Development of Cell-Permeable Adenylosuccinate Lyase Inhibitor
Abstract
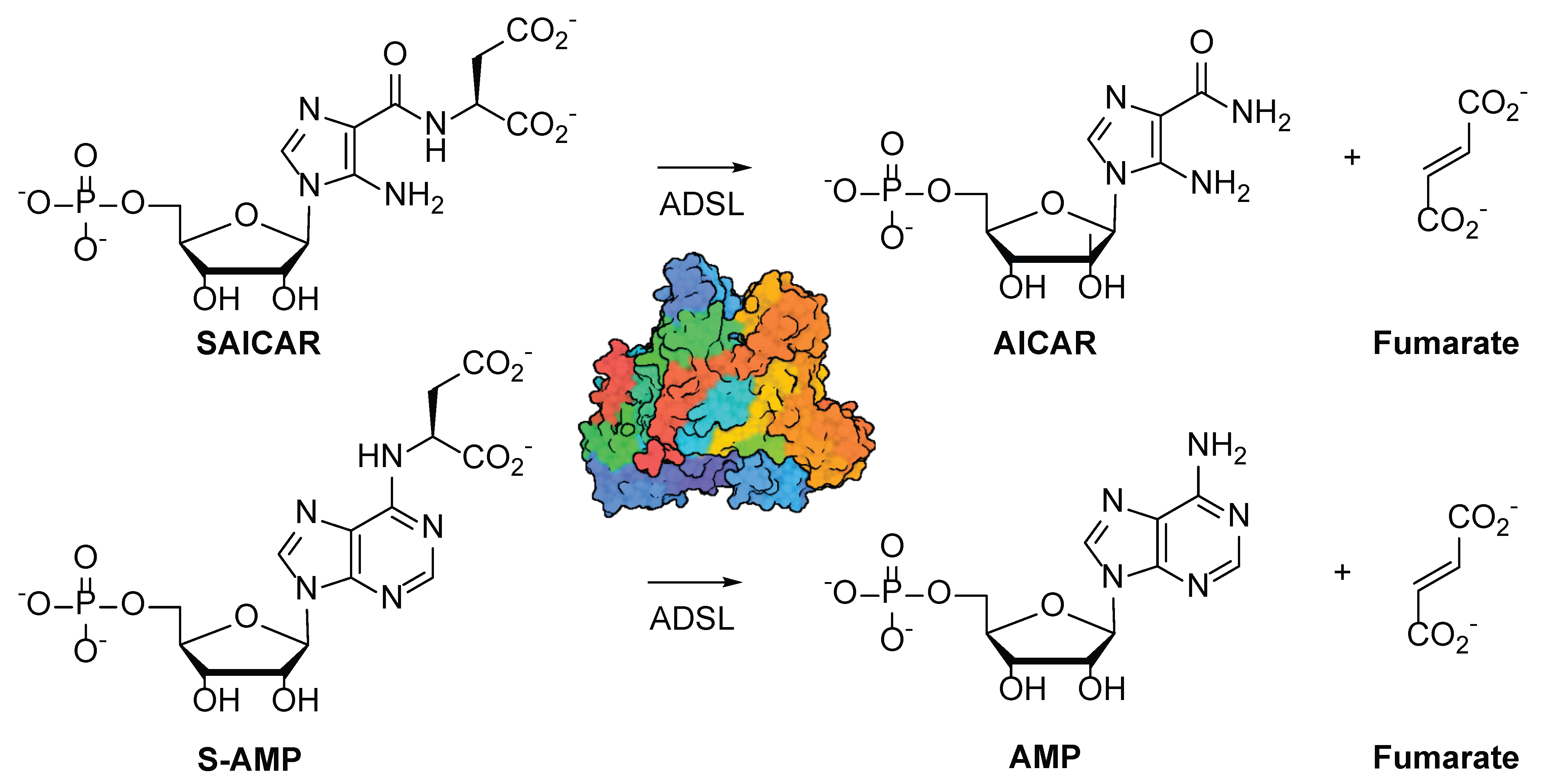
1. Introduction
2. Materials and Methods
2.1. Small Molecules Compounds and Proteins
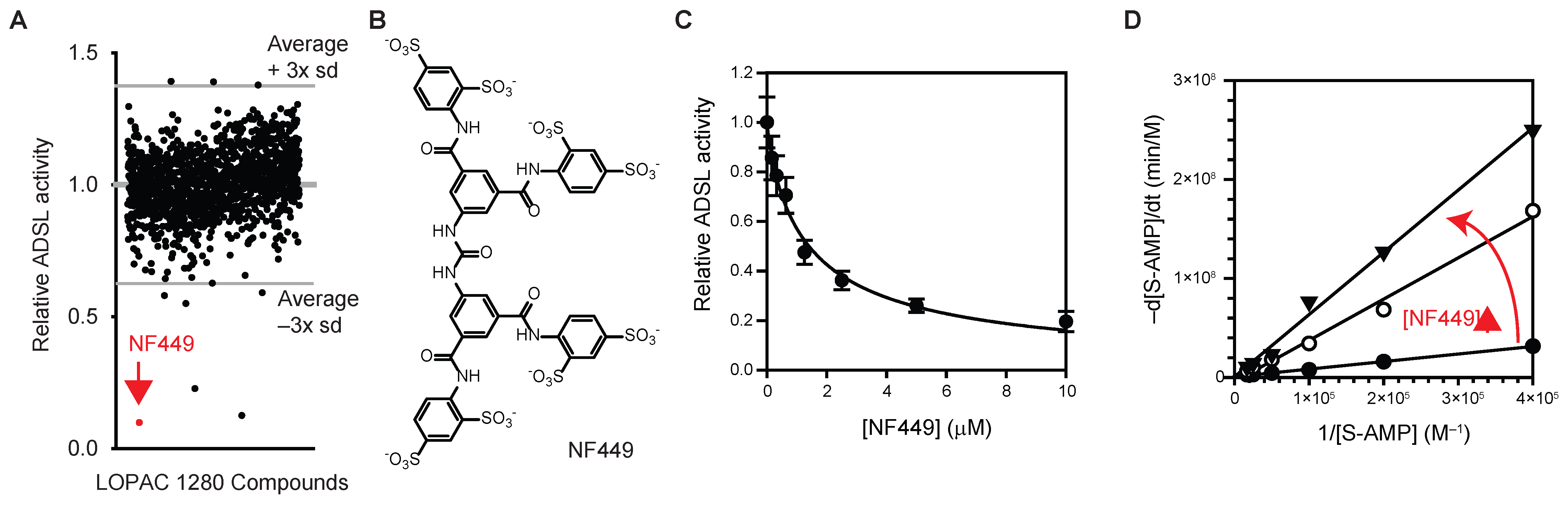
2.2. Screening of LOPAC® 1280 Compounds
2.3. ADSL Enzyme Activity Assay
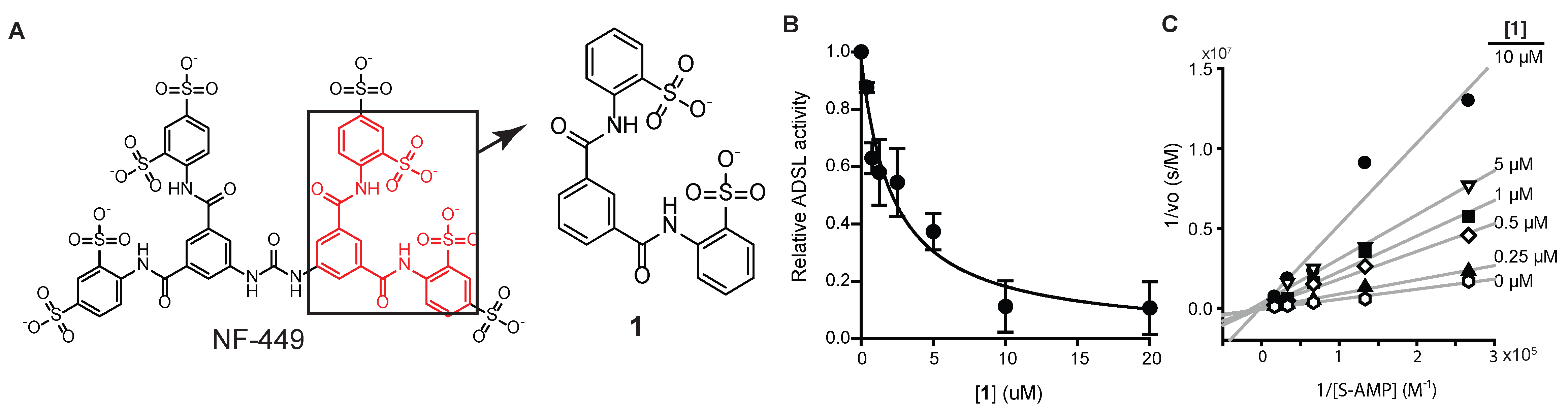
2.4. Synthesis of 2,2’-(1,3-Phenylenebis(carbonylimino))bisbenzenesulfonate (Compound 1)
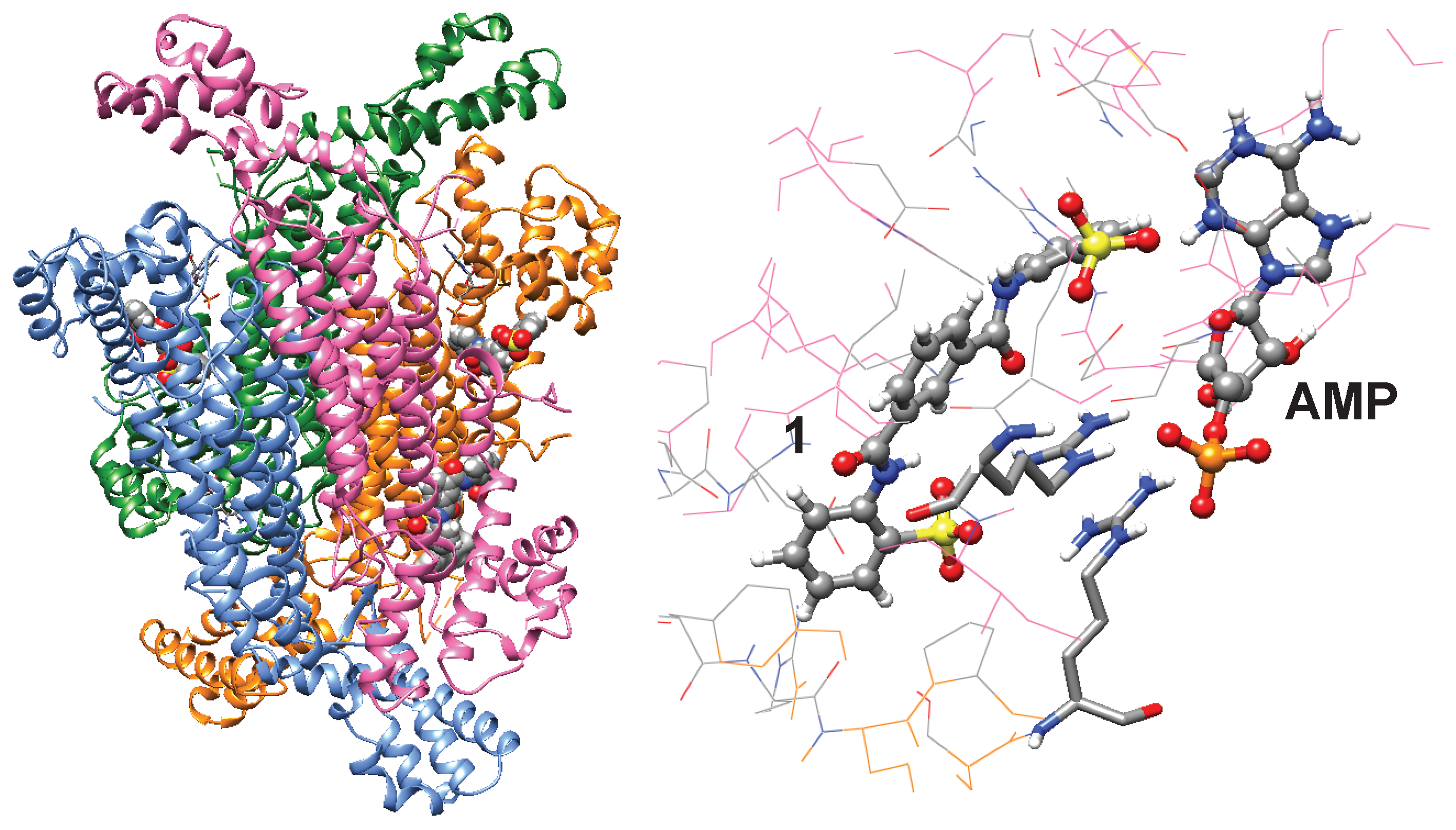
2.5. Blind Docking
2.6. HeLa Cell Experiments
3. Results
3.1. Identification of NF-449 as an ADSL Inhibitor
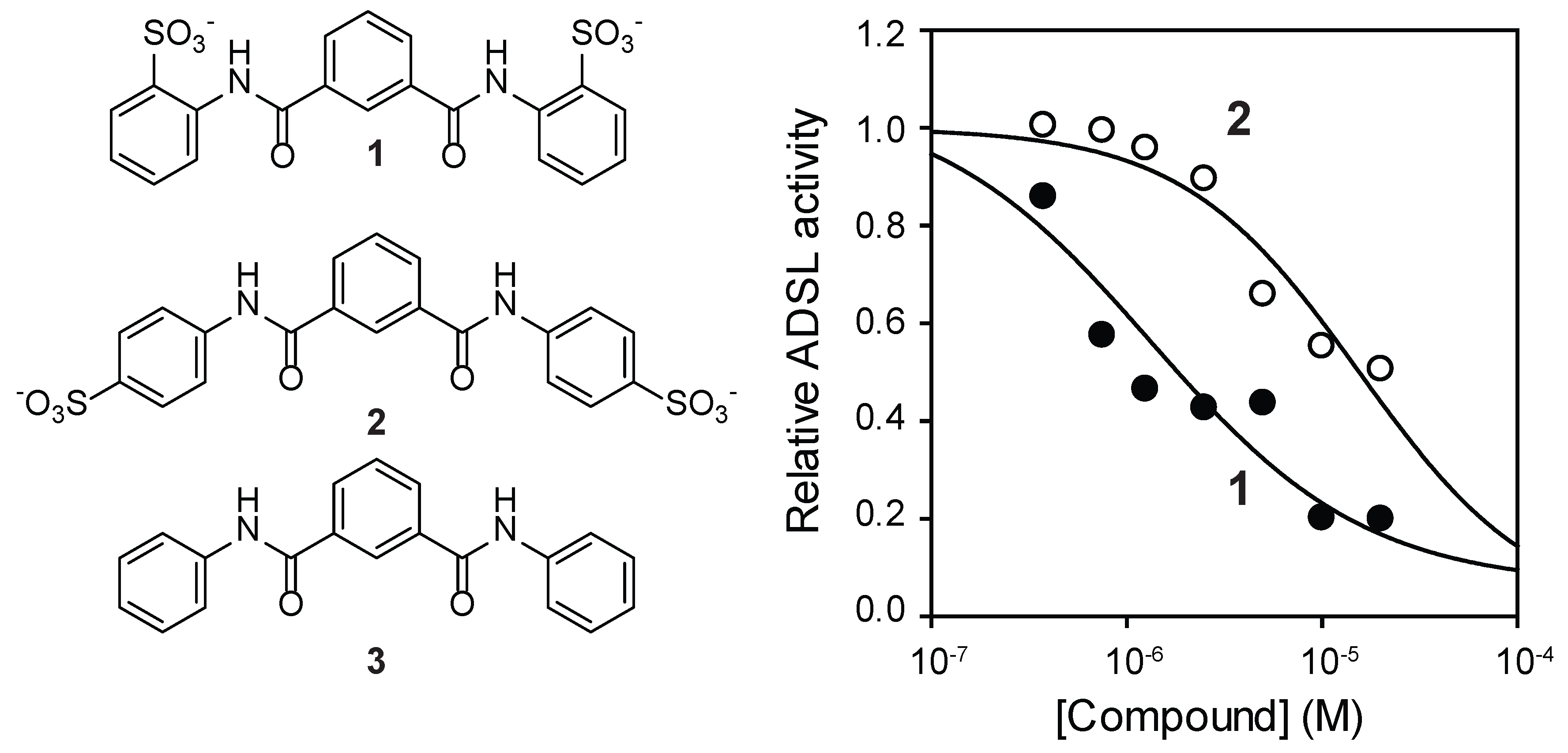
3.2. Identification of an NF-449 Fragment as an ADSL Inhibitor
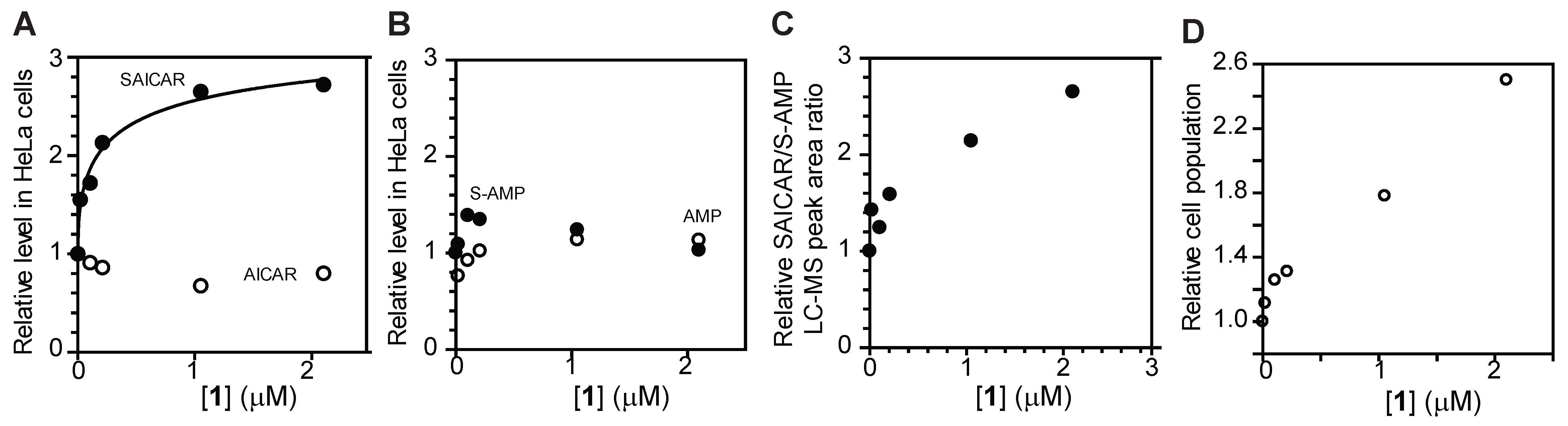
3.3. Cellular Effects of Compound 1
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADSL | Adenylosuccinate lyase |
| EC50 | Half-maximal effective concentration |
| SAICAR | N-Succinyl-5-aminoimidazole-4-carboxamide-1-ribose-5’-monophosphate |
| AICAR | 5-Aminoimidazole-4-carboxamide-1-ribose-5’-monophosphate |
| S-AMP | Adenylosuccinate |
