Antidepressant and Related Neurobiological and Neurophysiological Effects of Add-On Transcranial Direct Current Stimulation in Major Depressive Disorder with Residual Symptoms: A Randomized, Double-Blind Clinical Trial Protocol
Abstract
1. Introduction
1.1. Background
1.2. Aim and Hypothesis
- (i)
- This randomized, sham-controlled, double-blind trial primarily aims to evaluate the effects of tDCS as an adjunct to pharmacological treatment in MDD outpatients who continue to experience residual depressive symptoms despite adequate SSRI therapy.
- (ii)
- Secondary outcomes are as follows: to assess the impact of tDCS on cognitive performance; to examine pre- and post-tDCS variations in laboratory, neurophysiological, and neuropsychological parameters, including cortical excitability, hemodynamic measures, and neuroplasticity factors, which may underlie the long-term efficacy of the treatment.
- (i)
- The primary hypothesis is that participants receiving active tDCS will demonstrate a significant reduction in residual symptoms as measured by the Hamilton Depression Rating Scale—17 (HDRS-17).
- (ii)
- Secondary hypotheses are as follows: the active tDCS group is expected to show a significant reduction in cognitive symptomatology compared to the sham group, as measured by the Montreal Cognitive Assessment (MoCA), Frontal Assessment Battery (FAB), and Stroop Color–Word Interference Test (Stroop T); to show a significant change in serum concentrations of neurotrophic factors (BDNF, VEGF, Nrg1, Ang, IGF1, NGF) and inflammatory cytokines (IL-6, TNF-α), and a correlation with the persistence or remission of residual depressive symptoms. Furthermore, a correlation is expected between the reduction of residual symptoms following tDCS and changes in cortical excitability parameters, as assessed through TMS measures, as well as between improvements in residual depressive symptoms following tDCS and hemodynamic parameters assessed by transcranial Doppler sonography (TCD).
2. Experimental Design
2.1. Study Population
- (i)
- Age between 18 and 65 years.
- (ii)
- Residual depressive symptoms despite at least 4 weeks of stable SSRI treatment at an antidepressant dosage, described as follows: fluoxetine 20 mg/day; sertraline 100 mg/day; paroxetine 20 mg/day; citalopram 20 mg/day; escitalopram 10 mg/day; fluvoxamine 100 mg/day. The approximate equivalent dose for each molecule (often referenced to fluoxetine 20 mg as a standard unit) [56] is as follows: fluoxetine 20 mg; sertraline 50 mg; paroxetine 20 mg; citalopram 20 mg; escitalopram 10 mg; fluvoxamine 100 mg.
- (iii)
- A Hamilton Depression Rating Scale–17 (HDRS-17) score > 7 at screening. The HDRS-17 cut-off was selected because a score ≤ 7 is conventionally regarded as remission, whereas scores above this threshold indicate the persistence of residual symptoms. The use of HDRS-17, therefore, provides a standardized and widely accepted method to capture patients who, although they may have improved with treatment, continue to experience a clinically meaningful symptom burden that prevents full remission.
- (iv)
- Capacity to provide written informed consent and comply with all study procedures.
- (i)
- Cognitive impairment: Mini-Mental State Examination (MMSE) < 18 or Clinical Dementia Rating (CDR) > 2;
- (ii)
- Neurological disorders: history of stroke, multiple sclerosis, major head injury, epilepsy, or other clinically significant neurological conditions;
- (iii)
- Psychiatric comorbidity: diagnosis of schizophrenia, schizoaffective disorder, bipolar disorder, other primary psychiatric conditions, and personality disorders (excluding comorbid anxiety disorders if secondary to MDD);
- (iv)
- Severe medical conditions: acute or chronic illnesses not adequately controlled (e.g., uncontrolled hypertension, diabetes, or systemic diseases);
- (v)
- Endocrine disorders, vitamin deficiencies, or use of medications known to impair mood or cognition;
- (vi)
- Substance use: alcohol abuse or illicit substance dependence within the past 6 months;
- (vii)
- Contraindications to neurostimulation: history of craniotomy, presence of implanted medical devices (e.g., pacemakers, deep brain stimulators), metallic splinters or prostheses, or personal/family history of epilepsy;
- (viii)
- Pregnancy or lactation.
