that bias variation along particular axes of morphospace. For example, shared developmental mechanisms can produce similar body shapes across independently evolving populations. These biases are shaped by plastic responses to variations in diet and the mechanical stresses they impose, as well as by the environmental conditions experienced during early developmental stages, such as those of eggs and hatchlings. Thus, shared molecular mechanisms likely underpin both the plastic responses and evolutionary adaptations observed in these species.
Brakefield [
46] argues that developmental bias can shape both the pace and direction of evolution, leaving its imprint on patterns of biodiversity. For him, a comprehensive evo–devo approach, integrating evolutionary and developmental genetics, can provide a framework for experimentally analyzing these biases. Such analyses can reveal how shared genetic or developmental pathways among morphological traits contribute to the emergence of diversity. Moreover, Brakefield emphasizes that developmental bias itself evolves, which influences variation in evolvability. He further asserts that modern methodologies enable the assessment of the relative contributions of natural selection and genetic or developmental architecture in shaping organic diversity, particularly in cases of adaptive radiations [
47] and parallel evolution.
Darwin’s finches are a classic example of adaptive radiation, where 18 species have diversified ecologically and morphologically from a common ancestor that arrived in the Galapagos archipelago approximately two million years ago [
47]. Speciation occurred as populations on different islands diverged in traits like body size and beak morphology. These differences were further increased when populations coexisted on a single island. Natural selection played a key role in this process, increasing ecological differences during periods of food scarcity, such as droughts. Genomic studies have revealed genetic variation in two important transcription factor genes associated with beak morphology. Beaks are essential functional traits that play a central role in the ecological diversification of Darwin’s finches by facilitating food gathering and processing. Divergence in beak traits, known as character displacement, occurs when two coexisting species compete for limited resources and evolve away from each other to reduce competition.
Darwin’s finches primarily breed within their own species, recognizing potential mates by their species-specific song and appearance. However, occasional hybridization occurs because hybrids are both viable and fertile. This gene exchange through hybridization supposedly provides an alternative route to the formation of new species. When hybrids differ sufficiently in traits like song and appearance, they may only breed with each other, forming a distinct hybrid group through introgressive hybridization. Speciation in sexually reproducing species occurs when barriers to gene exchange arise. These barriers may act before mating (premating) or after fertilization. In Darwin’s finches, a premating barrier is formed by sexual imprinting, where young finches learn the song and morphology of their species early in life, and later use these traits to select mates. However, this barrier can break down if young finches mistakenly learn the song of another species, potentially leading to hybridization. Introgressive hybridization offers another path to speciation. Hybrids that are sufficiently distinct from their parental species in traits such as song and appearance may form a separate group that only breeds within itself. As a result, this process shows how both natural selection and gene flow can interact to drive the diversification of species.
The survival of the luckiest framework can frame developmental bias and parallel evolution as outcomes shaped by chance interactions between environmental pressures, genetic predispositions, and the dual forces of natural and sexual selection. While developmental systems may constrain the range of possible phenotypes, it is the stochastic nature of environmental changes and population dynamics that determines which traits emerge and persist. For example, the similar body shapes observed in cichlid fishes across two lakes may reflect random environmental factors, such as resource availability or predation pressures, that coincidentally favor analogous traits in each setting.
The interplay between natural and sexual selection further amplifies this dynamic. Traits like body shape that improve resource acquisition or predator avoidance may simultaneously influence mate selection. In environments where certain shapes confer a survival advantage, individuals with these traits are more likely to reproduce, reinforcing the trait’s prevalence through sexual selection. For instance, a streamlined body may not only aid in foraging or escaping predators, but also signal fitness to potential mates, which creates a feedback loop between survival and reproductive success.
Furthermore, the diversification of Darwin’s finches illustrates the pivotal role of chance events and interactions in shaping evolutionary outcomes. Instead of attributing adaptive radiation solely to deterministic forces like natural selection, random genetic variations and unforeseen environmental pressures open up opportunities for certain traits to emerge and endure. For example, beak variations among finches may initially arise through fortuitous mutations that happen to align with ecological demands during periods of resource scarcity. In such scenarios, the luckiest individuals thrive and pass on their traits. Hybridization, too, can be viewed through this lens as an outcome of chance encounters where barriers to gene exchange break down unpredictably, enabling the formation of new hybrid groups. Therefore, the interplay of randomness, adaptability, and selective pressures shapes evolutionary trajectories in ways that are often contingent on historical and ecological contexts. While developmental systems set the stage for developmental variation, it is luck—mediated by environmental randomness and reproductive dynamics—that plays a decisive role in determining which traits dominate. Unlike the extended synthesis, which attributes parallel evolution to developmental biases as deterministic forces, the survival of the luckiest approach suggests the contingent nature of evolutionary outcomes.
