Effects of Different Centrifugation Parameters on Equilibrium Solubility Measurements
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
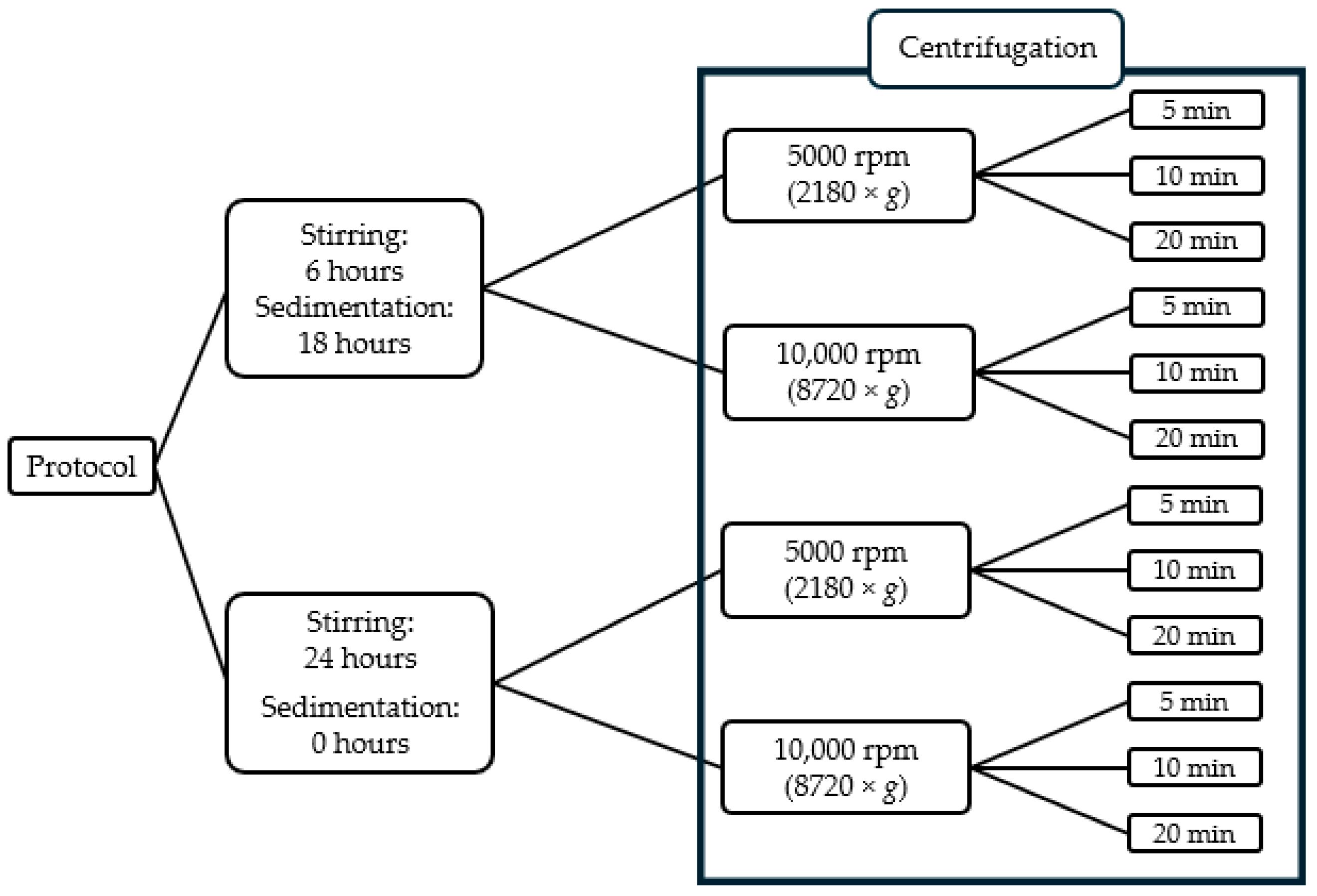
2.2. Determination of Equilibrium Solubility Using Saturation Shake-Flask Method
2.3. Statistical Analysis
3. Results and Discussion
3.1. Determination of Reference Equilibrium Solubility Values
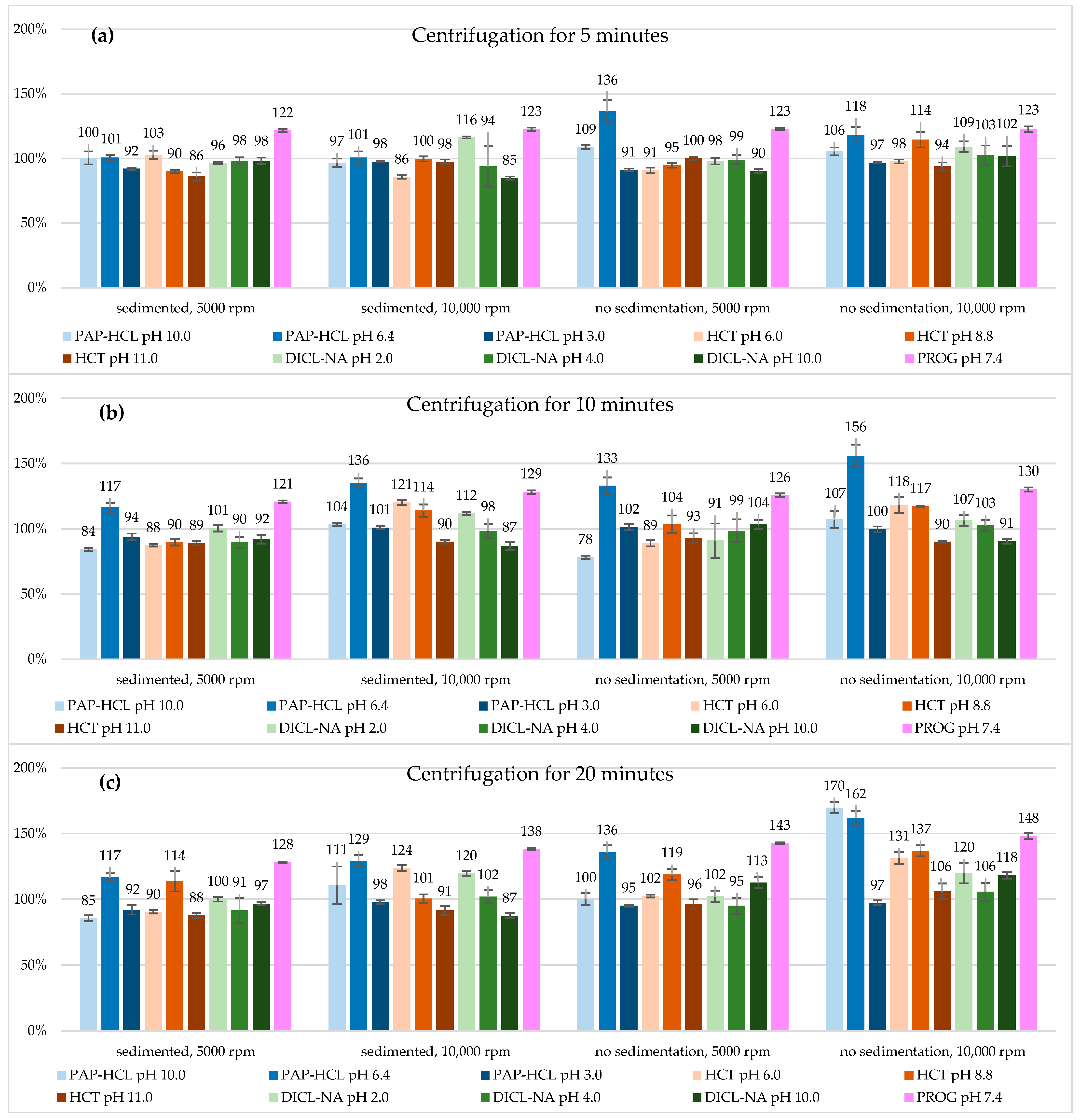
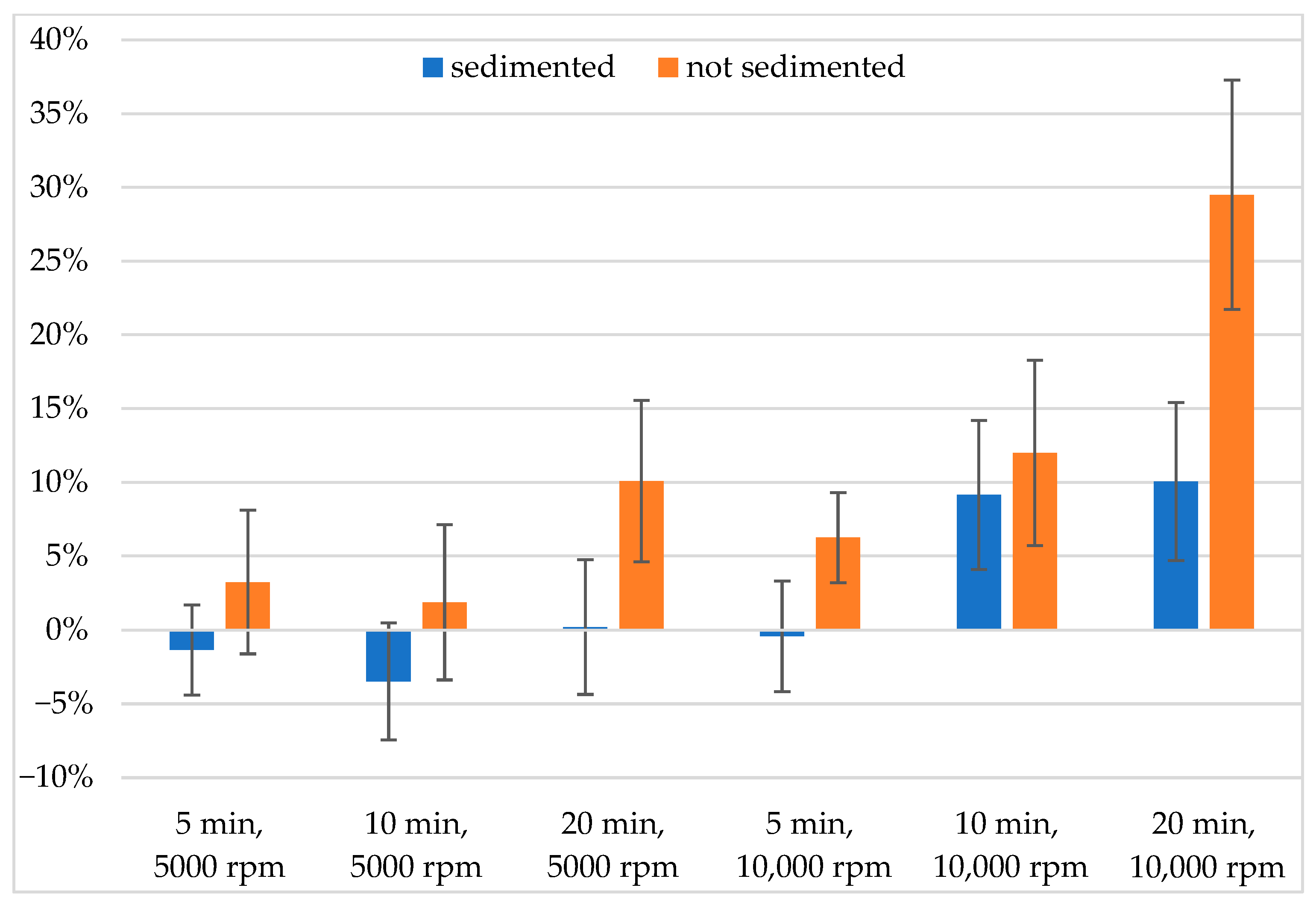
3.2. The Effect of Centrifugation
3.3. Comparison of Filtration and Centrifugation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SSF | Saturation shake-flask |
| API | Active pharmaceutical ingredient |
| BCS | Biopharmaceutics classification system |
| PROG | Progesterone |
| DICL-NA | Diclofenac sodium |
| HCT | Hydrochlorothiazide |
| PAP-HCL | Papaverine hydrochloride |
| Ssed | Solubility of samples sedimented before centrifugation |
| Sstir | Solubility of samples continuously stirred before centrifugation |
References
- Avdeef, A. Multi-Lab Intrinsic Solubility Measurement Reproducibility in CheqSol and Shake-Flask Methods. ADMET DMPK 2019, 7, 210–219. [Google Scholar] [CrossRef]
- Alsenz, J.; Kansy, M. High Throughput Solubility Measurement in Drug Discovery and Development. Adv. Drug Deliv. Rev. 2007, 59, 546–567. [Google Scholar] [CrossRef]
- Ono, A.; Matsumura, N.; Kimoto, T.; Akiyama, Y.; Funaki, S.; Tamura, N.; Hayashi, S.; Kojima, Y.; Fushimi, M.; Sudaki, H.; et al. Harmonizing Solubility Measurement to Lower Inter-Laboratory Variance—Progress of Consortium of Biopharmaceutical Tools (CoBiTo) in Japan. ADMET DMPK 2019, 7, 183–195. [Google Scholar] [CrossRef]
- Avdeef, A. Often Neglected Steps in Transforming Drug Solubility from Single Measurement in Pure Water to Physiologically-Appropriate Solubility-PH: Commentary. ADMET DMPK 2025, 13, 2626. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Takano, R.; Furumoto, K.; Shiraki, K.; Takata, N.; Hayashi, Y.; Aso, Y.; Yamashita, S. Rate-Limiting Steps of Oral Absorption for Poorly Water-Soluble Drugs in Dogs; Prediction from a Miniscale Dissolution Test and a Physiologically-Based Computer Simulation. Pharm. Res. 2008, 25, 2334–2344. [Google Scholar] [CrossRef]