2.2. Equipment
3. Procedure
3.1. Clinical and Psycho-Cognitive Assessment
3.2. Laboratory Assessment
3.3. Neurosonological Assessment
- (i)
- Peak systolic velocity (PSV): the maximal velocity during systole, reflecting arterial inflow;
- (ii)
- End-diastolic velocity (EDV): the velocity during end-diastole, influenced by downstream resistance;
- (iii)
- Mean blood flow velocity (MBFV): calculated as the time-averaged velocity over the cardiac cycle, representing global perfusion of the insonated vessel;
- (iv)
- Pulsatility index (PI): calculated as (PSV—EDV)/MBFV, serving as a marker of cerebrovascular resistance and arterial compliance;
- (v)
- Resistivity index (RI): calculated as (PSV—EDV)/PSV, also reflecting vascular resistance.
3.4. Neurophysiological Assessment
- (i)
- Resting motor threshold (rMT): defined as the minimum stimulus intensity required to evoke MEPs of ≥50 μV in at least 5 of 10 trials at rest, reflecting global corticospinal excitability.
- (ii)
- MEP amplitude: an index of corticospinal tract integrity and responsiveness.
- (iii)
- Central motor conduction time (CMCT): derived from the latency difference between spinal and cortical stimulation, reflecting conduction efficiency.
- (iv)
- Contralateral silent period (cSP): measured during tonic muscle contraction and reflecting GABA-B-mediated intracortical inhibition.
- (v)
- Ipsilateral silent period (iSP): elicited by suprathreshold stimulation of M1 during contraction of the homolateral FDI, serving as a marker of transcallosal inhibition.
3.5. Randomization and Blinding
3.6. Interventions and Study Duration
3.7. Sample Size and Statistical Analysis
3.8. Table and Figure
4. Expected Results
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| tDCS | transcranial direct current stimulation |
| MDD | major depressive disorder |
| NVU | neurovascular unit |
| DLPFC | dorsolateral prefrontal cortex |
| SSRIs | serotonin reuptake inhibitors |
| SNRIs | serotonin and norepinephrine reuptake inhibitors |
| HPA | hypothalamic–pituitary–adrenal axis |
| VEGF | vascular endothelial growth factor |
| IL-6 | interleukin 6 |
| TNF-α | tumor necrosis factor α |
| BDNF | brain-derived neurotrophic factor |
| NSCs | neural stem cells |
| NTs | neurotrophic factors |
| NGF | nerve growth factor |
| IGF-1 | insulin-like growth factor 1 |
| HIF-1 α | hypoxia-inducible factor-1 α |
| NRG1 | neuregulin-1 |
| NMDA | N-methyl-D-aspartate |
| sgACC | subgenual anterior cingulate cortex |
| fMRI | functional magnetic resonance |
| CRP | C-reactive protein |
| TrkB | tyrosine protein kinase B |
| MoCA | Montreal Cognitive Assessment |
| FAB | Frontal Assessment Battery |
| rMT | resting motor threshold |
References
- Kraus, C.; Kadriu, B.; Zarate, C.A., Jr.; Kasper, S. Prognosis and improved outcomes in major depression: A review. Transl. Psychiatry 2019, 9, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Maj, M.; Stein, D.J.; Parker, G.; Zimmerman, M.; Fava, G.A.; De Hert, M.; Demyttenaere, K.; McIntyre, R.S.; Widiger, T.; Wittchen, H. The clinical characterization of the adult patient with depression aimed at personalization of management. World Psychiatry 2020, 19, 269–293. [Google Scholar] [CrossRef] [PubMed]