2.9. Epigenetic Inheritance and Environmental Influence
Epigenetics refers to modifications that influence gene activity without altering the underlying DNA sequence. These changes often involve chemical molecules, such as methyl groups or histone modifications, that regulate gene expression. While many epigenetic marks are developmentally programmed or triggered by environmental factors—such as diet, exposure to pollutants, or social interactions [
48,
49,
50]—some are influenced by the DNA sequence itself. For instance, specific nucleotide motifs can recruit chromatin-modifying proteins, guiding where certain marks are deposited [
51]. At the same time, variation in the establishment or maintenance of epigenetic marks—particularly during early development—can introduce stochastic differences in gene expression among genetically identical individuals [
32]. This dual character of epigenetic regulation—partially sequence-guided, environmentally responsive, and occasionally stochastic—makes it a rich and dynamic contributor to phenotypic diversity.
The extended synthesis interprets epigenetic inheritance as a mechanism through which environmental conditions can shape evolutionary trajectories. For instance, plants exposed to drought may develop physiological adaptations that are passed to their offspring via epigenetic modifications, enabling subsequent generations to cope better with similar stressors. Thus, epigenetic changes serve as active responses to environmental pressures, offering a rapid, non-genetic mechanism of adaptation that complements traditional genetic inheritance and contributes to guiding evolutionary processes [
16,
32].
However, an alternative explanation remains consistent with the survival of the luckiest framework: while epigenetic responses to environmental stress—such as drought—are often repeatable across individuals and populations, their long-term evolutionary impact is still shaped by contingent factors. In drought-adapted plants, for example, exposure to water stress can reliably trigger specific epigenetic modifications that enhance survival [
49,
51]. Yet, the evolutionary significance of these changes depends not just on their immediate functional benefit, but also on whether they are inherited and selected for across generations. Only in environments where drought conditions persist—and where the epigenetically modified individuals also achieve greater reproductive success—will these traits become embedded in population-level patterns. Thus, while the mechanistic response is environmentally guided and repeatable, the fixation of such traits over time still involves a probabilistic interplay between environmental persistence, heritability, and selective dynamics.
The interplay between natural and sexual selection adds another dimension to this interpretation. While epigenetic changes like drought tolerance are primarily driven by natural selection, they may also influence reproductive success. Plants that exhibit enhanced survival traits may produce more seeds or attract more pollinators, indirectly affecting their reproductive fitness. This creates a feedback loop where traits favored by natural selection are further reinforced through sexual selection dynamics, amplifying their prevalence in the population.
Despite being non-genetic, epigenetic inheritance is not fully random. While some epimutations arise stochastically, many epigenetic responses are environmentally induced and exhibit partial repeatability across individuals and generations [
49,
50]. For instance, studies in plants and animals show that stress-related epigenetic modifications can recur under similar environmental conditions, and in some cases, these changes are transmitted across multiple generations [
51,
52]. This indicates that epigenetic systems are sensitive yet constrained, allowing for both environmentally responsive variation and inheritance patterns that selection can act upon.
The survival of the luckiest framework accommodates this dual nature by interpreting epigenetics as a source of probabilistic variation—intermediate between deterministic genetic inheritance and pure randomness. Rather than treating epigenetic mechanisms as fully directed adaptations or as noise, the framework emphasizes that they increase the range of phenotypic outcomes available to selection, especially under fluctuating environments. This broadens the scope of traits that may confer fitness advantages, making it more likely that some individuals—by chance—possess combinations of traits that align with emerging ecological pressures. In this way, epigenetic inheritance enhances the evolutionary sampling process without implying foresight or developmental predestination.
2.10. Inclusive Inheritance
Proponents of the extended synthesis take the unconventional view that inheritance extends beyond a simple transfer of genes and cytoplasmic resources. Instead, they argue that it is a dynamic, time-distributed developmental process through which various resources are made available to the next generation. They suggest that many extragenetic inheritance processes should not be dismissed as noise, fine-tuning, or ornamental additions, but rather seen as essential mechanisms for rapid adaptive responses. In this perspective, parent–offspring similarity is viewed not as the primary goal of inheritance, but as a byproduct of a system designed to enable accurate adaptation to environmental challenges [
17]. Inclusive inheritance expands the concept of inheritance beyond genes to include transgenerational epigenetic inheritance, physiological inheritance, ecological inheritance, social transmission (behavioral), and cultural inheritance. As a result, acquired traits can influence evolution by shaping phenotypic variation subject to selection, altering environments, and contributing to heritability [
16]. Thus, this perspective may be regarded as neo-Lamarckian.
For example, foraging techniques in birds and migratory routes in mammals are passed on through learning, rather than genes, while ecological inheritance involves environmental modifications, such as beaver dams or coral reefs, that influence the survival and reproduction of future generations. These diverse pathways are viewed as fundamental drivers of evolutionary change, shaping adaptation and influencing the dynamics of populations by extending the scope of heritable traits beyond genetic material [
32].