- Sugano, K. Fraction of a Dose Absorbed Estimation for Structurally Diverse Low Solubility Compounds. Int. J. Pharm. 2011, 405, 79–89. [Google Scholar] [CrossRef]
- Bergström, C.A.S.; Holm, R.; Jørgensen, S.A.; Andersson, S.B.E.; Artursson, P.; Beato, S.; Borde, A.; Box, K.; Brewster, M.; Dressman, J.; et al. Early Pharmaceutical Profiling to Predict Oral Drug Absorption: Current Status and Unmet Needs. Eur. J. Pharm. Sci. 2014, 57, 173–199. [Google Scholar] [CrossRef]
- Dahan, A.; Beig, A.; Lindley, D.; Miller, J.M. The Solubility–Permeability Interplay and Oral Drug Formulation Design: Two Heads Are Better than One. Adv. Drug Deliv. Rev. 2016, 101, 99–107. [Google Scholar] [CrossRef]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug Solubility: Importance and Enhancement Techniques. ISRN Pharm. 2012, 2012, 195727. [Google Scholar] [CrossRef]
- Sugano, K.; Okazaki, A.; Sugimoto, S.; Tavornvipas, S.; Omura, A.; Mano, T. Solubility and Dissolution Profile Assessment in Drug Discovery. Drug Metab. Pharmacokinet. 2007, 22, 225–254. [Google Scholar] [CrossRef]
- Lovette, M.A.; Albrecht, J.; Ananthula, R.S.; Ricci, F.; Sangodkar, R.; Shah, M.S.; Tomasi, S. Evaluation of Predictive Solubility Models in Pharmaceutical Process Development-an Enabling Technologies Consortium Collaboration. Cryst. Growth Des. 2022, 22, 5239–5263. [Google Scholar] [CrossRef]
- Hokkala, E.; Strachan, C.J.; Agopov, M.; Järvinen, E.; Semjonov, K.; Heinämäki, J.; Yliruusi, J.; Svanbäck, S. Thermodynamic Solubility Measurement without Chemical Analysis. Int. J. Pharm. 2024, 653, 123890. [Google Scholar] [CrossRef]