- Concerto, C.; Chiarenza, C.; Di Francesco, A.; Natale, A.; Privitera, I.; Rodolico, A.; Trovato, A.; Aguglia, A.; Fisicaro, F.; Pennisi, M.; et al. Neurobiology and Applications of Inositol in Psychiatry: A Narrative Review. Curr. Issues Mol. Biol. 2023, 45, 1762–1778. [Google Scholar] [CrossRef] [PubMed]
- Karrouri, R.; Hammani, Z.; Benjelloun, R.; Otheman, Y. Major depressive disorder: Validated treatments and future challenges. World J. Clin. Cases 2021, 9, 9350–9367. [Google Scholar] [CrossRef]
- Rodolico, A.; Cutrufelli, P.; Di Francesco, A.; Aguglia, A.; Catania, G.; Concerto, C.; Cuomo, A.; Fagiolini, A.; Lanza, G.; Mineo, L.; et al. Efficacy and safety of ketamine and esketamine for unipolar and bipolar depression: An overview of systematic reviews with meta-analysis. Front. Psychiatry 2024, 15, 1325399. [Google Scholar] [CrossRef]
- Pastuszak, M.; Cubała, W.J.; Kwaśny, A.; Mechlińska, A. The Search for Consistency in Residual Symptoms in Major Depressive Disorder: A Narrative Review. J. Pers. Med. 2024, 14, 828. [Google Scholar] [CrossRef]
- Uher, R.; Mors, O.; Rietschel, M.; Rajewska-Rager, A.; Petrovic, A.; Zobel, A.; Henigsberg, N.; Mendlewicz, J.; Aitchison, K.J.; Farmer, A.; et al. Early and delayed onset of response to antidepressants in individual trajectories of change during treatment of major depression: A secondary analysis of data from the genome-based therapeutic drugs for depression (GENDEP) study. J. Clin. Psychiatry 2011, 72, 1478–1484. [Google Scholar] [CrossRef]
- Frodl, T. Recent advances in predicting responses to antidepressant treatment. F1000Research 2017, 6, 619. [Google Scholar] [CrossRef]
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef]
- Troubat, R.; Barone, P.; Leman, S.; Desmidt, T.; Cressant, A.; Atanasova, B.; Brizard, B.; El Hage, W.; Surget, A.; Belzung, C.; et al. Neuroinflammation and depression: A review. Eur. J. Neurosci. 2012, 53, 151–171. [Google Scholar] [CrossRef]
- Hassamal, S. Chronic stress, neuroinflammation, and depression: An overview of pathophysiological mechanisms and emerging anti-inflammatories. Front. Psychiatry 2023, 14, 1130989. [Google Scholar] [CrossRef]
- Liu, Y.-Z.; Wang, Y.-X.; Jiang, C.-L. Inflammation: The common pathway of stress-related diseases. Front. Hum. Neurosci. 2017, 11, 316. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Ji, C.; Shao, A. Neurovascular Unit Dysfunction and Neurodegenerative Disorders. Front. Neurosci. 2020, 14, 334. [Google Scholar] [CrossRef]
- Wu, S.; Yin, Y.; Du, L. Blood–Brain Barrier Dysfunction in the Pathogenesis of Major Depressive Disorder. Cell. Mol. Neurobiol. 2022, 42, 2571–2591. [Google Scholar] [CrossRef] [PubMed]
- Dion-Albert, L.; Dudek, K.A.; Russo, S.J.; Campbell, M.; Menard, C. Neurovascular adaptations modulating cognition, mood, and stress responses. Trends Neurosci. 2023, 46, 276–292. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Liu, J.; Han, Y.-S.; Liu, L.; Tang, L.; Yang, H.; Meng, P.; Zhao, H.-Q. Abnormal Glu/mGluR2/3/PI3K pathway in the hippocampal neurovascular unit leads to diabetes-related depression. Neural Regen. Res. 2021, 16, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-H.; Liu, J.; Li, W.; Liu, L.; Yang, H.; Meng, P.; Han, Y.-S. Structural and functional damage to the hippocampal neurovascular unit in diabetes-related depression. Neural Regen. Res. 2019, 14, 289–297. [Google Scholar] [CrossRef]