The survival of the luckiest framework can interpret inclusive inheritance as a phenomenon driven by random processes and the combined influence of natural and sexual selection. For instance, behaviors like foraging techniques or migratory patterns likely originated as random individual innovations that were initially advantageous in specific environmental contexts. These behaviors persisted because they conferred survival benefits, but their transmission through cultural or social learning depended on chance encounters and the success of the individuals who first exhibited them. Similarly, ecological inheritance, such as the environmental modifications created by beavers or corals, arose from chance variations in behavior or structural development that happened to align with environmental pressures.
Natural and sexual selection further refine these inherited traits. For example, a bird’s foraging technique or a mammal’s migratory success may improve survival, but these behaviors can also play a role in mate selection. Individuals that exhibit superior skills or behaviors, such as effective resource gathering or successful navigation, may be perceived as more attractive mates, reinforcing the transmission of these traits across generations. In the case of ecological inheritance, the modified environments created by one generation may indirectly influence sexual selection by altering mate availability or competition dynamics in subsequent generations.
2.11. Genetic Accommodation
Genetic accommodation refers to the process by which natural selection refines the form, regulation, and phenotypic integration of novel traits. This process reflects the dynamic relationship between genotype and phenotype, where environmental inputs influence gene expression, trait development, and phenotypic integration. Phenotypic plasticity and epigenetic modifications can shape evolution in two key ways: first, by promoting evolutionary responses to environmental changes through population persistence or by exposing cryptic genetic variation to selection; and second, by enabling genetic accommodation to stabilize and improve adaptive phenotypic traits over time [
53].
The extended synthesis views genetic accommodation as a process where phenotypic traits initially produced by plastic responses to environmental changes are gradually stabilized through genetic evolution. For instance, if environmental stress induces a trait such as color change in lizards for camouflage, and this trait improves survival, natural selection favors individuals exhibiting it. Over time, genetic mutations may fix the trait, making it less dependent on environmental triggers. This process suggests the dynamic interaction between phenotypic plasticity and genetic evolution, positioning environmental influences as central to evolutionary change [
16,
20].
The survival of the luckiest framework can provide an alternative interpretation of genetic accommodation. The emergence of a plastic trait like camouflage coloration in lizards occurs as a random developmental response to environmental stress. The persistence of this trait depends on the chance alignment of environmental conditions and selective pressures that favor individuals exhibiting the trait. While natural selection gradually stabilizes the trait through genetic mutations, its initial occurrence and subsequent spread are heavily influenced by luck.
Furthermore, the interplay between natural and sexual selection plays a pivotal role in shaping the trajectory of genetic accommodation. For example, a lizard’s color change may not only enhance survival by improving camouflage, but also impact mate selection. If the new coloration becomes a signal of fitness, individuals exhibiting the trait may gain a reproductive advantage, further reinforcing its prevalence. This combined effect creates a feedback loop: traits shaped by natural selection are further refined and enhanced by sexual selection, which increases their chances of becoming fixed in the population.
2.12. Evolvability
Evolvability refers to the capacity or potential of biological systems to adapt and diversify over time. This concept raises important questions, such as whether organisms or their traits differ in their ability to evolve and whether evolvability itself can evolve, allowing organisms to become better or worse at adapting to changing conditions. Understanding evolvability provides insights into how systems generate and maintain the variation necessary for evolutionary processes [
54,
55].
The extended synthesis interprets evolvability as an evolved characteristic that improves a population’s ability to generate heritable phenotypic variation. Mechanisms such as modularity, robustness, and phenotypic plasticity are seen as contributors to this capacity, allowing populations to adapt to changing environments. For example, the modular organization of genetic and developmental systems enables traits to evolve independently, minimizing harmful pleiotropic effects and fostering adaptive innovation. As a result, evolvability is considered a fundamental property of evolutionary systems, shaping the ability of organisms to respond to selection pressures [
16,
56,
57].
From the survival of the luckiest framework, evolvability can emerge as a contingent property shaped by stochastic processes and the interplay between natural and sexual selection. The modular organization of genetic systems or the presence of plastic traits may initially arise through chance mutations or random developmental variations. Whether these mechanisms improve evolvability depends on their interaction with specific environmental pressures and selective forces. For instance, individuals with greater modularity may be luckier in navigating environmental shifts, as they can adapt more readily to changes without important negative trade-offs.
The interaction between natural and sexual selection further influences the evolution of evolvability. Traits that improve an individual’s capacity for adaptation, such as phenotypic plasticity, may also affect mate selection. For example, individuals demonstrating flexibility or resilience in the face of environmental stressors might be perceived as more desirable mates, reinforcing the prevalence of these traits through sexual selection. This dual reinforcement means that mechanisms driving evolvability are influenced by survival pressures and further refined by reproductive dynamics.