- Rahimpour, E.; Moradi, M.; Sheikhi-Sovari, A.; Rezaei, H.; Rezaei, H.; Jouyban-Gharamaleki, V.; Kuentz, M.; Jouyban, A. Comparative Drug Solubility Studies Using Shake-Flask Versus a Laser-Based Robotic Method. AAPS PharmSciTech 2023, 24, 207. [Google Scholar] [CrossRef]
- Veseli, A.; Kristl, A.; Žakelj, S. Proof-of-Concept for a Miniaturized Shake-Flask Biopharmaceutical Solubility Determination by Sonic Mixing. Pharmazie 2020, 75, 626–631. [Google Scholar] [PubMed]
- Völgyi, G.; Csicsák, D.; Takács-Novák, K. Right Filter-Selection for Phase Separation in Equilibrium Solubility Measurement. Eur. J. Pharm. Sci. 2018, 123, 98–105. [Google Scholar] [CrossRef]
- Veseli, A.; Žakelj, S.; Kristl, A. A Review of Methods for Solubility Determination in Biopharmaceutical Drug Characterization. Drug Dev. Ind. Pharm. 2019, 45, 1717–1724. [Google Scholar] [CrossRef]
- Baka, E.; Comer, J.E.A.; Takács-Novák, K. Study of Equilibrium Solubility Measurement by Saturation Shake-Flask Method Using Hydrochlorothiazide as Model Compound. J. Pharm. Biomed. Anal. 2008, 46, 335–341. [Google Scholar] [CrossRef]
- Shoghi, E.; Fuguet, E.; Bosch, E.; Ràfols, C. Solubility–PH Profiles of Some Acidic, Basic and Amphoteric Drugs. Eur. J. Pharm. Sci. 2013, 48, 291–300. [Google Scholar] [CrossRef]
- Kawakami, K.; Miyoshi, K.; Ida, Y. Impact of the Amount of Excess Solids on Apparent Solubility. Pharm. Res. 2005, 22, 1537–1543. [Google Scholar] [CrossRef]
- Völgyi, G.; Baka, E.; Box, K.J.; Comer, J.E.A.; Takács-Novák, K. Study of PH-Dependent Solubility of Organic Bases. Revisit of Henderson-Hasselbalch Relationship. Anal. Chim. Acta 2010, 673, 40–46. [Google Scholar] [CrossRef]
- Bou-Chacra, N.; Melo, K.J.C.; Morales, I.A.C.; Stippler, E.S.; Kesisoglou, F.; Yazdanian, M.; Löbenberg, R. Evolution of Choice of Solubility and Dissolution Media After Two Decades of Biopharmaceutical Classification System. AAPS J. 2017, 19, 989–1001. [Google Scholar] [CrossRef]