- Campagno, K.E.; Mitchell, C.H. The P2X7 Receptor in Microglial Cells Modulates the Endolysosomal Axis, Autophagy, and Phagocytosis. Front. Cell. Neurosci. 2021, 15, 645244. [Google Scholar] [CrossRef]
- Young, J.J.; Bruno, D.; Pomara, N. A review of the relationship between proinflammatory cytokines and major depressive disorder. J. Affect. Disord. 2014, 169, 15–20. [Google Scholar] [CrossRef]
- Liu, F.; Yang, Y.; Fan, X.-W.; Zhang, N.; Wang, S.; Shi, Y.-J.; Hu, W.-J.; Wang, C.-X. Impacts of inflammatory cytokines on depression: A cohort study. BMC Psychiatry 2024, 24, 195. [Google Scholar] [CrossRef]
- Chang, J.; Jiang, T.; Shan, X.; Zhang, M.; Li, Y.; Qi, X.; Bian, Y.; Zhao, L. Pro-inflammatory cytokines in stress-induced depression: Novel insights into mechanisms and promising therapeutic strategies. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2024, 131, 110931. [Google Scholar] [CrossRef] [PubMed]
- Zádor, F.; Joca, S.; Nagy-Grócz, G.; Dvorácskó, S.; Szűcs, E.; Tömböly, C.; Benyhe, S.; Vécsei, L. Pro-inflammatory cytokines: Potential links between the endocannabinoid system and the kynurenine pathway in depression. Int. J. Mol. Sci. 2021, 22, 5903. [Google Scholar] [CrossRef]
- Hussain, G.; Akram, R.; Anwar, H.; Sajid, F.; Iman, T.; Han, H.S.; Raza, C.; De Aguilar, J.-L.G. Adult neurogenesis. Neural Regen. Res. 2024, 19, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Zhang, J. Neuroinflammation, memory, and depression: New approaches to hippocampal neurogenesis. J. Neuroinflamm. 2023, 20, 283. [Google Scholar] [CrossRef]
- Emanueli, C.; Salis, M.B.; Pinna, A.; Graiani, G.; Manni, L.; Madeddu, P. Nerve growth factor promotes angiogenesis and arteriogenesis in ischemic hindlimbs. Circulation 2022, 106, 2257–2262. [Google Scholar] [CrossRef]
- Schirò, G.; Iacono, S.; Ragonese, P.; Aridon, P.; Salemi, G.; Balistreri, C.R. A Brief Overview on BDNF-Trk Pathway in the Nervous System: A Potential Biomarker or Possible Target in Treatment of Multiple Sclerosis? Front. Neurol. 2022, 13, 917527. [Google Scholar] [CrossRef]
- Nakamura, K.; Tan, F.; Li, Z.; Thiele, C.J. NGF activation of TrkA induces vascular endothelial growth factor expression via induction of hypoxia-inducible factor-1α. Mol. Cell. Neurosci. 2011, 46, 498–506. [Google Scholar] [CrossRef]
- Mahar, I.; MacIsaac, A.; Kim, J.J.; Qiang, C.; Davoli, M.A.; Turecki, G.; Mechawar, N. Effects of neuregulin-1 administration on neurogenesis in the adult mouse hippocampus, and characterization of immature neurons along the septotemporal axis. Sci. Rep. 2016, 6, 30467. [Google Scholar] [CrossRef]
- Mori, A.; Nishioka, Y.; Yamada, M.; Nishibata, Y.; Masuda, S.; Tomaru, U.; Honma, N.; Moriyama, T.; Ishizu, A. Brain-derived neurotrophic factor induces angiogenin secretion and nuclear translocation in human umbilical vein endothelial cells. Pathol. Res. Pract. 2018, 214, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Levada, O.A.; Troyan, A.S. Insulin-like growth factor-1: A possible marker for emotional and cognitive disturbances, and treatment effectiveness in major depressive disorder. Ann. Gen. Psychiatry 2017, 16, 38. [Google Scholar] [CrossRef]