While some interpretations within the extended evolutionary synthesis view evolvability as a trait shaped by selection, recent contributions—such as those by Lala et al. [
17]—acknowledge that evolvability can also arise through nonadaptive mechanisms, including historical contingencies and the self-organizing dynamics of developmental systems. In contrast, the survival of the luckiest framework emphasizes the stochastic foundations of evolvability even more strongly. From this perspective, evolvability emerges not as an inevitable outcome of adaptation, but as a byproduct of chance interactions among genetic variation, environmental perturbations, and the probabilistic nature of developmental pathways. Although this view may converge with some strands of extended synthesis thinking, it diverges in emphasizing that evolvability need not be fine-tuned by selection; it can instead reflect the persistence of traits that, by sheer compatibility with variable contexts, happen to facilitate further change.
2.13. Constructive Neutral Evolution
Constructive neutral evolution proposes that neutral evolutionary processes can lead to the development of novel structures and increased complexity. Evolution progresses through a stepwise process involving mechanisms such as biased variation, epistatic interactions, and excess capacities. These mechanisms, rather than neutral allele fixations alone, drive directional and constructive outcomes in evolutionary change. In contrast to the modern synthesis, which emphasizes natural selection as the primary creative force in evolution, constructive neutral evolution explores the role of nonselective factors in generating evolutionary innovation and directionality.
In addition, mutation is viewed not simply as a source of raw materials for evolution, but as an active agent introducing novelty, while selection acts as a stochastic sieve, filtering traits rather than directly shaping them [
58,
59]. As a result, many features of genomic architecture, gene structure, and developmental pathways are best understood through the influence of nonadaptive forces such as genetic drift and mutation. Thus, the extended synthesis acknowledges that stochastic processes like mutation and genetic drift are fundamental drivers of evolutionary change, operating alongside natural selection, rather than merely under its direction.
Furthermore, biological characteristics like complexity, modularity, and evolvability may arise as indirect byproducts of processes occurring at more fundamental levels of biological organization [
60]. For instance, the evolution of spliceosomal introns in eukaryotic genomes is seen as a result of molecular interactions that were initially neutral, but became entrenched over time through redundancy and molecular scaffolding. These traits, while nonadaptive in origin, may eventually acquire functions that make them indispensable, showing how evolutionary complexity can emerge without direct adaptive pressures. This view challenges the modern synthesis that prioritizes natural selection as the dominant force in evolution [
16].
From the survival of the luckiest perspective, while traits like spliceosomal introns may originate from neutral mutations, their persistence and eventual entrenchment depend on further random environmental and genetic conditions. For example, the fixation of molecular interactions could arise from chance genetic drift in small populations or from a random alignment of conditions that allow these traits to persist without deleterious effects. These processes are not deterministic, but are shaped by the probabilistic nature of evolutionary trajectories.
Moreover, the interplay between natural and sexual selection can further influence these traits’ trajectories. For instance, molecular changes that appear neutral at first might later influence phenotypes related to survival or reproduction. A mutation that stabilizes molecular interactions could indirectly enhance the fitness of an organism by improving cellular efficiency, making the organism more likely to survive. In turn, this survival advantage could affect mate choice, with individuals exhibiting such traits becoming more attractive mates, even if the trait itself remains functionally neutral. This perspective diverges from the extended synthesis by emphasizing the role of chance in the origin and persistence of traits associated with constructive neutral evolution. While these traits may acquire functions over time, their emergence is not guided by deterministic developmental processes, but rather by the stochastic interplay of genetic drift, environmental randomness, and the reinforcing dynamics of natural and sexual selection.
Kimura’s [
61] neutral theory of molecular evolution famously proposed that most evolutionary change at the molecular level is driven not by positive selection, but by the random fixation of selectively neutral mutations via genetic drift. This theory challenged the adaptationism of the modern evolutionary synthesis; yet, over time, drift has been partially integrated into an expanded version of that framework. The survival of the luckiest view builds on Kimura’s insight into the importance of stochasticity, but extends it by incorporating interactions between natural and sexual selection, environmentally induced epigenetic variation, and contingent trait fixation. While Kimura focused on molecular neutrality, the survival of the luckiest perspective emphasizes the emergence and persistence of phenotypic traits—including nonadaptive or initially neutral ones—via chance alignment with selective forces in specific ecological and demographic contexts. Like neutral theory, the survival of the luckiest approach underscores the limits of deterministic explanations in evolution, but it adds explanatory power by situating randomness within broader ecological and developmental dynamics.