| AMP | Adenosine 5’-monophosphate |
References
- Spiegel, E.K.; Colman, R.F.; Patterson, D. Adenylosuccinate lyase deficiency. Mol. Genet. Metab. 2006, 89, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Jurecka, A.; Zikanova, M.; Kmoch, S.; Tylki-Szymanska, A. Adenylosuccinate lyase deficiency. J. Inherit. Metab. Dis. 2014, 38, 231–242. [Google Scholar] [CrossRef]
- Balasubramaniam, S.; Duley, J.A.; Christodoulou, J. Inborn errors of purine metabolism: Clinical update and therapies. J. Inherit. Metab. Dis. 2014, 37, 669–686. [Google Scholar] [CrossRef]
- Ariyananda Lde, Z.; Lee, P.; Antonopoulos, C.; Colman, R.F. Biochemical and biophysical analysis of five disease-associated human adenylosuccinate lyase mutants. Biochemistry 2009, 48, 5291–5302. [Google Scholar] [CrossRef] [PubMed]
- Zikanova, M.; Skopova, V.; Hnizda, A.; Krijt, J.; Kmoch, S. Biochemical and structural analysis of 14 mutant adsl enzyme complexes and correlation to phenotypic heterogeneity of adenylosuccinate lyase deficiency. Hum. Mutat. 