Analysis of the Effect of the Tablet Matrix on the Polymorphism of Ibuprofen, Naproxen, and Naproxen Sodium in Commercially Available Pharmaceutical Formulations
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
- -
- Fourteen products (Ibumax, Ibupar forte, Ibuprex Max, Ibuprofen Aflofarm, Ibuprofen TZF, Ibuprom, Ibuprom Max, Ibuprom RR MAX, Ibuprom Ultramax, Iburapid, Ibuprofen Max PolfaŁódź, MIG, Nurofen, Nurofen Forte) contained ibuprofen as the only active ingredient.
- -
- Eight products (Acatar Zatoki, Ibum Zatoki Max, Ibuprom Zatoki, Ibuprom Zatoki Max, Infex Zatoki, Metafen Zatoki, Modafen Extra Grip, Nurofen Zatoki) contained ibuprofen and pseudoephedrine hydrochloride as the second active ingredient.
- -
- One product (Ibuprom Zatoki Tabs) contained ibuprofen and phenylephrine hydrochloride as the second active ingredient.
- -
- Two products (APAP intense, Metafen) contained ibuprofen and paracetamol as the second active ingredient.
- -
- Six products contained naproxen (Anapran EC, Apo Napro 250, Apo Napro 500, Naproxen Hasco, Naproxen Hasco 500, Naproxen Polfarmex).
- -
- Six products contained naproxen sodium (Aleve, Anapran, Nalgesin, Nalgesin Forte, Nalgesin Mini, Naxii).
2.2. Differential Scanning Calorimetry
2.3. Spectroscopic Methods
2.4. X-Ray Diffraction
3. Results
3.1. Ibuprofen Products
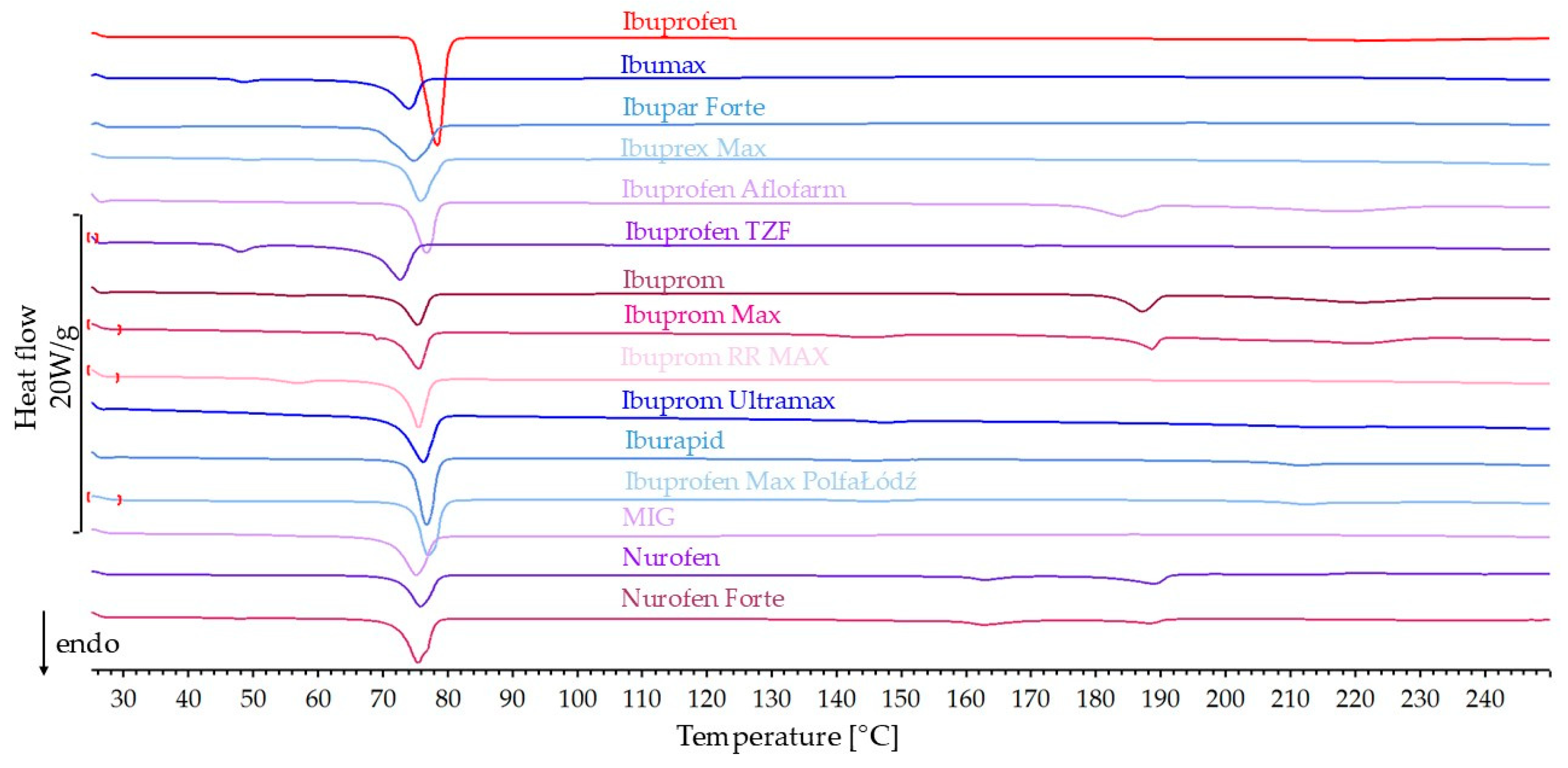
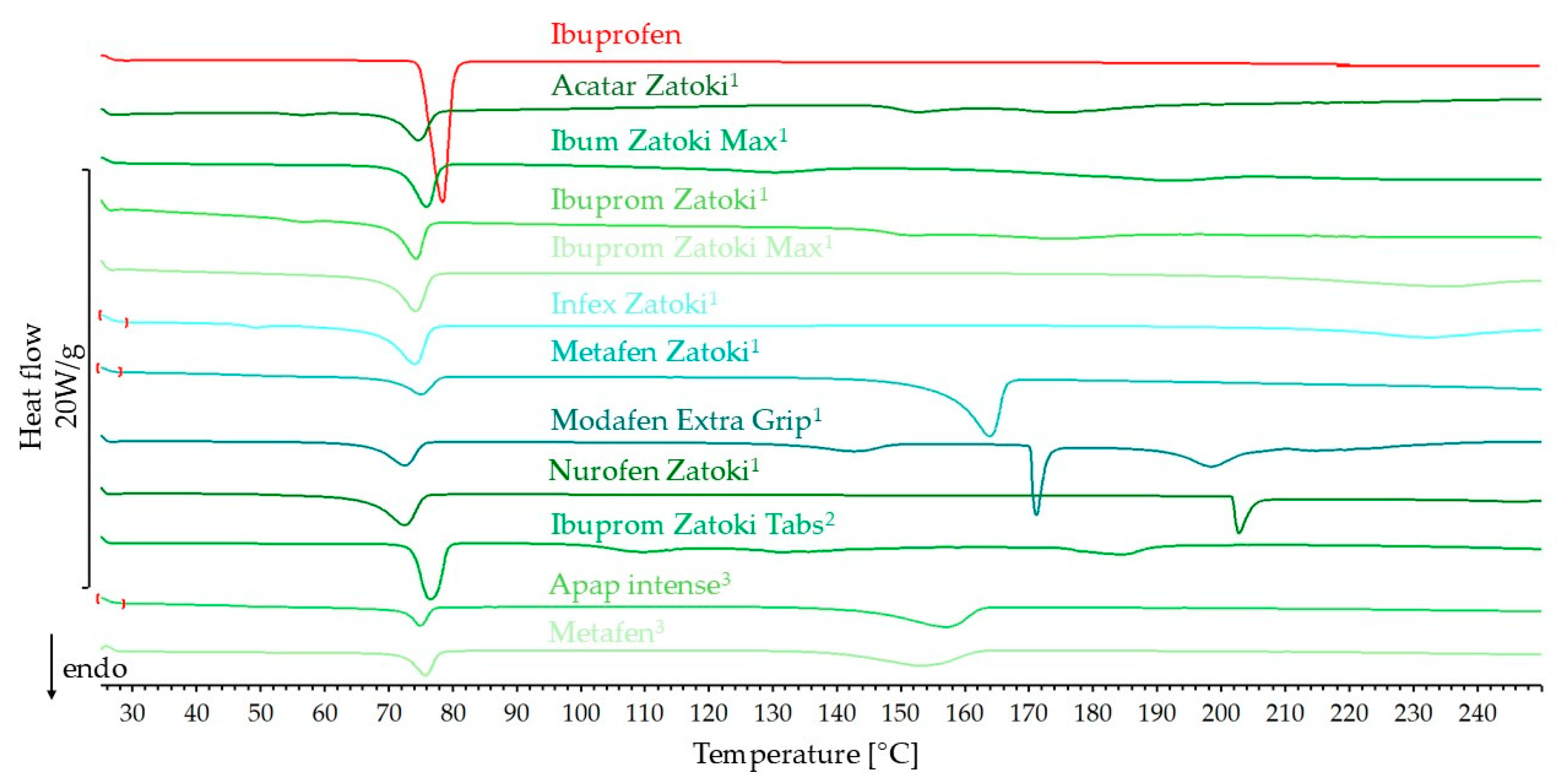
3.1.1. DSC Results
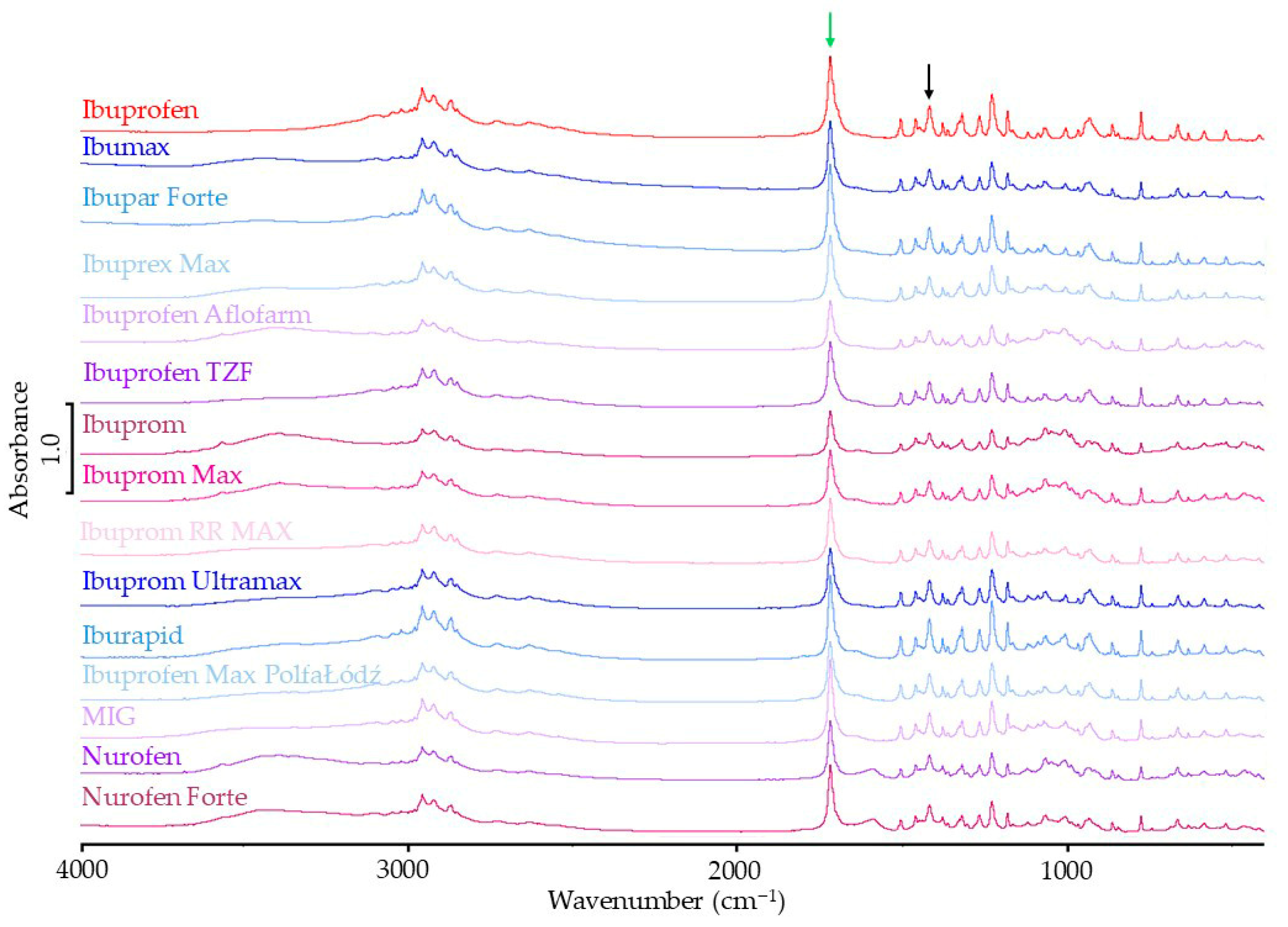
3.1.2. FTIR Results
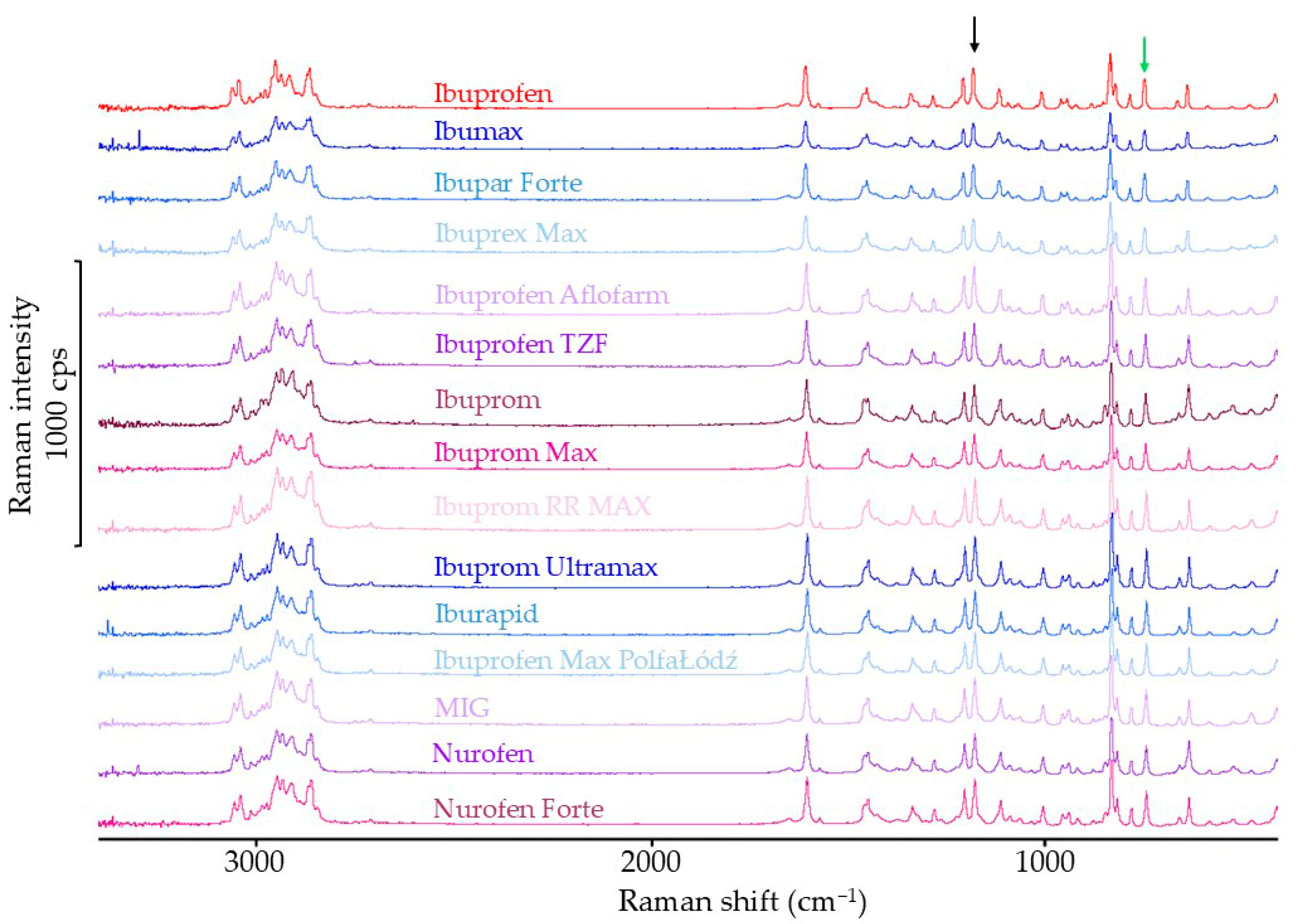
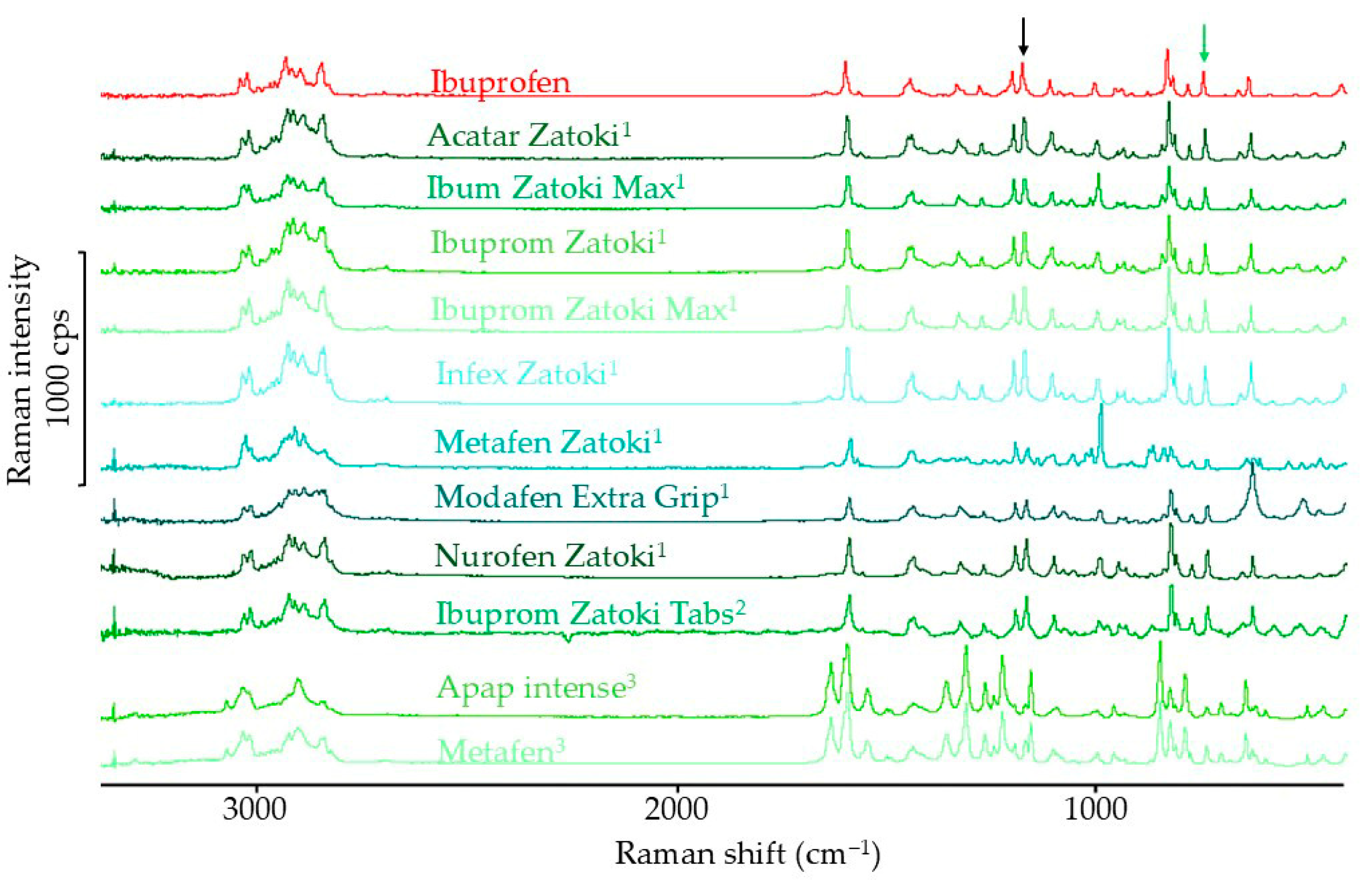
3.1.3. Raman Spectroscopy Results
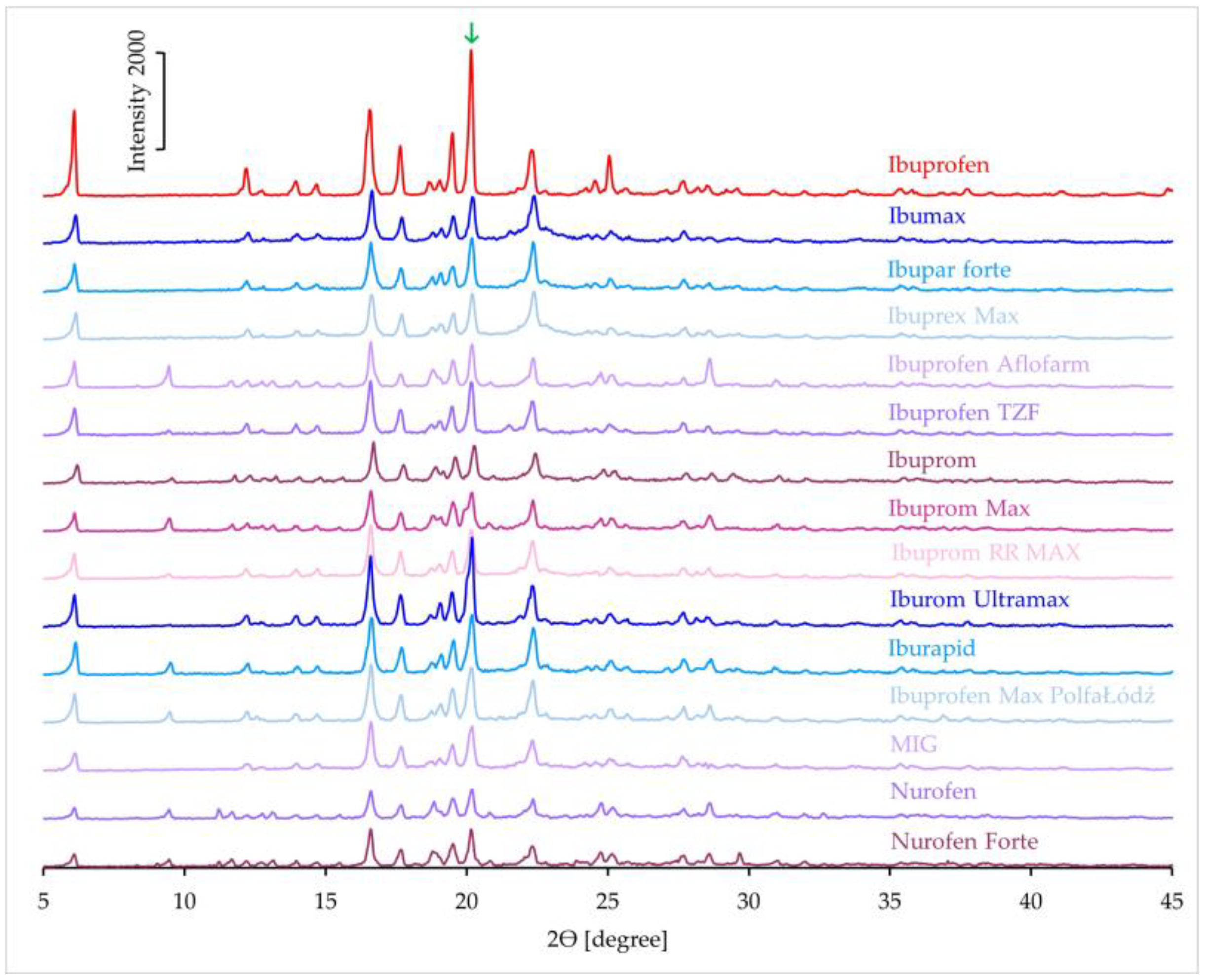
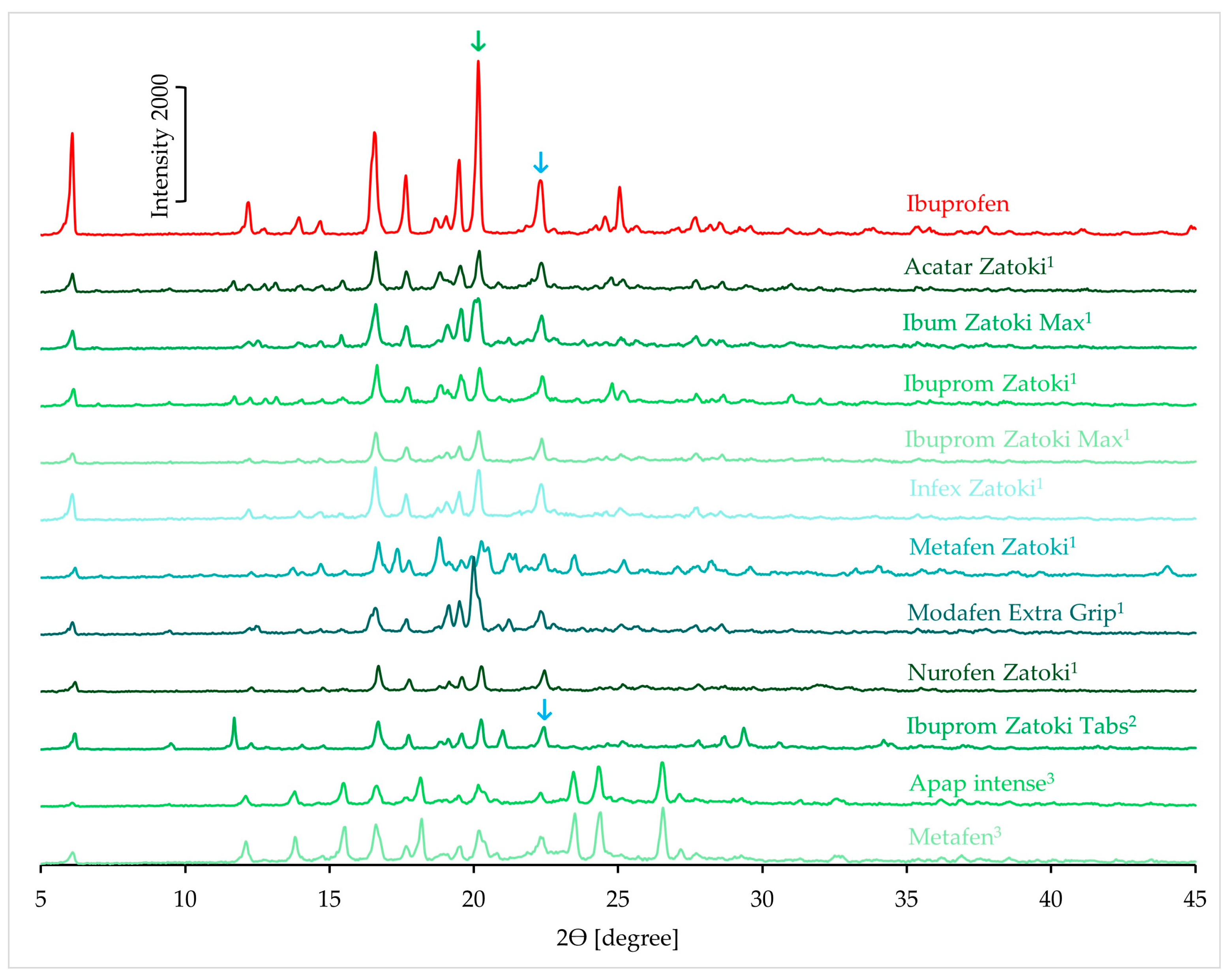
3.1.4. XRD Results
3.2. Naproxen Products