2.14. Exaptation and Co-Option
Exaptation, referring to traits that improve fitness today without having been originally shaped by natural selection for their current role, is seen as a mechanism of evolutionary innovation that repurposes existing traits to meet new environmental challenges, often driving significant adaptive changes [
16]. Gould and Vrba [
28] introduced this concept to distinguish such features from adaptations, which are traits built by natural selection specifically for their current function. They argued that many features of organisms, initially nonadaptive, become co-opted for new uses in descendants. For example, feathers in birds likely evolved for thermal regulation or display, and were later co-opted for flight. Similarly, cellular structures or proteins may gain secondary functions that differ from their original purpose.
Wagner [
62] argues that evolution by natural selection tends to favor robust solutions that enable organisms to survive and reproduce effectively. This robustness, he suggests, not only ensures stability, but also creates opportunities for future evolutionary innovation. Rejecting the redundancy theory, which attributes robustness to surplus biological parts, Wagner proposes that robustness arises because many mutations lead to changes that have minimal effects on an organism’s fitness. By allowing mutations to accumulate without detrimental impacts, robustness provides the conditions for traits to persist over time, eventually becoming available for exaptation, where traits originally serving one purpose are co-opted for entirely new functions.
From the survival of the luckiest perspective, traits like feathers or molecular structures may emerge in response to specific environmental pressures, but their repurposing for new functions often hinges on stochastic events. For instance, the initial evolution of feathers for insulation or display likely arose due to random mutations or environmental conditions that favored such traits. Their later role in flight reflects a contingent alignment of selective pressures, where individuals with feathers happened to gain a survival advantage in locomotion under changing environmental contexts. Natural and sexual selection interact dynamically in this process. While natural selection may favor the survival advantages of a repurposed trait, sexual selection often amplifies its prevalence. For example, feathers initially used for display might have been selected for their visual appeal, influencing mate choice. As they were co-opted for flight, their dual roles in survival and reproduction likely reinforced their fixation within populations.
2.15. Genetic Drift and Population Bottlenecks
Again, it is important to note that the extended synthesis acknowledges the role of chance in evolution, with mechanisms such as genetic drift and population bottlenecks also playing a significant role. Genetic drift causes random fluctuations in allele frequencies, often leading to the fixation or loss of traits independently of their adaptive value, particularly in small populations. Population bottlenecks, which drastically reduce population size, amplify the effects of drift, further affecting genetic diversity and possibly driving evolutionary change [
63]. These processes show how stochastic events can influence evolutionary trajectories, complementing the effects of natural selection [
16].
Of course, from the survival of the luckiest framework, genetic drift and population bottlenecks exemplify the critical role of chance in evolutionary dynamics. However, an additional level of randomness arises from the interaction between natural and sexual selection. In small populations undergoing bottlenecks, the survival of individuals—and the traits they carry—is often determined by random environmental events, rather than adaptive advantages. For instance, individuals that survive a catastrophic event, such as a natural disaster, may do so not because of superior fitness, but due to sheer luck in their location or timing of exposure.
Therefore, the interplay between natural and sexual selection becomes particularly important after bottlenecks. Traits that persist due to drift may later influence reproductive success within the reduced population. For example, a trait that survives by chance during a bottleneck might become associated with mate selection in subsequent generations, either because of its rarity or because of its incidental connection to survival. This dual reinforcement ensures that traits shaped by drift and bottlenecks can gain evolutionary importance, even if they were initially neutral or maladaptive.
2.16. Symbiosis and Mutualism
Proponents of the extended synthesis argue that symbiosis, including mutualism (cooperation between species [
64]), plays a central role in directing developmental biases toward specific evolutionary trajectories, and serves as a key driver of evolutionary innovation. Organisms function as holobionts, composed of both zygote-derived cells and microbial consortia. The development, physiology, and immunity of animals are shaped by complex interactions between these components. Microbial symbionts act as agents of phenotypic plasticity, guiding organisms along particular developmental paths. This plasticity may result in genetic assimilation, either through the incorporation of microbial genes into host genomes, or through the maternal transmission of microbes. Furthermore, microbial symbionts can drive niche construction by remodeling host anatomy or physiology to influence evolutionary outcomes [
65].
Relationships such as those between nitrogen-fixing bacteria and leguminous plants, or the symbiotic origin of mitochondria in eukaryotic cells, show how interspecies cooperation can drive significant evolutionary change. These partnerships, seen as mutualistic adaptations, benefit both organisms and generate new selection pressures that stabilize and promote their coevolution over time. In this way, interdependence becomes a fundamental evolutionary mechanism that shapes ecological niches and fosters complex adaptations [
16].