2010, 31, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Zigman, T.; Petkovic Ramadza, D.; Simic, G.; Baric, I. Inborn Errors of Metabolism Associated with Autism Spectrum Disorders: Approaches to Intervention. Front. Neurosci. 2021, 15, 673600. [Google Scholar] [CrossRef]
- Fenton, A.R.; Janowitz, H.N.; Franklin, L.P.; Young, R.G.; Moro, C.A.; DeGennaro, M.V.; McReynolds, M.R.; Wang, W.; Hanna-Rose, W. A Caenorhabditis elegans model of adenylosuccinate lyase deficiency reveals neuromuscular and reproductive phenotypes of distinct etiology. Mol. Genet. Metab. 2023, 140, 107686. [Google Scholar] [CrossRef]
- Dutto, I.; Gerhards, J.; Herrera, A.; Souckova, O.; Skopova, V.; Smak, J.A.; Junza, A.; Yanes, O.; Boeckx, C.; Burkhalter, M.D.; et al. Pathway-specific effects of ADSL deficiency on neurodevelopment. eLife 2022, 11, e70518. [Google Scholar] [CrossRef]
- Rea, V.; Van Raay, T.J. Using Zebrafish to Model Autism Spectrum Disorder: A Comparison of ASD Risk Genes Between Zebrafish and Their Mammalian Counterparts. Front. Mol. Neurosci. 2020, 13, 575575. [Google Scholar] [CrossRef]