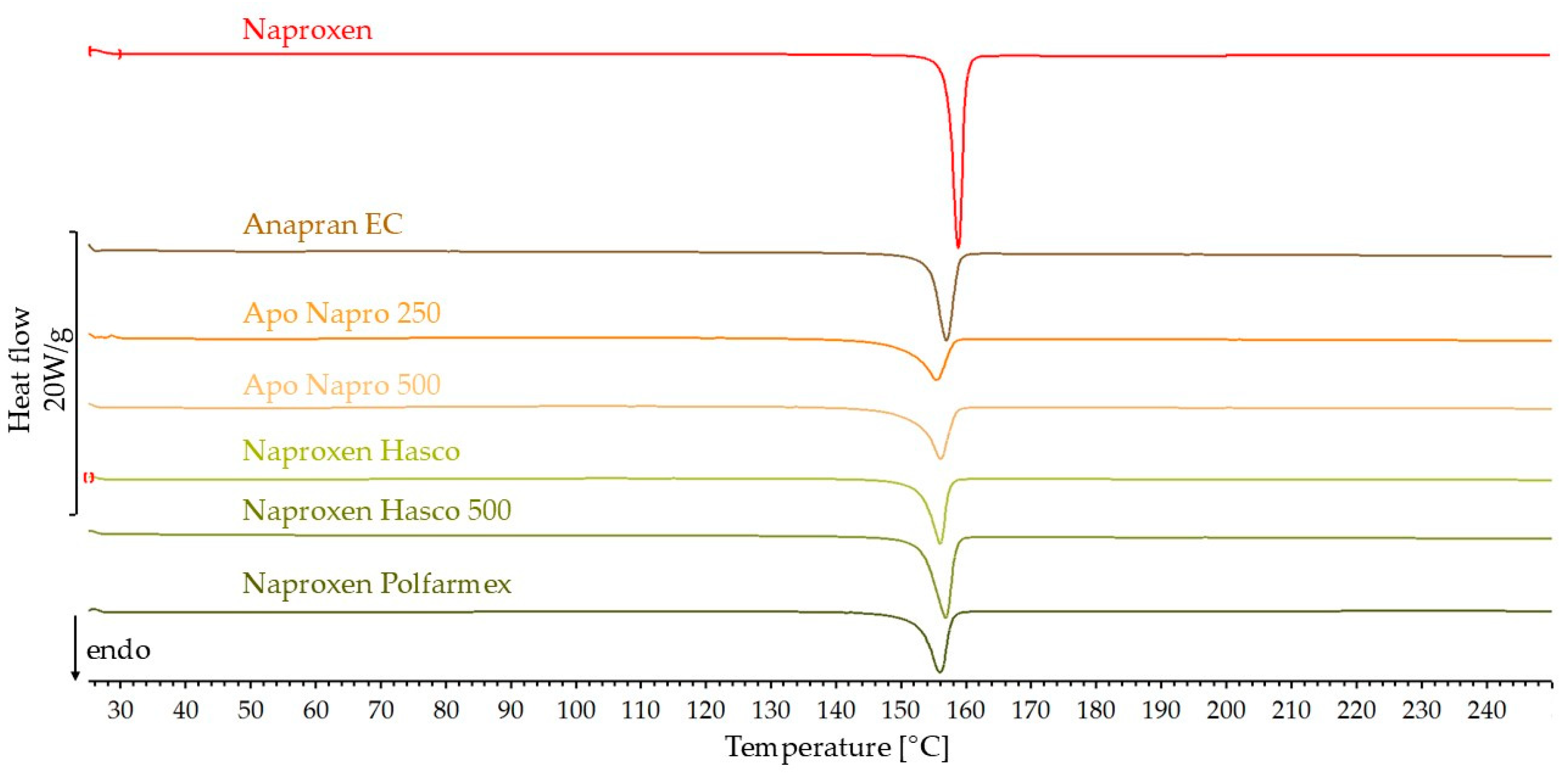
3.2.1. DSC Results
3.2.2. FTIR Results
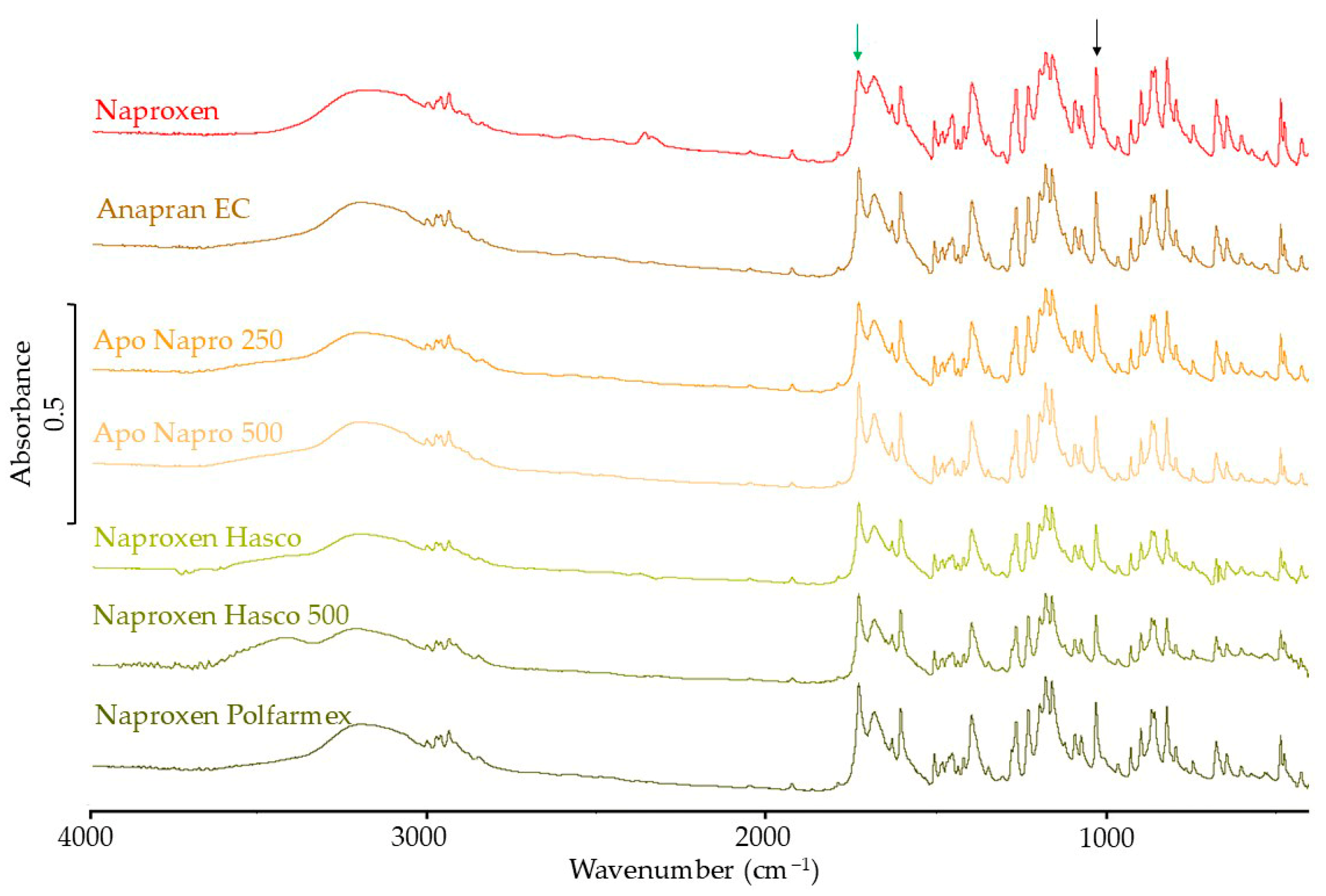
3.2.3. Raman Spectroscopy Results
3.2.4. XRD Results
3.3. Naproxen Sodium Products
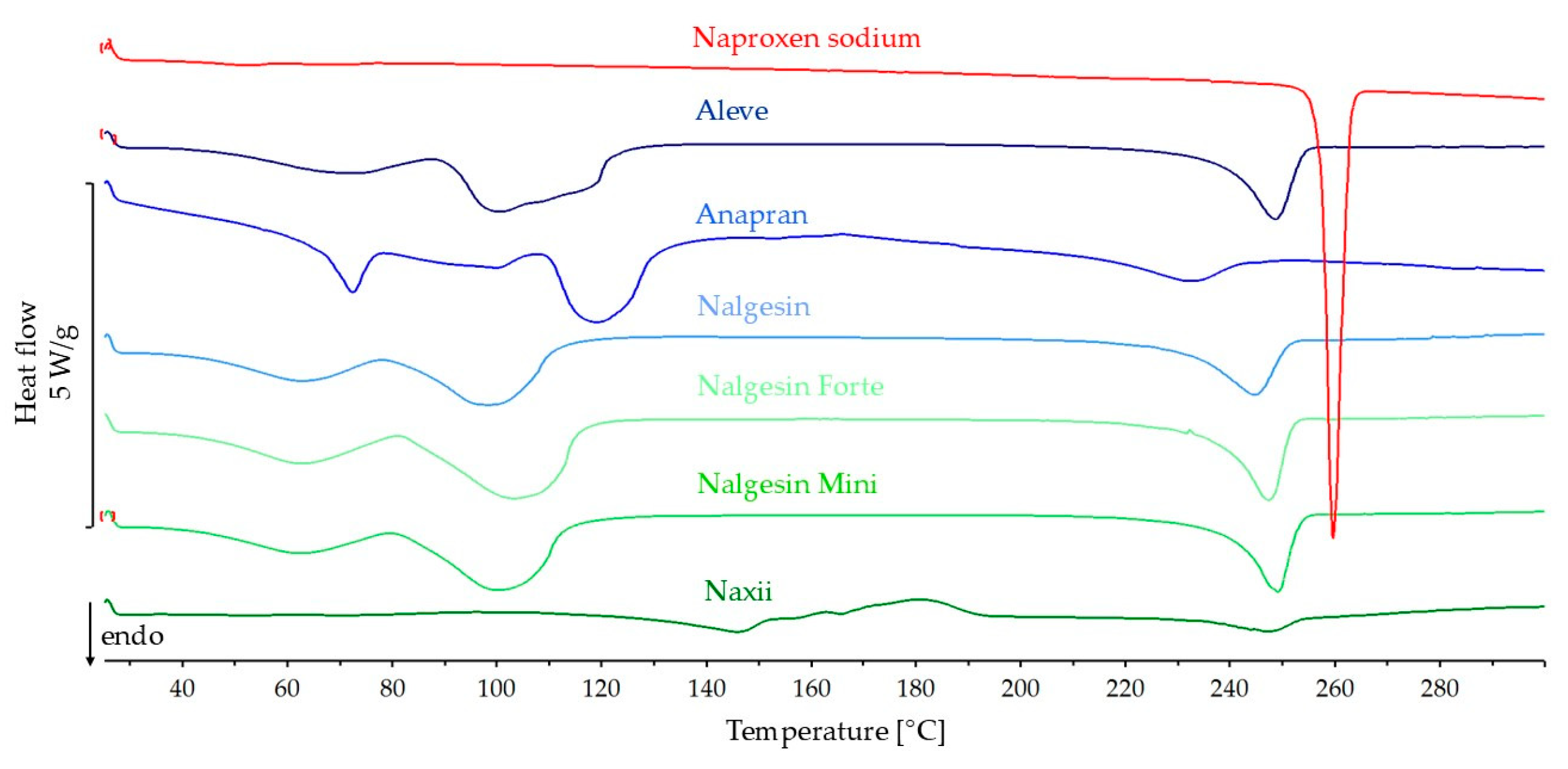
3.3.1. DSC Results
3.3.2. FTIR Results
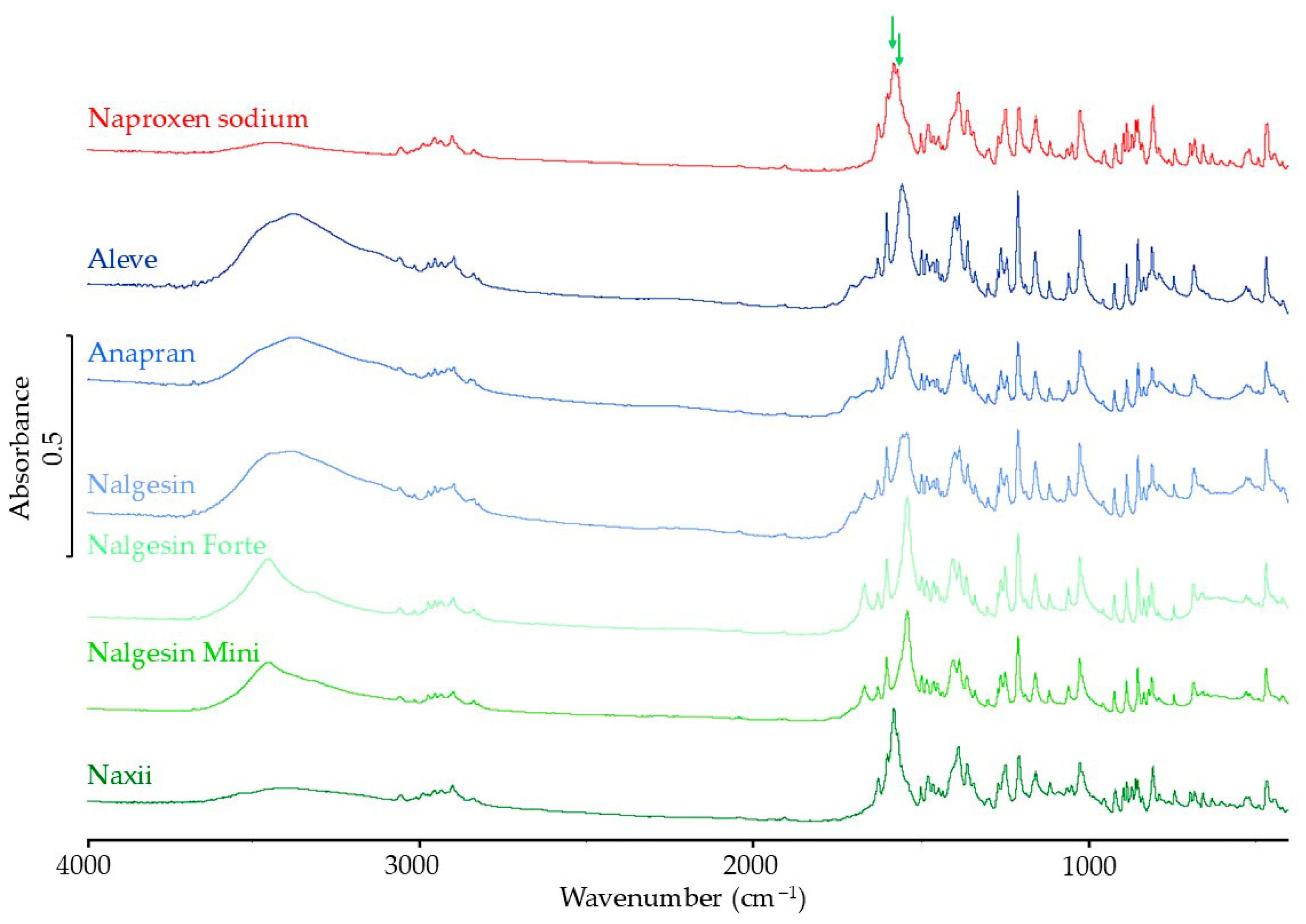
3.3.3. Raman Spectroscopy Results
3.3.4. XRD Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pifferi, G.; Restani, P. The safety of pharmaceutical excipients. Il Farm. 2003, 58, 541–550. [Google Scholar] [CrossRef]
- Makkad, S.; Sheikh, M.; Shende, S.; Jirvankar, P. Pharmaceutical Excipients: Functions, Selection Criteria, and Emerging Trends. Int. J. Pharm. Investig. 2025, 15, 361–376. [Google Scholar] [CrossRef]
- Veronica, N.; Heng, P.W.S.; Liew, C.V. Ensuring Product Stability—Choosing the Right Excipients. J. Pharm. Sci. 2022, 111, 2158–2171. [Google Scholar] [CrossRef]
- Rowe, R.C.; Sheskey, P.J.; Quinn, M.E. Handbook of Pharmaceutical Excipients, 6th ed.; Section: Preface; Pharmaceutical Press: London, UK, 2009. [Google Scholar]
- Bagal, N.N.; Subhedar, R.S.; Chougale, N.B. A Review On Excipient Used In Preparation/Formulation Of Solid Dosage Form. Int. J. Pharm. Sci. 2024, 2, 1711–1727. [Google Scholar] [CrossRef]
- Yu, D.; Hoag, S.W. The impact of diluents on the compaction, dissolution, and physical stability of amorphous solid dispersion tablets. Int. J. Pharm. 2024, 654, 123924. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Barker, J.; ElShaer, A. Pharmaceutical Excipients and Drug Metabolism: A Mini-Review. Int. J. Mol. Sci. 2020, 21, 8224. [Google Scholar] [CrossRef] [PubMed]
- O’Driscoll, C.M.; Griffin, B.T. Biopharmaceutical challenges associated with drugs with low aqueous solubility—The potential impact of lipid-based formulations. Adv. Drug Deliv. Rev. 2008, 60, 617–624. [Google Scholar] [CrossRef]