- Song, J. BDNF Signaling in Vascular Dementia and Its Effects on Cerebrovascular Dysfunction, Synaptic Plasticity, and Cholinergic System Abnormality. J. Lipid Atheroscler. 2024, 13, 122–138. [Google Scholar] [CrossRef]
- Esposito, E.; Licastro, E.; Cuomo, O.; Lo, E.H.; Hayakawa, K.; Pignataro, G. Postconditioning promotes recovery in the neurovascular unit after stroke. Front. Cell. Neurosci. 2023, 17, 1260389. [Google Scholar] [CrossRef]
- Duman, R.S.; Li, N. A neurotrophic hypothesis of depression: Role of synaptogenesis in the actions of NMDA receptor antagonists. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 2475–2484. [Google Scholar] [CrossRef]
- Yang, T.; Nie, Z.; Shu, H.; Kuang, Y.; Chen, X.; Cheng, J.; Yu, S.; Liu, H. The Role of BDNF on Neural Plasticity in Depression. Front. Cell. Neurosci. 2020, 14, 82. [Google Scholar] [CrossRef]
- Pardossi, S.; Fagiolini, A.; Cuomo, A. Variations in BDNF and Their Role in the Neurotrophic Antidepressant Mechanisms of Ketamine and Esketamine: A Review. Int. J. Mol. Sci. 2024, 25, 13098. [Google Scholar] [CrossRef] [PubMed]
- Nejati, V.; Salehinejad, M.A.; Nitsche, M.A. Interaction of the Left Dorsolateral Prefrontal Cortex (l-DLPFC) and Right Orbitofrontal Cortex (OFC) in Hot and Cold Executive Functions: Evidence from Transcranial Direct Current Stimulation (tDCS). Neuroscience 2018, 369, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Qiao, D.; Li, Y.; Zhang, X.; Wen, Y.; Zhang, X.; Ma, L.; Li, G.; Yang, C.; Liu, Z. Exploring the connectivity of dorsolateral prefrontal cortex and the modulatory impact of transcranial magnetic stimulation in adolescents with depression: A focus on pain-related cognitive processing. BMC Psychiatry 2024, 24, 852. [Google Scholar] [CrossRef] [PubMed]
- Fonseka, T.M.; MacQueen, G.M.; Kennedy, S.H. Neuroimaging biomarkers as predictors of treatment outcome in Major Depressive Disorder. J. Affect. Disord. 2018, 233, 21–35. [Google Scholar] [CrossRef]
- Castrén, E.; Monteggia, L.M. Brain-Derived Neurotrophic Factor Signaling in Depression and Antidepressant Action. Biol. Psychiatry 2021, 90, 128–136. [Google Scholar] [CrossRef]
- Nadar, P.M.; Merrill, M.A.; Austin, K.; Strakowski, S.M.; Halpern, J.M. The emergence of psychoanalytical electrochemistry: The translation of MDD biomarker discovery to diagnosis with electrochemical sensing. Transl. Psychiatry 2022, 12, 372. [Google Scholar] [CrossRef]
- Spampinato, C.; Aguglia, E.; Concerto, C.; Pennisi, M.; Lanza, G.; Bella, R.; Cantone, M.; Pennisi, G.; Kavasidis, I.; Giordano, D. Transcranial magnetic stimulation in the assessment of motor cortex excitability and treatment of drug-resistant major depression. IEEE Trans. Neural Syst. Rehabil. Eng. 2013, 21, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Brunoni, A.R.; Baeken, C.; Machado-Vieira, R.; Gattaz, W.F.; Vanderhasselt, M.-A. BDNF blood levels after non-invasive brain stimulation interventions in major depressive disorder: A systematic review and meta-analysis. World J. Biol. Psychiatry 2015, 16, 114–122. [Google Scholar] [CrossRef]