- Bergström, C.A.S.; Luthman, K.; Artursson, P. Accuracy of Calculated PH-Dependent Aqueous Drug Solubility. Eur. J. Pharm. Sci. 2004, 22, 387–398. [Google Scholar] [CrossRef]
- Abuhassan, Q.; Khadra, I.; Pyper, K.; Halbert, G.W. Small Scale in Vitro Method to Determine a Bioequivalent Equilibrium Solubility Range for Fasted Human Intestinal Fluid. Eur. J. Pharm. Biopharm. 2021, 168, 90–96. [Google Scholar] [CrossRef]
- Csicsák, D.; Szolláth, R.; Kádár, S.; Ambrus, R.; Bartos, C.; Balogh, E.; Antal, I.; Köteles, I.; Tőzsér, P.; Bárdos, V.; et al. The Effect of the Particle Size Reduction on the Biorelevant Solubility and Dissolution of Poorly Soluble Drugs with Different Acid-Base Character. Pharmaceutics 2023, 15, 278. [Google Scholar] [CrossRef]
- Inês Silva, M.; Khadra, I.; Pyper, K.; Halbert, G.W. Small Scale in Vitro Method to Determine a Potential Bioequivalent Equilibrium Solubility Range for Fed Human Intestinal Fluid. Eur. J. Pharm. Biopharm. 2022, 177, 126–134. [Google Scholar] [CrossRef]
- Takács-Novák, K.; Urac, M.; Horváth, P.; Völgyi, G.; Anderson, B.D.; Avdeef, A. Equilibrium Solubility Measurement of Compounds with Low Dissolution Rate by Higuchi’s Facilitated Dissolution Method. A Validation Study. Eur. J. Pharm. Sci. 2017, 106, 133–141. [Google Scholar] [CrossRef]
- Takács-Novák, K.; Szoke, V.; Völgyi, G.; Horváth, P.; Ambrus, R.; Szabó-Révész, P. Biorelevant Solubility of Poorly Soluble Drugs: Rivaroxaban, Furosemide, Papaverine and Niflumic Acid. J. Pharm. Biomed. Anal. 2013, 83, 279–285. [Google Scholar] [CrossRef]
- Bergström, C.A.S.; Avdeef, A. Perspectives in Solubility Measurement and Interpretation. ADMET DMPK 2019, 7, 88–105. [Google Scholar] [CrossRef]
- Barzegar-Jalali, M.; Jafari, P.; Hemmati, S.; Jouyban, A. Equilibrium Solubility Investigation and Thermodynamic Aspects of Paracetamol, Salicylic Acid and 5-Aminosalicylic Acid in Polyethylene Glycol Dimethyl Ether 250+Water Mixtures. J. Mol. Liq. 2023, 372, 121185. [Google Scholar] [CrossRef]