- Antonio, M.; Calvo, N.L.; Maggio, R.M. Chemometric study of the excipients’ influence on polymorphic-behavior. Mefenamic acid as case of study. J. Pharm. Biomed. Anal. 2019, 170, 8–15. [Google Scholar] [CrossRef]
- Bárdos, V.; Szolláth, R.; Tőzsér, P.; Mirzahosseini, A.; Sinkó, B.; Angi, R.; Takács-Novák, K. Study of the Influence of Pharmaceutical Excipients on the Solubility and Permeability of BCS Class II Drugs. Sci. Pharm. 2025, 93, 19. [Google Scholar] [CrossRef]
- Kawabata, Y.; Wada, K.; Nakatani, M.; Yamada, S.; Onoue, S. Formulation Design for Poorly Water-Soluble Drugs Based on Biopharmaceutics Classification System: Basic Approaches and Practical Applications. Int. J. Pharm. 2011, 420, 32. [Google Scholar] [CrossRef]
- Newman, A.; Zografi, G. What Are the Important Factors That Influence API Crystallization in Miscible Amorphous API–Excipient Mixtures during Long-Term Storage in the Glassy State? Mol. Pharm. 2021, 19, 378–391. [Google Scholar] [CrossRef]
- Patil, H.; Tiwari, R.V.; Repka, M.A. Hot-Melt Extrusion: From Theory to Application in Pharmaceutical Formulation. AAPS PharmSciTech 2016, 17, 20–42. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Shuai, S.; Zhang, H.; Liu, N.; Wang, Z.; Jin, L.; Zheng, A. Application of rheology to hot melt extrusion: Theory and practice. Int. J. Pharm. 2025, 680, 125742. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, X.; Meng, J.; Lu, L.; Du, S.; Xu, H.; Wu, S. Study on the Effect of Polymer Excipients on the Dispersibility, Interaction, Solubility, and Scavenging Reactive Oxygen Species of Myricetin Solid Dispersion: Experiment and Molecular Simulation. ACS Omega 2022, 7, 1514–1526. [Google Scholar] [CrossRef] [PubMed]