- Banerjee-Basu, S.; Packer, A. SFARI Gene: An evolving database for the autism research community. Dis. Model. Mech. 2010, 3, 133–135. [Google Scholar] [CrossRef]
- Zurlo, G.; Liu, X.; Takada, M.; Fan, C.; Simon, J.M.; Ptacek, T.S.; Rodriguez, J.; von Kriegsheim, A.; Liu, J.; Locasale, J.W.; et al. Prolyl hydroxylase substrate adenylosuccinate lyase is an oncogenic driver in triple negative breast cancer. Nat. Commun. 2019, 10, 5177. [Google Scholar] [CrossRef]
- Park, H.; Ohshima, K.; Nojima, S.; Tahara, S.; Kurashige, M.; Hori, Y.; Okuzaki, D.; Wada, N.; Ikeda, J.I.; Morii, E. Adenylosuccinate lyase enhances aggressiveness of endometrial cancer by increasing killer cell lectin-like receptor C3 expression by fumarate. Lab. Investig. 2018, 98, 449–461. [Google Scholar] [CrossRef]
- Taha-Mehlitz, S.; Bianco, G.; Coto-Llerena, M.; Kancherla, V.; Bantug, G.R.; Gallon, J.; Ercan, C.; Panebianco, F.; Eppenberger-Castori, S.; von Strauss, M.; et al. Adenylosuccinate lyase is oncogenic in colorectal cancer by causing mitochondrial dysfunction and independent activation of NRF2 and mTOR-MYC-axis. Theranostics 2021, 11, 4011–4029. [Google Scholar] [CrossRef]