- Nicoletti, V.G.; Fisicaro, F.; Aguglia, E.; Bella, R.; Calcagno, D.; Cantone, M.; Concerto, C.; Ferri, R.; Mineo, L.; Pennisi, G.; et al. Challenging the Pleiotropic Effects of Repetitive Transcranial Magnetic Stimulation in Geriatric Depression: A Multimodal Case Series Study. Biomedicines 2023, 11, 958. [Google Scholar] [CrossRef]
- Yu, T.-H.; Wu, Y.-J.; Chien, M.-E.; Hsu, K.-S. Transcranial direct current stimulation induces hippocampal metaplasticity mediated by brain-derived neurotrophic factor. Neuropharmacology 2019, 144, 358–367. [Google Scholar] [CrossRef]
- McClintock, S.M.; Martin, D.M.; Lisanby, S.H.; Alonzo, A.; McDonald, W.M.; Aaronson, S.T.; Husain, M.M.; O’REardon, J.P.; Weickert, C.S.; Mohan, A.; et al. Neurocognitive effects of transcranial direct current stimulation (tDCS) in unipolar and bipolar depression: Findings from an international randomized controlled trial. Depress. Anxiety 2020, 37, 261–272. [Google Scholar] [CrossRef]
- Maiworm, M. The relevance of BDNF for neuroprotection and neuroplasticity in multiple sclerosis. Front. Neurol. 2024, 15, 1385042. [Google Scholar] [CrossRef] [PubMed]
- Brennan, S.; McLoughlin, D.M.; O’COnnell, R.; Bogue, J.; O’COnnor, S.; McHugh, C.; Glennon, M. Anodal transcranial direct current stimulation of the left dorsolateral prefrontal cortex enhances emotion recognition in depressed patients and controls. J. Clin. Exp. Neuropsychol. 2017, 39, 384–395. [Google Scholar] [CrossRef]
- Lefaucheur, J.-P.; Wendling, F. Mechanisms of action of tDCS: A brief and practical overview. Neurophysiol. Clin. 2019, 49, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Zheng, E.Z.; Wong, N.M.L.; Yang, A.S.Y.; Lee, T.M.C. Evaluating the effects of tDCS on depressive and anxiety symptoms from a transdiagnostic perspective: A systematic review and meta-analysis of randomized controlled trials. Transl. Psychiatry 2024, 14, 295. [Google Scholar] [CrossRef]
- Pennisi, M.; Lanza, G.; Cantone, M.; Ricceri, R.; Spampinato, C.; Pennisi, G.; Di Lazzaro, V.; Bella, R. Correlation between Motor Cortex Excitability Changes and Cognitive Impairment in Vascular Depression: Pathophysiological Insights from a Longitudinal TMS Study. Neural Plast. 2016, 2016, 8154969. [Google Scholar] [CrossRef]
- Concerto, C.; Lanza, G.; Cantone, M.; Pennisi, M.; Giordano, D.; Spampinato, C.; Ricceri, R.; Pennisi, G.; Aguglia, E.; Bella, R. Different patterns of cortical excitability in major depression and vascular depression: A transcranial magnetic stimulation study. BMC Psychiatry 2013, 13, 300. [Google Scholar] [CrossRef]
- Zanardi, R.; Poletti, S.; Prestifilippo, D.; Attanasio, F.; Barbini, B.; Colombo, C. Transcranial direct current stimulation: A novel approach in the treatment of vascular depression. Brain Stimul. 2020, 13, 1559–1565. [Google Scholar] [CrossRef] [PubMed]
- Bikson, M.; Grossman, P.; Thomas, C.; Zannou, A.L.; Jiang, J.; Adnan, T.; Mourdoukoutas, A.P.; Kronberg, G.; Truong, D.; Boggio, P.; et al. Safety of Transcranial Direct Current Stimulation: Evidence Based Update 2016. Brain Stimul. 2016, 9, 641–661. [Google Scholar] [CrossRef]