- Brokmann, F.; Luthe, K.; Hartmann, J.; Müller, L.; Klammt, F.; Hoffmann, C.; Weitschies, W.; Rosenbaum, C. Hot Melt Extrusion as Continuous Manufacturing Technique to Produce Bilayer Films Loaded with Paracetamol or Lactase. Pharmaceuticals 2025, 18, 310. [Google Scholar] [CrossRef] [PubMed]
- Rawlinson, C.F.; Williams, A.C.; Timmins, P.; Grimsey, I. Polymer-mediated disruption of drug crystallinity. Int. J. Pharm. 2007, 336, 42–48. [Google Scholar] [CrossRef]
- Leyk, E.; Wesolowsk, M. Interactions Between Paracetamol and Hypromellose in the Solid State. Front. Pharmacol. 2019, 10, 14. [Google Scholar] [CrossRef]
- Adibkia, K.; Barzegar-Jalali, M.; Maheri-Esfanjani, H.; Ghanbarzadeh, S.; Shokri, J.; Sabzevari, A.; Javadzadeh, Y. Physicochemical characterization of naproxen solid dispersions prepared via spray drying technology. Powder Technol. 2013, 246, 448–455. [Google Scholar] [CrossRef]
- Davis, T.D.; Peck, G.E.; Stowell, J.G.; Morris, K.R.; Byrn, S.R. Modeling and Monitoring of Polymorphic Transformations During the Drying Phase of Wet Granulation. Pharm. Res. 2004, 21, 860–866. [Google Scholar] [CrossRef]
- Arce, F.; Schuman, Y.; Gawel, J.; Garmise, R.; Abebe, A.; Desai, D. An Evaluation of Wet Granulation Process Selection for API Prone to Polymorphic Form Conversion in the Presence of Moisture and Heat. Pharm. Res. 2024, 41, 595–607. [Google Scholar] [CrossRef]
- Mallick, S.; Pattnaik, S.; Swain, K.; De, P.K.; Saha, A.; Ghoshal, G.; Mondal, A. Formation of physically stable amorphous phase of ibuprofen by solid state milling with kaolin. Eur. J. Pharm. Biopharm. 2008, 68, 346–351. [Google Scholar] [CrossRef]