- McKnight, S.L. Please keep me 2uned to PKM2. Mol. Cell 2014, 53, 683–684. [Google Scholar] [CrossRef][Green Version]
- Keller, K.E.; Doctor, Z.M.; Dwyer, Z.W.; Lee, Y.S. SAICAR induces protein kinase activity of PKM2 that is necessary for sustained proliferative signaling of cancer cells. Mol. Cell 2014, 53, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Keller, K.E.; Tan, I.S.; Lee, Y.S. SAICAR stimulates pyruvate kinase isoform M2 and promotes cancer cell survival in glucose-limited conditions. Science 2012, 338, 1069–1072. [Google Scholar] [CrossRef] [PubMed]
- Casey, P.J.; Lowenstein, J.M. Inhibition of adenylosuccinate lyase by L-alanosyl-5-aminoimidazole-4-carboxylic acid ribonucleotide (alanosyl-AICOR). Biochem. Pharmacol. 1987, 36, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Yu, J. Alanosine (UCSD). Curr. Opin. Investig. Drugs 2001, 2, 1623–1630. [Google Scholar]
- Bulusu, V.; Srinivasan, B.; Bopanna, M.P.; Balaram, H. Elucidation of the substrate specificity, kinetic and catalytic mechanism of adenylosuccinate lyase from Plasmodium falciparum. Biochim. Biophys. Acta 2009, 1794, 642–654. [Google Scholar] [CrossRef]
- Winder, W.W. AMP-activated protein kinase: Possible target for treatment of type 2 diabetes. Diabetes Technol. Ther. 2000, 2, 441–448. [Google Scholar] [CrossRef]
- Lee, P.; Colman, R.F. Expression, purification, and characterization of stable, recombinant human adenylosuccinate lyase. Protein. Expr. Purif. 2007, 51, 227–234. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Nguyen, T.H.; Pham, T.N.H.; Huy, N.T.; Bay, M.V.; Pham, M.Q.; Nam, P.C.; Vu, V.V.; Ngo, S.T. Autodock Vina Adopts More Accurate Binding Poses but Autodock4 Forms Better Binding Affinity. J. Chem. Inf. Model. 2020, 60, 204–211. [Google Scholar] [CrossRef]
- Hulsmann, M.; Nickel, P.; Kassack, M.; Schmalzing, G.; Lambrecht, G.; Markwardt, F. NF449, a novel picomolar potency antagonist at human P2X1 receptors. Eur. J. Pharmacol. 