However, the survival of the luckiest framework can interpret symbiosis and mutualism as outcomes shaped by random encounters and the contingent interplay of natural and sexual selection. The initial establishment of a symbiotic relationship, such as nitrogen fixation in legumes or the incorporation of mitochondria into eukaryotic cells, likely arises from random interactions between organisms in specific environmental contexts. For instance, the mutualistic association between a plant and bacteria may begin when a chance encounter provides immediate survival benefits, such as improved nutrient acquisition, to both parties. The persistence of this relationship depends on the alignment of selective pressures favoring individuals that maintain and enhance the partnership. Furthermore, plants that form more effective partnerships with nitrogen-fixing bacteria likely experience increased fitness through improved growth and reproduction, making them more competitive in attracting pollinators or producing viable offspring. Similarly, traits associated with symbiosis in animals or microbes could influence reproductive success by signaling adaptability to potential mates, which reinforces these mutualistic traits through sexual selection.
2.17. Horizontal Gene Transfer
Horizontal gene transfer refers to the process by which genetic material is transferred between organisms, bypassing traditional vertical inheritance from parent to offspring. In microbial evolution, horizontal gene transfer plays a dominant role, with rates of gene gain and loss comparable to those of point mutations, and far exceeding duplication rates. It is essential for the survival of microbial populations, countering processes like Muller’s ratchet, which leads to the accumulation of harmful mutations. Some bacteria and archaea have evolved specialized mechanisms for horizontal gene transfer, such as plasmids and viruses. Episodes of massive horizontal gene transfer have been pivotal in the emergence of major groups of organisms, including multiple archaeal phyla and eukaryotes [
66,
67,
68,
69,
70,
71,
72].
The extended synthesis considers horizontal gene transfer a crucial mechanism for evolutionary innovation and adaptation, particularly among microbes. Processes such as the transfer of antibiotic resistance genes between bacteria or the acquisition of photosynthetic genes by eukaryotic algae from cyanobacteria illustrate how genetic material can move laterally between species. Horizontal gene transfer enables rapid adaptation and innovation, creating new opportunities for organisms to respond to environmental pressures and expand into new ecological niches. This process is seen as a departure from the linear inheritance of traits, suggesting a nontraditional path of evolutionary change [
16].
In the survival of the luckiest framework, horizontal gene transfer is also an evolutionary process driven by random interactions and shaped by the combined forces of natural and sexual selection. The initial occurrence of horizontal gene transfer can be viewed as a random event, such as the accidental uptake of genetic material from one organism by another, facilitated by environmental conditions like proximity or stress. However, the persistence and evolutionary impact of the transferred genes depend on whether they confer a survival advantage under specific environmental pressures. For example, bacteria that acquire antibiotic resistance through horizontal gene transfer may survive in environments with high antibiotic exposure, while others perish [
70].
Natural and sexual selection interact to amplify the impact of horizontal gene transfer. Genes acquired in this way that enhance survival—such as those conferring antibiotic resistance or photosynthetic capabilities—can boost an organism’s reproductive success. In microbial populations, individuals with these advantageous genes may outcompete others for resources or reproduce more effectively, spreading the traits throughout the group. In more complex organisms, horizontally transferred traits may also influence mate choice, further reinforcing their prevalence through sexual selection.
3. Discussion
To provide a transparent basis for comparing the survival of the luckiest framework with the extended synthesis, we adopt four widely recognized theory virtues: parsimony, explanatory scope, coherence with empirical mechanisms, and empirical testability [
73]. First, in terms of parsimony, survival of the luckiest invokes only two well-characterized processes—natural and sexual selection—whereas the extended synthesis proliferates distinct concepts (developmental bias, niche construction, extragenetic inheritance, etc.), each requiring its own ontological commitments. Second, with respect to explanatory scope, our framework explains diverse phenomena—from cavefish troglomorphism to domestication syndromes and rapid adaptive radiations—through a single mechanism of chance alignments between selection regimes, whereas the extended synthesis typically appeals to different mechanisms for each case. Third, with regard to coherence with empirical data, the survival of the luckiest model is grounded in recent demonstrations that environmental epigenetics generates heritable variation at frequencies that are orders of magnitude above genetic mutation rates, and that these epimutations can be transmitted across generations [
52]. Finally, regarding empirical testability, by focusing on measurable overlaps—such as how epigenetic marks simultaneously influence mating success and survival—this framework suggests clear, falsifiable predictions, whereas many extended synthesis concepts remain difficult to operationalize. Altogether, these criteria show that survival of the luckiest not only unifies previously disparate observations, but does so with greater theoretical economy and empirical traction.