- Yang, Z.; Hu, Y.; Tang, G.; Dong, M.; Liu, Q.; Lin, X. Development of ibuprofen dry suspensions by hot melt extrusion: Characterization, physical stability and pharmacokinetic studies. J. Drug Deliv. Sci. Technol. 2019, 54, 101313. [Google Scholar] [CrossRef]
- Biedrzycka, K.; Marcinkowska, A. The Use of Hot Melt Extrusion to Prepare a Solid Dispersion of Ibuprofen in a Polymer Matrix. Polymers 2023, 15, 2912. [Google Scholar] [CrossRef] [PubMed]
- Al-Zoubi, N.; Odeh, F.; Partheniadis, I.; Gharaibeh, S.; Nikolakakis, I. Spray drying of naproxen and naproxen sodium for improved tableting and dissolution—physicochemical characterization and compression performance. Pharm. Dev. Technol. 2020, 26, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Orszulak, L.; Włodarczyk, P.; Hachuła, B.; Lamrani, T.; Jurkiewicz, K.; Tarnacka, M.; Hreczka, M.; Kamiński, K.; Kamińska, E. Inhibition of naproxen crystallization by polymers: The role of topology and chain length of polyvinylpyrrolidone macromolecules. Eur. J. Pharm. Biopharm. 2025, 207, 114581. [Google Scholar] [CrossRef] [PubMed]
- Saitman, A. Chapter 13—Overview of Analytical Methods in Drugs of Abuse Analysis: Gas Chromatography/Mass Spectrometry, Liquid Chromatography Combined With Tandem Mass Spectrometry and Related Methods. In Critical Issues in Alcohol and Drugs of Abuse Testing, 2nd ed.; Dasgupta, A., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 157–171. [Google Scholar] [CrossRef]
- Varghese, S.; Ghoroi, C. Improving the wetting and dissolution of ibuprofen using solventless co-milling. Int. J. Pharm. 2017, 533, 145–155. [Google Scholar] [CrossRef]
- Gera, T.; Smausz, T.; Kopniczky, J.; Galbács, G.; Ambrus, R.; Szabó-Révész, P.; Hopp, B. Production of ibuprofen in crystalline and amorphous forms by Pulsed Laser Deposition (PLD). Appl. Surf. Sci. 2019, 493, 359–367. [Google Scholar] [CrossRef]