2003, 470, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Linares, I.; Perez-Sanchez, H.; Cecilia, J.M.; Garcia, J.M. High-Throughput parallel blind Virtual Screening using BINDSURF. BMC Bioinform. 2012, 13, S13. [Google Scholar] [CrossRef] [PubMed]
- Toth, E.A.; Yeates, T.O. The structure of adenylosuccinate lyase, an enzyme with dual activity in the de novo purine biosynthetic pathway. Structure 2000, 8, 163–174. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Lee, Y.-S. Development of Cell-Permeable Adenylosuccinate Lyase Inhibitor. Methods Protoc. 2025, 8, 126. https://doi.org/10.3390/mps8060126
Hu Y, Lee Y-S. Development of Cell-Permeable Adenylosuccinate Lyase Inhibitor. Methods and Protocols. 2025; 8(6):126. https://doi.org/10.3390/mps8060126
Chicago/Turabian StyleHu, Yijia, and Young-Sam Lee. 2025. "Development of Cell-Permeable Adenylosuccinate Lyase Inhibitor" Methods and Protocols 8, no. 6: 126. https://doi.org/10.3390/mps8060126
APA StyleHu, Y., & Lee, Y.-S. (2025). Development of Cell-Permeable Adenylosuccinate Lyase Inhibitor. Methods and Protocols, 8(6), 126. https://doi.org/10.3390/mps8060126