- Dressman, J.B.; Reppas, C. In Vitro–in Vivo Correlations for Lipophilic, Poorly Water-Soluble Drugs. Eur. J. Pharm. Sci. 2000, 11, S73–S80. [Google Scholar] [CrossRef]
- Avdeef, A.; Fuguet, E.; Llinàs, A.; Ràfols, C.; Bosch, E.; Völgyi, G.; Verbic, T.; Boldyreva, E.; Takács-Novák, K. Equilibrium Solubility Measurement of Ionizable Drugs—Consensus Recommendations for Improving Data Quality. ADMET DMPK 2016, 4, 117–178. [Google Scholar] [CrossRef]
- Monteiro, P.F.; Silva-Barcellos, N.M.; Caldeira, T.G.; Reis, A.C.C.; Ribeiro, A.S.; de Souza, J. Effects of Experimental Conditions on Solubility Measurements for BCS Classification in Order to Improve the Biowaiver Guidelines. Braz. J. Pharm. Sci. 2021, 57, e181083. [Google Scholar] [CrossRef]
- Avdeef, A. Suggested Improvements for Measurement of Equilibrium Solubility-PH of Ionizable Drugs. ADMET DMPK 2015, 3, 84–109. [Google Scholar] [CrossRef]
- Chen, T.-M.; Shen, H.; Zhu, C. Evaluation of a Method for High Throughput Solubility Determination Using a Multi-Wavelength UV Plate Reader. Comb. Chem. High Throughput Screen 2012, 5, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Qiu, W.; Gao, W. Adsorption of Estrone in Microfiltration Membrane Filters. Chem. Eng. J. 2010, 165, 819–826. [Google Scholar] [CrossRef]
- Liu, X.; Müllertz, A.; Bar-Shalom, D.; Berthelsen, R. Effect of Centrifugation Speed on the Measured Equilibrium Solubility of Poorly Water-Soluble Compounds in Viscous Solvents. J. Drug Deliv. Sci. Technol. 2020, 59, 101853. [Google Scholar] [CrossRef]
- Lipinski, C.A. Drug-like Properties and the Causes of Poor Solubility and Poor Permeability. J. Pharmacol. Toxicol. Methods 2000, 44, 235–249. [Google Scholar] [CrossRef]
- Waring, M.J.; Arrowsmith, J.; Leach, A.R.; Leeson, P.D.; Mandrell, S.; Owen, R.M.; Pairaudeau, G.; Pennie, W.D.; Pickett, S.D.; Wang, J.; et al. An Analysis of the Attrition of Drug Candidates from Four Major Pharmaceutical Companies. Nat. Rev. Drug Discov. 2015, 14, 475–486. [Google Scholar] [CrossRef]