- Benezra, S.A.; McRae, J.W. Pseudoephedrine Hydrochloride. In Analytical Profiles of Drug Substances; Florey, K., Ed.; Academic Press: Cambridge, MA, USA, 1979; pp. 489–507. [Google Scholar] [CrossRef]
- de Oliveira, G.G.G.; Feitosa, A.; Loureiro, K.; Fernandes, A.R.; Souto, E.B.; Severino, P. Compatibility study of paracetamol, chlorpheniramine maleate and phenylephrine hydrochloride in physical mixtures. Saudi. Pharm. J. 2017, 25, 99–103. [Google Scholar] [CrossRef]
- Ramukutty, S.; Ramachandran, E. Growth, spectral and thermal studies of ibuprofen crystals. Cryst. Res. Technol. 2012, 47, 31–38. [Google Scholar] [CrossRef]
- Özakar, R.S.; Yaman, M.E.; Özakar, E.; Atila, A.; Atila, N.E.; Kadioglu, Y. A new triple combination for upper respiratory tract infections: Preparation and evaluation of sustained-release pellet formulations containing erdosteine, ibuprofen and pseudoephedrine HCl. Farmacia 2022, 70, 816–830. [Google Scholar] [CrossRef]
- Shaik, A.P.; Adapa, S. Formulation and Evaluation of Phenylephrine Nasal Gels. IJCPMS 2024, 4, 1–10. [Google Scholar] [CrossRef]
- Habiba, U.; Alam, A.; Rahman, S.; Shamim, S.; Piya, A. IR spectra of paracetamol. BJSIR 2021, 56, 255–262. [Google Scholar] [CrossRef]
- Ziaee, A.; O’Dea, S.; Howard-Hildige, A.; Padrela, L.; Potter, C.; Iqbal, J.; Albadarin, A.B.; Walker, G.; O’Reilly, E.J. Amorphous solid dispersion of ibuprofen: A comparative study on the effect of solution based techniques. Int. J. Pharm. 2019, 572, 118816. [Google Scholar] [CrossRef] [PubMed]
- (+)-Pseudoephedrine Hydrochloride. Available online: https://spectrabase.com/spectrum/2WU4s2HC21f (accessed on 23 June 2025).