Of note, the survival of the luckiest framework rejects the notion that sexual selection anticipates future conditions. Instead, it posits that the interaction between natural and sexual selection preserves and amplifies phenotypic diversity through two key mechanisms. First, environmental epigenetics generates heritable, yet often context-sensitive, variation at rates ~10⁵-fold higher than those of genetic mutations [
52], creating an expansive and dynamic trait reservoir. Mutational load is not a problem, because epigenetic modifications—unlike genetic mutations—are typically reversible, dynamically regulated, and filtered by selection in each generation. They do not accumulate irreversibly or propagate unchecked across lineages. Moreover, their effects are often conditional on environmental cues, allowing maladaptive variants to be pruned efficiently before imposing population-wide burdens. Second, opposing selective pressures—sexual selection’s amplifying feedback and natural selection’s stabilizing feedback—jointly act as complementary filters, maintaining traits that may be neutral, costly, or latent under current conditions, but potentially advantageous under future ones. This dual sampling of trait space ensures a broader phenotypic landscape than either process could achieve alone. Empirical examples include rapid epigenetic adaptation in fish [
74], clonal divergence in snails [
75], and phenotypic shifts in Darwin’s finches [
76], where pre-existing variation enables survival under environmental change. Crucially, this model requires no teleology; it reflects the statistical nature of evolution, where stochastic trait variation increases the probability of pre-adaptation without predictive design.
In the survival of the luckiest approach, the origin of complex phenotypes under selection can stem from pre-existing variation rather than teleological emergence, operating through three key mechanisms: (1) epigenetic plasticity, where environmental cues induce heritable regulatory changes that affect multiple developmental pathways (e.g., cavefish sensory trade-offs [
27]); (2) nonlinear developmental systems that amplify minor perturbations—genetic or epigenetic—into coordinated trait complexes (e.g., Pax6-mediated eye reduction [
22]); and (3) trait covariance, where selection of one feature indirectly shifts others through developmental or pleiotropic linkage (e.g., domestication syndrome [
18]). These mechanisms do not imply foresight, but illustrate how selection can act on phenotypic outcomes that emerge from historically contingent systems. Empirical examples—from rapid divergence in clonal lineages [
75] to adaptation in novel environments [
74]—show that complex traits can arise and persist under selection without requiring pre-adaptive design or long-term planning.
The role of randomness in evolution, as emphasized in this framework, complements and extends perspectives introduced by the neutral theory of molecular evolution [
61]. Kimura argued that most molecular changes arise from the fixation of neutral mutations via genetic drift, rather than from positive selection. While the modern evolutionary synthesis largely emphasizes deterministic, adaptive processes, neutral theory—and its central mechanism, drift—highlight that evolutionary change can occur independently of adaptive value. In this sense, drift occupies a complex position: while conceptually distinct from the adaptive focus of the modern evolutionary synthesis, it is sometimes treated as part of an expanded modern evolutionary synthesis canon. The survival of the luckiest approach builds on this legacy by exploring how stochastic processes—including drift, epigenetic variation, and the contingent overlap of natural and sexual selection—contribute to evolutionary outcomes. Like Kimura’s theory, it underscores the need to move beyond rigid, adaptationist models toward a more probabilistic understanding of evolutionary change.
The phrase “survival of the luckiest” has previously appeared in the context of mass extinction research, most notably in the work of Raup [
77] and Gould [
78], where it describes how species survival during catastrophic events can hinge more on chance than on adaptive superiority. Our use of the term differs both in scope and emphasis: we apply it to the stochastic outcomes that arise from interactions between natural and sexual selection, rather than from extinction dynamics. Here, “survival of the luckiest” serves as a conceptual model for how overlapping selective regimes can amplify randomness at the phenotypic level. This process unfolds within evolutionary time, not at extinction boundaries, and emphasizes how selection itself—through contingent and competing pressures—can produce outcomes that are probabilistic, rather than deterministic. This perspective complements paleontological findings that emphasize the role of contingency in extinction dynamics, where evolutionary outcomes are shaped as much by historical accident as by adaptation. By acknowledging that randomness operates across multiple hierarchical levels—from genes to species—we connect our model of selection interaction to broader debates on the inherent unpredictability of evolutionary history.
Ball [
79] argues that if there is anything resembling a “language of life”, it will not be found in the genome, which bears little resemblance to any instruction manual created by humans. He notes that when biologists are asked why decoding genomes has provided limited insight into the processes of life, they often explain that the reality has turned out to be far more complex than initially expected. Recent research in molecular and cell biology, Ball observes, has revealed a richer and more astonishing view, moving beyond the mechanical metaphor. This shifts the burden of control away from the genome. For him, we should now focus on principles and processes of self-organization. These processes, by operating without the need for rigid genetic control, avoid the fragility that such strict dependence might entail.
While Ball’s perspective does not contradict the modern synthesis of evolutionary theory, which posits that evolution shapes organisms through the genetic transmission of information between generations, it challenges the notion of genes as “selfish” agents: genes serve as tools, rather than masters. A key reason the machine analogy fails, he explains, is that life operates at the molecular scale, where noise, randomness, and unpredictability dominate. Instead of resisting these influences, life embraces and utilizes them. It thrives on noise, diversity, chance events, and fluctuations—factors that are integral to its functioning, and could not be otherwise characterized. Note that Ball’s perspective can be accommodated within our survival of the luckiest approach to evolution.