- Amidon, G.L.; Lennernäs, H.; Shah, V.P.; Crison, J.R. A Theoretical Basis for a Biopharmaceutic Drug Classification: The Correlation of in Vitro Drug Product Dissolution and in Vivo Bioavailability. Pharm. Res. Off. J. Am. Assoc. Pharm. Sci. 1995, 12, 413–420. [Google Scholar] [CrossRef]
- Avdeef, A. Absorption and Drug Development: Solubility, Permeability, and Charge State. In Absorption and Drug Development: Solubility, Permeability, and Charge State; John Wiley and Sons: Hoboken, NJ, USA, 2012; ISBN 9781118057452. [Google Scholar]
- Stuart, M.; Box, K. Chasing Equilibrium: Measuring the Intrinsic Solubility of Weak Acids and Bases. Anal. Chem. 2005, 77, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, D.; Bi, J.; Liu, X.; Lyu, Y.; Verkerk, R.; Dekker, M. Micelle Separation Conditions Based on Particle Size Strongly Affect Carotenoid Bioaccessibility Assessment from Juices after in Vitro Digestion. Food Res. Int. 2022, 151, 110891. [Google Scholar] [CrossRef] [PubMed]
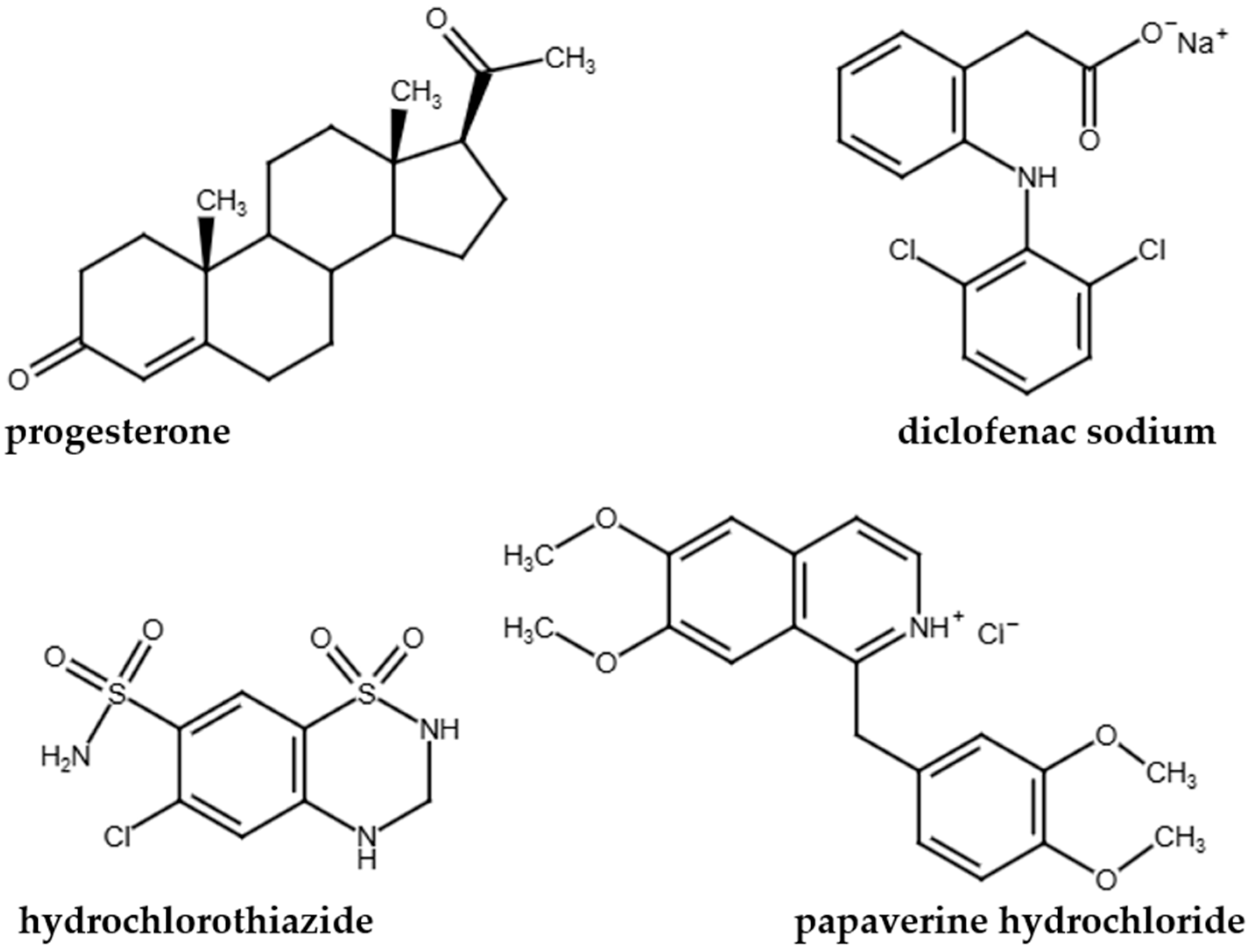
| pKa | logP | |
|---|---|---|
| PROG | - | 3.48 |
| DICL-NA | 3.99 | 4.51 |
| HCT | 8.75, 9.96 | −0.03 |
| PAP-HCL | 6.39 | 2.95 |
| pH | Solubility (µg/mL) | |
|---|---|---|
| PROG | 7.4 | 8.98 ± 0.09 |
| DICL-NA | 2.0 | 1.67 ± 0.05 |
| 4.0 | 2.72 ± 0.16 | |
| 10.0 | 9473 ± 327 | |
| HCT | 6.0 | 585 ± 19 |
| 8.8 | 892 ± 18 | |
| 11.0 | 57,362 ± 85 | |
| PAP-HCL | 3.0 | 40,397 ± 930 |
| 6.4 | 24.2 ± 1.1 | |
| 10.0 | 16.9 ± 1.7 |
| pH | S (µg/mL) | 5000 rpm | 10,000 rpm | |||||
|---|---|---|---|---|---|---|---|---|
| 5 | 10 | 20 | 5 | 10 | 20 | |||
| PROG | 7.4 | Ssed | 10.9 ± 0.09 * | 10.9 ± 0.09 * | 11.5 ± 0.06 * | 11.0 ± 0.12 * | 11.6 ± 0.11 * | 12.4 ± 0.06 * |
| Sstir | 11.0 ± 0.06 * | 11.3 ± 0.14 * | 12.8 ± 0.05 * | 11.0 ± 0.18 * | 11.7 ± 0.14 * | 13.3 ± 0.20 * | ||