- Olds, W.J.; Sundarajoo, S.; Selby, M.; Cletus, B.; Fredericks, P.M.; Izake, E.L. Noninvasive, Quantitative Analysis of Drug Mixtures in Containers Using Spatially Offset Raman Spectroscopy (SORS) and Multivariate Statistical Analysis. Appl. Spectrosc. 2012, 66, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, C.H.; Holmes, S.T.; Levin, K.; Veinberg, S.L.; Schurko, R.W. Characterization of ephedrine HCl and pseudoephedrine HCl using quadrupolar NMR crystallography guided crystal structure prediction. Faraday Discuss. 2025, 255, 88–118. [Google Scholar] [CrossRef]
- Patil, M.P.; Mali, R.P.; Kothmire, S.D.; Udavant, P.B.; Kshirsagar, S.J. Spray dried chitosan microspheres of phenylephrine hydrochloride for nasal delivery: Development and in vitro evaluation. Pharm. Reson. 2018, 1, 28–33. [Google Scholar]
- Jendrzejewska, I.; Goryczka, T.; Pietrasik, E.; Klimontko, J.; Jampilek, J. X-ray and Thermal Analysis of Selected Drugs Containing Acetaminophen. Molecules 2020, 25, 5909. [Google Scholar] [CrossRef]
- Löbmann, K.; Laitinen, R.; Grohganz, H.; Strachan, C.; Rades, T.; Gordon, K.C. A theoretical and spectroscopic study of co-amorphous naproxen and indomethacin. Int. J. Pharm. 2013, 453, 80–87. [Google Scholar] [CrossRef]
- Ueda, H.; Bøtker, J.P.; Edinger, M.; Löbmann, K.; Grohganz, H.; Müllertz, A.; Rades, T.; Østergaard, J. Formulation of co-amorphous systems from naproxen and naproxen sodium and in situ monitoring of physicochemical state changes during dissolution testing by Raman spectroscopy. Int. J. Pharm. 2020, 587, 119662. [Google Scholar] [CrossRef]
- Rojek, B.; Wesolowski, M. DSC supported by factor analysis as a reliable tool for compatibility study in pharmaceutical mixtures. J. Therm. Anal. Calorim. 2019, 138, 4531–4539. [Google Scholar] [CrossRef]
- Leyva-Porras, C.; Cruz-Alcantar, P.; Espinosa-Solís, V.; Martínez-Guerra, E.; Piñón-Balderrama, C.I.; Compean Martínez, I.; Saavedra-Leos, M.Z. Application of Differential Scanning Calorimetry (DSC) and Modulated Differential Scanning Calorimetry (MDSC) in Food and Drug Industries. Polymers 2020, 12, 5. [Google Scholar] [CrossRef]





| Ibuprofen | ||||||
|---|---|---|---|---|---|---|
| Product Name | API Content in Product [%] | Heat of Melting [J/g] | Percentage of Predicted Heat of Melting | Onset [°C] | Peak [°C] | |
| Predicted | Measured | |||||
| Ibuprofen | –128.1 | 74.7 | 77.0 | |||
| Ibumax | 54.6 | −69.9 | −54.2 | 77.5 | 69.5 | 73.7 |
| Ibupar forte | 62.3 | −79.8 | −72.6 | 91.0 | 69.5 | 74.5 |
| Ibuprex Max | 56.2 | −72.0 | −66.7 | 92.6 | 72.6 | 75.4 |
| Ibuprofen Aflofarm | 44.0 | −56.3 | −61.2 | 108.8 | 73.5 | 76.2 |
| Ibuprofen TZF | 67.9 | −86.9 | −62.3 | 71.6 | 68.1 | 72.3 |
| Ibuprom | 39.4 | −50.5 | −42.9 | 85.1 | 71.9 | 75.0 |
| Ibuprom Max | 46.7 | −59.8 | −53.0 | 88.6 | 71.4 | 75.1 |
| Ibuprom RR MAX | 59.5 | −76.2 | −62.6 | 82.2 | 72.4 | 75.0 |
| Ibuprom Ultramax | 77.3 | −99.0 | −81.3 | 82.2 | 71.7 | 75.7 |
| Iburapid | 71.6 | −91.6 | −78.3 | 85.4 | 74.0 | 76.0 |
| Ibuprofen Max PolfaŁódź | 71.8 | −91.9 | −73.3 | 79.8 | 74.3 | 76.5 |
| MIG | 60.3 | −77.3 | −69.1 | 89.5 | 71.2 | 74.6 |
| Nurofen | 46.9 | −60.0 | −50.6 | 84.4 | 72.4 | 75.5 |
| Nurofen Forte | 46.6 | −59.6 | −69.5 | 116.6 | 72.1 | 74.9 |
| Ibuprofen with different API | ||||||
| Acatar Zatoki 1 | 37.5 | −48.0 | −39.3 | 81.8 | 70.2 | 74.5 |
| Ibum Zatoki Max 1 | 48.6 | −62.3 | −50.2 | 80.6 | 71.9 | 75.5 |
| Ibuprom Zatoki 1 | 37.7 | −48.3 | −43.3 | 89.6 | 70.2 | 74.0 |
| Ibuprom Zatoki Max 1 | 54.2 | −69.5 | −60.5 | 87.1 | 69.5 | 73.8 |
| Infex Zatoki 1 | 53.3 | −68.3 | −65.3 | 95.6 | 68.4 | 73.7 |
| Metafen Zatoki 1 | 28.5 | −36.5 | −25.7 | 70.4 | 70.2 | 74.8 |
| Modafen Extra Grip 1 | 34.4 | −44.1 | −34.9 | 79.3 | 67.2 | 72.3 |
| Nurofen Zatoki 1 | 54.6 | −70.0 | −25.7 | 36.8 | 70.2 | 74.8 |
| Ibuprom Zatoki Tabs 2 | 48.6 | −62.2 | −61.6 | 98.9 | 73.5 | 76.1 |
| APAP intense 3 | 20.8 | −26.6 | −20.0 | 75.2 | 72.1 | 74.8 |
| Metafen 3 | 28.4 | −36.3 | −27.4 | 75.4 | 72.3 | 75.5 |
| Naproxen | ||||||
| Naproxen | –170.9 | 156.6 | 157.1 | |||
| Anapran EC | 82.7 | –141.4 | –114.9 | 81.3 | 153.9 | 155.6 |
| Apo Napro 250 | 81.0 | –138.4 | −90.8 | 65.6 | 150.9 | 154.7 |
| Apo Napro 500 | 79.9 | –136.6 | –102.9 | 75.3 | 152.3 | 155.3 |
| Naproxen Hasco | 92.5 | –158.1 | −90.4 | 57.1 | 152.6 | 155.0 |
| Naproxen Hasco 500 | 91.7 | –156.8 | –122.6 | 78.2 | 152.7 | 155.7 |
| Naproxen Polfarmex | 89.8 | –153.5 | −99.8 | 65.0 | 152.4 | 155.1 |
| Naproxen sodium | ||||||
| Naproxen sodium | –136.9 | 256.8 | 257.9 | |||
| Aleve | 69.4 | −95.0 | −62.3 | 65.6 | 239.2 | 248.4 |
| Anapran | 67.8 | −92.7 | −42.0 | 45.3 | 213.6 | 231.4 |
| Nalgesin | 62.5 | −85.6 | −59.7 | 69.8 | 231.2 | 244.5 |
| Nalgesin Forte | 69.7 | −95.4 | −75.8 | 79.5 | 238.4 | 247.0 |
| Nalgesin Mini | 68.1 | −93.2 | −64.8 | 69.5 | 240.6 | 248.9 |
| Naxii | 67.3 | −92.1 | –16.2 | 17.6 | 235.3 | 246.4 |