- Bjekić, J.; Živanović, M.; Stanković, M.; Paunović, D.; Konstantinović, U.; Filipović, S.R. The subjective experience of transcranial electrical stimulation: A within-subject comparison of tolerability and side effects between tDCS, tACS, and otDCS. Front. Hum. Neurosci. 2024, 18, 1468538. [Google Scholar] [CrossRef]
- De Smet, S.; Nikolin, S.; Moffa, A.; Suen, P.; Vanderhasselt, M.-A.; Brunoni, A.R.; Razza, L.B. Determinants of sham response in tDCS depression trials: A systematic review and meta-analysis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 109, 110261. [Google Scholar] [CrossRef]
- Hayasaka, Y.; Purgato, M.; Magni, L.R.; Ogawa, Y.; Takeshima, N.; Cipriani, A.; Barbui, C.; Leucht, S.; Furukawa, T.A. Dose equivalents of antidepressants: Evidence-based recommendations from randomized controlled trials. J. Affect. Disord. 2015, 180, 179–184. [Google Scholar] [CrossRef]
- Snyder, H.R. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: A meta-analysis and review. Psychol. Bull. 2013, 139, 81–132. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; Slachevsky, A.; Litvan, I.; Pillon, B. The FAB A frontal assessment battery at bedside. Neurology 2000, 55, 1621–1626. [Google Scholar] [CrossRef]
- Rock, P.L.; Roiser, J.P.; Riedel, W.J.; Blackwell, A.D. Cognitive impairment in depression: A systematic review and meta-analysis. Psychol. Med. 2014, 44, 2029–2040. [Google Scholar] [CrossRef] [PubMed]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool For Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2005, 53, 696–699. [Google Scholar] [CrossRef] [PubMed]
- Aaslid, R.; Markwalder, T.-M.; Nornes, H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J. Neurosurg. 1982, 57, 769–774. [Google Scholar] [CrossRef]
- Ivelja, M.P.; Ivic, I.; Dolic, K.; Mestrovic, A.; Perkovic, N.; Jankovic, S. Evaluation of cerebrovascular reactivity in chronic hepatitis C patients using transcranial color Doppler. PLoS ONE 2019, 14, e0218206. [Google Scholar] [CrossRef]
- Markus, H.S.; Harrison, M.J. Estimation of Cerebrovascular Reactivity Using Transcranial Doppler, Including the Use of Breath-Holding as the Vasodilatory Stimulus. Stroke 1992, 23, 668–673. [Google Scholar] [CrossRef]
- Puglisi, V.; Bramanti, A.; Lanza, G.; Cantone, M.; Vinciguerra, L.; Pennisi, M.; Bonanno, L.; Pennisi, G.; Bella, R. Impaired cerebral haemodynamics in vascular depression: Insights from transcranial doppler ultrasonography. Front. Psychiatry 2018, 9, 316. [Google Scholar] [CrossRef]
- Rossini, P.M.; Burke, D.; Chen, R.; Cohen, L.G.; Daskalakis, Z.; Di Iorio, R.; Di Lazzaro, V.; Ferreri, F.; Fitzgerald, P.B.; George, M.S.; et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application: An updated report from an I.F.C.N. Committee. Clin. Neurophysiol. 2015, 126, 1071–1107. [Google Scholar] [CrossRef] [PubMed]
- Faro, A.; Giordano, D.; Kavasidis, I.; Pino, C.; Spampinato, C.; Cantone, M.G.; Lanza, G.; Pennisi, M. An Interactive Tool for Customizing Clinical Transacranial Magnetic Stimulation (TMS) Experiments; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Nasseri, P.; Nitsche, M.A.; Ekhtiari, H. A framework for categorizing electrode montages in transcranial direct current stimulation. Front. Hum. Neurosci. 2015, 9, 54. [Google Scholar] [CrossRef] [PubMed]