| DICL-NA | 2.0 | Ssed | 1.61 ± 0.02 | 1.68 ± 0.04 | 1.67 ± 0.03 | 1.94 ± 0.01 | 1.87 ± 0.02 | 2.00 ± 0.03 |
| Sstir | 1.64 ± 0.04 | 1.52 ± 0.22 | 1.71 ± 0.07 | 1.82 ± 0.07 | 1.78 ± 0.07 | 2.00 ± 0.13 | ||
| 4.0 | Ssed | 2.67 ± 0.08 | 2.44 ± 0.12 | 2.49 ± 0.27 | 2.55 ± 0.06 | 2.68 ± 0.15 | 2.78 ± 0.13 | |
| Sstir | 2.70 ± 0.10 | 2.69 ± 0.24 | 2.58 ± 0.16 | 2.79 ± 0.20 | 2.80 ± 0.11 | 2.88 ± 0.18 | ||
| 10.0 | Ssed | 9304 ± 245 | 8724 ± 304 | 9164 ± 131 | 8048 ± 116 * | 8236 ± 297 | 8275 ± 187 | |
| Sstir | 8555 ± 161 | 9812 ± 318 | 10,678 ± 410 | 9655 ± 760 | 8597 ± 176 | 11,200 ± 251 * | ||
| HCT | 6.0 | Ssed | 601.80 ± 18.64 | 512.21 ± 5.14 * | 528.58 ± 7.18 | 502.08 ± 8.22 * | 704.99 ± 11.19 * | 723.59 ± 13.80 * |
| Sstir | 531 ± 12.3 | 521 ± 14.2 | 599 ± 6.4 | 571 ± 9.5 | 692 ± 35.6 * | 769 ± 26.5 * | ||
| 8.8 | Ssed | 803 ± 10.0 | 801 ± 20.7 | 1015 ± 70.2 | 890 ± 17.1 | 1019 ± 41.6 | 897.35 ± 28.2 | |
| Sstir | 845 ± 16.2 | 926 ± 60.8 | 1060 ± 36.9 * | 1021 ± 52.8 | 1048 ± 5.32 * | 1221 ± 36.9 * | ||
| 11.0 | Ssed | 49,333 ± 1778 | 51,165 ± 957 | 50,270 ± 1099 | 55,950 ± 980 | 51,790 ± 786 | 52,425 ± 1933 | |
| Sstir | 57,479 ± 658 | 53,479 ± 2079 | 55,100 ± 2240 | 53,783 ± 1808 | 51,700 ± 328 | 60,781 ± 3462 | ||
| PAP-HCL | 3.0 | Ssed | 37,200 ± 337 * | 37,980 ± 1090 | 37,070 ± 1396 * | 39,453 ± 300 | 40,863 ± 430 | 39,540 ± 561 |
| Sstir | 36,847 ± 382 * | 41,063 ± 922 | 38,398 ± 279 | 39,063 ± 197 | 40,360 ± 905 | 39,273 ± 777 | ||
| 6.4 | Ssed | 24.4 ± 0.48 | 28.2 ± 0.78 | 28.2 ± 0.73 | 24.4 ± 1.17 | 32.8 ± 0.81 * | 31.2 ± 1.08 * | |
| Sstir | 33.0 ± 2.11 * | 32.3 ± 1.54 * | 32.9 ± 1.26 * | 28.6 ± 1.55 | 37.8 ± 2.07 * | 39.1 ± 1.34 * | ||
| 10.0 | Ssed | 17.0 ± 0.86 | 14.3 ± 0.17 | 14.4 ± 0.38 | 16.3 ± 0.57 | 17.5 ± 0.18 | 18.7 ± 2.41 | |
| Sstir | 18.4 ± 0.28 | 13.3 ± 0.20 | 16.9 ± 0.79 | 17.8 ± 0.50 | 18.2 ± 1.12 | 28.7 ± 0.70 * | ||
| Sedimented | Not Sedimented | ||||||
|---|---|---|---|---|---|---|---|
| 5 min | 10 min | 20 min | 5 min | 10 min | 20 min | ||