| FTIR Spectra | Raman Spectra | |||
|---|---|---|---|---|
| Sample Name | Standardized Intensity of Band 1721 cm−1 | Percentage of Reference Intensity of Band in Spectra | Standardized Intensity of Band 1113 cm−1 | Percentage of Reference Intensity of Band in Spectra |
| Ibuprofen | 2.17 | - | 0.78 | - |
| Ibumax | 2.08 | 95.9 | 0.50 | 63.7 |
| Ibupar forte | 2.16 | 99.5 | 0.62 | 79.1 |
| Ibuprex Max | 2.06 | 94.9 | 0.58 | 73.9 |
| Ibuprofen Aflofarm | 1.81 | 83.4 | 0.72 | 92.4 |
| Ibuprofen TZF | 2.09 | 96.3 | 0.73 | 93.4 |
| Ibuprom | 1.64 | 75.6 | 0.58 | 75.0 |
| Ibuprom Max | 1.81 | 83.4 | 0.65 | 83.6 |
| Ibuprom RR MAX | 2.11 | 97.2 | 0.60 | 76.9 |
| Ibuprom Ultramax | 1.73 | 79.7 | 0.74 | 95.5 |
| Iburapid | 1.80 | 82.9 | 0.74 | 94.8 |
| Ibuprofen Max PolfaŁódź | 1.60 | 73.7 | 0.70 | 89.9 |
| MIG | 2.23 | 102.8 | 0.65 | 83.5 |
| Nurofen | 1.89 | 87.1 | 0.62 | 78.9 |
| Nurofen Forte | 1.97 | 90.8 | 0.65 | 83.2 |
| Ibuprofen with different API | ||||
| Acatar Zatoki 1 | 1.55 | 71.4 | 0.52 | 66.9 |
| Ibum Zatoki Max 1 | 1.64 | 75.6 | 0.64 | 82.4 |
| Ibuprom Zatoki 1 | 1.75 | 80.6 | 0.52 | 67.2 |
| Ibuprom Zatoki Max 1 | 1.59 | 73.3 | 0.71 | 91.4 |
| Infex Zatoki 1 | 1.59 | 73.3 | 0.66 | 84.0 |
| Metafen Zatoki 1 | 1.11 | 51.2 | 0.52 | 67.0 |
| Modafen Extra Grip 1 | 1.05 | 48.4 | 0.47 | 59.7 |
| Nurofen Zatoki 1 | 1.56 | 71.9 | 0.63 | 80.7 |
| Ibuprom Zatoki Tabs 2 | 1.50 | 69.1 | no | no |
| Ibuprofen (stardadized to 780 cm−1) | 2.54 | - | ||
| APAP intense 3 | 1.79 | 70.5 | no | no |
| Metafen 3 | 1.79 | 70.5 | no | no |
| Product Name | Intensity of XRD Peak 20.2 (2θ [Degree]) | Percentage of Predicted Peak Intensity | |
|---|---|---|---|
| Predicted | Measured | ||
| Ibuprofen | 3068 | ||
| Ibumax | 1675 | 1004 | 59.9 |
| Ibupar Forte | 1911 | 1134 | 59.3 |
| Ibuprex Max | 1724 | 988 | 57.3 |
| Ibuprofen Aflofarm | 1350 | 918 | 68.0 |
| Ibuprofen TZF | 2083 | 1136 | 54.4 |
| Ibuprom | 1209 | 807 | 66.8 |
| Ibuprom Max | 1433 | 827 | 57.7 |
| Ibuprom RR MAX | 1825 | 1050 | 57.5 |
| Ibuprom Ultramax | 2372 | 1863 | 78.6 |
| Iburapid | 2197 | 1274 | 58.0 |
| Ibuprofen Max PolfaŁódź | 2203 | 1173 | 53.2 |
| MIG | 1850 | 944 | 51.0 |
| Nurofen | 1439 | 630 | 43.8 |
| Nurofen Forte | 1430 | 803 | 56.2 |
| Acatar Zatoki 1 | 1151 | 735 | 63.9 |
| Ibum Zatoki Max 1 | 1491 | 913 | 61.2 |
| Ibuprom Zatoki 1 | 1157 | 690 | 59.7 |
| Ibuprom Zatoki Max 1 | 1663 | 582 | 35.0 |
| Infex Zatoki 1 | 1635 | 899 | 55.0 |
| Metafen Zatoki 1 | 874 | 658 | 75.3 |
| Modafen Extra Grip 1 | 1055 | 685 | 64.9 |
| Nurofen Zatoki 1 | 1675 | 468 | 27.9 |
| Intensity of XRD peak 22 (2θ [degree]) | |||
| Ibuprofen | 183 | ||
| Ibuprom Zatoki Tabs 2 | 89 | 85 | 95.6 |
| APAP intense 3 | 38 | 99 | 260.1 |
| Metafen 3 | 52 | 222 | 427.2 |
| FTIR Spectra | Raman Spectra | |||
|---|---|---|---|---|
| Standardized Intensity of Band 1728 cm−1 | Percentage of Reference Intensity of Band in Spectra | Standardized Intensity of Band 760 cm−1 | Percentage of Reference Intensity of Band in Spectra | |
| Naproxen | 1.40 | - | 0.46 | - |
| Anapran EC | 1.17 | 83.6 | 0.45 | 97.3 |
| Apo Napro 250 | 1.02 | 72.9 | 0.43 | 93.2 |
| Apo Napro 500 | 1.32 | 94.3 | 0.43 | 93.1 |
| Naproxen Hasco | 1.16 | 82.9 | 0.44 | 96.3 |
| Naproxen Hasco 500 | 1.09 | 77.9 | 0.45 | 98.2 |
| Naproxen Polfarmex | 1.09 | 77.9 | 0.45 | 97.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leyk, E.; Środa, M.; Maślanka, G.; Nowaczyk, P.; Orzołek, A.; Grodzka, H.; Kurek, A.; Knut, O.; Michalak, J.; Płachciak, J.; et al. Analysis of the Effect of the Tablet Matrix on the Polymorphism of Ibuprofen, Naproxen, and Naproxen Sodium in Commercially Available Pharmaceutical Formulations. Methods Protoc. 2025, 8, 99. https://doi.org/10.3390/mps8050099
Leyk E, Środa M, Maślanka G, Nowaczyk P, Orzołek A, Grodzka H, Kurek A, Knut O, Michalak J, Płachciak J, et al. Analysis of the Effect of the Tablet Matrix on the Polymorphism of Ibuprofen, Naproxen, and Naproxen Sodium in Commercially Available Pharmaceutical Formulations. Methods and Protocols. 2025; 8(5):99. https://doi.org/10.3390/mps8050099
Chicago/Turabian StyleLeyk, Edyta, Marcin Środa, Gracjan Maślanka, Patrycja Nowaczyk, Amelia Orzołek, Hanna Grodzka, Aleksandra Kurek, Olaf Knut, Julia Michalak, Jonatan Płachciak, and et al. 2025. "Analysis of the Effect of the Tablet Matrix on the Polymorphism of Ibuprofen, Naproxen, and Naproxen Sodium in Commercially Available Pharmaceutical Formulations" Methods and Protocols 8, no. 5: 99. https://doi.org/10.3390/mps8050099
APA StyleLeyk, E., Środa, M., Maślanka, G., Nowaczyk, P., Orzołek, A., Grodzka, H., Kurek, A., Knut, O., Michalak, J., Płachciak, J., & Plenis, A. (2025). Analysis of the Effect of the Tablet Matrix on the Polymorphism of Ibuprofen, Naproxen, and Naproxen Sodium in Commercially Available Pharmaceutical Formulations. Methods and Protocols, 8(5), 99. https://doi.org/10.3390/mps8050099