- Razza, L.B.; Palumbo, P.; Moffa, A.H.; Carvalho, A.F.; Solmi, M.; Loo, C.K.; Brunoni, A.R. A systematic review and meta-analysis on the effects of transcranial direct current stimulation in depressive episodes. Depress. Anxiety 2020, 37, 594–608. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhao, B.; Sun, X.; Ding, K.; Sun, J.; Tao, S. High-definition transcranial direct current stimulation (HD-tDCS) in major depressive disorder with anxious distress—A study protocol for a double-blinded randomized sham-controlled trial. Trials 2024, 25, 320. [Google Scholar] [CrossRef]
- Chan, A.-W.; Boutron, I.; Hopewell, S.; Moher, D.; Schulz, K.F.; Collins, G.S.; Tunn, R.; Aggarwal, R.; Berkwits, M.; Berlin, J.A.; et al. SPIRIT 2025 statement: Updated guideline for protocols of randomised trials. The Lancet 2025, 389, e081477. [Google Scholar] [CrossRef]
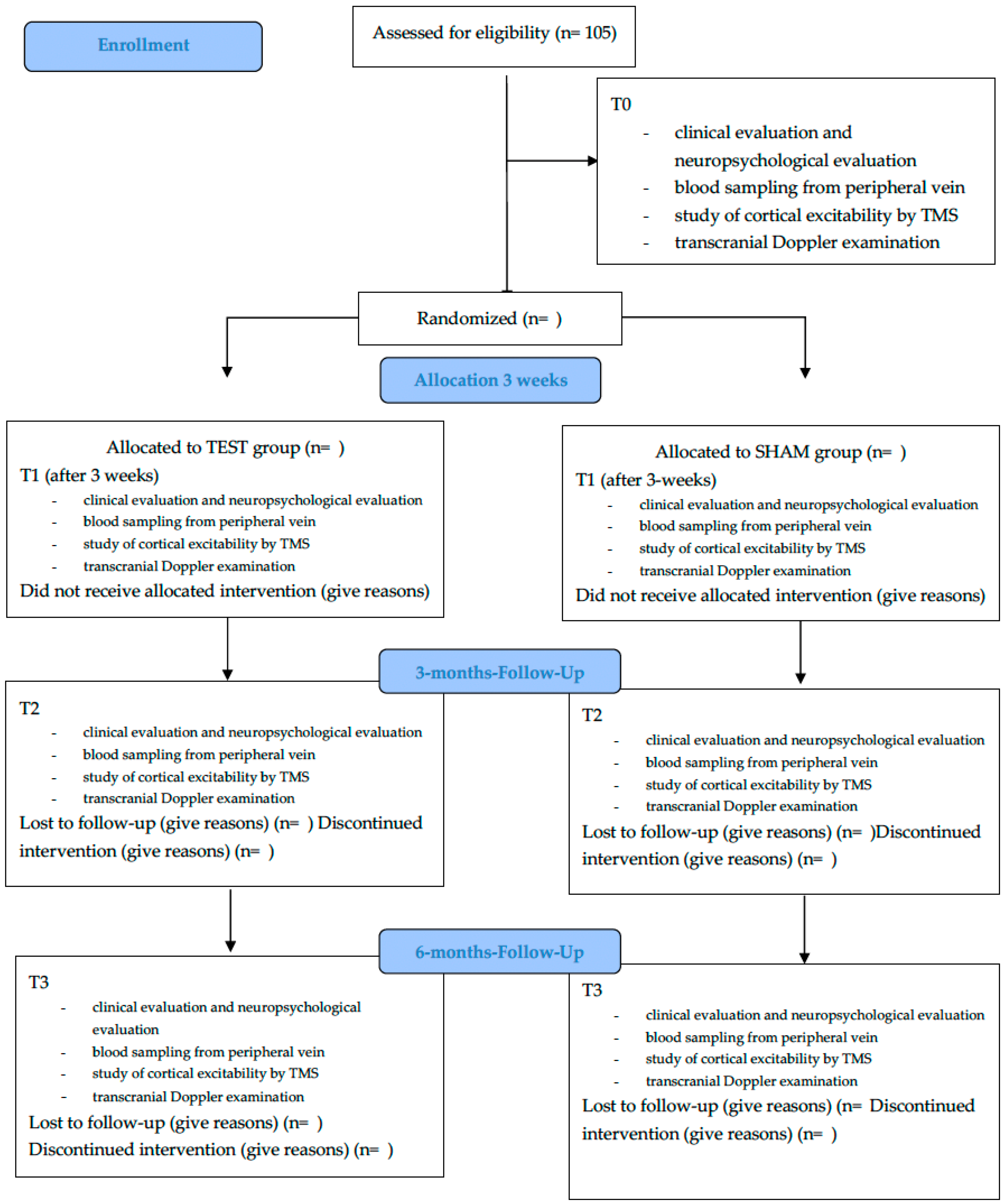
| Assessment Type | Baseline (T0) | Post-Treatment, 3 Weeks (T1) | 3-Month Follow-Up (T2) | 6-Month Follow-Up (T3) |
|---|---|---|---|---|
| Clinical evaluation | ✔ | ✔ | ✔ | ✔ |
| Cognitive assessment | ✔ | ✔ | ✔ | ✔ |
| Serum biomarker dosage | ✔ | ✔ | ✔ | ✔ |
| Cortical excitability by TMS | ✔ | ✔ | ✔ | ✔ |
| Cerebral hemodynamics by TCD | ✔ | ✔ | ✔ | ✔ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Concerto, C.; Bella, F.; Chiarenza, C.; Rodolico, A.; Di Francesco, A.; Ciancio, A.; Lanzafame, S.; Spigarelli, R.; Mineo, L.; Petralia, A.; et al. Antidepressant and Related Neurobiological and Neurophysiological Effects of Add-On Transcranial Direct Current Stimulation in Major Depressive Disorder with Residual Symptoms: A Randomized, Double-Blind Clinical Trial Protocol. Methods Protoc. 2025, 8, 117. https://doi.org/10.3390/mps8050117
Concerto C, Bella F, Chiarenza C, Rodolico A, Di Francesco A, Ciancio A, Lanzafame S, Spigarelli R, Mineo L, Petralia A, et al. Antidepressant and Related Neurobiological and Neurophysiological Effects of Add-On Transcranial Direct Current Stimulation in Major Depressive Disorder with Residual Symptoms: A Randomized, Double-Blind Clinical Trial Protocol. Methods and Protocols. 2025; 8(5):117. https://doi.org/10.3390/mps8050117
Chicago/Turabian StyleConcerto, Carmen, Fabrizio Bella, Cecilia Chiarenza, Alessandro Rodolico, Antonio Di Francesco, Alessia Ciancio, Stefania Lanzafame, Riccardo Spigarelli, Ludovico Mineo, Antonino Petralia, and et al. 2025. "Antidepressant and Related Neurobiological and Neurophysiological Effects of Add-On Transcranial Direct Current Stimulation in Major Depressive Disorder with Residual Symptoms: A Randomized, Double-Blind Clinical Trial Protocol" Methods and Protocols 8, no. 5: 117. https://doi.org/10.3390/mps8050117
APA StyleConcerto, C., Bella, F., Chiarenza, C., Rodolico, A., Di Francesco, A., Ciancio, A., Lanzafame, S., Spigarelli, R., Mineo, L., Petralia, A., Ferri, R., Libra, M., Bella, R., Pennisi, M., Lanza, G., & Signorelli, M. S. (2025). Antidepressant and Related Neurobiological and Neurophysiological Effects of Add-On Transcranial Direct Current Stimulation in Major Depressive Disorder with Residual Symptoms: A Randomized, Double-Blind Clinical Trial Protocol. Methods and Protocols, 8(5), 117. https://doi.org/10.3390/mps8050117