| average | 5000 rpm | 7% | 11% | 12% | 10% | 12% | 13% |
| 10,000 rpm | 8% | 14% | 15% | 9% | 16% | 30% | |
| SEM | 5000 rpm | 0.03 | 0.04 | 0.05 | 0.05 | 0.05 | 0.05 |
| 10,000 rpm | 0.04 | 0.05 | 0.05 | 0.03 | 0.06 | 0.08 | |
| Filtration [16] | Centrifugation | ||||
|---|---|---|---|---|---|
| Closest to Reference (100%) | Result Range | Closest to Reference (100%) | Result Range | ||
| papaverine HCl | pH 10.0 | 95% | 62–95% | 100% | 78–170% |
| pH 6.4 | 95% | 42–106% | 101% | 101–162% | |
| pH 3.0 | 95% | 85–95% | 100% | 91–102% | |
| hydrochlorothiazide | pH 6.0 | 102% | 83–103% | 98% | 86–131% |
| pH 8.8 | 100% | 75–100% | 100% | 90–137% | |
| pH 11.0 | 100% | 65–119% | 100% | 86–106% | |
| diclofenac-Na | pH 2.0 | 93% | 0–164% | 100% | 90–120% |
| pH 4.0 | 103% | 0–103% | 99% | 90–106% | |
| pH 10.0 | 99% | 64–99% | 98% | 85–118% | |
| progesterone | pH 7.4 | 98% | 55–98% | 121% | 121–148% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szolláth, R.; Bárdos, V.; Stifter-Mursits, M.; Angi, R.; Mazák, K. Effects of Different Centrifugation Parameters on Equilibrium Solubility Measurements. Methods Protoc. 2025, 8, 116. https://doi.org/10.3390/mps8050116
Szolláth R, Bárdos V, Stifter-Mursits M, Angi R, Mazák K. Effects of Different Centrifugation Parameters on Equilibrium Solubility Measurements. Methods and Protocols. 2025; 8(5):116. https://doi.org/10.3390/mps8050116
Chicago/Turabian StyleSzolláth, Rita, Vivien Bárdos, Marcell Stifter-Mursits, Réka Angi, and Károly Mazák. 2025. "Effects of Different Centrifugation Parameters on Equilibrium Solubility Measurements" Methods and Protocols 8, no. 5: 116. https://doi.org/10.3390/mps8050116
APA StyleSzolláth, R., Bárdos, V., Stifter-Mursits, M., Angi, R., & Mazák, K. (2025). Effects of Different Centrifugation Parameters on Equilibrium Solubility Measurements. Methods and Protocols, 8(5), 116. https://doi.org/10.3390/mps8050116