In contrast, the developmental perspective of the extended synthesis appears to be in conflict with Ball’s argument that the “book of life” cannot be rewritten as a set of instructions, regardless of how complex they may be. While the extended synthesis shifts focus from a gene-centric view to developmental processes, epigenetic mechanisms, and environmental interactions, it still aims to identify overarching principles that dictate evolutionary outcomes. This approach contrasts with Ball’s view that life thrives on unpredictability, noise, and randomness, resisting attempts to distill its complexity into deterministic frameworks. By seeking to replace the genome-centric “instruction manual” metaphor with one grounded in developmental pathways, the extended synthesis risks imposing new constraints on understanding life—constraints that Ball suggests are incompatible with the stochastic, self-organizing, and emergent nature of biological systems [
9].
In particular, the survival of the luckiest framework resonates with Ball’s emphasis on self-organization and emergence in life processes. It suggests that evolutionary outcomes do not solely stem from deterministic genetic pathways; instead, they arise from the interaction between natural and sexual selection, which injects an additional element of randomness. This added randomness enables the self-organization of adaptive patterns under selective pressures. By incorporating stochastic elements, the survival of the luckiest framework reflects the emergent nature of evolutionary systems, where complex traits and behaviors develop from simple, random interactions. The survival of the luckiest framework extends Ball’s perspective by proposing that the core source of this randomness is the interplay between natural and sexual selection. This approach suggests how the contingent nature of evolutionary processes—guided by principles of self-organization [
9]—shapes biological diversity without strict reliance on deterministic genetic control. Ultimately, it reframes evolution as a dynamic interplay of chance and necessity, where luck, rather than design, plays a critical role in determining life’s trajectories.
Therefore, the interaction between natural and sexual selection adds an extra layer of randomness to evolutionary dynamics—an element that both the modern and extended syntheses overlook. Individual interactions, whether driven by mate choice or survival pressures, give rise to complex population-level patterns that cannot be predicted by examining these interactions in isolation [
9]. These stochastic processes generate feedback loops that amplify minor genetic variations, ultimately leading to emergent evolutionary outcomes. In this way, chance events at both the micro- and macro-scales can drive adaptive innovations, suggesting that life relies on unpredictable, self-organizing processes, rather than on strictly deterministic genetic control [
79].
As a result, the survival of the luckiest framework bridges the gap between the modern synthesis and the understanding of evolution as a product of self-organization and emergence. This approach retains the foundational concepts of natural and sexual selection, emphasizing their interaction, while placing luck at the forefront. By doing so, it opens the door to exploring how evolutionary processes arise through self-organization and emergent dynamics, offering a parsimonious and integrative perspective.
The survival of the luckiest framework does not serve as an outright replacement for either the modern or extended synthesis; instead, it acts as an intermediary extension between the two. It preserves the essential tenets of the modern synthesis—most notably, the importance of natural and sexual selection—while also integrating elements from the extended synthesis, such as developmental processes, epigenetics, and niche construction. However, rather than stressing deterministic developmental biases, this approach conceptualizes evolution as inherently stochastic, with random interactions between natural and sexual selection often playing a decisive role in evolutionary outcomes.
Of course, to substantiate this framework, empirical research is required—particularly in the fields of population genetics, agent-based modeling, and experimental evolution—to illustrate how the additional layer of randomness arising from the interplay between natural and sexual selection affects evolution. Moreover, our critique of the extended synthesis should be refined to avoid sweeping generalizations, since its proponents already acknowledge the role of stochasticity, albeit not the specific randomness generated by the interplay of natural and sexual selection. Similarly, the modern synthesis tends to overlook this extra stochastic element.
Enhancing the methodological support for this framework would involve comparative predictive modeling, simulations that incorporate stochastic selection, and case studies that document the persistence of neutral mutations due to this additional randomness. It is also essential to clearly differentiate between luck and genetic drift—while drift alters allele frequencies in finite populations in the absence of selection, “luck” here refers specifically to the extra randomness emerging from the interaction between natural and sexual selection. This distinction underlines how chance events within selection dynamics can influence evolution in ways that go beyond mere neutral evolution.
Finally, we believe that this framework holds significant implications for our understanding of adaptive evolution, predictability, and fitness landscapes. If luck indeed plays a critical role in shaping evolutionary trajectories, then evolution may be inherently less predictable than previously assumed, and adaptations might not always lead to “optimal” fitness solutions, but rather to survival advantages that emerge stochastically, potentially disrupting straightforward selective pathways. Expanding upon these implications can provide a broader perspective on evolution as an emergent, self-organizing process influenced by both chance and necessity.